Abstract
The idiom heart of the matter refers to the focal point within a topic and, with regard to health and longevity, the heart is truly pivotal for quality of life. Societal trends worldwide continue toward increased percent body fat and decreased physical activity with coincident increases in chronic diseases including cardiovascular disease (CVD) as the top global cause of death along with insulin resistance, accelerated aging, cancer. Although long-term survival rates for CVD patients are grim, intense research efforts continue to improve both prevention and treatment options. Pharmacological interventions remain the predominant interventional strategy for mitigating progression and managing symptoms, but cellular therapies have the potential to cure or even mediate remission of CVD. Adult stem cells are the most studied cellular therapy in both preclinical and clinical investigation. This review will focus on the advanced therapeutic strategies to augment products and methods of delivery, which many believe heralds the future of clinical investigations. Advanced preclinical strategies using adult stem cells are examined to promote synergism between preclinical and clinical research, streamline implementation, and improve this imminent matter of the heart.
Keywords: Cardiac, Stem Cells, Enhancement, Regeneration
Introduction
Ensuring an effective means of regenerating cardiac tissue in damaged and/or aged hearts would provide a revolutionary means for restoration of function to pump oxygen-enriched blood through the body. Pharmacologics are well-established in the cardiovascular community for controlling multiple syndromes associated with cardiovascular dysfunction including but not limited to high blood pressure, arrhythmia, balanced Na+/Ca2+ exchange, low cardiac output and stroke volume, and prevention of sudden cardiac death. Unfortunately, current pharmacological strategies for treating heart failure usually serve to delay death rather than provide hope for restoration of functional tissue and hemodynamic performance to the heart.
The quest to restore compromised function and regenerate lost tissue in the failing heart led to implementation of clinical trials using adult stem cells in the past fifteen years, rooted in successful utilization of stem cells since discovery of remarkable regenerative potential in the latter half of the twentieth century. Multiple clinical trials using various adult stem cells with varied trial design highlight the desperate need for new approaches to both the stem cell therapy treatment and the clinical trial design to further advance cardiac regenerative medicine. Numerous laboratories are pursuing diverse preclinical studies to improve therapeutic strategies involving stem cell-mediated treatment of heart failure. This review focuses upon established and visionary preclinical research strategies to augment adult cellular therapeutic products and how such products will advance clinical research intended to improve function in the failing myocardium.
Designing for Cardiac Regenerative Medicine
Classification of cardiac regenerative products with the Food and Drug Administration (FDA) can be organized into drug, device, biological product or combination product. Statutory definitions of FDA products are set forth in sections 201(g) and 201(h) of the Federal Food, Drug and Cosmetic Act (FD&C Act) and codified in Title 21 Chapter 9 of the United States Code [1–3]. Specific to regenerative products in the body, the FDA is likely to follow guidelines outlined in “Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)” in Title 21 of the Code of Federal Regulations, Part 1271 [4]. Regulatory clarification regarding classification will evolve with approval of additional cell therapies and advancement to the clinic [5]. Researchers should remain mindful of the importance of designing therapeutic products that demonstrate the necessary safety and efficacy required by the FDA.
As knowledge and understanding of cardiac regenerative medicine advances, described as “generational” advances [6–8], refinements are incorporated into design and evaluation of products and delivery methods. Potential products can be evaluated during preclinical stages in vitro or in vivo whereas clinical outcomes are focused on patient outcomes. Selected primary in vitro analysis include proliferation, cell-cycle behavior, anti-senescence, migration, non-oncogenic transformation, cardiomyogenesis, secretome release, and co-culture response. Despite an impressive array of ex vivo metrics, in vivo preclinical performance is absolutely essential to determine potential success of the product as a therapeutic option. Unfortunately, meaningful determinations of important metrics such as cardiomyogenesis, proliferation, and a host of other measurements in the context of clinical trials with patients receiving stem cell treatments are impossible. Indeed, claims of regeneration based solely upon imaging cannot be validated without tissue-based analyses. Many early phase clinical trials are intended to assess safety and feasibility in relatively small patient cohorts without sufficient power for significant determinations of endpoints such as quality of life, long term adverse events, survival, and functional endpoints. As such, definitive research, novel approaches, and defining in vivo mechanisms remain critically important to allow for development, optimization, and implemention of efficacious therapeutic strategies.
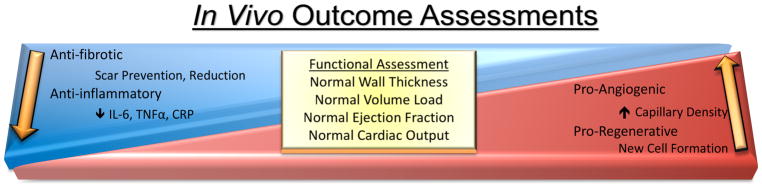
Benefits of the product are determined by a combination of responses to the therapeutic product including internal cellular responses, external endogenous responses to the product upon introduction into the system, external conditions of delivery and host response [6]. Primary benchmarks referenced to evaluate cellular product impact include durability, persistence, and endogenous response. The local microenvironment of the injection is influenced by inflammatory reaction, cell communication between product to endogenous tissue, and formation of new tissue. Another facet for critical consideration is the design and performance of the surgery including method of delivery and frequency of treatment. Lastly, and most important in evaluating a therapeutic product, is host condition and the host’s potential response to the therapeutic treatment, such as the severity of heart disease, biological age, and prognosis for functional recovery. Measurable outcomes are broader and more expansive in experimental models relative to limited analyses in clinical trials. Specifically, experimental research studies can encompass detailed cellular and molecular characterizations of inflammation, fibrosis, angiogenesis, and cellular regeneration along with functional assessments, such as wall thickness, load volume, ejection fraction and cardiac output (Figure 1). In comparison, clinical assessments or cellular and molecular mechanisms are narrow in scope or absent because of restricted sample availability from patients. Thus, clinical outcomes beyond functional assessments include standardized testing for patient quality of life and tracking for occurrences of adverse events. With a plethora of factors influencing outcomes, the complexity of efficacy evaluation presents a challenge to rigorously and fully evaluate regenerative therapy (Figure 1).
Figure 1. In Vivo Outcome Assessment when testing cardiac therapeutic products.
A mixture of form and functional attributes are measured to assess the overall effectiveness of a cardiac therapeutic option.
Enhancing Cellular Products
The desire to regenerate is fundamentally tied to the pathos of humanity since early civilization. Ancient Greek mythology tells the story of Prometheus who advanced human civilization by introducing mastery of fire; in return, Zeus punished Prometheus by chaining him to a rock where an eagle would peck out his liver every day and the liver would regenerate every night for eternity. The etymology of the name prometheus meaning forethought is serendipitous to the myth considering that the liver is one of the few naturally regenerative organs within the human body. Unlike the adult liver, the adult myocardium is ill-equipped for recovery of lost function or repopulation of damaged tissue. As such, cardiac tissue regeneration remains an important initiative for the medical research community as ischemic heart disease is the top cause of death and related complications, such as stroke and hypertensive heart disease, are additional top ten causes of death worldwide [9]. Cellular therapies for regenerative therapy are still in product development stages, but no commercial products are available in the United States [6].
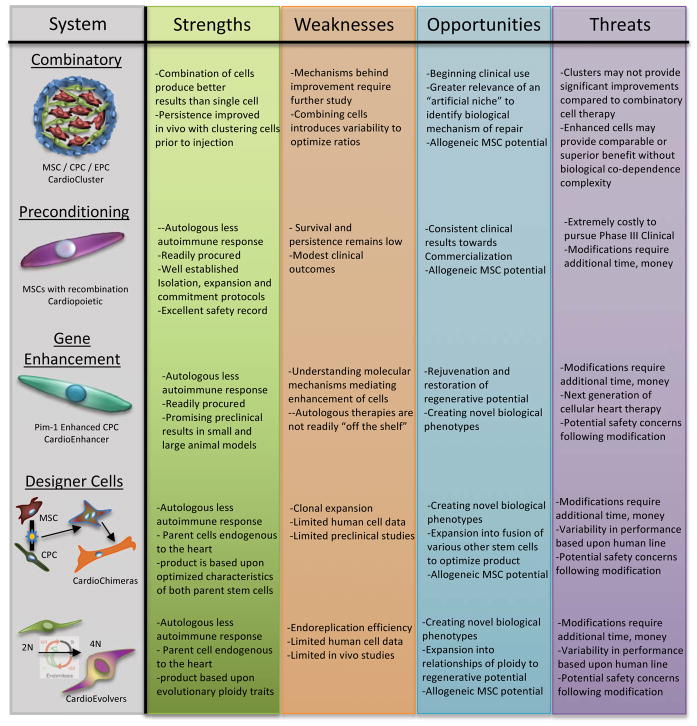
To improve upon cardiac regenerative products, understanding the state of endogenous cardiac cellular responses in normal aging as well as disease is critical [10,11]. Challenges in the heart with age and/or disease are to 1) preserve existing functional myocardium, 2) generate new, healthy cardiomyocytes, and 3) integrate new cardiomyocytes into the tissue with necessary vascular supply to fuel their function. New myocyte formation occurs, albeit at a very small, slow but steady rate of 0.45–1%, in normal physiologic circumstances within the adult heart [12]. The origin of new adult cardiomyocytes is still under investigation although the rate of cardiomyogenesis is demonstrably low under normal circumstances in the adult mammalian heart. Prevailing theories focus upon either preexisting cardiomyocytes [13,14] or adult stem cells [15–17] as the source, but reconciliation of these divergent origins for new myocytes may not require a single answer since the heart may possess more than one cardiomyogenic cell type, similar to the liver having multiple origins of new hepatocytes. Mechanisms of liver regeneration and cellular origins involved continue to be delineated [18], with origins including preexisting hepatocytes undergoing chromatin reduction to rapidly respond to new cell formation [19], and non-hepatocyte cells undergoing a progenitor state before transformation into hepatocytes [18,20]. Thus, the pursuit of clinically meaningful myocardial regeneration most likely also benefits from contributions of multiple cell types. Because clinical outcomes thus far have been less desirable than hoped or hyped, new strategies involving multiple cell types together with engineered approaches to augment regenerative potential are poised at the vanguard of next generation approaches to enhance cardiac cell therapies. Considering cellular products strictly, a number of enhancement strategies are in preclinical research intended to improve cardiac function in a diseased state. Cellular enhancement strategies can be categorized as combinatory, preconditioning and genetic enhancement with each strategy having its strengths and challenges towards clinical application. Enhanced therapeutic products discussed in this review are summarized in a Strengths Weaknesses Opportunities Threats (SWOT) analysis (Figure 2). Next generation approaches may evolve from observing basic mechanistic underpinnings of regeneration in organisms where such process are normal biological activities. Recapitulating regenerative capabilities of lower vertebrates using human cells and tissues has been frustratingly difficult. However, such biological activity is not beyond our organismal capabilities as humans do possess regenerative potential well into adulthood as evidenced in liver, lung, skin, and many other tissues. But specific constraints and barriers to regeneration in human myocardium have evolved consequential to functional performance. The challenge for cardiovascular researchers is to define those evolutionary boundaries, with the promise of future advancement resting upon innovation, creativity, and unnatural answers to enhance and promote myocardial regeneration in the human heart.
Figure 2. SWOT Analysis of Adult Stems Cells Enhancement Strategies for Cardiovascular Therapy.
The SWOT analysis focus is the individual adult stem cell therapeutic treatment options based on current clinical trial results and ongoing pre-clinical research.
Combinatory Cell Therapy
Combinatory cell therapy evolved from initial investigations using a single adult stem cell product to treat heart failure. The rationale behind combinatory adult stem cell therapy is hinged upon a synergistic effect occurring between the two cell types leading towards better functional and structural improvement within the failing heart compared to either cell type alone. Mesenchymal Stem Cells (MSCs) are known to secrete cytokines towards the survival and continued function of cardiomyocytes [21,22], inhibition of fibrosis [22,23]. Cardiac Stem Cells (CSCs), also referred to as Cardiac Progenitor Cells (CPCs), are known to assist in mitigating damage and preserving cardiomyocytes after infarction [24–26], as well as preserving and/or repairing vascularization [27,28] through release of secretome [29,30]. Multiple groups have demonstrated fusion between cardiomyocytes and CPCs [30,31] as well as formation of new myocytes from CPCs [15,17,31], both contributing to the functional value of CSC/CPC as a therapeutic treatment. Large-animal swine preclinical myocardial infarction modeling using the combinatorial approach of 200M MSCs with 1M CSC demonstrated cardiac recovery to nearly baseline [32], with a 21.1% scar size reduction compared to a reduction of 10.4% or 9.9% in CSCs and MSCs, respectively [32]. A follow-up large-animal (swine) preclinical study of ischemia/reperfusion injury with a three-month delay after injury before treatment with allogeneic MSCs, MSCs and CSCs or placebo demonstrated significant reduction in scar size of the treatment groups at three months post cell therapy [33]. A second follow-up study focused upon combining allogeneic MSCs and CSCs in a large-animal (swine) model [34]. Results at three months post injection demonstrate a reduced scar size in all treatment groups with ejection fraction improved in all cell treatment groups compared to placebo [34]. These large animal studies provide insight into study design at the clinical level.
The clinical trial Prospective Randomized Study Of MEsenchymal Stem Cell THErapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) was a double blind (participant, investigator) study with randomized treatment groups (clinicaltrials.gov: NCT00587990). Treatment groups included six patients with chronic ischemic cardiomyopathy undergoing coronary artery bypass grafting (CABG) with either 20 or 200 million autologous MSCs [23]. Magnetic resonance imaging was used to measure scar, perfusion wall thickness and contractility at baseline, 3, 6 and 18 months after treatment. After 18 months, overall left ventricle ejection fraction (LVEF) was improved (+9.4±1.7%) and scar mass was decreased (47.5±8.1%), compared to baseline [23]. Similar to PROMETHEUS, The Stem Cell Infusion in Patients with Ischemic CardiOmyopathy (SCIPIO) trial was an open label study with randomized treatment groups (clinicaltrials.gov: NCT00474461) performed in CABG patients with post-infarction EF of ≤40% [24]. CSCs were isolated from the patient’s right atrial appendage and re-infused intracoronarily 4±1 months after CABG surgery. 20 patients in total were treated with 1 million autologous CSCs and 13 patients did not receive cellular treatment. By one year, CSC-treated patients demonstrated an improved LVEF (10.3±2.2%) and a decrease in scar mass (43.2%) compared to non-treated patients (no significant change) [24]. A two-year follow-up study revealed that LVEF continued to improve over baseline in treated patients (11.9±2.7%) compared to nontreated patients (3.66±4.5) and scar mass continued to decrease (46.4%) [25]. The successful demonstration of MSCs and CPCs in clinical trials prompted advancing combinatory cellular therapy, which demonstrated improved results over a single cellular therapy in preclinical studies.
Two clinical trials are currently focused on the combinatory cell approach using MSCs and CSCs. The Combination Of MeseNchymal and C-kit+ Cardiac StEm Cells as Regenerative Therapy for Heart Failure (CONCERT-HF) is a multi-center, randomized, quadruple blinded (participant, care provider, investigator, outcomes assessor) Phase 2 clinical study evaluating the safety, feasibility and efficacy of autologous 150M MSCs and 5M CSCs, alone or in combination, compared to placebo, and administered transendocardially in subjects with ischemic heart failure (clinicaltrials.gov: NCT02501811). This study is currently underway. The Transendocardial Autologous Cells (hMSC) or (hMSC) and (hCSC) is Ischemic Heart Failure Trial (TAC-HFT-II) is a randomized single blinded (participant) Phase I/II placebo-controlled safety and efficacy study in patients with chronic ischemic left ventricular dysfunction and heart failure secondary to MI (clinicaltrials.gov: NCT02503280). In this trial, patients will receive either 200M MSCs, a mixture of 199M MSC with 1M CSC or a placebo; the trial is currently scheduled to begin enrollment in 2025.
In an alternative combinatory approach, the use of CSCs with saphenous vein-derived pericytes (SCPs) was tested in a small animal model [35]. Results demonstrated a reduction in infarction size with improved vascularization but not statistically improved contractility in comparison to the individual cellular therapy. An advanced combinatory approach is the use of CPCs, cardiac MSCs and endothelial progenitor cells (EPCs), which can be concurrently isolated from a single human cardiac biopsy [36]. With the concurrent isolation of these three stem cells, an autologous three-dimensional ‘CardioCluster’ can be formed through an in vitro mixing of well-defined cell types in predetermined three-dimensional architecture and diameter prior to injection [37]. CardioClusters provide a cellular scaffold, which allows cells to engraft better and persist longer within the tissue post injection compared to injecting individual cells (Figure 2). Preclinical in vivo studies are underway and highly anticipated given the known improved vascularization and cardiac repair and preservation mediated by EPCs [38,39]. Combinatory therapy approaches are demonstrating to be a safe and improved method of treating heart failure with immediate clinical implications.
In vitro Preconditioning
Adoptive transfer of in vitro expanded stem cells has, in general, demonstrated preclinical challenges of poor engraftment and persistence. Clinically, although improved over no treatment, patients rarely if ever recover substantial heart function after stem cell therapy. This presents the opportunity to improve the stem cell therapy treatment through augmentation of the cells in vitro during expansion, prior to delivery, as well as after adoptive transfer. One method to enhance these cells is through preconditioning by treatments with growth factors, anti-aging compounds, or hypoxia to induce higher secretome release, modifying their function and improving their therapeutic efficacy after injection. Most research on preconditioning of stem cells to date is in preclinical models but evidence of clinical effectiveness prompts further exploration of this enhancement strategy.
Preconditioning stem cells with growth factors is rooted in research from the late 2000’s. As bone-marrow MSCs are a popular choice for study due to their ease of isolation and purported efficacy in allogeneic settings, a number of preclinical preconditioning experiments were performed. Bone marrow MSCs were preconditioned with transforming growth factor-alpha (TGF-α) to promote vascular endothelial growth factor (VEGF) as well as paracrine activity via a p38 mitogen-activated protein kinase (MAPK) dependent mechanism [40]. Using an ex-vivo ischemia/reperfusion model in Sprague-Dawley rats, bone marrow MSCs from C57BL/6J mice that were expanded in vitro and preconditioned with TGF-α or TGF-α with a p38 MAPK inhibitor and injected into the ex-vivo hearts prior to ischemia and compared to a placebo and non-preconditioned MSCs. Results observed include an improved left ventricular developed pressure and greater +dP/dt along with −dP/dt at end-reperfusion after TGF-α MSCs injection compared to the p38 MAPK inhibitor TGF-α MSCs injection. Additionally, VEGF production was increased in the TGF-α preconditioned MSC and downregulated in p38 MAPK negated TGF-α MSCs. In another study bone marrow MSCs from Fisher 344 rats were preconditioned with: fibroblast growth factor (FGF) −2, insulin-like growth factor (IGF) −1 and bone morphogenetic protein (BMP) −2 and the combinatory growth-factor preconditioned MSCs were used in an adoptive transplant of a rat myocardial infarction model. [41]. Results demonstrate a statistically significant reduced scar size after eight weeks in the growth-factor preconditioned MSC-injected hearts, compared to the untreated MSC-injected hearts along with a statistically significant improved fractional shortening by four weeks, sustained through eight weeks, in the combinatory growth-factor preconditioned MSCs compared to non-preconditioned MSCs or placebo group. The group also found enhanced gap junction formation in the growth-factor preconditioned MSC group and cited this as a probable mechanism as to why the growth-factor preconditioned MSC group performed better than the non-treated MSC or placebo group [41]. In vitro experimentation has also demonstrated IGF-1 and FGF-2 preconditioning improves functional characteristics of diabetes-impaired MSCs through upregulation of VEGF and Angiopoietin 1 and downregulation of p16Ink4a, indicating improved angiogenesis potential [42].
Preconditioning of bone marrow MSCs into cardiac progenitor phenotypes prior to injection has also led to extensive preclinical and clinical research with attendant strengths and weaknesses (Figure 2). Terzic and colleagues used a defined cocktail of recombinant TGF-β1, BMP-4, IGF-1, FGF-2, Activin-A, retinoic acid, α-thrombin and interleukin (IL) −6 to transform hMSCs into cardiac progenitor-like cells, coined ‘cardiopoietic’ stem cells [43]. These cardiopoietic stem cells were then injected into infarcted myocardium of nude mice and followed over one year, with comparison to hMSCs and saline control injected mice. One-year results demonstrated a reduced global fibrosis and scar size in cardiopoietic-treated hearts 8±6% and 3±2%, respectively, compared to naïve hMSC-treated hearts at 32±4% and 12±2%, respectively. Absolute LVEF was statistically improved in cardiopoietic-treated hearts compared to baseline after infarction with an improvement from 35% to 45% at one year, with a peak at two months post treatment at 55%. Naïve hMSC-treated hearts resulted in little LVEF improvement over baseline at one year and no statistical difference was found between saline and naïve hMSC-treated hearts. This study led to a randomized, single blinded (outcomes assessor) Phase II/III clinical trial Cardiopoietic stem Cell therapy in heart failURE (C-CURE) which focused on the safety, feasibility and efficacy of using cardiopoietic stem cells for treating ischemic cardiomyopathy heart failure patients (clinicaltrials.gov: NCT00810238). After exclusion of a potential 320 screened patients, 21 patients received cardiopoietic hMSCs and 15 patients received typical standard of care treatment [44]. Follow-up was performed at six months and two years with the study completed in 2012. Results at six months revealed a 7% LVEF improvement in the treatment group from 27.5±1.0% to 34.5±1.1%, whereas the LVEF was unchanged in the control group (27.8±2.0% to 28.0±1.8%). Additional improvements with the 6-min walk distance and LV end-systolic volume were documented. The subsequent Phase III Congestive Heart Failure CArdiopoietic Regenerative Therapy (CHART-1) clinical trial, held in multiple European countries and Israel (clinicaltrials.gov: NCT01768702), was randomized, double blinded (participant and outcomes assessor) and focused upon using a “guided cardiopoiesis” approach, meaning the cardiopoietic stem cells were delivered using an endoventricular injection catheter with key assessment points at 52 and 104 weeks post-injection [45]. Three hundred fifteen patients were enrolled in the study, which was officially completed in August 2017. Reported results at thirty-nine weeks were primarily neutral but LVEF appeared to improve in patients with the cell therapy [46]. Sixty percent of the cell therapy treatment group was reported to exhibit statistically significant positive outcomes and post-hoc analyses are now identifying key attributes of this patient subpopulation to better define candidates for stem cell therapy treatment. The trial serves as a basis for designing the CHART-2 US trial, which is not yet scheduled to begin enrollment.
An alternative approach to preconditioning with growth factors is use of an environmental stimulus. After an infarction, the ischemic region is infiltrated with inflammatory cells and signals with a substantial concentration of reactive oxygen species (ROS) [47]. This results in a challenging microenvironment for endogenous cells as well as transfused cells. Mimicking the ischemic environment rich in ROS through preconditioning stem cells with hydrogen peroxide was hypothesized to render adoptively transferred cells more tolerant and communicative with the ischemic environment in vivo. Indeed, CPCs preconditioned with hydrogen peroxide improve their therapeutic effect over non-preconditioned CPCs in infarcted rat hearts by stimulating neoangiogenesis in the infarction border zone [48]. Interestingly, MSCs pretreated with hydrogen peroxide resulted in a decrease of focal adhesion proteins and less adherence in the infarcted heart region of Sprague-Dawley rats [49]. To improve adhesion and migration of these preconditioned hydrogen peroxide MSCs the cells were co-injected with free radical scavenger N-acetyl-L-cysteine, which resulted in reduced fibrosis and infarction size. An alternative preconditioning microenvironment to ROS provided by hydrogen peroxide is the use of hypoxia, which mimics the infarction region insofar as a lack of oxygen. Preconditioning MSCs in a hypoxic environment and injecting these cells into the peri-infarction region of rats resulted in increased pro-angiogenesis, statistically smaller infarctions and improved systolic and diastolic pressure in the left ventricle, measured six weeks after infarction [50]. Likewise, hypoxia preconditioned CPCs demonstrated better survival seven days after injection into C57BL/6 mice as well as improved LVEF and FS, as compared to normoxic CPCs or placebo [51]. The use of environmental stimulus preconditioning technique typically demonstrated improved functional effects in the infarcted heart as compared to non-treated cells, but the transience of such preconditioning approaches remains a concern. Therefore, other studies have pursued permanent modification to improve cellular function and response to adoptive transfer in search of superior in vivo results.
Gene Enhancement
Lentiviral vectors in gene therapy are a method in which genes can be inserted, deleted or modified in cells using a lentivirus. Lentivirus is a retrovirus with a single stranded RNA genome with a reverse transcriptase enzyme that allows for transcription of the viral genetic material upon entering the cell. Lentiviral vectors integrate into sites away from transcriptional regulatory sites, making them a safe therapeutic option [52] and have, in fact, become incorporated into clinical therapies [53] for advanced forms of HIV infections [54], Parkinson’s disease [55], and inherited disorders affecting hematopoietic cells [56]. This past August the FDA approved the first lentiviral gene therapy available in the US for the treatment of refractory B-cell acute lymphoblastic leukemia (ALL), a cancer of the bone marrow and blood, in patients up to 25 years old [57].
Chimeric antigen receptor (CAR) T-cell immunotherapy mediated by Tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corp) has generated excitement using autologous T cells collected during a leukapheresis procedure and genetically modified to insert a CAR protein. The modified T-cells are capable of identifying CD19-expressing normal and malignant cells and after these modified T-cells are reintroduced into the patient in a single treatment, the modified T-cells target and eliminate CD19-expressing cells. The first pediatric global CAR-T cell therapy was an open label Phase 2, single arm, multicenter trial with twenty-five centers involved throughout the US, EU, Canada, Australia and Japan (clinicaltrials.gov: NCT02228096). Sixty-eight patients were infused and 63 were evaluated for efficacy with 83% of the patients achieving complete remission or complete remission with incomplete blood count recovery within three months of infusion with no minimal residual disease, indicating potential relapse, detected among patients [58]. One of the main side effects is cytokine release syndrome (CRS), causing high fever and flu-like symptoms, as well as neurological events, both of which can be life-threatening. In response to treat CAR, the FDA approved Actemra (tocilizumab), which was resolved 69% of the time with one or two doses within two weeks after treatment [59]. This ground-breaking treatment demonstrates the advancement of gene therapy treatments involving permanent ex vivo cell modification from the clinic as a tractable commercial strategy as well as the effectiveness of a gene therapeutic strategy for mitigating human disease.
Gene therapy using adult stem cells to treat heart failure is a therapeutic approach with preclinical data dating back to early 2000’s. Rat MSCs genetically enhanced with prosurvival gene Akt1 demonstrated improved cardiac output and a blunting of lost myocardial volume and remodeling, compared to control lacZ MSCs and saline treated ischemic rat myocardium, in a dose-dependent manner [60]. Similarly, nuclear Protein kinase B (Akt) overexpression in murine CPCs demonstrated increased proliferation and paracrine factor secretion in vitro as well as increased recruitment of endogenous ckit+ cells in vivo, compared to non-enhanced CPCs [61]. Stromal-derived factor-1 (SDF-1) is a chemokine required for homing and increasing survival of progenitor cells. MSCs engineered to overexpress SDF-1 were found to improve cardiac function 5 weeks after injection by nearly 240% compared to saline control and 70% in non-engineered MSCs within an acute myocardial infarction rat model [62]. MSCs engineered to overexpress CXCR4, the cognate receptor for SDF-1, were also found to decrease ventricular remodeling in response to myocardial infarction in a rat model after 30 days [63]. Mechanistically, the basis for potentiation of reparative responses mediated by such modifications can include enhanced survival, proliferation, persistence, engraftment, or secretion from adoptively transferred cells.
Few, if any, preclinical ex vivo genetic modifications for cardioreparative therapy are better documented for mechanism and efficacy than the proto-oncogene Pim-1 (Proviral Integration Moloney Kinase 1). Pim-1 is a highly conserved serine/threonine kinase unique by constitutive activation, meaning it is active in its nascent translated form rather than having to become “activated” like most kinases [64,65]. Pim-1 activity is regulated by concerted control of gene transcription, mRNA translation and protein degradation. The target phosphorylation consensus sequence for Pim-1 is found in proteins mediating transcription, cell growth, proliferation, and survival [66,67]. Transgenic mouse experiments [68–70] and cell culture experiments [71–74] demonstrate Pim-1 exerts potent synergistic activity with c-MYC, p21Cip1/WAF1, STAT3, JNK, and survivin, as well as with other proteins particularly when the protein function involves proliferation and cell survival. Pim-1’s role in cell survival is related to the fact that it is a downstream target of Akt kinase and Akt-dependent survival is attributed, in part, to the interaction of Pim-1 with Bad, a primary apoptotic initiator [75]. Pim-1 also regulates activity independent of the Akt pathway, with regulation of the ASK1 proapoptotic pathway [76] as well as p38 MAPK [77]. Pim-1 is naturally found within cardiac tissues.
Pim-1 is produced in response to stress or pathologic injury in the myocardium. Pim-1 is also expressed in stem cells [78] upon activation as well as in endothelial [79] and vascular smooth muscle cells [80]. Primary downstream molecular targets of Pim-1 regulate cell survival and mitotic activity [81]. Pim-1 expression is higher in fetal human CPCs (hCPCs) and correlation exists between Pim-1 expression and youthful phenotypic characteristics of hCPCs, regardless of human cell line or age [82]. Pim-1 enhanced human CPCs (hCPCeP) demonstrate youthful characteristics that provide statistically significant improvement over other stem cell therapy treatments for myocardial infarction [82,83], with numerous strengths (Figure 2). Salutary effects of Pim-1 modification on cardiac progenitor cells in vitro proliferation, normal karyotyped diploid content [83] and sustained telomere lengths were consistent with suppression of p53 and p16 and blunting of the senescent phenotype [82]. Using adult SCID mice, human CPCs rejuvenated with Pim-1 overexpression were delivered by intramyocardial injection concurrent with myocardial infarction and compared to non-enhanced hCPCs or vehicle alone [83]. Persistence, expansion, and integration of Pim-1 enhanced hCPCs into myocardial tissue translate into progressive improvement in myocardial structure and function up to 20 weeks post-delivery relative to control hCPCs or vehicle [83]. The in vitro and small animal in vivo findings using Pim-1 enhanced hCPCs warranted further studies using a large animal model.
In a large animal model using adult female Yorkshire swine, the swine underwent closed-chest ischemic reperfusion myocardial infarction [84] and received cell therapy two weeks after infarction challenge [85]. Swine treated with Pim-1 enhanced hCPCs exhibit ~3-fold decrease in scar mass compared to non-enhanced hCPCs at 8 weeks post-injection at −29.2±2.7% versus −8.4±0.7%, respectively (p<0.003). Pim-1 enhanced hCPC treated animals also had a sustained significantly increased ejection fraction at both 4 and 8 weeks post injection, compared to improvements in the hCPC-treated animals only found at 4 weeks post injection. Importantly, cardiac output and ejection fraction by 8 weeks post injection was comparable with the pre-infarction measurement in swine treatment with Pim1 enhanced-hCPCs. Mechanoenergetic recoupling improved in the Pim1 group. Importantly to note, biopsies were taken from each slice to include infarct zone (IZ), border zone (BZ), and remote zone (RZ) for 30 biopsies from each heart and examined by an experienced pathologist blinded to the treatment groups. Transmural infarcts were observed in all hearts characterized by densely collagenized scar tissue with mild-to-moderate cellularity. Histological evaluation findings for oncogenic transformation, tumorigenicity, teratomas, ectopic tissue formation or similar neoplastic plastic processes were all negative for all CPC treated animals on whole body necropsy either at 4 or 8 weeks following study product administration [85] Durability of repair, demonstrated safety and lack of oncogenic activity and superior improvement of myocardial hemodynamic performance in both the small and large animal, supports use of Pim-1 enhanced hCPCs in a clinical setting. With the advancements in lentiviral vectors and CAR-T therapy, ex vivo autologous gene therapy such as Pim-1 modification is gaining acceptance as a safe and effective means to treating disease.
Designer Cells
As an adjective, “designer” refers to the aesthetics of an object in which it may perform a specific function or combat a specific problem. For example, a designer baby is a baby genetically engineered for specially selected traits, whereas designer drugs are structural analogues with similar properties and effects to the parent drug intended to enhance performance while avoiding detection or classification (e.g. professional sports doping). In these instances, the intent is deliberate modification of the original agent in order to enhance fitness and performance. A similar strategy can also be employed to develop designer cells as genetically engineered cellular products with modified properties to serve as enhanced therapeutic agents to combat human disease and assist in repair and regeneration. This avant-garde approach relies heavily upon design thinking [86] and will lead to new scientific discoveries and understandings with basic science to provide new, clinically relevant opportunities to cure heart failure. Such next generation approaches also resonate with the Precision Medicine Initiative, taking into account an individual’s variability in genes, environment and lifestyle when defining prevention strategies and treatment for specific diseases.
Design of therapeutic cells is intended to overcome clinical challenges revealed from testing multiple stem cell types, expanded in vitro and used in an autologous or allogeneic manner, with safe but marginal outcomes overall [6]. Endogenous stem cell reparative capabilities are likely compromised by aging and heart disease [9, 87–89], highlighting the need to develop novel methodologies to improve stem cell treatment through identification and optimization of important cellular and in vivo performance characteristics [90,91]. Designing cells to optimize their performance empowers capabilities to deal with challenges of the harsh microenvironment and compromised tissue structure typical for pathologic injury or age [92,93]. Conceptually, designing cellular therapeutic products for cardiac repair and regeneration represents “cutting edge” engineering to overcome limitations inherent in cell therapy and optimize induction of repair and regenerate the pathologically injured heart [94,95]. Two examples of designer approaches involving innovative adaptations of adult stem cells are provided below.
One approach explored in creating designer cells is the formation of a hybrid stem cell [94], based upon the idea of fusing two stem cells and selecting clonally expanded cell lines demonstrating the most beneficial characteristics to treat the myocardium. Fusion has been identified to naturally occur in vivo, specifically with respect to stem cells in the heart [31,96]. The mechanism behind endogenous fusion and any benefits, specific to these fused cells in vivo, has not yet been identified. Chemically-induced fusion of FVB mouse MSCs with mouse CPCs using Sendai virus resulted in a hybrid stem cell, termed ‘CardioChimera’, demonstrating properties of both parent cells [97], with various strengths, weaknesses, opportunities and threats as a therapeutic product (Figure 2). These distinct and clonally derived cells have an in vitro proliferation rate similar to CPCs and statistically faster to MSCs with a secretome profile enhanced to MSCs and statistically higher than CPCs. Treatment of infarction injury with CardioChimeras normalized left ventricular wall structure and sustained improved cardiac function for at least 18 weeks after treatment, compared to either of the parent cells or a combination of the two parents. These initial studies were performed using chimeric murine lines, and translational research is ongoing to determine the potential of human CardioChimeras.
A second approach to design a novel stem cell with enhanced reparative potential draws upon observations of DNA content and ploidy [95]. Increasing DNA and chromatin content have been hypothesized as a strategic biological evolutionary approach to overcome challenges imposed by environmentally stressful environments that mimics highly regenerative life, such as plants [98,99] and lower vertebrate organisms including fish [100,101] and newts [102]. Zebrafish, a frequently used model organism, are capable of regenerating 20% of the heart after ventricular resection [103]. Zebrafish are also known to have undergone whole genomic duplication [101], making their DNA content equivalent to that present in a polyploid organism. Also, certain species undergo genome duplication in response to environmental stress, possibly as an adaptive strategy for easing the burden of regeneration and adaptation to stress [104]. Analysis of CPC ploidy content shows that mouse CPCs possess mononuclear tetraploid content, whereas human CPCs possess mononuclear diploid content [105]. This fundamental biological difference between species may provide insight regarding differentially greater improvement in small mammal models relative to clinical outcomes revealed by meta-analysis [106], although cell treatment is demonstrably better than no treatment in a clinical setting [107]. Current research regarding stem cell ploidy is focused upon investigating mechanisms supporting improved regenerative capacity when tetraploid cells are used in adoptive transfer studies as well as developing “designer” human tetraploid stem cells, termed ‘CardioEvolver’ to mimic lower vertebrates and test the possibility that higher ploidy will confer a functional advantage (Broughton and Sussman, in preperation), with associated strengths, weaknesses opportunities and threats as a therapeutic product (Figure 2). Futuristic approach using designer cells requires substantial basic scientific research and concomitant mechanistic understanding before achieving clinical implementation. Nevertheless, benefits of expanding knowledge and understanding of genomic content and how specific genes influence regenerative capacity will further the potential to heal the human heart during pathologic injury as well as potentially offer advanced longevity options impacting whole body processes.
Perspective
After over a decade of myocardial regenerative research studies, the initial optimism and enthusiasm that fueled rapid and widespread adoption of cellular therapies for heart failure has given way to more pragmatic, realistic, and achievable goals. Indeed, substantial progress and increased understanding has developed that guides future directions of experimental cell therapy studies. However, it comes as no surprise to anyone familiar with myocardial biology that the process of endogenous mammalian cardiac regeneration is extremely inefficient with debatable relevance for clinical treatment as currently implemented. These realities, while challenging, should not be misinterpreted as a basis for abandoning the concept of myocardial cell therapy. Instead, the clear message from the cumulative literature is that large mammals do not possess reparative potential of lower vertebrates and that dependence upon endogenous reparative mechanisms to mediate clinically meaningful regeneration is a long shot, especially in the typical large market target population of elderly patients in need of such interventional approaches. Truthfully, accepting that humans are not zebrafish or mice is one of the few universal consensus facts that all researchers engaged in regenerative therapy can agree upon. Given that reality, attention turns toward the differences that define the remarkable reparative potential of lower vertebrates relative to humans. Reproducibly demonstrable regeneration can be studied in organisms such as zebrafish or neonatal mice, indicating that such capabilities are natural and biologically normal in those specific systems. Continued translational research spanning invertebrate through small mammals and humans will undoubtedly blaze new trails for unraveling differences in regulatory control of cardiomyogenesis in adults. Nevertheless, expecting larger mammals to recapitulate the impressive regenerative capabilities of lower species remains a disappointing endeavor for one primary reason: the imposition of a demand to respond unnaturally and contrary to the biology of the adult mammalian myocardium. Therefore, it stands to reason that promotion of regeneration in a context where such processes do not naturally occur will by necessity require an unnatural solution. Of course, even the protocol of delivering exogenous cells to injured or aged tissue is in itself unnatural – and also evidently insufficient to yield desired results. Even as cell therapy continues to evolve, concurrent post stem cell generational findings including cell products or cell product components (secretomes, exosomes, modified versions of them, etc.) could ultimately be a legacy of stem cell biomedicine. Additional layers of “outside the box” strategies concurrently applied to the basic premise of cell therapy do indeed yield additional benefits as covered in this review. From proven preclinical approaches of combinatorial cell treatments, preconditioning cells, and genetic engineering to the next generation of “designer” cells, future advances in cell therapy will undoubtedly need to confront inherent limitations by incorporation of visionary concepts not present in normal human myocardial biology.
Acknowledgments
Sources of Funding
M.A. Sussman is supported by NIH grants: R01HL067245, R37HL091102, R01HL105759, R01HL113647, R01HL117163, 01HL085577, and R01HL122525, as well as an award from the Fondation Leducq. K.M. Broughton is supported by NIH grant F32HL136196.
Abbreviations
- CSCs
Cardiac Stem Cells
- CPCs
Cardiac Progenitor Cells
- EPCs
Endothelial Progenitor Cells
- MSCs
Mesenchymal Stem Cells
- SCPs
Saphenous Vein-derived Pericytes
- CABG
coronary artery bypass grafting
- LVEF
left ventricle ejection farction
- C-CURE
Cardiopoietic stem Cell therapy in heart failURE
- CHART-1
Congestive Heart Failure CArdiopoietic Regenerative Therapy
- CONCERT-HF
Combination Of MeseNchymal and C-kit+ Cardiac StEm Cells as Regenerative Therapy for Heart Failure
- PROMETHEUS
Prospective Randomized Study Of MEsenchymal Stem Cell THErapy in Patients Undergoing Cardiac Surgery
- SCIPIO
Stem Cell Infusion in Patients with Ischemic CardiOmyopathy
- TAC-HFT-II
Transendocardial Autologous Cells (hMSC) or (hMSC) and (hCSC) is Ischemic Heart Failure Trial
- Akt
Protein kinase B
- BMP
bone morphogenetic protein
- CAR
Chimeric antigen receptor
- c-MYC
cellular Myelocytomatosis Viral Oncogene Homolog
- FGF
fibroblast growth factor
- IGF
insulin-like growth factor
- IL
interleukin
- JNK
Jun Amino-terminal Kinases
- MAPK
mitogen-activated protein kinase
- Pim-1
Proviral Integration Moloney Kinase 1
- SDF-1
Stromal-derived factor-1
- STAT3
Signal Transducer and Activator of Transcription 3
- TGF-α
transforming growth factor-alpha
- VEGF
vascular endothelial growth factor
- WAF1
Wild-type P53-Activated Fragment 1
Footnotes
Disclosures
M.A. Sussman is a founder with substantial interest in CardioCreate Inc. K.M. Broughton has a substantial interest in CardioCreate Inc.
References
- 1.United States Code. Supplement 5, Title 21 - FOOD AND DRUGS. 2006:25–449. [Google Scholar]
- 2.United State Food and Drug Association. Importation of Active Pharmaceutical Ingredients (APIs) Requirements FD&C Act 201(g) & (p) [21 USC 321(g) & (p)] Definitions; generally (Tab B).
- 3.Food and Drug Administration. Draft Guidance. 2011. Guidance for Industry and FDA Staff: Classification of Products as Drugs and Devices & Additional Product Classification Issues. [Google Scholar]
- 4.FDA. Small Entity Compliance Guide. 2007. Guidance for Industry: Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) [Google Scholar]
- 5.Halme DG, Kessler DA. FDA regulation of stem-cell–based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 6.Broughton KM, Sussman MA. Empowering adult stem cells for myocardial regeneration V2. 0. Circ Res. 2016;118(5):867–880. doi: 10.1161/CIRCRESAHA.115.305227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambria E, Steiger J, Günter J, Bopp A, Wolint P, Hoerstrup SP, Emmert MY. Cardiac regenerative medicine: the potential of a new generation of stem cells. Transfusion Medicine and Hemotherapy. 2016;43(4):275–81. doi: 10.1159/000448179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanz-Ruiz R, Climent AM, Fernández-Santos ME, Arranz AV, Ibañes EG, Vázquez-Álvarez ME, Fernández-Avilés F. General Overview of the 14th International Symposium on Stem Cell Therapy and Cardiovascular Innovations: Working Progress of a Global Initiative in 2017; 2017; pp. 1040–43. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart disease and stroke statistics-2016 update: A Report from the American Heart Association. Circulation. 2016;133(4):447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 10.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 11.Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovascular research. 1992;26(2):101–8. doi: 10.1093/cvr/26.2.101. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, Zhou L. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation. 2017;136(9):834–48. doi: 10.1161/CIRCULATIONAHA.116.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci. 2003;100(18):10440–5. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfò M, Agosti V. Adult c-kit pos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Smith AJ, Torella A, Nadal-Ginard B, Vicinanza C, Terracciano CM, Indolfi C, Torella D, Britti D, Cianflone E, Marino F, Lewis FC. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell death and differentiation. 2017;24:2101. doi: 10.1038/cdd.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preziosi ME, Monga SP, Michalopoulos GK, DeFrances MC, Kuramitsu K, Sverdlov DY, Liu SB, Luedde T, Kaplowitz N, Schwabe RF, Bernardi M. Update on the Mechanisms of Liver Regeneration. In Seminars in Liver Disease Thieme Medical Publishers. 2017;37(2):141–151. doi: 10.1055/s-0037-1601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan AW, Taylor MH, Hickey RD, Newell AEH, Lenzi ML, Olson SB, Finegold MJ, Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467(7316):707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raven A, Lu WY, Man TY, Ferreira-Gonzalez S, O’Duibhir E, Dwyer BJ, Thomson JP, Meehan RR, Bogorad R, Koteliansky V, Kotelevtsev Y. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547(7663):350–4. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell stem cell. 2012;10(3):244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–35. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 23.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous Mesenchymal Stem Cells Produce Concordant Improvements in Regional Function, Tissue Perfusion, and Fibrotic Burden When Administered to Patients Undergoing Coronary Artery Bypass Grafting The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) Trial. Circ Res. 2014;114(8):1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Bolli R, Chugh AR, D’Amario D, et al. Effect of cardiac stem cells in patients with ischemic cardiomyopathy: interim results of the SCIPIO trial up to 2 years after therapy. Circulation. 2012;126(23):2784. [Google Scholar]
- 26.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, Higuchi K, Siatskas C, Lee P, Lim H, Zhang J. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci. 2006;103(7):2304–9. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nature medicine. 2003;9(6):702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 28.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clinical Investigations. 2001;107(11):1395. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clinical Investigation. 2009;119(8):2204. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Zhu W, Bender I, Gong W, Kwak IY, Yellamilli A, Hodges TJ, Nemoto N, Zhang J, Garry DJ, van Berlo JH. Pathologic Stimulus Determines Lineage Commitment of Cardiac C-kit+ Cells. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.030137. CIRCULATIONAHA-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation. 2013;127(2):213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. Journal of the American College of Cardiology. 2015 Nov 3;66(18):1990–9. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natsumeda M, Florea V, Rieger AC, Tompkins BA, Banerjee MN, Golpanian S, Fritsch J, Landin AM, Kashikar ND, Karantalis V, Loescher VY. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. Journal of the American College of Cardiology. 2017 Nov 21;70(20):2504–15. doi: 10.1016/j.jacc.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avolio E, Meloni M, Spencer HL, et al. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116(10):e81–e94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]
- 36.Monsanto MM, White KS, Kim T, Wang BJ, Fisher K, Ilves K, Khalafalla FG, Casillas A, Broughton K, Mohsin S, Dembitsky WP. Concurrent Isolation of Three Distinct Cardiac Stem Cell Populations from a Single Human Heart Biopsy. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.116.310494. CIRCRESAHA-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monsanto MM, White KS, Wang J, Fisher K, Sussman MA. CardioClusters: Harnessing the Power of Multi-Lineage Cardiac Stem Cells. Circulation. 2016:A19532. [Google Scholar]
- 38.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci. 2000;97(7):3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of molecular and cellular cardiology. 2005;39(5):733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33(1):24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 41.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. JACC. 2008;51(9):933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Khan M, Akhtar S, Mohsin S, Khan SN, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem cells and development. 2010;20(1):67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 43.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. JACC. 2010;56(9):721–34. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. JACC. 2013;61(23):2329–38. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 45.Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B, Metra M, Filippatos G, Hajjar R, Behfar A, Homsy C. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur J Heart Failure. 2016;18(2):160–8. doi: 10.1002/ejhf.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur J Heart Failure. 2017;38(9):648–60. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews Cardiology. 2014;11(5):255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pendergrass KD, Boopathy AV, Seshadri G, Maiellaro-Rafferty K, Che PL, Brown ME, Davis ME. Acute preconditioning of cardiac progenitor cells with hydrogen peroxide enhances angiogenic pathways following ischemia-reperfusion injury. Stem cells and development. 2013;22(17):2414–24. doi: 10.1089/scd.2012.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song H, Cha MJ, Song BW, Kim IK, Chang W, Lim S, Choi EJ, Ham O, Lee SY, Chung N, Jang Y. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28(3):555–63. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 50.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135(4):799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 51.Yan F, Yao Y, Chen L, Li Y, Sheng Z, Ma G. Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1alpha-CXCR4 axis. PloS one. 2012;7(7):e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modlich U, Navarro S, Zychlinski D, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretro- viral vectors. Mol Ther. 2009;17:1919–28. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schambach A, Baum C. Clinical application of lentiviral vectors— concepts and practice. Curr Gene Ther. 2008;8:474–82. doi: 10.2174/156652308786848049. [DOI] [PubMed] [Google Scholar]
- 54.Mautino MR. Lentiviral vectors for gene therapy of HIV-1 infection. Curr Gene Ther. 2002;2:23. doi: 10.2174/1566523023348165. [DOI] [PubMed] [Google Scholar]
- 55.Lundberg C, Bjorklund T, Carlsson T, et al. Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr Gene Ther. 2008;8:461–473. doi: 10.2174/156652308786847996. [DOI] [PubMed] [Google Scholar]
- 56.Woods NB, Ooka A, Karlsson S. Development of gene therapy for hematopoietic stem cells using lentiviral vectors. Leukemia. 2002;16:563–569. doi: 10.1038/sj.leu.2402447. [DOI] [PubMed] [Google Scholar]
- 57.US Food and Drug Administration. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. FDA. 2017 [ https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm574154.htm]
- 58.Novartis: receives first ever FDA approval for a CAR-T cell therapy, Kymriah (TM)(CTL019), for children and young adults with B-cell ALL that is refractory or has relapsed at least twice. Novartis. 2017 Aug 30; [ https://novartis.gcs-web.com/novartis-receives-fda-approval-for-KymriahTM]
- 59.US Food and Drug Administration. FDA approval brings first gene therapy to the United States. FDA; 2017. [ https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm] [Google Scholar]
- 60.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 61.Fischer KM, Din S, Gude N, Konstandin MH, Wu W, Quijada P, Sussman MA. Cardiac Progenitor Cell Commitment Is Inhibited by Nuclear Akt Expression. Circ Res. 2011;108(8):960–70. doi: 10.1161/CIRCRESAHA.110.237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. The FASEB Journal. 2007;21(12):3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 63.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Molecular Therapy. 2008;16(3):571–9. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 64.Siddiqi S, Sussman MA. Cell and gene therapy for severe heart failure patients: the time and place for Pim-1 kinase. Expert review of cardiovascular therapy. 2013;11(8):949–57. doi: 10.1586/14779072.2013.814830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 66.Domen J, Von ML, Hermans A, Breuer M, Grosveld G, Berns A. Comparison of the human and mouse PIM-1 cDNAs: nucleotide sequence and immunological identification of the in vitro synthesized PIM-1 protein. Oncogene Research. 1987;1(1):103–12. [PubMed] [Google Scholar]
- 67.van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 68.Moroy T, Grzeschiczek A, Petzold S, Hartmann KU. Expression of a PIm-1 transgene accelerates lymphoproliferation and inhibits apoptosis in lpr/lpr mice. Proc Natl Acad Sci US A. 1993;90:10734–10738. doi: 10.1073/pnas.90.22.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baron BW, Anastasi J, Hyjek EM, Bies J, Reddy PL, Dong J, Joseph L, Thirman MJ, Wroblewski K, Wolff L, Baron JM. PIM1 gene cooperates with human BCL6 gene to promote the development of lymphomas. Proc Natl Acad Sci US A. 2012;109:5735–5739. doi: 10.1073/pnas.1201168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narlik-Grassow M, Bianco-Aparicio C, Cecilia Y, Perez M, Munoz-Galvan S, Canamero M, Renner O, Canero A. Conditional transgenic expression of Pim1 kinase in prostate induces inflammation-dependent neoplasia. PLoS One. 2013;8:e60277. doi: 10.1371/journal.pone.0060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS. Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta. 2002;1593:45–55. doi: 10.1016/s0167-4889(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS. Pim-1 associates with protein complexes necessary for mitosis. Chromosoma. 2002;111:80–95. doi: 10.1007/s00412-002-0192-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Wang Z, Li X, Magnuson NS. Pim-1 kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 74.Weirauch U, Beckman N, Thomas M, Grunweller A, Huber K, Bracher F, Hartmann RK, Aigner A. Functional role and therapeutic potential of the pim-1 kinase in colon carcinoma. Neoplasia. 2013;15:783–794. doi: 10.1593/neo.13172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dautry F, Weil D, Yu J, Dautry-Varsat A. Regulation of pim and myb mRNA accumulation by interleukin 2 and interleukin 3 in murine hematopoietic cell lines. J Biol Chem. 1988;263(33):17615–20. [PubMed] [Google Scholar]
- 76.Gu JJ, Wang Z, Reeves R, Magnuson NS. PIM1 phosphorylates and negatively regulates ASK1-mediated apoptosis. Oncogene. 2009;28(48):4261–71. doi: 10.1038/onc.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Didichenko SA, Spiegl N, Brunner T, Dahinden CA. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood. 2008;112(10):3949–58. doi: 10.1182/blood-2008-04-149419. [DOI] [PubMed] [Google Scholar]
- 78.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–4483. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S. Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood. 2004;103:4536–4544. doi: 10.1182/blood-2003-11-3827. [DOI] [PubMed] [Google Scholar]
- 80.Katakami N, Kaneto H, Hao H, Umayahara Y, Fujitani Y, Sakamoto K, Gorogawa S, Yasuda T, Kawamori D, Kajimoto Y, Matsuhisa M, Yutani C, Hori M, Yamasaki Y. Role of pim-1 in smooth muscle cell proliferation. J Biol Chem. 2004;279:54742–54749. doi: 10.1074/jbc.M409140200. [DOI] [PubMed] [Google Scholar]
- 81.Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005;37:726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, Sussman MA. Rejuvenation of Human Cardiac Progenitor Cells With Pim-1 KinaseNovelty and Significance. Circ Res. 2013;113(10):1169–79. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohsin S, Khan M, Toko H, et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nature Protocols. 2012;7:1479–1496. doi: 10.1038/nprot.2012.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kulandavelu S, Karantalis V, Fritsch J, et al. Pim1 kinase overexpression enhances ckit+ cardiac stem cell cardiac repair following myocardial infarction in swine. J Am Col Cardiol. 2016;68(22):2454–64. doi: 10.1016/j.jacc.2016.09.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cross N. Designerly ways of knowing. Design studies. 1982;3(4):221–7. [Google Scholar]
- 87.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nature reviews Drug discovery. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 88.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350(6265):1193–8. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 89.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nature Reviews Immunology. 2015;15(2):117–29. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golpanian S, Schulman IH, Ebert RF, et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem cells translational medicine. 2016;5(2):186–91. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017;38(33):2532–46. doi: 10.1093/eurheartj/ehx248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 2016;96(3):1127–68. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixit P, Katare R. Challenges in identifying the best source of stem cells for cardiac regeneration therapy. Stem cell research & therapy. 2015;6(1):26. doi: 10.1186/s13287-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quijada P, Sussman MA. Making it stick: chasing the optimal stem cells for cardiac regeneration. Expert Rev Cardiovasc Ther. 2014;12(11):1275–88. doi: 10.1586/14779072.2014.972941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Broughton KM, Sussman MA. Myocardial Regeneration for Humans—Modifying Biology and Manipulating Evolution—. Circ J. 2017;81(2):142–8. doi: 10.1253/circj.CJ-16-1228. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110(25):3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 97.Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Gude NA, Sussman MA. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ Res. 2015;117(8):695–706. doi: 10.1161/CIRCRESAHA.115.306838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Current Biology. 2008;18(10):R435–44. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 99.Masterson J. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science. 1994;264(5157):421–3. doi: 10.1126/science.264.5157.421. [DOI] [PubMed] [Google Scholar]
- 100.Leggatt RA, Iwama GK. Occurrence of polyploidy in the fishes. Rev Fish Biol Fisheries. 2003;13:237–246. [Google Scholar]
- 101.Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–1679. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mescher AL, Tassava RA. Denervation effects on DNA replication and mitosis during the initiation of limb regeneration in adult newts. Developmental biology. 1975;44(1):187–97. doi: 10.1016/0012-1606(75)90386-3. [DOI] [PubMed] [Google Scholar]
- 103.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 104.Kondrashov FA. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc R Soc B The Royal Society. 2012;279(1749):5048–5057. doi: 10.1098/rspb.2012.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Broughton K, Khieu T, Nguyen N, Mohsin S, Khubli D, Quijada P, Wang J, Monsanto M, Gude N, Sussman M. Chromosome Content of Cardiac Progenitor Cells Influences Regenerative Potential of the Heart. Circulation. 2016:A18049–A18049. [Google Scholar]
- 106.Zwetsloot PP, Végh AM, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, Doevendans PA, Chamuleau SA. Cardiac Stem Cell Treatment in Myocardial Infarction. Circ Res. 2016;118(8):1223–32. doi: 10.1161/CIRCRESAHA.115.307676. [DOI] [PubMed] [Google Scholar]
- 107.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-Analysis of Cell Therapy Trials for Patients with Heart Failure-An Update. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.116.304386. CIRCRESAHA-114. [DOI] [PubMed] [Google Scholar]