Abstract
Background
The Lake Louise Criteria (LLC) was established in 2009 and is the recommended cardiac magnetic resonance (CMR) imaging criterion for diagnosing patients with suspected myocarditis. Subsequently, newer parametric imaging techniques which can quantify T1, T2, and the extracellular volume (ECV) have been developed and may provide additional utility in the diagnosis of myocarditis. However, whether their diagnostic accuracy is superior to LLC remains unclear. In this meta-analysis, we compared the diagnostic performance of native T1, T2, ECV to LLC in diagnosing acute myocarditis.
Methods and Results
We searched PubMed for published studies of LLC, native T1, ECV, and T2 diagnostic criteria used to diagnose acute myocarditis. Seventeen studies were included, with a total of 867 myocarditis patients and 441 control subjects. Pooled sensitivity, specificity, and diagnostic odds ratio (DOR) of all diagnostic tests were assessed by bivariate analysis. LLC had a pooled sensitivity of 74%, specificity of 86% and DOR of 17.7. Native T1 had a significantly higher sensitivity than LLC (85% vs 74%, p = 0.025). Otherwise, there was no significant difference in sensitivity, specificity, and DOR when comparing LLC to native T1, T2, or ECV.
Conclusions
Native T1, T2, and ECV mapping provide comparable diagnostic performance to LLC. Although only native T1 had significantly better sensitivity than LLC, each technique offers distinct advantages for evaluating and characterizing myocarditis as compared to the LLC.
Keywords: CMR, Myocarditis, LLC, native T1, T2, ECV
Introduction
Myocarditis has a significant global impact with an estimated prevalence of 22 in 100,000 patients annually1. Specifically, myocarditis continues to be an important cause of sudden cardiac death and non-ischemic dilated cardiomyopathy (DCM). Data suggests that up to 20–40% of sudden cardiac death of young adults is due to myocarditis2,3. In addition, endomyocardial biopsy (EMB) has shown that 9% of DCM is attributed to myocarditis4. Nevertheless, myocarditis poses as a clinically challenging diagnosis due to its heterogenous manifestations. The Lake Louise criteria (LLC) is currently the recommended diagnostic cardiac magnetic resonance (CMR) imaging criteria for patients with suspected myocarditis5. LLC uses tissue-based CMR markers consisting of T2-weighted (T2w) ratio, early gadolinium enhancement (EGE), and late gadolinium enhancement (LGE). These parameters assess for myocardial edema, hyperemia/capillary leak, and fibrosis/necrosis respectively. Since the inception of LLC, quantitative imaging with T1 and T2 mapping have made significant advancements in assessing diffuse myocardial injury6–8. Novel techniques such as native T1 and T2 mapping or extracellular volume (ECV) calculations have been shown to provide additional diagnostic information in patients with myocarditis9–11. While some studies have shown quantitative mapping techniques are superior to LLC11–13, their performance across the literature remains unclear.
Methods
By email request, the data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Search Strategy and Selection
This meta-analysis was conducted according to standard guidelines from the Meta-analysis of Observational Studies in Epidemiology14, the Preferred Reporting Items for Systematic Reviews and Meta-analyses documents15, and the Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies16. We performed a systematic search for published studies evaluating LLC, native T1, T2, and ECV diagnostic criteria for acute myocarditis using PubMed (search last updated January 2018).
Key words used were “myocarditis” AND “lake” OR “louise” OR “mapping” OR “T1” OR “T2” OR “ECV” OR “MRI” OR “MR” OR “CMR”. Abstracts were independently reviewed and selected by two investigators (JAP and YL) based on the following eligibility criteria:
Pertaining to LLC, native T1 mapping, T2 mapping, or ECV
Investigating diagnosis of acute myocarditis in human adults
Complete analytic study in English
LLC was defined based on the combined use of EGE, T2w, and LGE with a positive result defined as having 2 of 3 positive criteria5. Studies were considered eligible for inclusion if the majority of patients had suspected acute viral myocarditis. Studies pertaining to chronic or autoimmune myocarditis were excluded. Only complete analytic studies published in peer-reviewed journals were included. Case reports, editorials, and reviews were excluded. Abstracts from meetings were excluded due to limited information regarding data.
Data Extraction
Data from each study was independently extracted by two investigators (JAP and YL). Studies were excluded if they contained: (a) overlapping subjects with other studies, (b) incomplete data, or (c) unconventional methods. Any disagreements were resolved by consensus or by consultation with a third reviewer (MS). In the case of overlapping studies, the included study was chosen based on quality of methodology, sample size, and year. Complete data consisted of sufficient information to calculate sensitivity, specificity and accuracy for acute myocarditis. Positive LLC had to be defined as showing evidence of myocarditis in two out of three criteria. Quantitative parametric tests were considered appropriate if it calculated a global mean and defined positive based on a cut-off for the global mean. Reference tests for myocarditis could be based on either clinical criteria or EMB.
Study Quality
The quality of included studies was assessed by two investigators (JAP and MS) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) instrument17. It consists of a list of 14 questions with closed-ended questions (yes, no, or unclear). The items included in this instrument covered patient spectrum, reference standard, disease progression bias, verification bias, review bias, clinical review bias, incorporation bias, test execution, study withdrawal, and indeterminate results. Publication bias was assessed by visual analysis of funnel plots and using the Peter's and Egger's methods18,19.
Statistical Analysis
Dichotomous variables are presented as percentages and continuous variables as mean ± standard deviation or median [interquartile range]. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) and their 95% confidence intervals were calculated with an exact method for binomial proportions using the F-distribution method20. Both univariate and bivariate pooling was performed. Univariate method was performed using MetaDiSc, version 1.4 freeware package (Universidad Complutense, Madrid, Spain). Pooled estimates of sensitivity, specificity, PPV, and NPV were determined by weighting the studies by their sample sizes21. Likelihood ratios (LR) and diagnostic odds ratios (DOR) were pooled using a random-effects model with the DerSimonanian-Laird method21. Heterogeneity between studies was assessed visually from Forest plots of the individual parameters and using the Cochran’s Q index and the inconsistency index (I2). Significant statistical heterogeneity was defined based on having both p[Cochran’s Q] < 0.05 and I2 > 50%. Bivariate analysis and comparison of pooled sensitivity, specificity, and DOR estimates between the diagnostic techniques (LLC, T1, T2, and ECV) was performed as described by Reitsma et al22 and Van Houwelingen et al23 using SAS/STAT software, version 9.4, of the SAS System for Windows (SAS Institute Inc., Cary, North Carolina). The bivariate approach tests for significance differences between imaging parameters while incorporating possible correlation between sensitivity and specificity. Statistical significance for hypothesis testing set at the α < 0.05, 2-tailed level. Bivariate analysis was not performed for PPV and NPV due to their dependence on disease prevalence and the lack of well-validated bivariate pooling methods. Meta-regression and sensitivity analyses (including exclusion of 1 study at a time) were conducted to explore heterogeneity.
Results
Search Results
Our literature search identified 806 relevant abstracts; of these, 33 abstracts were considered eligible for data extraction. Sixteen studies were excluded for overlapping patient cohorts, insufficient data, or unconventional methodology. Figure 1 shows the summary of our literature search. A total of 17 studies were included for analysis (Table 1). The sequences and cut-offs used in each study can be found in Supplemental Table 1.
Figure 1.
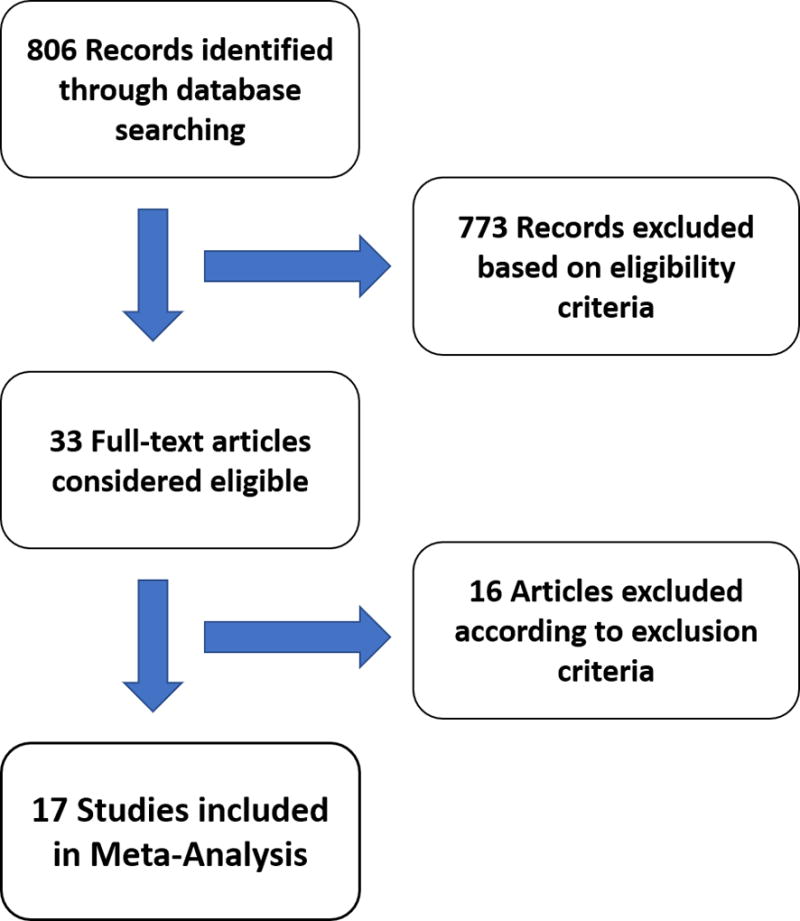
Flow diagram of the review process
Table 1.
Characteristics of Included Studies
| First Author | Year Published |
Subjects (n) |
Study Design | Validation | Parameters | Scanner | Vendor | Field Strength (Tesla) |
Interval from Admission to CMR (days) |
Interval from Onset to CMR (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baeßler12 | 2017 | 84 | Retrospective | Clinical | LLC, T2 | Achieva | Philips | 1.5 | n/a | 4.8 ± 4.4‡ |
| Galea24 | 2017 | 54 | Retrospective | EMB | LLC | Magnetom Avanto | Siemens | 1.5 | n/a | 9.5 ± 5.1‡ |
| Imbriaco25 | 2017 | 61 | Not Reported | Clinical | LLC | Gyroscan Intera | Philips | 1.5 | 6.8 ± 4‡ | n/a |
| Luetkens26 | 2017 | 83 | Prospective | Clinical | LLC | Ingenia | Philips | 1.5 | 2.7 ± 1.9‡ | n/a |
| Nadjiri27 | 2017 | 171 | Retrospective | Clinical* | LLC, ECV, T1 | Magnetom Avanto | Siemens | 1.5 | n/a | n/a |
| von Knobelsdorff-Brenkenhoff28 | 2017 | 36 | Prospective | Clinical | ECV, T1, T2 | Magnetom Avanto | Siemens | 1.5 | n/a | < 7 |
| Lurz11 | 2016 | 61 | Prospective | EMB | LLC, ECV, T1, T2 | Intera CV | Philips | 1.5 | < 1.5 | n/a |
| Luetkens13 | 2016 | 84 | Prospective | Clinical | LLC, ECV, T1, T2 | Ingenia | Philips | 1.5 | 2.6 ± 1.9‡ | n/a |
| Schwab29 | 2016 | 78 | Retrospective | Clinical | LLC | Intera CV | Philips | 1.5 | 1–17 | n/a |
| Hinojar30 | 2015 | 101 | Prospective | Clinical | T1 | Achieva | Philips | 1.5 & 3.0 | n/a | 2–8 |
| Bohnen9 | 2015 | 31 | Not Reported | EMB | T2 | Achieva | Philips | 1.5 | 3 [1–6]† | n/a |
| Radunski10 | 2014 | 125 | Not Reported | Clinical | LLC, ECV, T1, T2 | Achieva | Philips | 1.5 | n/a | 14 [7–49]† |
| Luetkens31 | 2014 | 66 | Prospective | Clinical | LLC, ECV, T1 | Ingenia | Philips | 3.0 | 2.6 ± 2.2‡ | n/a |
| Ferreira32 | 2014 | 110 | Prospective | Clinical | T1 | Avanto | Siemens | 1.5 | 3 [1–6]† | n/a |
| Lurz33 | 2012 | 70 | Prospective | EMB | LLC | Intera CV | Philips | 1.5 | n/a | 3 [1–7]† |
| Chu34 | 2012 | 45 | Not Reported | Clinical | LLC | Magnetom Avanto | Siemens | 1.5 | n/a | 7 ± 10‡ |
| Abdel-Aty35 | 2005 | 48 | Not Reported | EMB | LLC | Signa CV | GE | 1.5 | n/a | 5.6 ± 4.2‡ |
Expressed as median with interquartile range
Expressed as mean with standard deviation
Final diagnosis based on troponin > 10 times upper limit of normal
ECV = extracellular volume; EMB = endomyocardial biopsy; LLC = Lake Louise Criteria; n = number.
Clinical Characteristics
The 17 included studies had a total of 1308 subjects, of whom either had myocarditis (Table 2) or were part of the control group (Tables 3). The myocarditis group included 867 subjects with a sample-weighted mean age of 42 and 72% male. The control group included 441 subjects with a sample-weighted mean age of 39 and 67% male. The two groups had similar sample-weighted mean body mass index (26 vs 25 kg/m2 for myocarditis and control respectively) and heart rate (72 vs 67 bpm for myocarditis and control respectively). The myocarditis group had lower sample-weighted mean ejection fraction (54 vs 62% for myocarditis and control respectively).
Table 2.
Myocarditis Group Characteristics
| First Author | Subjects (n) |
Age (yrs) | Male (%) |
HR (bpm) |
BMI (kg/m2) |
LVEDVI (ml/m2) |
LVESVI (ml/m2) |
Troponin (T or I) |
Elevated Troponin (%) |
LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baeßler12 | 67 | 37 ± 14‡ | 73 | 65 | 25 | 84 | 33 | 3 (T) | 55 | 62 ± 7‡ |
| Galea24 | 34 | 41 ± 18‡ | 76 | n/a | n/a | 87 | 44 | n/a | 44 | 53 ± 12‡ |
| Imbriaco25 | 49 | 44 ± 16‡ | 75 | n/a | n/a | n/a | n/a | 242 (I) | 43 | 47 ± 15‡ |
| Luetkens26 | 48 | 44 ± 19‡ | 56 | 70 | 26 | n/a | 72 | 6.6 (I) | n/a | 55 ± 11‡ |
| Nadjiri27 | 153 | 47 ± 16‡ | 68 | n/a | n/a | n/a | n/a | n/a | 10 | n/a |
| von Knobelsdorff-Brenkenhoff28 | 18 | 25 [23–38]† | 78 | n/a | n/a | 90 | n/a | n/a | n/a | 60 [57–63]† |
| Lurz11 | 43 | 40 [29–56]† | 72 | n/a | n/a | n/a | n/a | 0.23 (T) | 77 | 48 [28–54]† |
| Luetkens13 | 34 | 45 ± 19‡ | 50 | 70 | 26 | n/a | 71 | 4.7 (I) | n/a | 56 ± 12‡ |
| Schwab29 | 43 | 35 ± 15‡ | 88 | n/a | 26 | 82 | 32 | n/a | 100 | 60 [54–66]† |
| Hinojar30 | 61 | 48 ± 17‡ | 60 | 70 | 26 | 94 | 51 | n/a | 95 | 70 ± 21‡ |
| Bohnen9 | 16 | 52 [37–62]† | 75 | 89 | 27 | 149 | 108 | 0.07 (T) | n/a | 31 [22–37]† |
| Radunski10 | 104 | 44 [33–58]† | 76 | 71 | 25 | 101 | 60 | 0.04 (T) | n/a | 42 [28–57]† |
| Luetkens31 | 24 | 35 ± 33‡ | 75 | 68 | 27 | 128 | n/a | 7.2 (I) | n/a | 60 ± 9‡ |
| Ferreira32 | 60 | 41 ± 16‡ | 75 | n/a | n/a | n/a | n/a | 4.5 (I) | n/a | 64 ±12‡ |
| Lurz33 | 53 | 44 ± 17‡ | 87 | n/a | n/a | n/a | n/a | n/a | 52 | 52 [31–62]† |
| Chu34 | 35 | 40 ± 17‡ | 77 | 68 | 26 | 102 | 49 | 1.1 (T) | n/a | 52 ± 11‡ |
| Abdel-Aty35 | 25 | 44 ± 17‡ | 72 | n/a | n/a | n/a | n/a | n/a | 92 | 57 ± 13‡ |
Expressed as median with interquartile range
Expressed as mean with standard deviation
Data included all patients with clinical suspicion of acute myocarditis regardless validation test.
BMI = body mass index, bpm = beats per minute; HR = heart rate; I = troponin I (ng/ml); LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end diastolic index; LVESVI = left ventricular end systolic index; n = number; T = troponin T (ng/ml); yrs = years.
Table 3.
Control Group Characteristics
| First Author | Subjects (n) |
Age (yrs) | Male (%) |
HR (bpm) |
BMI (kg/m2) |
LVEDVI (ml/m2) |
LVESVI (ml/m2) |
Troponin (T or I) |
Elevated Troponin (%) |
LVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Baeßler12 | 17 | 36 ± 12‡ | 65 | 63 | 24 | 81 | 29 | n/a | n/a | 65 ± 5‡ |
| Galea24 | 20 | 50 ± 15‡ | 65 | n/a | n/a | 78 | 38 | n/a | 65 | 52 ± 12‡ |
| Imbriaco25 | 12 | 47 ± 16‡ | 58 | n/a | n/a | n/a | n/a | 1 (I) | 25 | 54 ± 14‡ |
| Luetkens26 | 35 | 41 ± 17‡ | 66 | 66 | 25 | n/a | 73 | U (I) | n/a | 61 ± 3‡ |
| Nadjiri27 | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| von Knobelsdorff-Brenkenhoff28 | 18 | 27 [24–35]† | 78 | n/a | n/a | 90 | n/a | n/a | n/a | 61 [60–63]† |
| Lurz11 | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Luetkens13 | 50 | 39 ± 17‡ | 60 | 66 | 25 | n/a | 73 | U (I) | n/a | 61 ± 13‡ |
| Schwab29 | 35 | 35 ± 14‡ | 89 | n/a | 25 | 76 | 25 | n/a | 0 | 69 [58–81]† |
| Hinojar30 | 40 | 45 ± 15‡ | 53 | 68 | 25 | 74 | 30 | n/a | 0 | 61 ± 5‡ |
| Bohnen9 | 15 | 38 [28–63]† | 80 | 76 | 27 | 152 | 113 | 0.04 (T) | n/a | 25 [18–34]† |
| Radunski10 | 21 | 34 [28–47]† | 81 | 65 | 25 | 80 | 28 | 0.005 (T) | n/a | 59 [55–66]† |
| Luetkens31 | 42 | 39 ± 10‡ | 64 | 65 | 25 | 128 | n/a | U (I) | n/a | 63 ± 6‡ |
| Ferreira32 | 50 | 41 ± 13‡ | 74 | n/a | n/a | n/a | n/a | n/a | n/a | 72 ± 6‡ |
| Lurz33 | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Chu34 | 10 | 40 ± 16‡ | 50 | 66 | 24 | 82 | 32 | n/a | n/a | 61 ± 6‡ |
| Abdel-Aty35 | 23 | 29 ± 10‡ | 57 | n/a | n/a | n/a | n/a | n/a | n/a | 64 ± 5‡ |
Expressed as median with interquartile range
Expressed as mean with standard deviation
BMI = body mass index, bpm = beats per minute; HR = heart rate; I = troponin I (ng/ml); LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end diastolic index; LVESVI = left ventricular end systolic index; n = number; T = troponin T (ng/ml); U = Undetectable; yrs = years.
Diagnostic Performance
The univariate and bivariate meta-analysis results are included Table 4 and Table 5, respectively. The Forest plots of the univariate sensitivity and specificity estimates are presented in Figure 2. The bivariate comparison showed that native T1 had significantly higher sensitivity than LLC (85% vs 74%, p = 0.025). Otherwise, there was no significant difference in sensitivity, specificity, and DOR when comparing LLC to native T1, T2, or ECV. Native T1 had the highest point estimate DOR of 36.6 and a high point estimate specificity of 86%. T2 had a point estimate sensitivity of 76%, specificity of 82%, and DOR of 14.4. ECV had the lowest point estimate specificity of 76% and DOR of 10.5 with a moderate sensitivity of 77%.
Table 4.
Univariate Diagnostic Estimates
| Parameter | Studies | Subjects | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Positive LR | Negative LR | Diagnostic OR |
|---|---|---|---|---|---|---|---|---|---|
| LLC | 13 | 1022 | 75 [71–78] | 87 [84–90] | 88 [85–91] | 73 [69–76] | 6.2 [3.1–12.3] | 0.31 [0.25–0.39] | 24.0 [10.1–56.8] |
| ECV | 6 | 533 | 76 [70–81] | 76 [70–81] | 72 [66–77] | 79 [74–84] | 3.2 [2.6–4.1] | 0.32 [0.25–0.42] | 11.4 [6.6–19.7] |
| T1 | 8 | 694 | 83 [79–87] | 87 [83–90] | 86 [81–89] | 85 [81–88] | 6.2 [3.4–11.0] | 0.15 [0.07–0.32] | 44.1 [18.4 – 105.4] |
| T2 | 6 | 421 | 71 [65–76] | 84 [76–89] | 90 [85–93] | 58 [51–65] | 4.1 [2.4–7.0] | 0.29 [0.18–0.47] | 18.6 [10.0–34.5] |
Expressed as pooled estimate with 95% confidence interval.
ECV = extracellular volume; LLC = Lake Louise Criteria; LR = likelihood ratio; NPV = negative predictive value; OR = odds ratio; PPV = positive predictive value.
Table 5.
Bivariate Diagnostic Estimates
| Modality | Sensitivity | Specificity | Diagnostic OR |
|---|---|---|---|
| LLC | 74 [67–80] | 86 [77–92] | 17.7 [9.4–33.2] |
| ECV | 77 [66–85] | 76 [60–87] | 10.5 [4.6–23.6] |
| T1 | 85 [78–90]† | 86 [76–93] | 36.6 [17.1–78.5]‡ |
| T2 | 76 [65–84] | 82 [68–91] | 14.4 [6.1–34.2] |
Expressed as pooled estimate with 95% confidence interval.
p < 0.05 vs LLC
p < 0.05 vs ECV
ECV = extracellular volume; LLC = Lake Louise Criteria; OR = odds ratio.
Figure 2.
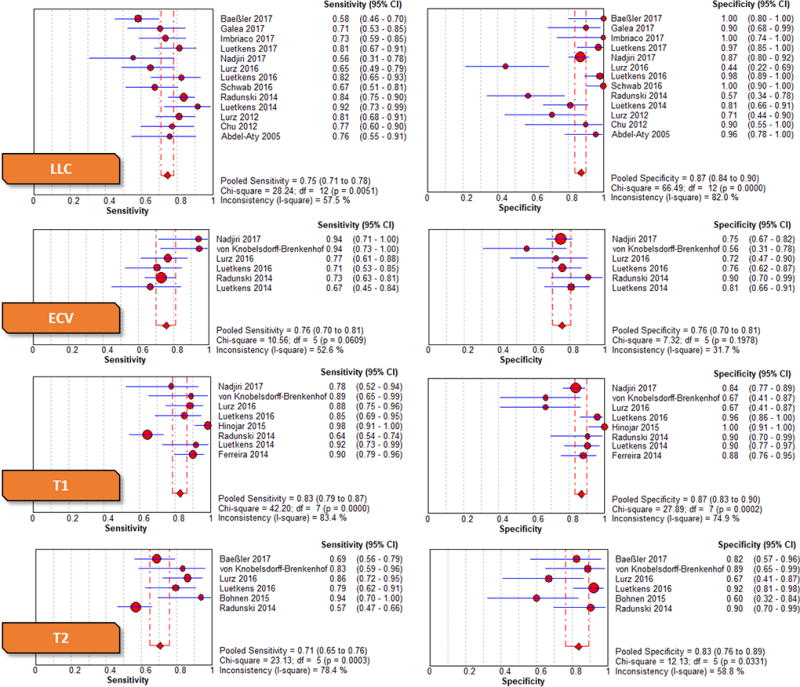
Forest plots with univariate pooled sensitivities and specificities across all imaging parameters
Significant heterogeneity was seen for LLC sensitivity (p[Cochran’s Q] < 0.05, I2 = 57.5%) and specificity (p[Cochran’s Q] < 0.05, I2 = 82.0%), T1 sensitivity (p[Cochran’s Q] < 0.05, I2 = 83.4%) and specificity (p[Cochran’s Q] < 0.05, I2 = 74.9%), and T2 sensitivity (p[Cochran’s Q] < 0.05, I2 = 78.4%) and specificity (p[Cochran’s Q] < 0.05, I2 = 58.8%). For the meta-regression, we used publication year, age, gender, and ejection fraction as the covariates and found no significant correlation with DOR for all imaging tests. Other clinical variables were not included due to insufficient reporting. Sensitivity analysis showed that exclusion of Radunski et al10 significantly reduced heterogeneity for T1 sensitivity (p[Cochran’s Q] = 0.11, I2 = 41.6%) and T2 sensitivity (p[Cochran’s Q] = 0.08, I2 = 51.5%). T1 sensitivity remained significantly higher than LLC after exclusion of Radunski et al10 (89% vs 74%, p < 0.001).
Quality and Bias Assessment
The selected studies had overall high-quality scores in all the 14 items of the QUADAS questionnaire (Supplemental Table 2). Egger’s test suggested presence of publication bias for LLC and ECV but Peter’s test did not demonstrate evidence of significant publication bias for any of the parameters.
Discussion
In this study we demonstrate that native T1, T2, and ECV mapping are comparable to LLC with only native T1 sensitivity being significantly better. Each quantitative imaging parameter may offer unique advantages depending on the clinical question. Utilization of a single parameter such as native T1 could potentially simplify the diagnostic criteria for assessing acute myocarditis.
Lake Louise Criteria
LLC was originally designed to detect different types of injuries that occur during myocarditis5. Beginning with an initial insult from either direct injury or activation of the innate immune system, myocardial inflammation leads to increased membrane permeability resulting in intracellular edema, hyperemia with capillary leakage, and eventually irreversible injury36. In the LLC criteria, LGE imaging provides an assessment of irreversible injury whereas EGE and T2w imaging provide an assessment of inflammation and edema. However, as EGE and T2w images may be prone to artifacts and misinterpretation, generalizing the application of the LLC criteria in routine clinical practice is challenging. When using univariate pooling of LLC components (Supplemental Table 3), LGE demonstrates the highest point estimate for diagnostic accuracy, and is the main driver of LLC performance, primarily due to its high specificity in patients with irreversible injury or necrosis5. The low sensitivity of LGE stems from its inability to identify subtle edema and reversible injury associated with early phases of inflammation33. Additionally, because gadolinium contrast agents can only assess the extracellular space, LGE cannot detect intracellular edema which is also thought to occur in early stages of myocarditis13. The sensitivity of LGE is similar to that of EGE and the T2w ratio, and the combination of these three parameters increases the sensitivity of the LLC as compared to LGE alone. Though EGE and T2w provide some incremental improvement in performance, they present with many technical challenges. Both are susceptible to artifact, especially from respiratory motion and arrhythmias5. EGE is also dependent on slice orientation and segment selection31. T2w often has low signal-to-noise ratios and regions of signal inhomogeneities that can obscure myocardial edema5. Finally, in patients with underlying skeletal myositis, T2w or EGE ratios can result in false negatives when they rely on reference signal intensities from skeletal muscle. In those cases, myocarditis must be assessed based on regional changes in T2w signal intensities or global elevations of absolute EGE signal intensities.
In the MyoRacer-Trial11 involving biventricular myocardial biopsies, LLC exhibited inferior diagnostic performance to native T1, ECV, and T2 mapping. However, it is unclear whether this holds true for other studies due to variations in methods and sample populations. In addition, many studies have opted to use only T2w and LGE for diagnosis of myocarditis as it has shown comparable accuracy30,32,34. In the study by von Knobelsdorff-Brenkenhoff et al28, they combined various parameters including native T1 mapping, T2 mapping, ECV, LGE, and T2w ratio. They found that the best combination was LGE and T2w ratio, with a diagnostic accuracy of 88.9%. The best combinations that incorporated quantitative parameters was LGE with native T1 or T2 mapping with native T1, which both had a diagnostic accuracy of 86.1%.
T2 Mapping
T2 mapping demonstrated reasonable diagnostic accuracy to other modalities. Primarily detecting free water content, T2 relaxations times are most elevated during the acute phase of myocarditis and gradually normalizes over months. This feature may be useful for staging and monitoring recovery28. In patients with symptoms lasting more than two weeks, T2 mapping is considered the only technique that adequately discriminates between myocarditis and non-inflammatory cardiomyopathies validated by EMB9,11. Other techniques such as ECV and native T1 are not specific enough to detect inflammation in patients with confounding fibrosis11.
Extracellular Volume Mapping
Commonly used as a surrogate marker for fibrosis, ECV can also detect extracellular expansion from sustained inflammation7. In fact, inflammation of the myocardium has been recently shown to confound the correlation between ECV and fibrosis37. In this study, ECV had the lowest point estimate for specificity and DOR. At best, studies have shown that ECV is comparable to LLC10,28. The main advantage of ECV as compared to LGE is its ability to assess diffuse fibrosis and inflammation beyond focal areas of fibrosis. ECV measurement is relatively insensitive to field strength, as compared to native T1. In addition, Radunski et al10 showed that ECV can be combined with LGE to improve diagnostic accuracy to 90%. Specifically, global ECV can improve the sensitivity by identifying diffuse myocardial injury in patients with negative LGE.
Native T1 Mapping
Native T1 had excellent diagnostic performance compared to the other parameters. Given that both edema and extracellular expansion contribute to T1 prolongation, native T1 mapping is capable of detecting myocarditis at various stages. Furthermore, unlike most gadolinium contrast imaging, native T1 is dependent on both intracellular and extracellular/interstitial factors38. During the acute phases of myocarditis in which edema is most prevalent, native T1 offers both excellent sensitivity and specificity when the optimal cut-off is chosen13,30,31. However, as the early inflammation subsides and subsequent fibrosis occurs, native T1 prolongation becomes less specific to myocarditis11,28. Therefore, native T1 struggles to discriminate between inflammatory and non-inflammatory etiologies in patients with chronic symptoms, especially given that many cardiac pathologies progress to diffuse fibrosis9,11. Additionally, there are a number of limitations with native T1 such as variation in sequences, different sensitivities to T2 effects, lack standardization and normal values, and partial dependence on heart rate. A recent SCMR consensus statement on CMR mapping of T1, T2, T2*, and ECV discusses these challenges and provides clinical recommendations for parametric mapping with CMR39. Currently, there is no consensus on optimal cut-offs for T1 mapping when diagnosing myocarditis, especially given that absolute T1 values depend on the CMR sequence and algorithm for T1 calculation40. Until a cut-off is determined, T1 mapping is probably best used either with site specific reference values, or when combined with incremental thresholding, providing similar visual information as LGE without the need for gadolinium41.
Limitations
The studies included in this meta-analysis had significant variability in duration of symptoms, severity of disease, and type of validation tests. The time from symptom onset/admission to CMR ranged from 1 to 49 days, which may impact the prevalence of edema and thus could affect test performance. In addition, some studies included patients in severe cardiac dysfunction with ejection fractions as low as 22%. Patients with such severe myocarditis or new-onset heart failure often reflect more subacute disease rather than acute myocarditis9,10. As a result, this subpopulation of myocarditis patients can present with less edema and more fibrosis, which can distort the diagnostic performance of parametric mapping. In particular, the study by Radunski et al10 contributed significant heterogeneity to our study with much lower native T1 and T2 sensitivities. Their patient population represented more subacute myocarditis with a median time from onset to CMR of 14 days, which could result in partial resolution of inflammation and edema. This likely explained why heterogeneity was reduced in the sensitivity analysis when this study was removed.
There are additional factors that influence the meta-analysis results. The type of validation test can dramatically affect the study designs. Clinical criteria based on patient history, abnormal biomarkers, and absence of other causes is useful for diagnosing myocarditis but provides no definitive pathological evidence of the presence of myocarditis. EMB continues to be the gold-standard reference test for interpreting the results of these CMR studies as they accurately correlate the physiological findings. Variability in cut-off values and field strengths can also influence diagnostic performance. Of the 3 parameters, native T1 is the most field strength dependent with normal myocardium having a native T1 that is roughly 200ms longer at 3T. Another limitation of this meta-analysis is that all studies were conducted using scanners from only two vendors, largely due to the availability of parametric mapping sequences, and the results may not be generalizable to other vendors. Additionally, our meta-analysis was conducted using PubMed and does not include studies that may be exclusively found in other databases such as EMBASE, Scopus, and The Cochrane Library.
Finally, we expected typical sources of bias such as small-study effects, decline effect, and early-extreme bias to influences our results42. Small-study effects, which we assessed using Egger’s and Peter’s test, was most likely the largest potential source of bias in our meta-analysis given the large number of single center studies. Regarding decline effect and early-extreme bias, meta-regression showed that publication year was not a significant covariate of diagnostic performance.
Conclusion
In diagnosing patients with acute myocarditis, native T1, T2, and ECV mapping were shown to be comparable to LLC. Native T1 had significantly better sensitivity than LLC. Our results suggest that incorporation of quantitative CMR parameters may improve accuracy, provide additional disease characterization, and help guide management. Furthermore, only needing to assess a single parameter such as native T1 could simplify the diagnosis of myocarditis as compared to using the LLC. Further research is needed to investigate the optimal combinations for assessing different presentations of myocarditis.
Supplementary Material
Clinical Perspective Summary.
Established nearly a decade ago, the Lake Louise Criteria (LLC) is the recommended cardiac magnetic resonance imaging criterion for diagnosing patients with suspected myocarditis. However, advances in quantitative imaging techniques for T1, T2, and extracellular volume (ECV) have demonstrated comparable diagnostic utility in myocarditis. In the present meta-analysis, we pooled 17 studies for a total of about 13,000 subjects to compare the diagnostic performance of native T1, T2, and ECV to LLC in identifying acute myocarditis. The principle finding is that only native T1 offered a significantly better sensitivity than LLC. Otherwise, there are no other significant differences in sensitivity and specificity between quantitative imaging modalities and LLC. This study validates our hypothesis that native T1, T2, and ECV mapping provide comparable diagnostic performance to LLC. This finding implies that clinicians should carefully consider both the technical and diagnostic advantages when selecting which modalities to include in the evaluation of myocarditis.
Acknowledgments
Sources of Funding
NIH R01
Disclosures
Dr. Salerno receives research support from Siemens Healthcare. Dr. Salerno has a research grant from Astra Zeneca and is a consultant to Locus Health and IBM Watson.
References
- 1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips M, Robinowitz M, Higgins JR, Boran KJ, Reed T, Virmani R. Sudden cardiac death in Air Force recruits: a 20-year review. JAMA. 1986;256:2696–2699. doi: 10.1001/jama.1986.03380190066026. [DOI] [PubMed] [Google Scholar]
- 3.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy: the Johns Hopkins experience with 1,278 patients. Medicine. 1999;78:270–283. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellman P, Wilson JR, Xue H, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, Part 1: evaluation of an automated method. J Cardiovasc Magn Reson. 2012;14:63. doi: 10.1186/1532-429X-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 9.Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging. 2015;8:e003073. doi: 10.1161/CIRCIMAGING.114.003073. [DOI] [PubMed] [Google Scholar]
- 10.Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC: Cardiovascular Imaging. 2014;7:667–675. doi: 10.1016/j.jcmg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel K, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol. 2016;67:1800–1811. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Baeßler B, Treutlein M, Schaarschmidt F, Stehning C, Schnackenburg B, Michels G, Maintz D, Bunck AC. A novel multiparametric imaging approach to acute myocarditis using T2-mapping and CMR feature tracking. J Cardiovasc Magn Reson. 2017;19:71. doi: 10.1186/s12968-017-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, Block W, Andrié R, Stehning C, Fimmers R, Gieseke J, Thomas DK, Schild HH, Naehle CP. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. European Heart Journal-Cardiovascular Imaging. 2015;17:154–161. doi: 10.1093/ehjci/jev246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao G, Lopez-Jimenez F, Boyd J, D'Amico F, Durant NH, Hlatky MA, Howard G, Kirley K, Masi C, Powell-Wiley TM, Solomonides AE, West CP, Wessel J American Heart Association Council on Lifestyle and Cardiometabolic Health, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Stroke Council. Methodological Standards for Meta-Analyses and Qualitative Systematic Reviews of Cardiac Prevention and Treatment Studies: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e172–e194. doi: 10.1161/CIR.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 17.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC medical research methodology. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 20.Blyth CR. Approximate binomial confidence limits. Journal of the American Statistical Association. 1986;81:843–855. doi: 10.2307/2289018. [DOI] [Google Scholar]
- 21.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC medical research methodology. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 24.Galea N, Francone M, Fiorelli A, Noce V, Giannetta E, Chimenti C, Frustaci A, Catalano C, Carbone I. Early myocardial gadolinium enhancement in patients with myocarditis: Validation of "Lake Louise consensus" criteria using a single bolus of 0.1mmol/Kg of a high relaxivity gadolinium-based contrast agent. Eur J Radiol. 2017;95:89–95. doi: 10.1016/j.ejrad.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Imbriaco M, Nappi C, Puglia M, De Giorgi M, Dell’Aversana S, Cuocolo R, Ponsiglione A, De Giorgi I, Polito MV, Klain M, Piscione F, Pace L, Cuocolo A. Assessment of acute myocarditis by cardiac magnetic resonance imaging: Comparison of qualitative and quantitative analysis methods. Journal of Nuclear Cardiology. 2017:1–9. doi: 10.1007/s12350-017-1109-3. [DOI] [PubMed] [Google Scholar]
- 26.Luetkens JA, Schlesinger-Irsch U, Kuetting DL, Dabir D, Homsi R, Doerner J, Schmeel FC, Fimmers R, Sprinkart AM, Naehle CP, Schild HH, Thomas D. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol. 2017;27:4661–4671. doi: 10.1007/s00330-017-4854-4. [DOI] [PubMed] [Google Scholar]
- 27.Nadjiri J, Nieberler H, Hendrich E, Greiser A, Will A, Martinoff S, Hadamitzky M. Performance of native and contrast-enhanced T1 mapping to detect myocardial damage in patients with suspected myocarditis: a head-to-head comparison of different cardiovascular magnetic resonance techniques. The international journal of cardiovascular imaging. 2017;33:539–547. doi: 10.1007/s10554-016-1029-3. [DOI] [PubMed] [Google Scholar]
- 28.von Knobelsdorff-Brenkenhoff F, Schuler J, Doganguzel S, Dieringer MA, Rudolph A, Greiser A, Kellman P, Schulz-Menger J. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2017;10:e005242. doi: 10.1161/CIRCIMAGING.116.005242. [DOI] [PubMed] [Google Scholar]
- 29.Schwab J, Rogg H, Pauschinger M, Fessele K, Bareiter T, Bär I, Loose R. Functional and morphological parameters with tissue characterization of cardiovascular magnetic imaging in clinically verified “infarct-like myocarditis”. Fortschr Röntgenstr. 2016;188:365–373. doi: 10.1055/s-0041-108200. [DOI] [PubMed] [Google Scholar]
- 30.Hinojar R, Foote L, Ucar EA, Jackson T, Jabbour A, Yu C, McCrohon J, Higgins DM, Carr-White G, Mayr M, Nagel E, Puntmann VO. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC: Cardiovascular Imaging. 2015;8:37–46. doi: 10.1016/j.jcmg.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, Fimmers R, Stehning C, Homsi R, Schwab JO, Schild HH, Naehle CP. Acute myocarditis: multiparametric cardiac MR imaging. Radiology. 2014;273:383–392. doi: 10.1148/radiol.14132540. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16:36. doi: 10.1186/1532-429X-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, Klingel K, Kandolf R, Schuler G, Gutberlet M, Thiele H. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC: Cardiovascular Imaging. 2012;5:513–524. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Chu GC, Flewitt JA, Mikami Y, Vermes E, Friedrich MG. Assessment of acute myocarditis by cardiovascular MR: diagnostic performance of shortened protocols. The international journal of cardiovascular imaging. 2013;29:1077–1083. doi: 10.1007/s10554-013-0189-7. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109 doi: 10.1161/01.CIR.0000127428.10985.C6. 2411-6-2416. [DOI] [PubMed] [Google Scholar]
- 36.Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circ Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 37.Lurz JA, Luecke C, Lang D, Besler C, Rommel KP, Klingel K, Kandolf R, Adams V, Schone K, Hindricks G, Schuler G, Linke A, Thiele H, Gutberlet M, Lurz P. CMR-Derived Extracellular Volume Fraction as a Marker for Myocardial Fibrosis: The Importance of Coexisting Myocardial Inflammation. JACC Cardiovasc Imaging. 2018;11:38–45. doi: 10.1016/j.jcmg.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19 doi: 10.1186/s12968-017-0389-8. 75-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–822. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira VM, Piechnik SK, Dall'armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14 doi: 10.1186/1532-429X-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanelli D, Costas R, Ioannidis JPA. Meta-assessment of bias in science. Proc Natl Acad Sci USA. 2017;114:3714–3719. doi: 10.1073/pnas.1618569114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


