Abstract
Human pain neuroimaging has exploded in the past 2 decades. During this time, the broader neuroimaging community has continued to investigate and refine methods. Another key to progress is exchange with clinicians and pain scientists working with other model systems and approaches. These collaborative efforts require that non-imagers be able to evaluate and assess the evidence provided in these reports. Likewise, new trainees must design rigorous and reliable pain imaging experiments. In this article we provide a guideline for designing, reading, evaluating, analyzing, and reporting results of a pain neuroimaging experiment, with a focus on functional and structural magnetic resonance imaging. We focus in particular on considerations that are unique to neuroimaging studies of pain in humans, including study design and analysis, inferences that can be drawn from these studies, and the strengths and limitations of the approach.
Perspective:
This article provides an overview of the concepts and considerations of structural and functional magnetic resonance neuroimaging studies. The primer is written for those who are not familiar with brain imaging. We review key concepts related to recruitment and study sample, experimental design, data analysis and data interpretation.
Keywords: Pain, magnetic resonance imaging, structural magnetic resonance imaging, functional magnetic resonance imaging, multivoxel pattern analysis, functional connectivity, guidelines
Understanding how pain is encoded in the brain has been a fundamental challenge for pain researchers. Despite the universality of acute pain and the high prevalence of chronic pain, we have yet to precisely characterize the mechanisms of pain perception and modulation in health and disease. The complexity of identifying these mechanisms stems from the multidimensional nature of pain—pain is a complex amalgam of sensory, affective, cognitive, and motor responses to dynamic internal and external states. The challenge of characterizing mechanisms increases as pain becomes chronic, with widespread plasticity of nociceptive and modulatory pathways contributing to the ongoing experience of pain. The rise of neuroimaging techniques offers the potential for breakthroughs in these efforts. Neuroimaging approaches (including functional magnetic resonance imaging [fMRI], positron emission tomography, electroencephalography, and other approaches) are extremely powerful tools that offer unique, noninvasive, in vivo views of central processes. Indeed, functional and structural neuroimaging studies have identified neural responses and features of acute,4,46,57,68,76,179,224,225 as well as chronic pain,3,33,57 and effects of pharmacological9,29,30,69,197,229 and nonpharmacological27,28,48,72,126,131,170,202,216,222,225,226,238 interventions.
Because of the promise of these new techniques, pain imaging is rapidly growing and will continue to expand as scanning facilities become more available and analysis software becomes increasingly user-friendly. Although brain imaging findings can provide important insight into central mechanisms, there are many aspects of study design and analysis that must be carefully considered and planned a priori to obtain a robust, reproducible result. Indeed, a recent systematic review of fMRI data showed that a data set could be processed through almost 7,000 unique pipelines, with almost 35,000 resulting maps.38 This highlights the importance for non-imagers and new trainees who read neuroimaging reports to be familiar with some of these considerations and how they may affect outcomes and inferences. In this article, we provide an overview of these considerations, and key questions that should be asked when reading an imaging report. We will not weigh in on the many exciting theoretical debates in the pain neuroimaging community, such as the specificity of pain-evoked cortical and subcortical responses or the feasibility of brain-based biomarkers for pain (see, for example Davis et al56). We instead provide a primer and guidelines to assist trainees and nonexperts in designing and reporting pain neuroimaging experiments as well as reading and evaluating articles.
Because of the brain’s key role in generating pain percepts, the ability to noninvasively examine brain function in vivo is critical. We focus specifically on functional and structural magnetic resonance imaging (MRI), because MRI is the most common tool used in human pain studies. Other promising human neuroimaging methods such as electroencephalography, magnetoencephalography, functional near-infrared spectroscopy, transcranial magnetic stimulation, positron emission tomography, and arterial spin labeling are beyond the scope of this review.
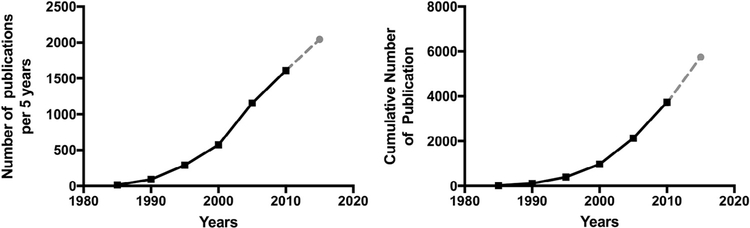
A cursory PubMed search with the search terms “(pain or nocicept*) AND brain AND MRI NOT review” on January 20, 2017 resulted in 4,895 studies (see Fig 1). A number of publications address methodological issues and statistical considerations associated with human neuroimaging in general,39,144,146,147,168,172,180,184 and we encourage neuroimagers to consult these reviews for additional guidelines, and more in-depth discussion of some of the technical considerations we delineate herein. Our aim is to address how these issues may specifically affect pain research. Our goal is to provide an introduction of particular use to a novice audience, including new trainees, clinicians, and/or non-imagers interested in evaluating studies on the neuroimaging of pain. We believe that awareness of methodological and inferential limitations can lead to positive advances. We focus on the elements that should be included in the Methods and Results sections of any report, and address inferences that may be drawn during the Discussion.
Figure 1.
Number of pain imaging studies since the advent of fMRI. These numbers were determined with a search on PubMed using the search terms: “(Pain or nocicept*) AND brain AND (fMRI OR MRI) NOT CT NOT PET NOT EEG NOT review.” The search was restricted to 5-year time windows. The left panel shows the number of publications per 5-year window. The panel on the right shows the cumulative number of studies over the years. The dashed line and the grey point have been extrapolated on the basis of the current number of studies published from 2015 to January 2017.
Recruitment and Sample
Sample Characteristics
After deciding on a research question, the first aspect any researcher considers is his or her research population and sample. It is important to acknowledge that neuroimaging experiments require unique constraints on enrollment, and many of these constraints might put a particular burden on neuroimaging studies of patient samples.
MRI scanners are large magnets with a narrow bore in which the participant lies during scanning. The participant’s brain is scanned with specialized coils embedded in a small head cage, or head coil. This provides several constraints that are rarely acknowledged but can substantially affect a study. Subjects must fit in the narrow bore, and therefore MRI studies are likely to exclude obese patients, despite the fact that obese individuals are more likely to report severe pain than normal and underweight counterparts.109 Because the MRI scanner uses radio waves, participants cannot have any ferromagnetic metal in their body, because there is a risk of the main magnetic field pulling on the metal, especially as the subject enters the bore of the scanner. Radio waves can also cause heating in tissues, and this can be exacerbated by any electric conducting materials including cables and wires. Furthermore, metal in the head and neck region can cause image quality issues, because these reflect the radio waves. These factors provide severe constraints on researchers interested in studying topics such as phantom pain in amputees or pain in individuals after certain surgical procedures, because these patients may have metallic (and possibly ferromagnetic) objects in their body that are not safe in a research MRI scanner. Studies of pain in other populations, such as the elderly, or patients with cardiac pain, may also be limited because of other types of incompatible implants or devices, including pacemakers or medication pumps. Finally, some patients may experience claustrophobia because of the narrow bore or confining coil surrounding their head, and so patients with comorbid anxiety disorders may be less likely to complete these experiments. These constraints mean that such studies might routinely exclude the most severe cases, which must be considered when drawing general inferences about pain populations. Studies must always measure and report complete characteristics of the sample as well as inclusion and exclusion criteria.
Because of the constraints on the participants that can be recruited for an imaging study, it is often tempting to recruit a convenience sample—usually healthy young adults from the university environment. However, such a sample is not representative of those disproportionately affected by chronic pain: women between the ages of 30 and 50 of lower socioeconomic position.205 Nonetheless, many neuroimaging experiments are not designed to isolate mechanisms of chronic pain, and may instead be interested in more general neural mechanisms, such as those involved in the psychological modulation of pain, or basic processes of nociception and acute pain. Convenience samples may be appropriate in these cases, because they allow investigators to isolate basic mechanisms underlying pain perception in healthy individuals. However, even studies of acute pain in healthy volunteers should carefully consider generalizability to the larger population, because convenience samples may lack diversity in age, educational background, socioeconomic status, race and ethnicity, depending on the study, and each of these factors has been shown to influence pain.36,66,125 All experiments should report full sample characteristics so that readers and reviewers can evaluate generalizability to the population.
Many experiments compare patients with pain disorders with matched control patients and compare results across groups. If healthy controls are not selected to match nonpain-related characteristics, there is a strong chance that differential findings between groups will reflect processes other than the pain disorder of interest (eg, group differences because of higher incidence of mood disorders, obesity, and comorbid chronic pains).215,218 It is strongly recommended that such matching be done by carefully selecting the control population, rather than attempting to find “clean” pain patients (eg, those free of comorbid psychopathology). Although capturing processes related to comorbid disorders complicates interpretation, and can lead to confounds, individuals without such comorbidities may not be representative of most pain patients. Potential confounds and comorbidities should be carefully considered, and if they are unavoidable, accounted for with appropriate experimental designs and statistics. Researchers must also carefully consider differential artifacts related but not germane to clinical presentation. For example, recent work indicates that resting state fMRI (rs-fMRI) studies are highly susceptible to even small motion artifacts, such that a group difference could emerge if there was a systematic difference between the groups in movement187,203 (as discussed in the section, Preprocessing). Factors such as discomfort from lying in the scanner, comorbid movement disorders, or psychiatric disorders such as anxiety might be more likely in pain populations and lead to greater motion in patient groups, resulting in differential between group effects related to movement rather than the measure of interest.203 Thus, head motion and potential group differences in motion must be carefully considered and quantified in rs-fMRI as well as taskbased fMRI studies, and data cleaning strategies should be carefully used to mitigate the contributions of such factors.203
Statistical Power and Sample Size
All studies must ensure adequate sample size and statistical power to reliably detect meaningful effects, and neuroimaging experiments are not unique in this regard. Neuroimaging studies are expensive to run, generally requiring hundreds or even thousands of dollars in scanning fees for each subject. It is therefore common for sample sizes to be smaller in neuroimaging studies than other types of experiments. Although small samples are common because of the financial burdens and additional constraints on neuroimaging studies, this has led to the publication of many studies that are likely to be underpowered for effects that might be of greatest clinical interest, such as individual differences in pain populations. Underpowered studies are less likely to detect true effects and more likely to find false positive results by overfitting data, thereby decreasing the likelihood that findings will replicate.34 Studies of chronic pain populations might be particularly susceptible to such effects because of the breadth of diagnostic categories (meaning that patients with similar diagnoses but divergent symptom profiles might be included in the same small sample; eg, fibromyalgia, which is commonly a diagnosis of exclusion,79 where symptom profiles can vary greatly) and heterogeneity in terms of comorbid disorders and medication use.
Because of the potential for false positive results in underpowered imaging studies, studies with small samples are coming under increased scrutiny. The onus will increasingly be placed on researchers to show that samples are adequately powered to detect expected effects. Such demonstrations must be on the basis of justifiable, a priori calculations, so researchers should always estimate power before conducting an fMRI study, and report how the sample size was determined. Statisticians have recently introduced several new, easily accessible fMRIspecific power analyses that make it easier to consider desired effect and sample sizes (eg, fmriPower.org,167 neuropowertools.org71) and will help to justify the funding of fully powered studies. These approaches can be used a priori to estimate sample size on the basis of expected effect size. Importantly, a number of these approaches require researchers to have preexisting imaging data, which is not always possible for new researchers or those using new paradigms. Power calculation on the basis of behavioral effect sizes may also be useful because these do not rely on previous imaging studies. These can be computed using data from pilot studies completed outside of the fMRI environment, which would substantially reduce costs.
The problem of underpowered studies can also be addressed by aggregating data via repositories and/or metaanalyses. Repositories facilitate data-sharing across imaging experiments. Some repositories host data from many different tasks, scanners, and populations. These repositories facilitate reproducibility, open science, and large-scale analyses that are relatively impervious to noise created by inconsistencies across individual experiments. Other repositories require contributors to collect brain images with standardized scanning protocols. These scans then undergo standardized preprocessing and analysis pipelines, allowing several groups with limited resources to pool brain imaging data and investigate larger cohorts of patients. Several pain imaging repositories (OpenPain [http://openpain.org]; Pain and Interoceptive Imaging Network [https://www.painrepository.org]136) have been established, and have proven to be successful. However, there are legal and ethical considerations that may affect a group’s ability to contribute to such repositories.
Finally, meta-analyses statistically test the distribution of findings across studies, which permits assessments of the consistency of effects and overcomes some of the limitations associated with small individual studies. These studies allow for a principled approach to determining the relationship between a particular brain region and behavior. Several pain imaging meta-analyses have been published, including meta-analyses of pain-evoked cortical responses,68,179 placebo analgesia,1,8 automated metaanalyses of brain activation associated with the term “pain,”234 and meta-analyses of brain responses and structural brain abnormalities in patients with chronic pain.48,80,143
Experimental Design
Consider the Context: Constraints of the MRI Environment
Neuroimaging experiments (especially MRI) take place in a unique environment, so studies must be carefully designed and made suitable for the imaging suite. As mentioned previously, this restrains the patient populations that can be studied. It also places substantial constraints on the types of experiments pain imagers can conduct. All equipment must be MRI-compatible and suited to the unique scanner environment. There are few commercially available MRI-compatible devices capable of delivering nociceptive stimuli, because these must be completely nonferromagnetic and must not introduce electrical noise during data collection. Even commercially available devices may have differential success at different scanners depending on considerations such as MRI field strength, sequence design, and even bore size. Thus, many pain researchers have examined brain responses associated with acute thermal and electrical pain, but few have examined cold pain, cold allodynia, or chemical pain. During the experiment, painful stimuli are usually controlled by simple computer tasks that coordinate stimulus presentation and synchronize timing with the MRI scan (see the section, Materials and Procedures). Experimenters often use these programs to present visual stimuli (eg, task instructions, cues) and to measure responses (eg, pain ratings). Visual stimuli are usually presented on a computer screen that patients view through goggles or via a mirror that sits atop the head coil and reflects images displayed on a projector screen. Participants provide pain ratings and make other responses using devices such as button boxes, joysticks, mouse-like trackballs, or by moving their hands5 because verbal responses are generally avoided because of the loud noises produced by the scanner and because head motion must be minimized. Thus, fMRI studies of pain may not capture the social and interpersonal elements that are likely to contribute to verbal pain ratings when a patient informs her doctor about her pain. This can be seen as a strength (eg, for researchers who investigate ascending nociceptive pathways and want to minimize social modulatory influences)60,140,141,211 or a weakness (eg, patient-provider interactions are thought to be a critical component of placebo analgesia,65,77,119,121 and few computer paradigms are able to capture these processes).
Head motion provides a third unique constraint afforded by the MRI scanner context. Participants cannot move their heads more than a few millimeters (typically <2 mm) during scanning, for fear of contaminating the data (see the section, Analysis). Some individuals, such as those with lower back pain, may not be able to lie still without pain, which provides additional constraints on patient samples and eligibility. In addition, painful stimuli that induce strong withdrawal responses such as startle and electric shock cause unique challenges because of task-related motion if not carefully mitigated37,237 (see the section, Preprocessing, for discussion of motion correction). Many researchers choose to familiarize subjects with the stimuli to minimize startle and other withdrawal-related behaviors, as well as to ensure stimuli are tolerable for the participants. This of course imposes limitations on the intensity of pain or novelty of the stimuli that can actually be administered in the scanner. Furthermore, it is unclear to what degree neural responses might reflect regulation of the prepotent withdrawal response even when the participant is able to voluntarily suppress motion. All of these considerations must be weighed in terms of the construct validity of painful stimuli presented within the scanning environment.
Another key consideration is the scanning parameters and sequences. Most sites will have “out of the box” sequences, but these should be selected with consideration, to best optimize the sequence to maximize signal from regions of interest. Although the technical aspects of these decisions are outside the scope of this review, it should be recognized that these considerations must be tailored to experimental hypotheses. We therefore recommend that researchers work closely with magnetic resonance physicists and experts to ensure that the selected sequences are ideally suited to the temporal resolution of their effect of interest and the particular anatomical region(s) of interest. For example, some brain stem structures might require multiple acquisitions of different contrasts for precise anatomical localization. Additionally, protocols might be adjusted for experimental efficiency. For example, a standard diffusion-weighted study to investigate white matter in the brain might acquire 64 diffusion-encoding directions, with b-shell of 1,000 s/mm2. However, this acquisition is more than might be needed for a standard fractional anisotropy (FA) map, which requires a minimum of 6 diffusion-encoding directions,17,18,165 resulting in an unnecessary cost and participant burden. These studyspecific considerations might also be balanced against the homogeneity requirements of data-sharing repositories, although collaborative and multisite studies also exist (eg, the Multi-Disciplinary Approach to the Study of Chronic Pelvic Pain45,138), which may permit more heterogeneity.
Materials and Procedures
Authors should outline everything that participants did from the start of the study until they finished. A study begins when a participant provides informed consent, as approved by a local institutional review board, which evaluates the ethics of the study. Informed consent and ethics approval should be acknowledged in any study, and authors should include important details, for example whether authorized deception was used in any studies that include misleading information (eg, studies of placebo analgesia), or whether patients were asked to refrain from taking their prescribed medication. In some cases, understanding the relative rate of participation is important and studies should report the number of people contacted for recruitment. Were subjects debriefed at the end of the study? For studies of individual differences and/or clinical severity, which questionnaires were administered (not just the ones relevant to the current report)? Similarly, were any tasks or relevant procedures administered outside of the main experiment that are not analyzed in the report? This is important information because additional tasks and measures might influence behavior in the main paradigm, and reviewers and readers should be able to evaluate potential confounds.
The description of task design should include all details necessary for the purpose of external replication, including the instructions, counterbalancing schemes, and task timing. What platform was used for experimental programming, and how did subjects provide responses? If decisions about task design were made on the basis of earlier pilot testing, it can be useful to report these details (eg, “We collected pain ratings 20 seconds after heat offset. Pilot testing revealed that there was no difference between ratings made immediately after offset vs after a delay. We chose the current design so as to reduce contamination of the blood-oxygen-level dependent (BOLD) response to heat”). It is also important to provide the exact instructions provided, as well as a description of the scales used to assess painful percepts, and other features of the stimulus. What were the exact instructions? What was the resolution of the scale? What were the anchors to the scale?
Some pain studies use nociceptive stimuli to elicit pain in healthy subjects and/or in chronic pain patients. Researchers can choose between several nociceptive stimulation paradigms. Thermal pain is the most well established and most common stimulus used in neuroimaging.68 This is likely because the paradigm works well in the scanner and is relatively convenient. However, it is a poor model of chronic pain, because heat pain is qualitatively dissimilar to pain experienced in most chronic pain conditions. Therefore, the researcher must ensure that inferences are appropriate. It should not be taken for granted, for instance, that acute heat pain is a valid model for chronic pain that is neuropathic or musculoskeletal in nature. Other acute pain models such as ischemia, muscular hypertonic saline injections, bladder filling, and visceral/rectal distention might be more appropriate for questions about the neural bases of musculoskeletal or visceral chronic pain disorders.6,55,96,132,135,177,178,214 However, not all pain experiments seek to model chronic pain; some focus on understanding neural processes associated with acute pain and its modulation. In this case, different considerations guide the evaluation of validity. Are experimental manipulations appropriate with regard to the psychological construct the researchers are testing? Is the chosen noxious stimulus appropriate for the questions of interest (eg, does thermal vs electrical vs laser pain activate different ascending fibers)?
Questionnaires are also often an important component to a study. These can serve as screening tools against exclusion criteria (eg, handedness, other neurological disorders, and MRI contraindications, such as claustrophobia), to characterize a chronic pain population (eg, the McGill Pain Questionnaire158,159), or as a measure or covariate of interest. When used judiciously, such measures can help strengthen inferences about the mental processes that particular patterns of activation might be subserving. However, each added questionnaire inflates the number of statistical tests and increases the chance for false positive results, if not carefully taken into account through multiple comparisons correction. As discussed in the section, GroupLevel Analyses, the issue of type I error is particularly germane to neuroimaging studies, and increases patient burden in terms of time spent on the study. Thus, the type and number of questionnaires included should be carefully considered on the basis of clear hypotheses. Questionnaires expected to have large amounts of overlapping variance (eg, the Fear of Pain Questionnaire157 and the Pain Catastrophizing Scale210) can increase the chance of incidental findings with little additional explanatory power added to the study.
There are 3 different approaches that can be used to investigate neuroimaging data: hypothesis-driven, exploratory, and reproduction/replication. A typical neuroimaging study uses these respective approaches to test a hypothesis, develop new hypotheses, and confirm (or reproduce) the findings. Hypotheses should be selected a priori, before any data are collected, on the basis of theory and previous data. When a hypothesis is generated, a pilot study is usually performed on a small sample size to ensure that the experimental paradigm is valid (ie, to ensure that the hypothesis is appropriately operationalized), and to determine the feasibility of the study. Small details may be adjusted at this time (eg, instructions, task timing, etc). Pilot studies are rarely submitted for publication, because they often use liberal statistical thresholds (because of low power). They allow researchers to adjust the paradigm before fMRI scanning, because scan time is costly and one wants to make sure the task is clear so that scanning can proceed uninterrupted. Pilot studies can also be used to estimate effect size, although it is known that small samples can overestimate effect size.34 Then, an independent sample (whose size has been determined using a power calculation) consisting entirely of new subjects should be acquired to formally test the hypothesis. Preregistration allows researchers and reviewers to distinguish between a priori, hypothesis-driven analyses and post hoc exploratory analyses, which are intended to generate new hypotheses. Several preregistration sites now exist (eg, Open Science Framework https://osf.io/registries/176), and several neuroscience and psychology journals now allow researchers to conduct preregistered reports (https://cos.io/rr/). We hope that interest in preregistration will grow within the pain community. We recognize that because of an emphasis on novelty among publication and funding outlets, the choice to follow a path from exploratory to replication studies is generally not in the hands of young or even senior investigators. However, because of the paucity of replication studies in fMRI, and its reliability being called into question,23,54 we join others in advocating for a funding and publication climate in which confirmatory neuroimaging studies are encouraged.11,164
Analysis
In this section we provide an overview of the analyses that should appear in a typical imaging report and provide some guidance about how choices might affect pain studies. We focus primarily on the Results section of a typical fMRI-BOLD investigation, because these are the most prevalent type of studies, although we acknowledge structural MRI studies where relevant. Other modalities may have different analysis steps, and specific literature should be consulted. Furthermore, trainees should refer to more comprehensive articles on how to analyze and report neuroimaging studies.173,184
Preprocessing
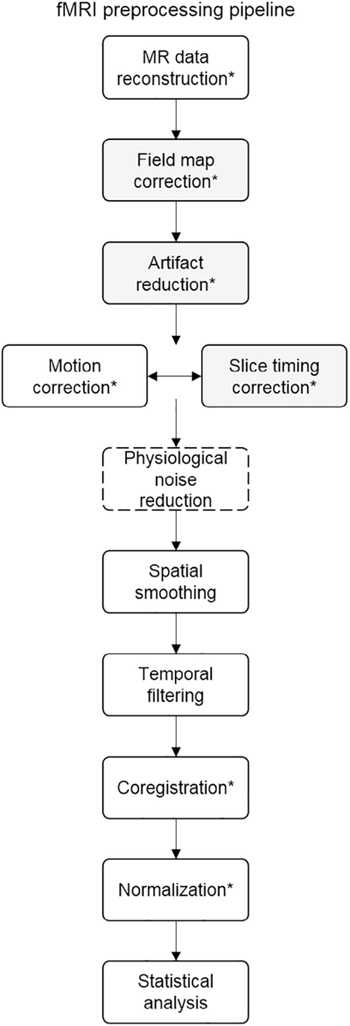
When data are collected, several steps are necessary to transform the data into the proper multidimensional format that can undergo statistical analysis. These transformations are grouped together and generally referred to as preprocessing. A summary of these steps is provided in Fig 3. The fMRI pipeline usually includes steps to: 1) remove the first set of volumes acquired (usually between 5 and 10), because it takes some time for the magnetic field to reach a steady state; 2) correct for the fact that fMRI data are collected in slices, and slice time acquisition differs within each time point (“slice time correction”); 3) test whether the head moved at any time point, which can induce artifacts, and correct for any head motion so that analyses capture to the same brain region over time (“motion correction”; the interested reader is referred to other sources for in-depth discussion of the problem with motion in MRI, and reviews of approaches to motion correction94,95,106,185,236); 4) remove high- or lowfrequency noise that can contaminate the signal or lead to spurious results (“temporal filtering”); 5) register the subject’s functional images with structural images (because the images are collected separately in time and head displacement might occur; “coregistration”); and 6) spatially transform the individual’s images to a standard template brain (eg, Montreal Neurological Institute template, Talaraich-Tournoux atlas, a group mean) with a specific stereotaxic space so that analyses are possible across individuals, who vary in neuroanatomy (“normalization”). The normalization step is also important for spatial specificity when reporting results of an fMRI study, and allows findings to be interpreted by other researchers and be included in subsequent meta-analyses. Several additional optional steps might occur at this stage (eg, correction for artifacts, spatial smoothing, etc), but details of these individual procedures are outside the scope of the current review.83,112,185
Figure 3.
fMRI preprocessing pipeline. This flow chart represents 1 common fMRI preprocessing pipeline. The inclusion/ exclusion of each step, as well as the order of steps, may vary on the basis of a study’s unique goals and analysis plan. Optional steps are shaded in gray, the step specific to rs-fMRI (but optional in task-based studies) has a dashed outline. Abbreviation: MR, magnetic resonance. *Steps requiring quality check. Adapted from: Poldrack et al.185
Preprocessing structural MRI (sMRI) data for gray matter analysis largely comprises: 1) removing scanner-induced noise from the structural T1-weighted brain images, and 2) segmenting tissues on the basis of image contrast, and/ or model the tissue of interest. Finally, the images are transformed and registered to a stereotaxic coordinate space for analysis. For example, for a gray matter analysis the B1 field is calculated to remove signal inhomogeneities. This allows for better tissue classification, and better estimates of gray matter volume or cortical thickness. For diffusion-weighted scans, which are used for white matter analysis, susceptibility artifacts, such as eddy currents induced by gradients coils, are corrected using various algorithms. Next a diffusion model (whether the tensor model to calculate fractional anisotropy, or a tractographic model) is calculated and applied to the data.
It is important to note that many of the algorithms used for these specific steps may vary as a function of software package (ie, SPM [http://www.fil.ion.ucl.ac.uk/spm/], vs AFNI [https://afni.nimh.nih.gov/] vs FSL [https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/]), as well as the fact that individual programs exist to optimize each of these steps. We highly recommend that new researchers/trainees carefully investigate the specifics and defaults of the analysis software. Default assumptions are not always transparent, and may not always suit your individual needs. For example, if a researcher is interested in effects in small regions like the periaqueductal gray or the hippocampus, he or she might want to minimally spatially smooth the data (which blurs the boundaries of image units [3-D pixels, or “voxels”]), rather than use the default values within a software package. Large smoothing kernels are suitable for analyses of cortical regions, but not for smaller, more discrete brain regions.193 As another example, the default settings of SPM (http://www.fil.ion.ucl.ac.uk/spm/) can restrict statistical analyses to regions where the calculated signal is greater than a set threshold. This can particularly affect voxels that are prone to signal loss from susceptibility artifacts in regions with tissue-air boundaries,43 such as the inferior temporal lobe and the orbitofrontal cortex and other ventral brain areas, which are of interest in many pain studies. These artifacts can be reduced through informed decisions about acquisition parameters.63,92,188,207,223 Neuroimagers benefit from developing expertise across multiple analysis packages and making informed decisions about which approach to use for a given analysis. Imagers should also develop practices that involve visualizing data at each stage of analysis, which can help to identify such issues as substantial dropout, missing data, and poor normalization or coregistration, among other important steps. Of course, when researchers elect to apply specific approaches, they should report their decision process and the reason that they opted for a given technique. The following 2 sections describe analysis considerations unique to taskbased fMRI and rs-fMRI, respectively.
Subject-Level Task-Based fMRI Statistical Analyses
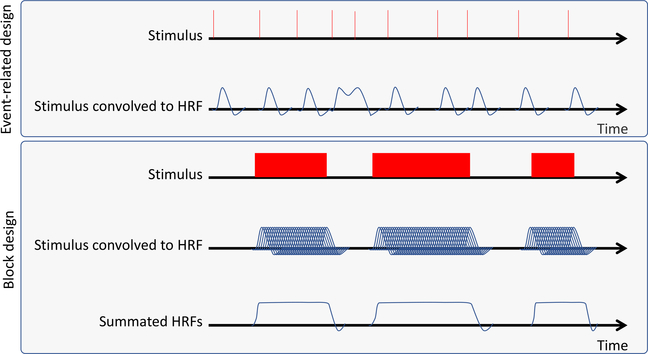
When fMRI data have been preprocessed, they can be analyzed in relation to the tasks that were administered during the fMRI session. The simplest way to think of the most common fMRI subject-level analysis approach, referred to as the general linear model (GLM), is that we test the correlation between each voxel’s activity and the events that occurred during the experiment. To accomplish this, the researcher must carefully track the timing of all the events in scanning sessions relative to the start of scanning, including the stimuli delivered (eg, noxious stimuli, images) and any responses collected. It is also important to record responses required for the analysis (eg, pain ratings). A model representing the onset and duration of the various stimuli, a time course of the scan, is constructed (Fig 2). This stimulus time course will then be transformed to represent an ideal response to a given stimulus by combining the moments the stimulus was presented with a wave-like function that captures the biological delay in the BOLD response, referred to as a hemodynamic response function, or HRF. There are several options for HRF models, and these require an a priori understanding of how hemodynamics vary on the basis of the population studied, stimulus type, and the brain region of interest (ROI). For example, HRF in the brainstem, an important region when studying descending modulation of pain, may differ from those in the cortex,99,127 and the HRF shape and timings are different between young adults and elderly individuals.64 When the events are combined, or “convolved” with the HRF, this generates an example time course of what the BOLD response would look like if activity within a voxel activation increased every time this stimulus was delivered (Fig 2). Different software packages model the HRF differently, and a researcher should understand how these differences can affect their results.
Figure 2.
Stimulus convolution. Here, a hypothetical pattern of stimuli is convolved with the HRF. In the upper panel, an eventrelated design is convolved to a double-gamma function to model the HRF. In the lower panel, the convolution of a block design study is shown. A prolonged stimulus would, in theory, elicit repeated HRFs. These are summated to produce a regressor to produce an idealized model of brain activity correlated to the stimulus. Note that the peak of the HRF is delayed with respect to the onset of the stimulus, and the offshoot is delayed with respect to the offset of the stimulus.
The researcher can simultaneously model several different stimulus types or conditions at this stage, and can test whether the magnitude of the response varies as a function of another variable (eg, whether responses to a noxious stimulus are larger in the trials when the participant rated the stimulus as more painful). This is called a “parametric analysis.”120 The different independent variables, or regressors, included in the GLM depend critically on the a priori design of the task, and the comparisons that the researchers planned to make. To enhance the ability to measure task-related activation (ie, activation that correlates with the design-based regressor created through the convolution steps described previously), researchers can include nuisance variables, or regressors of no interest (eg, head motion, intercepts for each block of data acquisition in case mean activation varies when the scanner stops and starts), to capture the noise in the MRI signal. When the design matrix is complete, it is regressed against every voxel in a “mass univariate” approach82 for each of the many hundred thousand or so voxels that make up standard fMRI whole brain acquisition. Effectively, this means that an analysis is performed for each voxel in the brain—leading to more than 100,000 statistical tests, and thus a high potential for false positive results (see the section, Group-Level Analyses, for discussion on correction for multiple comparisons). This subject-level analysis results in a coefficient (“beta”) for each voxel for each element of the design matrix (all stimulus conditions and all nuisance variables). The coefficient describes the strength of the relationship between that regressor (the experimental condition or conditions of interest) and the voxel’s time course of activation. This usually involves averaging across stimuli and tasks, although some pain researchers use single trial analyses169,189 to generate 1 beta for each event (per voxel).7,8 Researchers can also compute contrasts across these regressors at the subject-level (eg, to compare highand low-intensity stimulation) to generate voxelwise contrast values that describe whether a given voxel’s activation strongly differs on the basis of condition. Whole brain summary statistic maps of these voxelwise beta coefficients or contrast values are then passed to grouplevel analyses to facilitate statistical analyses across groups of subjects.
Subject-Level Rs-fMRI Analyses
Rs-fMRI is becoming increasingly popular in the pain neuroimaging community, because it allows the investigation of intrinsic brain functional connectivity associated with pain-related phenotypes, including painrelated characteristics (eg, severity, duration) as well as cognitions (eg, pain hypervigilance, fear of pain, catastrophizing).12–15,42,57,111,123,129,153 The preprocessing steps for rs-fMRI are similar to those of task-based connectivity, with a few notable exceptions (Fig 3). Rs-fMRI does not rely on a task-based regressor. Rather, time courses from spontaneous activity serve as a regressor. There are 2 primary approaches to the resting state analysis: seedbased connectivity or independent components analysis. The former relies on an a priori interest on the connectivity of a particular brain region, whose time course becomes the regressor. This approach requires additional nuisance regressors to correct for physiological noise, such as cardiac pulsatility and respiration-related motion. There are several approaches to correct for such noise,35 such as: 1) measuring heart rate and breathing rate and including these measures as regressors, 2) post hoc correction for physiological noise,93 3) regressing out time courses from white matter and cerebrospinal fluid,20 or 4) global signal regression. In contrast, the independent components analysis method does not rely on the time course of a particular brain region, but rather is a data-driven approach that groups correlated regions. In this method, artifacts can be identified on the basis of the spatiotemporal patterns of the resulting components, and artifactual components can be removed from the signal. When summary statistics maps of connectivity have been produced, a group-level analysis can be performed, similar to task-based data.
Group-Level Analyses
When each subject’s fMRI data have been preprocessed, and statistics are performed on individual data sets, a group-level analysis is undertaken. The purpose of this analysis is to identify brain regions that are significantly activated across participants or between groups. At this stage, univariate statistical tests can be performed, or multivariate statistics can beused. We focus primarily on traditional univariate statistics, which are tests that identify voxels in which there is significant activation as a function of the condition (orcontrast) of interest. Perhaps the most common group-level analysis is the onesample t-test on contrast maps across all participants to test whether activation at each voxel differs significantly as a function of the contrast (for example, whether all participants show greater activation in response to noxious, relative to innocuous, stimuli). This generates a whole-brain statistical map (eg, a distribution oft-statistics across voxels), which must then be appropriately thresholded for inference. Other standard group-level analyses include t-tests, which compare activation across groups (eg, patients vs controls), and correlation analyses, which identify the strength of the correlation between voxelwise activation and individual differences in some known parameter (eg, behavioral performance, symptom severity, or questionnaire measure).
As with any type of statistical analysis, one must ensure results are not driven by outliers. This is particularly important in small samples, and brain–behavior correlations, but is true of all experiments. As an example, with a small sample size, a correlation between pain duration and the thickness of the cortex in a particular brain region might be driven by a single subject, or a small subset of subjects. Results should be visualized (ie, scatter plots should be inspected to determine whether effects can be attributed to a small number of individuals). Alternatively, automated statistical techniques such as robust regression2,21,89,221 can reduce the contribution of outliers.
The group-level analyses outlined previously, when conducted on each voxel throughout the entire brain, require tens of thousands of statistical tests (ie, multiple comparisons). Because of the inherent nature of statistics, this will necessarily lead to a cumulative proportion of false positive results. Therefore, it is important to adjust or correct for this inflated rate of false positive results (ie, correct for multiple comparisons). There are 2 fundamental approaches to the multiple comparisons problems: 1) ROI analyses, which reduce the number of multiple comparisons on the basis of a priori hypotheses, or 2) multiple comparisons corrections, which correct for the number of tests performed. These are not mutually exclusive, because testing multiple ROIs will still require multiple comparisons correction. We also note that thresholds can be computed at the level of the voxel or the level of the cluster (for a review of thresholding methods, see Poldrack et al185). We describe each of these considerations in the following paragraphs.
To reduce the number of multiple comparisons, researchers can reduce the search area within the brain and restrain analyses to a priori ROIs. This excludes statistical tests from brain regions we do not expect to be implicated in the analysis, and reduces the number of comparisons, because tests are restricted to voxels within ROIs. When an analysis is performed on every voxel within an ROI, multiple comparisons corrections must still be conducted, but there will be substantially fewer comparisons for which to correct. Researchers can also extract various coefficients across the ROI, depending on their question of interest. For example, the mean of the time series across all voxels in the ROI can be extracted. Another, and perhaps better, option is to extract the first principal component of the time series in the voxels. The resulting eigenvalue is effectively a weighted average of the activation in the ROI, where atypical voxels are downweighted. When the metric of interest is extracted, a single statistical test can be conducted, which would therefore be evaluated with a standard P < .05 statistical threshold if data are combined (eg, averaged) across voxels within the ROI. However, if coefficients are extracted from multiple ROIs or multiple voxels within an ROI, multiple comparisons correction is required. So how do researchers identify appropriate ROIs? One way is to include a functional localizer—an fMRI scan or contrast that excludes the condition of interest. For example, if we want to determine how a painkiller affects painrelated brain activation, a baseline scan of pain-related activation, in which each participant receives high- and low-intensity stimulation, can be performed before analgesic administration. This generates a functional localizer scan, which can be analyzed with whole-brain statistics. Regions that show significant pain-related activation would then be used as ROIs for subsequent tests of analgesic-related reductions. In this case, ROIs are on the basis of the same subjects within the same scanner; one can even identify subject-specific ROIs if the localizer task is designed properly70,90 (ie, a substantial number of trials per condition during the localizer run; details are discussed elsewhere113,174,182). Researchers can also use a priori ROIs on the basis of the relevant literature. In this case, ROIs may be defined on the basis of brain atlases that are in standardized stereotaxic space, using either neuroanatomical boundaries or extracting data from a sphere or box placed at specific coordinates. There are many atlases, and researchers must be judicious in their selection of ROIs, on the basis of their aims. Alternatively, ROIs can be on the basis of meta-analyses (eg, with automated term-based meta-analyses such as through Neurosynth234). Regardless of how individual ROIs are selected, the rationale for such decisions must be reported and should be carefully evaluated.
If a researcher does not have strong a priori hypotheses about specific ROIs, or wants to conduct whole brain analyses, they must use multiple comparisons corrections to account for the number of tests and adjust P-value thresholds accordingly. Several appropriate methods exist. For example, familywise error correction and Bonferroni correction set adjusted P-values by dividing the threshold by the number of tests. This leads to a very stringent threshold. The false discovery rate (FDR), in contrast, is a more lenient method to correct for multiple comparisons, which controls for a false-positive proportion (ie, the fraction of detected voxels that are false positive; which is an unobservable metric).22 In FDR correction, a rate (q) between 0 and 1, is specified, which represents the maximum FDR that will be tolerated on average. Next, uncorrected P values of suprathreshold voxels are ranked on the basis of significance (from smallest/most significant to largest/least significant). Next, the voxel (or cluster) whose probability is greater than its ranking divided by the total number of tests, as corrected by a desired FDR (q-value) is identified. This voxel’s P value is set as the threshold for a given FDR.91 Each software package might implement corrections slightly differently (eg, some approaches account for the spatial contiguity of activation; cluster-wise correction), whereas others set thresholds on the basis of voxelwise values alone. Cluster correction is now advised231—especially for FDR correction44—although there are notable differences in how this is implemented in various packages73 and this is an active area of debate in the broader neuroimaging community.73,183 Comparisons across these approaches have been discussed elsewhere and we advise interested researchers to consult these articles for more thorough treatment of considerations involved in methods for multiple comparison corrections.24,73,150,151,171,231
Another form of multiple comparisons correction is to perform nonparametric tests, such as permutation testing, which do not make parametric assumptions about the data. Permutation testing resamples data and randomizes the assignment of observations. This process is repeated several times (usually >1,000) to empirically determine a null distribution of the data, and to determine whether the observed differences are significant on the basis of the data, and the α value is set to minimize falsepositive results. A notable advantage of permutation testing is that it identifies the false-positive rate on the basis of the data. However, permutation testing can be very computationally intensive. For a comprehensive review, see Hayasaka and Nichols.104
Finally, we note that recent studies have shed light on an inflated rate of type I errors in neuroimaging studies.73,105,110 This indicates that there is an abnormally large number of significant findings that are reported in these studies—implying that negative results are not being reported (the so-called “file-drawer issue,” where negative results are not published, and that only results that meet statistical significance are reported, despite a large number of statistical tests performed (p-hacking). The field has become increasingly aware of and vigilant against such practices, because they can hinder true progress, and perpetuate false-positive results.
Multivoxel Pattern Analyses
The analyses we reviewed are all referred to as mass univariate statistical tests, because each voxel is modeled as a single outcome in a statistical test, and multiple tests are conducted. In multivariate analyses, this relationship is switched—multiple voxels are modeled together rather than individually. One of the first multivariate analysis approaches was partial least squares,155 which identifies patterns, or distributed networks of brain activity related to a construct (such as a behavioral task). This approach was used by Seminowicz and Davis to investigate the neural underpinnings of pain–cognition interactions.198 More recently, this approach was used to compare networks dynamics with increasing cognitive loads in patients with fibromyalgia compared with controls.41
More recently, classifier-based multivoxel pattern analysis, or MVPA, have become increasingly popular in pain research (see Haxby103 for a review). In MVPA, algorithms from computer science and machine learning identify patterns across voxels that relate to a single construct. For example, MVPA has been used to test whether voxels within the anterior cingulate discriminate physical pain from other stimuli,49,140,219,230 or to identify features that can detect chronic pain.10,100,137,212 In MVPA, machine learning algorithms use a subset of the data to train a pattern of weights across voxels that discriminate between states (eg, acute pain vs other modality).161,191 The pattern is then tested against data that were not included in the training set to determine whether it can reliably discriminate between states (eg, predicting that the condition was acute pain). This is repeated iteratively with different sets of held-out data through “cross-validation,” which then allows researchers to assess how well the pattern predicts outcomes. MVPA is a robust method to recover information encoded in ensembles of voxels. There are different algorithms and approaches that can be used for MVPA, but these are outside the scope of this primer. For a review, please see Cohen et al.47
Functional Connectivity Analyses
Another way to move beyond the consideration of individual activation clusters, or “blobs,” is by acknowledging the network nature of brain activity through functional connectivity analyses. Functional connectivity refers to a family of techniques that examine temporal correlations in BOLD activity across brain regions (highly related to the rs-fMRI techniques reviewed previously). Critically, despite the term “connectivity,” functional connectivity does not rely on anatomical connections, but on simple correlations between the time courses of different regions. Functional connectivity approaches can be divided into several classes: those that assume static relationships between regions over time (“static connectivity”), those that assume that relationships vary over time (“dynamic connectivity”), and those that identify the influence of neural regions on each other (which can provide directional inferences in connections; “effective connectivity”). The simplest static connectivity measure is the seed-based analysis, in which researchers extract the time series from a single ROI (the “seed”) and test for correlations between that region’s activation and activation throughout the rest of the brain. The region’s activation is used as the predictor in a GLM, and this results in a map of the strength of the correlations at each voxel, which can be threshold according to the mass univariate approach described previously. For example, one study reported that patients with temporomandibular disorders (TMD) have abnormally stronger connectivity between the medial prefrontal cortex and the posterior cingulate cortex/precuneus. This strength of this abnormal connectivity was related to pain rumination in these patients.129
A more complex approach to static connectivity is to use principles from graph theory.19,32,88,98,206 In this framework, brain structures are called nodes, and the connections between them are called edges. A graph theoretical approach captures the structure of a network (a set of nodes) and allows researchers to identify a variety of metrics for the network elements, including the density of connections of the brain regions (node degree), the clustering of neighboring nodes, the distance needed to travel between 2 regions (path length, or efficiency), which regions are highly connected to many other regions (ie, hubs), and which hubs are crucial (centrality). Together, these can be used to map networks in pain, and how such networks may be altered in chronic pain conditions. Studies indicate that graph theoretical analysis of rs-fMRI can discriminate between placebo responders and nonresponders in osteoarthritis,102,213 and has potential in the development of translational biomarkers for pain.122 Additionally, these graph theoretical approaches have been used to investigate brain network abnormalities in chronic pain disorders.124,135,149,152 Whereas the details of these graph theoretical approaches are beyond the scope of this review, we refer readers to technical reviews of graph theoretical approaches to neuroimaging.19,32,75,88,206
Whereas static connectivity measures assume that regions vary over time but their correlations remain stable, dynamic connectivity approaches allow the correlations across regions to vary as well. Dynamic connectivity has been growing in popularity within the pain community.128 Although the field of dynamic connectivity is still rather new,114 one type of dynamic connectivity approach has existed for quite some time: the psychophysiological interaction (PPI).85 This is essentially an interaction analysis, whereby a researcher can ask whether a condition modulates the connectivity between 2 brain regions. For example, a PPI was used to show that there is stronger coupling between the periaqueductal grey (PAG) and the rostral anterior cingulate cortex (rACC) under placebo analgesia than under a control condition.26 PPI can also be used to examine whether altered connectivity is associated with a behavioral state. This approach has been used to show that increased ventrolateral prefrontal cortex (vlPFC) connectivity with the amygdala and nucleus accumbens during controllable pain was associated with reduced anxiety.195 In PPI analyses, the “dynamic” aspect is known and driven by the experimental design (eg, placebo blocks vs control blocks). In other approaches, dynamics are more fluid. Sliding window analyses examine connectivity across time by binning the task into specific chunks of time; this approach was used to show that individuals with more dynamic connectivity between the medial PFC (mPFC) and PAG were more apt to spontaneously disengage from pain.130 Finally, new approaches can also use purely data-driven approaches to identify moments when networks reconfigure. For example, statebased dynamic connectivity analyses use latent models to estimate whether brain networks remain stable or shift connections over time.190 This approach revealed dissociations among brain networks during remifentanil administration (eg, connectivity within networks associated with emotion remained stable, whereas connectivity within networks associated with pain reorganized as drug infusion increased).
In contrast to static and dynamic connectivity, effective connectivity tests the plausibility of directional brain networks models—that is, not only is the activity between 2 brain regions correlated, but the effect of a neural region on another.81,84,87,88 This allows for stronger inferences about the flow of information in the brain.156 Effective connectivity usually requires that a set of brain regions of interest be defined a priori, although some path analysis approaches such as voxelwise multilevel mediation analysis,7,220 allow for whole brain searches. One such method, dynamic causal modeling uses Bayesian statistics to adjudicate between physiologically plausible models of interactions between brain regions (or nodes).81,148 When a model has been selected, the algorithm can determine how a condition can modulate the connectivity strength (or edges) between the nodes that comprise the network. This method has been used to determine the brain networks underlying isosalient nociceptive and innocuous stimuli of other modalities.142 Mediation analysis tests whether the relationships between 2 variables is significantly reduced when a third, intervening variable is taken into account. Mediation can be used as a form of effective connectivity by testing whether the relationship between a brain region and behavior, or the correlation between 2 brain regions, can be partially explained by connections with another region. For example, this method has been used to show that connectivity between the ventral striatum and ventromedial prefrontal cortex mediate the effects of selfregulation on pain.232 Another study used mediation analysis to show that functional connectivity between the lateral prefrontal cortex and the PAG during rectal distention in healthy controls and patients with ulcerative colitis were mediated by the medial prefrontal cortex.154 Notably, such a relationship was not observed in patients with irritable bowel syndrome, although the difference between the 2 groups was not formally tested and cannot, therefore, be considered a group difference. Another example showed that the relationship between structural gray matter abnormalities in the supplementary motor area and pain-related helplessness in temporomandibular disorders was mediated by corticofugal motor white matter tracts.194
One might assume that these group-level analysis decisions are atheoretical with respect to pain mechanisms. This is not, however, entirely the case. As an example, one of the most basic historical debates in the field is whether pain is the product of “labeled lines” running from the periphery to pain-specific areas in the brain, or rather as a function of temporal and spatial patterns of activity throughout the neuroaxis. Labeled line theories date back centuries162 and are largely on the basis of neuroanatomic studies of spinal pathways and lesion studies. This locationist approach led to statistical analyses biased toward clusters of activation, such as traditional univariate GLM analyses. Local interactions or spatial patterns within these clusters are obscured by spatial blurring. As such, these analyses have traditionally led to more modular interpretations of neural processing (ie, that single regions have single functions)—a framework more consistent with a specificity account of neural pain processing. However, this account has largely been unable to identify specific brain regions that are necessary and/ or sufficient for the experience of pain. As described previously, MVPA has been used to increase specificity by searching for spatial patterns associated with particular psychological experiences. Such an analysis technique operates on the belief that activation more specific to pain will be identified by searching for unique patterns of spatial distribution, clearly more consistent with a pattern account. Similarly, studies using functional connectivity or changes in functional connectivity across different time epochs are likely to result in interpretations of pain as a function of temporal and spatial patterning, rather than the product of activation of any single specific pain center.
Model-Based Analyses
Another approach to analyze fMRI data is the use of models to identify brain regions or networks related to perceptual experiences and more abstract concepts, such as the hedonic value of pain. One approach to modeling perceptual (and thus subjective) experiences, perceptrelated fMRI, was developed by Porro and colleagues.186 This method requires a continuous rating of a measure of interest for the duration of the trial. This point-bypoint perceptual rating curve is used as a regressor of interest in the statistical model. This method has been used to investigate prickle,58 paradoxical heat,59 and heat pain intensity coding12 in healthy participants. It has also been used to investigate brain activity to experimental stimuli in chronic pain conditions.13,134
To investigate more complex behaviors, such as decisionmaking, valuations, and social interactions, neuroimagers have adopted engineering modeling approaches. Theoretical computational models of such processes are developed to estimate underlying computations and predictions about neural signals,61,62,201,233 and the dynamic interactions between theoretical signals. These models can generate predictive time-series that can be used as regressors in the statistical model to identify correlated brain activity, and identify regions underlying the process. Because of the complexity of pain, such approaches may be appropriate for pain research. Indeed, several families of computational models have been used to investigate the neural computations related to pain, including estimating the subjective value of pain and its relationship to cognitive factors and modulation.74,133,202,208,209,217,227,228,235
Interpreting Data and Making Inferences
The ability to observe changes in oxygenated blood flow throughout the brain while a subject is in a particular behavioral or cognitive state is extremely powerful. Interpretingtheseactivations,however,canbecomplicated, and the inferences that can be drawn are affected by a number of factors. We discuss some of these factors, and the limitations they pose on the interpretability of data.
Inference
Because of the high cost of neuroimaging studies, and the difficulty in recruiting chronic pain populations, most pain imaging studies tend to be cross-sectional. These studies are important and useful because they can provide information on mechanisms. For example, if a brain activation is significantly correlated with pain intensity, one might conclude that that the activity is pain-related. However, observed activation might reflect many other correlated mental processes, so control conditions and interpretations must be carefully considered before making such conclusions. For example, correlations with pain intensity could reflect processes involved in magnitude estimation,12 salience detection,115,139,166 defensive reactions,163 or other processes that accompany pain but are not specific to pain. One must ensure that the control condition is sufficient for making claims about differentiating between these processes. Furthermore, the exact relationship between BOLD activation and underlying neuronal activity is still an open area of investigation.25,107,108,175,204 Brain activation is inherently correlational, and authors often speculate on the biological basis of a finding. Whereas activations observed are related to the stimulus, one cannot infer causation from fMRI alone. In the absence of a task or intervention that can directly test a brain region (eg, eliciting a virtual lesion using transcranial magnetic stimulation),we can only infer associations, but not causal links. For example, higher dorsolateral PFC (dlPFC) activation might be associated with increased placebo analgesia (ie, reduced pain8,48,199), but if one wants to test whether the dlPFC causes placebo effects on pain, one must show that placebo effects are abolished when dlPFC activity is disrupted. Indeed, researchers have done exactly this,199 indicating that the dlPFC causally contributes to placebo analgesia. Although this limits the inferences that might be drawn from fMRI alone, it is also clear that activation-based fMRI studies help identify brain candidates for causal interventions, and thus their potential utility remains enormous.
A related inferential difficulty arises when group differences are observed between pain patients and healthy controls in cross-sectional studies. Without additional data, it is impossible to determine whether these differences preexisted the condition (and therefore represent predisposing or possibly causal factors) or whether they are related to the cumulative effects of living with a chronic condition. Nevertheless, such inferences are frequently implied. For example, in structural imaging, it is common to refer to observed group differences as “plasticity” or “changes,” implying (without support) that the differences represent cumulative effects of disease. Some groups have attempted to form stronger inferences by performing correlations with disease characteristics, such as pain intensity, duration, questionnaire data, and other metrics,57 but even when such correlations are found, it remains difficult to determine whether they are due to cumulative effects of pain or other long-term effects of chronic disease (eg, mood and behavioral changes). Furthermore, such inferences are still limited by their correlational nature. Modeling techniques, such as dynamic causal modeling86 and mediation analysis145 allow inferences about the direction of information flow in neural circuits during a mental process or across participants, but they are still limited in causal inferences because they rely on the statistical variance within a crosssectional data set. The strongest way to address this issue is to perform longitudinal studies in healthy subjects and/ or chronic pain patients—scanning subjects before and after a therapeutic intervention,97,118,131,200 or before the progression from acute to chronic pain.14,101 Although such designs pose challenges in terms of resources and time, they allow for stronger and more mechanistically informative inferences about temporal precedence.
Logical Fallacies in Neuroimaging
Reverse inference is the widespread tendency to ascribe a function to an observed pattern of activation (eg, “we observed activation in the visual cortex, therefore the participant is probably looking at something”). This issue has been discussed in depth in other reviews116,181 so will only be covered briefly. Reverse inference is tempting and, to some extent necessary, because the ultimate goal of scanning is elucidating mechanism. Caution is required, however, because reverse inference relies on a logical error (“affirming the consequent”). Despite the fact that the logic underlying such inferences is flawed, the probability that they are true can still be high if (as in the previously mentioned example of the visual cortex) the region in question is linked fairly exclusively with a particular function. In pain imaging, however, we frequently observe activation of regions (eg, insular and anterior cingulate cortices) that are involved in a wide variety of functions.234 As a practical example of how this might affect inferences in pain studies, there is a widespread tendency to interpret observed activations in the periaqueductal gray as an indication that descending modulation is taking place. Although such inferences can be strengthened by corroborating evidence (eg, correlation with reduced pain ratings), it is commonly forgotten that the PAG is involved in many functions also relevant to pain, including escape and avoidance responses,16 prediction coding,192 and emotion.16,31,196 Because of this ambiguity, it is critical that experiments be designed to include appropriate control conditions and supplementary measures to adjudicate between these competing accounts.
A second common error that affects inferences is the so-called “imager’s fallacy”: that a difference in significance implies a significant difference.53 Three instances of this fallacy in imaging studies are group comparisons, implied lateralization, and subregional specialization. Researchers often conduct separate analyses for each group in a between-groups study (eg, to examine activation within patients and activation within controls). In such cases, it is tempting to display separate thresholded group maps and draw qualitative comparisons on the basis of the extent of activation in each group. However, one cannot assume that a region was differentially activated simply because it meets a threshold for significance in one group and not in the other: apparent differences may simply reflect near-threshold differences (eg, the P value for a particular cluster was .049 for one group and .051 for the other). To conclude that there was a group difference, the groups must be formally compared using an appropriate statistical test (ie, an interaction analysis). Relatedly, when comparing a correlation between 2 groups where 1 group shows a significant correlation with a behavioral measure, but the second group does not have a significant correlation, it is tempting to conclude that there are group differences between these correlations. However, the correlation coefficients must be normalized using Fisher z-transformation,78 and then formally compared. A similar error is often made with respect to laterality claims: if significant activation is observed on one side of the brain, but not in the symmetric contralateral brain region, it is common to conclude that the effect in question is lateralized. Without formally comparing the activation between the 2 sides, however, there is no basis for ruling out that processing is bilateral. Similarly, it is common to use significant activations to draw inferences about specialization of subregions of a given structure. As an example, on the basis of anatomical tracing and other evidence, the insula has often been divided into a posterior region associated with sensory input and an anterior region involved in more abstract components of pain perception (eg, interoception).50–52,67,160 Neuroimaging findings can only support this functional gradient if formal statistical testing is performed to show such selective functional association.117
Conclusion
In this primer, we reviewed fundamental considerations in the design, analysis, description, and evaluation of a pain neuroimaging experiment. Investigators must make many careful decisions when designing experiments, and reports must be written so that reviewers and readers in the community can evaluate the work appropriately, whether or not the reviewer has expertise in imaging. We hope this primer provides a foundation for pain clinicians and trainees without advanced training in neuroimaging, and encourage interested potential investigators to continue to read about the unique considerations involved in fMRI experiments by consulting recent dialogues that address these issues and their implications for the broader neuroimaging community.
Acknowledgments
L.Y.A. is supported by the Intramural Research program of the NIH’s National Center for Complementary and Integrative Health and National Institute on Drug Abuse. M.M. is supported by an International Association for the Study of Pain (IASP) Early Career Award, and the Bertha Rosenstadt Endowment. T.V.S. was supported by a Marie Curie International Incoming Fellowship from the European Commission, and a British Academy Leverhulme Small Research Grant.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F: Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 34:738–752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen R: Modern Methods for Robust Regression. Toronto, Sage Publications, 2008 [Google Scholar]
- 3.Apkarian AV, Baliki MN, Farmer MA, Tétreault P, Vachon-Presseau E: Pain: Acute and Chronic, in Toga AW (ed): Brain Mapping. Waltham, Academic Press, 2015, pp 553–563 [Google Scholar]
- 4.Apkarian AV, Bushnell MC, Treede RD, Zubieta J: Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM: Imaging the pain of low back pain: Functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett 299:57–60, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ: Brain responses to changes in bladder volume and urge to void in healthy men. Brain 124:369–377, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Atlas LY, Bolger N, Lindquist MA, Wager TD: Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atlas LY, Wager TD: A meta-analysis of brain mechanisms of placebo analgesia: Consistent findings and unanswered questions. Handb Exp Pharmacol 225:37–69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD: Dissociable influences of opiates and expectations on pain. J Neurosci 32:8053–8064, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagarinao E, Johnson KA, Martucci KT, Ichesco E, Farmer MA, Labus J, Ness TJ, Harris R, Deutsch G, Apkarian AV, Mayer EA, Clauw DJ, Mackey S: Preliminary structural MRI based brain classification of chronic pelvic pain: A MAPP network study. Pain 155:2502–2509, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker M: 1,500 scientists lift the lid on reproducibility. Nature 533:452–454, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Baliki MN, Geha PY, Apkarian AV: Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101:875–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baliki MN, Geha PY, Fields HL, Apkarian AV: Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66:149–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV: Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15:1117–1119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV: Brain morphological signatures for chronic pain. PLoS One 6:e26010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandler R, Keay KA: Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 107:285–300, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Basser PJ, Jones DK: Diffusion-tensor MRI: Theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Basser PJ, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-diffusiontensor MRI. J Magn Reson B 111:209–219, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Bassett DS, Bullmore ET: Human brain networks in health and disease. Curr Opin Neurol 22:340–347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behzadi Y, Restom K, Liau J, Liu TT: A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Gal I: Outlier detection, in Maimon O, Rockach L (eds): Data Mining and Knowledge Discovery Handbook: A Complete Guide for Practitioners and Researchers. New York, NY, Kluwer Academic Publishers, 2005 [Google Scholar]
- 22.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300, 1995 [Google Scholar]
- 23.Bennett CM, Miller MB: How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci 1191:133–155, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Bennett CM, Wolford GL, Miller MB: The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 4:417–422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley WJ, Li JM, Snyder AZ, Raichle ME, Snyder LH: Oxygen level and lfp in task-positive and task-negative areas: Bridging BOLD fMRI and electrophysiology. Cereb Cortex 26: 346–357, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C: Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120:8–15, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bingel U, Schoell E, Buchel C: Imaging pain modulation in health and disease. Curr Opin Neurol 20:424–431, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Bingel U, Tracey I: Imaging CNS modulation of pain in humans. Physiol 23:371–380, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I: The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 3:70ra14, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Borsook D, Becerra LR: Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain 2:30, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buhle JT, Kober H, Ochsner KN, Mende-Siedlecki P, Weber J, Hughes BL, Kross E, Atlas LY, McRae K, Wager TD: Common representation of pain and negative emotion in the midbrain periaqueductal gray. Soc Cogn Affect Neurosci 8:609616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullmore E, Sporns O: Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Bushnell MC, Ceko M, Low LA: Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR: Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Caballero-Gaudes C, Reynolds RC: Methods for cleaning the BOLD fMRI signal. Neuroimage 154:128–149, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell CM, Edwards RR: Ethnic differences in pain and pain management. Pain Manag 2:219–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell LE, Hughes M, Budd TW, Cooper G, Fulham WR, Karayanidis F, Hanlon MC, Stojanov W, Johnston P, Case V, Schall U: Primary and secondary neural networks of auditory prepulse inhibition: A functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci 26:2327–2333, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Carp J: On the plurality of (methodological) worlds: Estimating the analytic flexibility of FMRI experiments. Front Neurosci 6:149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carp J: The secret lives of experiments: Methods reporting in the fMRI literature. Neuroimage 63:289–300, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Cauda F, Palermo S, Costa T, Torta R, Duca S, Vercelli U, Geminiani G, Torta DM: Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImage Clin 4:676–686, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceko M, Gracely JL, Fitzcharles MA, Seminowicz DA, Schweinhardt P, Bushnell MC: Is a responsive default mode network required for successful working memory task performance? J Neurosci 35:11595–11605, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA: Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp 36: 2075–2092, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen NK, Dickey CC, Yoo SS, Guttmann CR, Panych LP: Selection of voxel size and slice orientation for fMRI in the presence of susceptibility field gradients: Application to imaging of the amygdala. Neuroimage 19:817–825, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Chumbley JR, Friston KJ: False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage 44:62–70, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodriguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ, Group MRNS: The MAPP research network: A novel study of urologic chronic pelvic pain syndromes. BMC Urol 14:57, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coghill RC, Sang CN, Maisog JM, Iadarola MJ: Pain Intensity processing within the human brain: A bilateral, distributed mechanism. J Neurophysiol 82:1934–1943, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Cohen JD, Daw N, Engelhardt B, Hasson U, Li K, Niv Y, Norman KA, Pillow J, Ramadge PJ, Turk-Browne NB, Willke TL: Computational approaches to fMRI analysis. Nat Neurosci 20:304–313, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colloca L, Klinger R, Flor H, Bingel U: Placebo analgesia: Psychological and neurobiological mechanisms. Pain 154: 511–514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corradi-Dell’Acqua C, Tusche A, Vuilleumier P, Singer T: Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat Commun 7:10904, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craig AD: Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–505, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Craig AD: Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477:119–148, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Craig AD: Once an island, now the focus of attention. Brain Struct Funct 124:395–396, 2010 [DOI] [PubMed] [Google Scholar]
- 53.de Hollander G, Wagenmakers EJ, Waldorp L, Forstmann B: An antidote to the imager’s fallacy, or how to identify brain areas that are in limbo. PLoS One 9:e115700, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David SP, Ware JJ, Chu IM, Loftus PD, Fusar-Poli P, Radua J, Munafò MR, Ioannidis JP: Potential reporting bias in fMRI studies of the brain. PLoS One 8:e70104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis KD, Bushnell MC, Strigo IA, Duncan GH, Kwan CL, Diamant NE, Sarkar S, Gregory L, Aziz Q: Imaging visceral sensations, in Dostrovsky JO, Carr DB, Koltzenburg M (eds): Proceedings of the 10th World Congress on Pain, Vol. 24 Seattle, IASP Press, 2003, pp 261–276 [Google Scholar]
- 56.Davis KD, Flor H, Greely HT, Iannetti GD, Mackey S, Ploner M, Pustilnik A, Tracey I, Treede RD, Wager TD: Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nat Rev Neurol 13:624–638, 2017 [DOI] [PubMed] [Google Scholar]
- 57.Davis KD, Moayedi M: Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8:518–534, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Davis KD, Pope GE, Crawley AP, Mikulis DJ: Neural correlates of prickle sensation: A percept-related fMRI study. Nat Neurosci 5:1121–1122, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Davis KD, Pope GE, Crawley AP, Mikulis DJ: Perceptual illusion of “paradoxical heat” engages the insular cortex. J Neurophysiol 92:1248–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Davis KD, Wood ML, Crawley AP, Mikulis DJ: fMRI of human somatosensory and cingulate cortex during painful electrical nerve stimulation. Neuroreport 7:321, 1995 [PubMed] [Google Scholar]
- 61.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ: Model-based influences on humans’ choices and striatal prediction errors. Neuron 69:1204–1215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ: Cortical substrates for exploratory decisions in humans. Nature 441:876–879, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deichmann R, Gottfried JA, Hutton C, Turner R: Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19:430–441, 2003 [DOI] [PubMed] [Google Scholar]
- 64.D’Esposito M, Deouell LY, Gazzaley A: Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci 4:863–872, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J: Influence of context effects on health outcomes: A systematic review. Lancet 357:757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Dorner TE, Muckenhuber J, Stronegger WJ, Rasky E, Gustorff B, Freidl W: The impact of socio-economic status on pain and the perception of disability due to pain. Eur J Pain 15:103–109, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Dostrovsky JO, Craig AD: Ascending projection systems, in McMahon A, Koltzenburg M (eds): Wall and Melzack’s Textbook of Pain. Edinburgh, UK, Churchill Livingstone, 2006, pp 187–204 [Google Scholar]
- 68.Duerden EG, Albanese MC: Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum Brain Mapp 34:109–149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duff EP, Vennart W, Wise RG, Howard MA, Harris RE, Lee M, Wartolowska K, Wanigasekera V, Wilson FJ, Whitlock M, Tracey I, Woolrich MW, Smith SM: Learning to identify CNS drug action and efficacy using multistudy fMRI data. Sci Transl Med 7:274ra216, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Dunsmoor JE, Kragel PA, Martin A, LaBar KS: Aversive learning modulates cortical representations of object categories. Cereb Cortex 24:2859–2872, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Durnez J, Degryse J, Moerkerke B, Seurinck R, Sochat V, Poldrack RA, Nichols T: Power and sample size calculations for fMRI studies based on the prevalence of active peaks. bioRxiv, 2016. April 20; [Epub ahead of print] [Google Scholar]
- 72.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C: Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63:533–543, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Eklund A, Nichols TE, Knutsson H: Cluster failure: Why fMRI inferences for spatial extent have inflated falsepositive rates. Proc Natl Acad Sci U S A 113:7900–7905, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eldar E, Hauser TU, Dayan P, Dolan RJ: Striatal structure and function predict individual biases in learning to avoid pain. Proc Natl Acad Sci U S A 113:4812–4817, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE: Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:e1000381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farrell MJ, Laird AR, Egan GF: Brain activity associated with painfully hot stimuli applied to the upper limb: A metaanalysis. Hum Brain Mapp 25:129–139, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F: Biological, clinical, and ethical advances of placebo effects. Lancet 375:686–695, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fisher RA: On the probable error of a coefficient of correlation deduced from a small sample. Metron 1:3–32, 1921 [Google Scholar]
- 79.Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choiniere M, Ko G, Moulin DE, Panopalis P, Proulx J, Shir Y, National Fibromyalgia Guideline Advisory P.: 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Res Manag 18:119–126, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Friebel U, Eickhoff SB, Lotze M: Coordinate-based metaanalysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage 58:1070–1080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friston K, Zeidman P, Litvak V: Empirical Bayes for DCM: A group inversion scheme. Front Syst Neurosci 9:164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friston KJ, Jezzard P, Turner R: Analysis of functional MRI time-series. Hum Brain Mapp 1:153–171, 1994 [Google Scholar]
- 83.Friston KJ: Statistical Parametric Mapping the Analysis of Functional Brain Images. London, United Kingdom, Elsevier/Academic Press, 2007 [Google Scholar]
- 84.Friston KJ: Functional and effective connectivity: A review. Brain Connect 1:13–36, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ: Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229, 1997 [DOI] [PubMed] [Google Scholar]
- 86.Friston KJ, Harrison L, Penny W: Dynamic causal modelling. Neuroimage 19:1273–1302, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Friston KJ, Kahan J, Biswal B, Razi A: A DCM for resting state fMRI. Neuroimage 94:396–407, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friston KJ, Kahan J, Razi A, Stephan KE, Sporns O: On nodes and modes in resting state fMRI. Neuroimage 99:533547, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fritsch V, Da Mota B, Loth E, Varoquaux G, Banaschewski T, Barker GJ, Bokde AL, Bruhl R, Butzek B, Conrod P, Flor H, Garavan H, Lemaitre H, Mann K, Nees F, Paus T, Schad DJ, Schumann G, Frouin V, Poline JB, Thirion B, IMAGEN consortium: Robust regression for large-scale neuroimaging studies. Neuroimage 111:431–441, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Furl N, Henson RN, Friston KJ, Calder AJ: Top-down control of visual responses to fear by the amygdala. J Neurosci 33:17435–17443, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Genovese CR, Lazar NA, Nichols T: Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Glover GH, Law CS: Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med 46:515–522, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Glover GH, Li TQ, Ress D: Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 94.Godenschweger F, Kagebein U, Stucht D, Yarach U, Sciarra A, Yakupov R, Lusebrink F, Schulze P, Speck O: Motion correction in MRI of the brain. Phys Med Biol 61:R32–R56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goto M, Abe O, Miyati T, Yamasue H, Gomi T, Takeda T: Head motion and correction methods in resting-state functional MRI. Magn Reson Med Sci 15:178–186, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graven-Nielsen T, Jansson Y, Segerdahl M, Kristensen JD, Mense S, Arendt-Nielsen L, Sollevi A: Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur J Pain 7:93–102, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I: Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: A longitudinal voxelbased morphometric study. Arthritis Rheum 62:2930–2940, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O: Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Handwerker DA, Ollinger JM, D’Esposito M: Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21:1639–1651, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Harper DE, Shah Y, Ichesco E, Gerstner GE, Peltier SJ: Multivariate classification of pain-evoked brain activity in temporomandibular disorder. Pain Rep 1:e572, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV: Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 136:2751–2768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL: Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci 34:3924–3936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haxby JV: Multivariate pattern analysis of fMRI: The early beginnings. Neuroimage 62:852–855, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayasaka S, Nichols TE: Validating cluster size inference: Random field and permutation methods. Neuroimage 20: 2343–2356, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Head ML, Holman L, Lanfear R, Kahn AT, Jennions MD: The extent and consequences of p-hacking in science. PLoS Biol 13:e1002106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hedley M, Yan H: Motion artifact suppression: A review of post-processing techniques. Magn Reson Imaging 10:627–635, 1992 [DOI] [PubMed] [Google Scholar]
- 107.Heeger DJ, Ress D: What does fMRI tell us about neuronal activity? Nat Rev Neurosci 3:142–151, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Hipp JF, Siegel M: BOLD fMRI correlation reflects frequency-specific neuronal correlation. Curr Biol 25:1368–1374, 2015 [DOI] [PubMed] [Google Scholar]
- 109.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG: Comorbidity of obesity and pain in a general population: Results from the Southern Pain Prevalence Study. J Pain 8:430–436, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Holman L, Head ML, Lanfear R, Jennions MD: Evidence of experimental bias in the life sciences: Why we need blind data recording. PLoS Biol 13:e1002190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA: Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 1:e20, 14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huettel SA, McCarthy G, Song AW: Functional Magnetic Resonance Imaging. Sunderland, Mass., Sinauer Associates, 2009 [Google Scholar]
- 113.Hutchison JL, Hubbard NA, Brigante RM, Turner M, Sandoval TI, Hillis GA, Weaver T, Rypma B: The efficiency of fMRI region of interest analysis methods for detecting group differences. J Neurosci Methods 226:57–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hutchison RM: Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80:360–378, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iannetti GD, Mouraux A: From the neuromatrix to the pain matrix (and back). Exp Brain Res 205:1–12, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Iannetti GD, Salomons TV, Moayedi M, Mouraux A, Davis KD: Beyond metaphor: contrasting mechanisms of social and physical pain. Trends Cogn Sci 17:371–378, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, Weber B, Knecht S: The assessment of hemispheric lateralization in functional MRI–robustness and reproducibility. Neuroimage 33:204–217, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, Kadetoff D, Ingvar M: Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 153:1495–1503, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, Cook A, Gollub RL, Kong J, Kaptchuk TJ: Sharing pain and relief: Neural correlates of physicians during treatment of patients. Mol Psychiatry 19:392–398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnstone T, Salomons TV, Backonja MM, Davidson RJ: Turning on the alarm: The neural mechanisms of the transition from innocuous to painful sensation. Neuroimage 59: 1594–1601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE, Riess H, Kaptchuk TJ: Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med 71:789–797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Khalili-Mahani N, Rombouts SA, van Osch MJ, Duff EP, Carbonell F, Nickerson LD, Becerra L, Dahan A, Evans AC, Soucy JP, Wise R, Zijdenbos AP, van Gerven JM: Biomarkers, designs, and interpretations of resting-state fMRI in translational pharmacological research: A review of state-of-theart, challenges, and opportunities for studying brain chemistry. Hum Brain Mapp 38:2276–2325, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khan SA, Keaser ML, Meiller TF, Seminowicz DA: Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155:1472–1480, 2014 [DOI] [PubMed] [Google Scholar]
- 124.Kim H, Kim J, Loggia ML, Cahalan C, Garcia RG, Vangel MG, Wasan AD, Edwards RR, Napadow V: Fibromyalgia is characterized by altered frontal and cerebellar structural covariance brain networks. NeuroImage Clin 7:667, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim HS, Neubert JK, Miguel AS, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA: Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 109:488–496, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Kong J, Loggia ML, Zyloney C, Tu P, Laviolette P, Gollub RL: Exploring the brain in pain: Activations, deactivations and their relation. Pain 148:257–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kruggel F, von Cramon DY: Temporal properties of the hemodynamic response in functional MRI. Hum Brain Mapp 8:259–271, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kucyi A, Davis KD: The dynamic pain connectome. Trends Neurosci 38:86–95, 2014 [DOI] [PubMed] [Google Scholar]
- 129.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD: Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34:3969–3975, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kucyi A, Salomons TV, Davis KD: Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A 110:1869218697, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kucyi A, Salomons TV, Davis KD: Cognitive behavioral training reverses the effect of pain exposure on brain network activity. Pain 157:1895–1904, 2016 [DOI] [PubMed] [Google Scholar]
- 132.Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP: Cortical representation of the urge to void: A functional magnetic resonance imaging study. J Urol 174:1477–1481, 2005 [DOI] [PubMed] [Google Scholar]
- 133.Kurniawan IT, Seymour B, Vlaev I, Trommershauser J, Dolan RJ, Chater N: Pain relativity in motor control. Psychol Sci 21:840–847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD: Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology 65:1268–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA: Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 155:137–149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Labus JS, Naliboff B, Kilpatrick L, Liu C, Ashe-McNalley C, dos Santos IR, Alaverdyan M, Woodworth D, Gupta A, Ellingson BM, Tillisch K, Mayer EA: Pain and Interoception Imaging Network (PAIN): A multimodal, multisite, brainimaging repository for chronic somatic and visceral pain disorders. Neuroimage 124:1232–1237, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Labus JS, Van Horn JD, Gupta A, Alaverdyan M, Torgerson C, Ashe-McNalley C, Irimia A, Hong JY, Naliboff B, Tillisch K, Mayer EA: Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain 156:1545–1554, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ, Group MRNS: The MAPP research network: Design, patient characterization and operations. BMC Urol 14:58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Legrain V, Iannetti GD, Plaghki L, Mouraux A: The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 93:111–124, 2011 [DOI] [PubMed] [Google Scholar]
- 140.Liang M, Mouraux A, Hu L, Iannetti GD: Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nat Comm 4:1979, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liang M, Mouraux A, Iannetti GD: Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: evidence from dynamic causal modelling of fMRI data. J Neurosci 31:8976–8985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liang M, Mouraux A, Iannetti GD: Bypassing primary sensory cortices–a direct thalamocortical pathway for transmitting salient sensory information. Cereb Cortex 1:1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Lin CS: Brain signature of chronic orofacial pain: A systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS One 9:e94300, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lindquist MA: The statistical analysis of fMRI Data. Stat Sci 23:439–464, 2008 [Google Scholar]
- 145.Lindquist MA: Functional causal mediation analysis with an application to brain connectivity. J Am Stat Assoc 107: 1297–1309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lindquist MA, Mejia A: Zen and the art of multiple comparisons. Psychosom Med 77:114–125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lindquist MA, Meng Loh J, Atlas LY, Wager TD: Modeling the hemodynamic response function in fMRI: efficiency, bias and mis-modeling. Neuroimage 45:S187–S198, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Litvak V, Garrido M, Zeidman P, Friston K: Empirical Bayes for Group (DCM) Studies: A reproducibility study. Front Hum Neurosci 9:670, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu J, Zhao L, Lei F, Zhang Y, Yuan K, Gong Q, Liang F, Tian J: Disrupted resting-state functional connectivity and its changing trend in migraine suffers. Hum Brain Mapp 36: 1892–1907, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Logan BR, Geliazkova MP, Rowe DB: An evaluation of spatial thresholding techniques in fMRI analysis. Hum Brain Mapp 29:1379–1389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Logan BR, Rowe DB: An evaluation of thresholding techniques in fMRI analysis. Neuroimage 22:95–108, 2004 [DOI] [PubMed] [Google Scholar]
- 152.Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang PC, Huang L, Apkarian AV, Baliki MN: Global disruption of degree rank order: A hallmark of chronic pain. Sci Rep 6:34853, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA: Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin 7:347–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L: Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain 115:398–409, 2005 [DOI] [PubMed] [Google Scholar]
- 155.McIntosh AR, Bookstein FL, Haxby JV, Grady CL: Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3:143–157, 1996 [DOI] [PubMed] [Google Scholar]
- 156.McIntosh AR, Gonzalez-Lima F: Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp 2:2–22, 1994 [Google Scholar]
- 157.McNeil DW, Rainwater AJ 3rd.: Development of the Fear of Pain Questionnaire–III. J Behav Med 21:389–410, 1998 [DOI] [PubMed] [Google Scholar]
- 158.Melzack R: The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1:277–299, 1975 [DOI] [PubMed] [Google Scholar]
- 159.Melzack R: The short-form McGill Pain Questionnaire. Pain 30:191–197, 1987 [DOI] [PubMed] [Google Scholar]
- 160.Moayedi M: All roads lead to the insula. Pain 155:1920–1921, 2014 [DOI] [PubMed] [Google Scholar]
- 161.Moayedi M: Advances in multivariate pattern analysis for chronic pain: An emerging, but imperfect method. Pain Rep 1:e580, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moayedi M, Davis KD: Theories of pain: From specificity to gate control. J Neurophysiol 109:5–12, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Moayedi M, Liang M, Sim A, Haggard P, Iannetti GD: Laser-evoked vertex potentials predict defensive motor actions. Cereb Cortex 25:4789–4798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mogil JS, Macleod MR: No publication without confirmation. Nature 542:409–411, 2017 [DOI] [PubMed] [Google Scholar]
- 165.Mori S: Basics of diffusion measurement, in Mori S (ed): Introduction to Diffusion Tensor Imaging. Amsterdam. Boston, MA, Elsevier, 2007, pp 1–11 [Google Scholar]
- 166.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD: A multisensory investigation of the functional significance of the “pain matrix.” Neuroimage 54:2237–2249, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Mumford JA: A power calculation guide for fMRI studies. Soc Cogn Affect Neurosci 7:738–742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mumford JA, Poldrack RA: Modeling group fMRI data. Soc Cogn Affect Neurosci 2:251–257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mumford JA, Turner BO, Ashby FG, Poldrack RA: Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage 59: 2636–2643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Napadow V, Makris N, Liu J, Kettner NW, Kwong KK, Hui KK: Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp 24:193–205, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nichols T, Hayasaka S: Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res 12:419–446, 2003 [DOI] [PubMed] [Google Scholar]
- 172.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline JB, Proal E, Thirion B, Van Essen DC, White T, Yeo BT: Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 20:299–303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline JB, Proal E, Thirion B, Van Essen DC, White T, Yeo BT: Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 20:299–303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nieto-Castanon A, Fedorenko E: Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage 63:1646–1669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R: Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol 17:1275–1285, 2007 [DOI] [PubMed] [Google Scholar]
- 176.Nosek BA, Ebersole CR, DeHaven AC, Mellor DT: The Preregistration Revolution. Proc Natl Acad Sci U S A 115: 2600–2606, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Owen DG, Clarke CF, Bureau Y, Ganapathy S, Prato FS, St Lawrence KS: Measuring the neural response to continuous intramuscular infusion of hypertonic saline by perfusion MRI. J Magn Reson Imaging 35:669–677, 2012 [DOI] [PubMed] [Google Scholar]
- 178.Owen DG, Clarke CF, Ganapathy S, Prato FS, St Lawrence KS: Using perfusion MRI to measure the dynamic changes in neural activation associated with tonic muscular pain. Pain 148:375–386, 2010 [DOI] [PubMed] [Google Scholar]
- 179.Peyron R, Laurent B, Garcia-Larrea L: Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30:263–288, 2000 [DOI] [PubMed] [Google Scholar]
- 180.Poldrack RA: Imaging brain plasticity: Conceptual and methodological issues–a theoretical review. Neuroimage 12: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 181.Poldrack RA: Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci 10:59–63, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Poldrack RA: Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2:67–70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, Nichols TE, Poline JB, Vul E, Yarkoni T: Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18:115–126, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE: Guidelines for reporting an fMRI study. Neuroimage 40:409–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Poldrack RA, Mumford JA, Nichols TE: Handbook of Functional MRI Data Analysis. Cambridge, Cambridge University Press, 2011 [Google Scholar]
- 186.Porro CA, Lui F, Facchin P, Maieron M, Baraldi P: Perceptrelated activity in the human somatosensory system: Functional magnetic resonance imaging studies. Magn Reson Imaging 22:1539–1548, 2004 [DOI] [PubMed] [Google Scholar]
- 187.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE: Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH: Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage 21:291–301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rissman J, Gazzaley A, D’Esposito M: Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23:752–763, 2004 [DOI] [PubMed] [Google Scholar]
- 190.Robinson LF, Atlas LY, Wager TD: Dynamic functional connectivity using state-based dynamic community structure: Method and application to opioid analgesia. Neuroimage 108:274–291, 2015 [DOI] [PubMed] [Google Scholar]
- 191.Rosa MJ, Seymour B: Decoding the matrix: Benefits and limitations of applying machine learning algorithms to pain neuroimaging. Pain 155:864–867, 2014 [DOI] [PubMed] [Google Scholar]
- 192.Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD: Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci 17:16071612, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sacchet MD, Knutson B: Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage 66:270–277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Salomons TV, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD: Perceived helplessness is associated with individual differences in the central motor output system. Eur J Neurosci 35:1481–1487, 2012 [DOI] [PubMed] [Google Scholar]
- 195.Salomons TV, Nusslock R, Detloff A, Johnstone T, Davidson RJ: Neural emotion regulation circuitry underlying anxiolytic effects of perceived control over pain. J Cogn Neurosci 27:222–233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Satpute AB, Wager TD, Cohen-Adad J, Bianciardi M, Choi JK, Buhle JT, Wald LL, Barrett LF: Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci U S A 110:17101–17106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schweinhardt P, Bountra C, Tracey I: Pharmacological FMRI in the development of new analgesic compounds. NMR Biomed 19:702–711, 2006 [DOI] [PubMed] [Google Scholar]
- 198.Seminowicz DA, Davis KD: Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol 97:3651–3659, 2007 [DOI] [PubMed] [Google Scholar]
- 199.Seminowicz DA, Moayedi M: The dorsolateral prefrontal cortex in acute and chronic pain. J Pain 18:1027–1035, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, Newhouse PA, Filippi CG, Keefe FJ, Naylor MR: Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14:1573–1584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Seymour B, Daw N, Dayan P, Singer T, Dolan R: Differential encoding of losses and gains in the human striatum. J Neurosci 27:4826–4831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Seymour B, O’Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R: Opponent appetitiveaversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8:1234–1240, 2005 [DOI] [PubMed] [Google Scholar]
- 203.Siegel JS, Mitra A, Laumann TO, Seitzman BA, Raichle M, Corbetta M, Snyder AZ: Data quality influences observed links between functional connectivity and behavior. Cereb Cortex 27:4492–4502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Siero JC, Hermes D, Hoogduin H, Luijten PR, Ramsey NF, Petridou N: BOLD matches neuronal activity at the mm scale: A combined 7T fMRI and ECOG study in human sensorimotor cortex. Neuroimage 101:177–184, 2014 [DOI] [PubMed] [Google Scholar]
- 205.Smith BH, Torrance N: Epidemiology of chronic pain, in Colvin LA, Fallon M (eds): ABC of Pain. Chichester, UK, Blackwell, 2012, pp 1–4 [Google Scholar]
- 206.Sporns O, Tononi G, Edelman GM: Theoretical neuroanatomy and the connectivity of the cerebral cortex. Behav Brain Res 135:69–74, 2002 [DOI] [PubMed] [Google Scholar]
- 207.Stenger VA, Boada FE, Noll DC: Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)-weighted functional MRI. Magn Reson Med 44: 525–531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Story GW, Vlaev I, Dayan P, Seymour B, Darzi A, Dolan RJ: Anticipation and choice heuristics in the dynamic consumption of pain relief. PLoS Comput Biol 11:e1004030, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Story GW, Vlaev I, Seymour B, Winston JS, Darzi A, Dolan RJ: Dread and the disvalue of future pain. PLoS Comput Biol 9:e1003335, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sullivan MJ, Bishop S, Pivik J: The pain catastrophizing scale: Development and validation. Psychol Assess 7:524–532, 1995 [Google Scholar]
- 211.Summers PE, Ferraro D, Duzzi D, Lui F, Iannetti GD, Porro CA: A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. Neuroimage 50:1408–1415, 2010 [DOI] [PubMed] [Google Scholar]
- 212.Sundermann B, Burgmer M, Pogatzki-Zahn E, Gaubitz M, Stuber C, Wessolleck E, Heuft G, Pfleiderer B: Diagnostic classification based on functional connectivity in chronic pain: Model optimization in fibromyalgia and rheumatoid arthritis. Acad Radiol 21:369–377, 2014 [DOI] [PubMed] [Google Scholar]
- 213.Tetreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN: Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol 14:e1002570, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Thunberg J, Lyskov E, Korotkov A, Ljubisavljevic M, Pakhomov S, Katayeva G, Radovanovic S, Medvedev S, Johansson H: Brain processing of tonic muscle pain induced by infusion of hypertonic saline. Eur J Pain 9:185–194, 2005 [DOI] [PubMed] [Google Scholar]
- 215.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M: Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain 9:883–891, 2008 [DOI] [PubMed] [Google Scholar]
- 216.Villemure C, Bushnell CM: Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 95:195–199, 2002 [DOI] [PubMed] [Google Scholar]
- 217.Vlaev I, Seymour B, Dolan RJ, Chater N: The price of pain and the value of suffering. Psychol Sci 20:309–317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R: Chronic spinal pain and physical-mental comorbidity in the United States: Results from the national comorbidity survey replication. Pain 113:331–339, 2005 [DOI] [PubMed] [Google Scholar]
- 219.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E: An fMRI-based neurologic signature of physical pain. N Engl J Med 368:1388–1397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN: Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wager TD, Keller MC, Lacey SC, Jonides J: Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26:99–113, 2005 [DOI] [PubMed] [Google Scholar]
- 222.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD: Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303:1162–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 223.Weiskopf N, Hutton C, Josephs O, Deichmann R: Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: A whole-brain analysis at 3 T and 1.5 T. Neuroimage 33:493–504, 2006 [DOI] [PubMed] [Google Scholar]
- 224.Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I: Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30: 16324–16331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wiech K, Ploner M, Tracey I: Neurocognitive aspects of pain perception. Trends Cogn Sci 12:8, 2008 [DOI] [PubMed] [Google Scholar]
- 226.Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ: Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage 27:59–69, 2005 [DOI] [PubMed] [Google Scholar]
- 227.Wiech K, Tracey I: Pain, decisions, and actions: A motivational perspective. Front Neurosci 7:46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Winston JS, Vlaev I, Seymour B, Chater N, Dolan RJ: Relative valuation of pain in human orbitofrontal cortex. J Neurosci 34:14526–14535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I: Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage 16:999–1014, 2002 [DOI] [PubMed] [Google Scholar]
- 230.Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD: Separate neural representations for physical pain and social rejection. Nat Commun 5:5380, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Woo CW, Krishnan A, Wager TD: Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage 91:412–419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Woo CW, Roy M, Buhle JT, Wager TD: Distinct brain systems mediate the effects of nociceptive input and selfregulation on pain. PLoS Biol 13:e1002036, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wunderlich K, Dayan P, Dolan RJ: Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci 15:786–791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD: Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yoshida W, Seymour B, Koltzenburg M, Dolan RJ: Uncertainty increases pain: Evidence for a novel mechanism of pain modulation involving the periaqueductal gray. J Neurosci 33:5638–5646, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zaitsev M, Maclaren J, Herbst M: Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging 42:887–901, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zebardast N, Crowley MJ, Bloch MH, Mayes LC, Wyk BV, Leckman JF, Pelphrey KA, Swain JE: Brain mechanisms for prepulse inhibition in adults with Tourette syndrome: Initial findings. Psychiatry Res 214:33–41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC: Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J Neurosci 35:15307–15325, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]