Abstract
Prostate cancer (PC) is the most common type of cancer among men. Aggressive and metastatic PC results in life- threatening tumors, and represents one of the leading causes of mortality in men. Previous studies of atypical protein kinase C isoforms (aPKCs) have highlighted its role in the survival of cultured prostate cells via the nuclear factor (NF)-κB pathway. The present study showed that PKC-ι was overexpressed in PC samples collected from cancer patients but not in non-invasive prostate tissues, indicating PKC-ι as a possible prognostic biomarker for the progression of prostate carcinogenesis. Immunohistochemical staining further confirmed the association between PKC-ι and the prostate malignancy. The DU-145 and PC-3 PC cell lines, and the non-neoplastic RWPE-1 prostatic epithelial cell line were cultured and treated with aPKC inhibitors 2-acetyl-1,3-cyclopentanedione (ACPD) and 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide (ICA-1). Western blot data demonstrated that ICA-1 was an effective and specific inhibitor of PKC-ι and that ACPD inhibited PKC-ι and PKC-ζ. Furthermore, the two inhibitors significantly decreased malignant cell proliferation and induced apoptosis. The inhibitors showed no significant cytotoxicity towards the RWPE-1 cells, but exhibited cytostatic effects on the DU-145 and PC-3 cells prior to inducing apoptosis. The inhibition of aPKCs significantly reduced the translocation of NF-κB to the nucleus. Furthermore, this inhibition promoted apoptosis, reduced signaling for cell survival, and reduced the proliferation of PC cells, whereas the normal prostate epithelial cells were relatively unaffected. Overall, the results suggested that PKC-ι and PKC-ζ are essential for the progression of PC, and that ACPD and ICA-1 can be effectively used as potential inhibitors in targeted therapy.
Keywords: protein kinase C-ι, atypical protein kinase C inhibitors, prostate cancer, nuclear factor-κB, biomarkers
Introduction
The American Cancer Society estimates that 164,690 new cases of prostate cancer (PC) will be diagnosed in 2018. PC is the third leading cause of cancer-associated mortality among American men, following lung and colorectal cancer. It is estimated that ~1/7 men are diagnosed with the disease during their lifetime, with 1/39 succumbing to the disease (1).
The most common treatments for PC include radiation therapy, chemotherapy and hormone therapy. Radiation therapy is not always ideal as healthy living cells are damaged by the treatment, causing numerous side effects in patients. Hormone therapy requires surgical castration and the use of anti-androgens, which also have side effects and affect the patient’s lifestyle (2). In addition to the side effects that follow hormone therapy, patients often become castration-resistant, meaning the cancer is no longer affected by the treatment (2). Once this occurs, chemotherapeutics, including docetaxel, are used. Docetaxel only increases a patient’s survival rate by ~3 months. This is largely due to resistance that develops within the cancer cells (3).
The protein kinase C (PKC) family of isozymes transduce signals and control other proteins through phosphorylation (4). The PKC family comprises 14 known isozymes, which are found in varying ratios in the cytoplasmic and membrane fraction of cells depending on the type of tissue and its physiological state (5). PKC isozymes can be classified into three groups. Group I includes Ca2+-dependent isozymes: cPKC-α, cPKC-βl, cPKC-βll, and cPKC-γ. Isozymes in Group II (nPKC-δ, nPKC-ε, nPKC-η and PKC-θ) are Ca2+-independent. Group III includes the atypical (a)PKCs, including aPKC-ι (6), aPKC-ζ, aPKC-ζll (7) aPKC-µ (protein kinase D) and aPKC-ν (8), which are insensitive to diacylglycerol and calcium and do not bind to or become activated by phorbol esters. PKC regulates cellular functions, metabolism and proliferation by phosphorylating proteins in response to transmembrane signals from hormones, growth factors, neuro-transmitters, and pharmacological agents. The activation of PKC by various agonists, including radiation, results in the altered transcription of a considerable number of genes. PKCs are involved in the day-to-day functioning of normal cells, however, they can cause several adverse effects when disrupted. PKC is the major receptor for tumor-promoting phorbol esters, however, the extent to which PKC is involved in cellular malignancy remains to be fully elucidated. Various studies have indicated that increased tumorigenicity results from either the dysregulation of PKC activity, changes in PKC concentration, or both (9-13).
PKC isozymes have been implicated in carcinogenesis, however, investigation of the functional significance of these enzymes in human cancer has been limited so far. PKC isozymes α, β, δ, ε, γ, η, ζ, ι, and µ are expressed in the normal and cancerous prostate (14). LNCaP cells are a widely used model for investigation of PC. Besides classical PKC-α, the novel PKC-δ is overexpressed in LNCaP cells and provokes phorbol ester-induced apoptosis. In PC-3 and DU-145 cells, PKC-δ is involved in the cell motility and invasion of prostate tumor cells (15,16). In addition, PKC-ε alongside Akt promotes matrix adhesion-containing actin-filaments and β-intergins in recurrent PC cells (15). In DU-145 cells, the downregulation of PKC-ε prevents apoptosis (16). Therefore, PKC-ε may be targeted for prostate therapy. Finally, the PKC-µ and PKC-ζ mammalian target of rapamycin (mTOR)/p70 S6 kinase pathway is associated with the progression of androgen- dependent PC to androgen-independent PC (17-19). Therefore, understanding PKC isoforms in PC may contribute to possible novel diagnostic and therapeutic strategies.
PKCs is involved in tumor growth and formation when the levels of PKC are markedly altered (5). PKC-ι is a member of the PKC family located in chromosome 3 at 3q26.2, and is a human oncogene. PKCs can be overexpressed in various types of cancer, including ovarian, lung, head and neck cancer, and PC (20-22). Elevated levels of PKC-ι have been correlated with poorer prognosis. It was found that patients with lung cancer who had elevated levels of PKC-ι during the early stages were 10 times more likely to succumb to mortality from the disease, compared with those who had low levels of PKC-ι. PKC-ι is also involved in several oncogenic signaling path- ways (23). For these reasons, the in vitro effects of two novel aPKC inhibitors, 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide (ICA-1) and 2-acetyl-1,3-cyclopentanedione (ACPD), on the normal RWPE-1 cell line, and the DU-145 and PC-3 PC cell lines, were investigated in the present study. ICA-1 has been shown to target PKC-ι, whereas ACPD has been shown to target PKC-ι and PKC-ζ (24,25).
The nuclear factor (NF)-κB signaling pathway is involved in cancer propagation and dissemination in several types of cancer, however, its involvement in PC remains to be fully elucidated. The inhibitor of NF-κB kinase (IKK) complex is comprised of IKKα and IKKβ, both of which are necessary for the activation of NF-κB. In the present study, it was hypothesized that PKC-ι acts on IKKα/β, causing the release and translocation of NF-κB. Inhibition of this pathway following treatment with ICA-1 is expected allow normal apoptosis to take place with minimal effect on RWPE-1 cells, but with more marked effects in DU-145 and PC-3 cells.
The results of the present study showed a correlation between the presence of PKC-ι and PC. It also revealed the efficacy of ACPD and ICA-1 on PKC-ι and indicated the role of PKC-ι in the survival of PC. Cumulatively, the results led to the conclusion that the detection of PKC-ι may be used as a biomarker of prostate carcinogenesis and that PKC-ι inhibition may be an alternative therapy in patients with PC.
Materials and methods
ICA-1 was synthesized by Therachem Research Medilab (Jaipur, India) and ACPD was purchased from Sigma-Aldrich; EMD Millipore (Billerica, MA, USA)
The inhibitors were dissolved in sterile distilled water prior to use. Dulbecco’s phosphate-buffered saline without Mg2+ and Ca2+ (DPBS) was purchased from the American Type Culture Collection (Manassas, VA, USA). Trypsin-ethylenediaminetetraacetic acid (EDTA) solution was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Polyclonal primary antibodies were purchased from the following companies: Anti-PKC-ι mouse monoclonal (cat. no. 610176) and B-cell lymphoma 2 (Bcl-2; cat. no. 610538) from BD Transduction Laboratory (Lexington, KY, USA). PKC-ζ (cat. no. sc-17781), NF-κB p65 (cat. no. sc-372-G), inhibitor of NF-κBα (IκBα; cat. no. sc-1643), phosphorylated (phospho) IκBα (cat. no. sc-8404) β-actin (cat. no. sc-1616) goat polyclonal, PKC-α (cat. no. sc-8393) mouse monoclonal, cytochrome c (cat. no. sc-13156), survivin (cat. no. sc-17779) and caspase-3 (cat. no. sc-7272) from Santa Cruz Biotechnology Co., Ltd. (Santa Cruz, CA, USA), phosphorylated phosphatase and tensin homolog (PTEN; S380; cat. no. 9551), phosphorylated AKT (S473; cat. no. 4059S), phosphorylated IKKα/β (S176/180; cat. no. 2697), poly (ADP-ribose) polymerase (PARP; cat. no. 9532) and cleaved-PARP (cat. no. 9185) from Cell Signaling Technology Inc. (Danvers, MA, USA). β-catenin (cat. no. ab16051) from Abcam (Cambridge, MA, USA). Secondary antibodies were purchased from the following companies: Horseradish peroxidase (HRP) goat x mouse IgG (cat. no. JGM035146), HRP goat x rabbit IgG (cat. no. JGZ035144) from Accurate (Westbury, NY, USA); HRP bovine anti-goat IgG (cat. no. sc-2350 from Santa Cruz Biotechnology, Inc.. The RWPE-1 (ATCC® CRL-11609™) epithelial cells and DU-145 (ATCC® HTB-81™) human prostate carcinoma cells were purchased from the American Type Culture Collection. The PC-3 cells were acquired from Moffitt Cancer Center (Tampa, FL, USA).
Prostate tissue analysis
The protein for western blot analysis was extracted from human biopsy-derived benign prostate hyperplasia (BPH) tissues obtained from the Cooperative Human Tissue Network (Southern Division) at the University of Alabama (Birmingham, AL, USA). For the purposes of the present study, BPH was defined as a non-cancerous enlargement of the prostate gland. The BPH tissue samples were obtained from men of varying ages (57-80 years old) with a mean age of 67.6 years. Protein extraction from the fresh-frozen radical prostatectomy samples of patients with PC were obtained during surgery performed at the James A. Haley Veterans Hospital (Tampa, FL, USA) between May and August 2007. All patients provided informed consent as part of a clinical protocol approved by the Institutional Review Board (IRB no. 103023) of the University of South Florida (Tampa, FL, USA). The samples were placed on ice immediately following prostatectomy and were frozen in liquid nitrogen within 30-60 min post-prostatectomy. The prostate tissues (0.5-1 g) were re-suspended and sonicated in 2 ml homogenization buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 0.1% Tween-20, 1 mM ethylenediaminetetraacetic acid, 2 mM ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′,-tetraacetic acid, 0.1 mM orthovanadate, 1 mM NaF, 2 mM phenyl-methylsulfonly fluoride, 2.5 µg/ml leupeptin, 1 mM dithiothreitol and 0.15 U/ml aprotinin. The suspension was sonicated for three 15 sec cycles on ice. The prostate tissue suspensions or cell lysates were centrifuged at 40,000 g for 30 min at 4°C to obtain cell extracts. The proteins were quantified according to the Bradford method (26). Tissue extracts containing equal quantities of protein in each lane were run on 10% SDS-PAGE gels according to Laemmli (27) and the proteins were transblotted on nitrocellulose membrane according to Towbin et al (28). The tissue lysates (100 µg) were used for western blot analysis with antibodies against PKC-α (10 µg, 1:500 dilution), PKC-ι (5 µg, 1:4,000 dilution) and β-actin (10 µg, 1:500 dilution) and incubated for a duration of 1 h at room temperature. The secondary antibodies, obtained from Accurate (cat. nos. JGM035146 and JG2035744) were used at a dilution of 1:15,000 (1 µg) and incubated for 2 h at room temperaure. The blots were subjected to chemiluminescence and results were captured on X-ray film and developed by machine. Observable bands were scanned and quantified by densitometry analysis. The specimens used were from 10 patients with PC (one core with adenocarcinoma from each patient), eight patients with high grade prostate intraepithelial neoplasma (HGPIN; 1 core with HGPIN from each patient) and nine patients with BPH (1 core with from each patient), comprising a total of 27 samples. Samples obtained from cell cultures and examined by western blot analysis and were visualized digitally by the Protein Simple (San Jose, CA, USA) FlourChem system and analyzed with the accompanying alpha viewer software.
Immunohistochemical staining
For immunohistochemistry (IHC), de-identified, archival, unstained sections of full-core prostate needle biopsies (PNBs) were used, which were obtained for diagnostics in the systematic PC screening program at the James A. Haley Veterans Administration Medical Center. The PNB specimens examined were from 10 patients with PC (1 core with adenocarcinoma from each patient), eight patients with HGPIN (1 core with HGPIN from each patient) and nine patients with BPH (1 core with from each patient), comprising a total of 27 samples. Following the deparaffinization of the sections, and citrate microwave antigen retrieval, blocking was performed. For the detection of PKC-α, the sections were incubated with monoclonal anti-PKC-α antibody (1:100 dilution; BD Transduction Laboratories) for 60 min at room temperature, followed by washing and detection with the ‘EnVision’ detection system using mouse IgG polymer and DAB chromogen. For the detection of PKC-ι, separate sections were subsequently incubated overnight with purified mouse monoclonal anti-PKC-ι (1:200 dilution; BD Transduction Laboratories), followed by washing and a subsequent 30-min incubation at room temperature with 1:200 rat anti-mouse IgG2b. Final detection was performed using DAB chromogen. All sections were examined for PKC-α and PKC-ι and scored using the Allred semi-quantitative scoring system (29). The Allred score is a composite of the percentage of cells stained and the intensity of their staining. The percentage of cells stained, termed the proportion score, is classified between 0 and 5. The intensity of the cells stained is termed the intensity score and it is rated as 1, 2 or 3; thus, composite scores range between 0 and 8, with 0 being the lowest and 8 being the maximum score. The adenocarcinoma glands, glands with HGPIN, BPH glands, and stromal cells were scored separately using a microscope (Olympus, Tokyo, Japan).
Cell culture
The cells were grown as a monolayer in a T25 tissue culture flask with 5 ml of growth medium and were maintained in a 37°C incubator with 5% CO2. The E-MEM and F-12 growth media were obtained from American Type Culture Collection. The medium was supplemented with 10% fetal bovine serum (FBS) and a mix of the antibiotics penicillin (10,000 IU) and streptomycin (10,000 µg/ml) in a 100X concentration purchased from Corning Life Sciences (Tewksbury, MA, USA).
Cell proliferation assay
The RWPE-1, DU-145 and PC-3 cells were cultured in T25 cell culture flasks with 20,000 cells seeded into each. The flasks were treated with either ICA-1 or ACPD at doses of 1, 5, and 10 µM, in addition to an untreated control set for each. The treatment was repeated over the course of 72 h and samples were taken at 24-h intervals. The cells were trypsinized and placed into a 1.5 ml microcentrifuge tube at intervals of 24, 48, and 72 h. The tubes were inverted gently to maintain solution homogeneity and cell numbers were measured using a Cell Scepter counter (EMD Millipore).
4-[3-(4-iodophenyl)-2-(4-nitropheny))-2H-5-tetrazolio]-1,3- benzene disulfonate (WST-1) assay for cell viability and cytotoxicity
The WST-1 assay (in vitro) was performed by culturing ~4×103 cells/well (RWPE-1, DU-145 and PC-3 cells) in a 96-well plate. After 24 h, fresh media were supplied (200 µl/well) and the cells were treated with either an equal volume of sterile water (vehicle control) or with 10 µM of ICA-1 or 10 µM of ACPD, which was identified as the optimal working concentration for the PC cell lines for the two inhibitors. Additional doses were supplied every 24 h during a 3-day incubation period. At the end of day 3, media were removed and fresh media (100 µl) were added with WST-1 reagent (10 µl) to each well. The absorbance was measured at 450 nm every 1 h up to 8 h using the Synergy HT microplate reader from Biotek Instruments, Inc., (Winooski, VT, USA). The detailed procedure was performed as described by Ratnayake et al (30).
Cell sub-fractionation
The DU-145 and PC-3 cells were cultured in a T25 cell culture flasks (20,000 cells seeded into each). The flasks were treated with either ICA-1 or ACPD at a dose 10 µM, with an untreated control set for each. The treatment was repeated over the course of 72 h and samples were taken at 24-h intervals. At 30 min prior to harvesting, the cells were exposed to 10 nM of tumor necrosis factor (TNF)-α. The NE-PER Nuclear and Cytoplasmic Extraction kit from Thermo Fisher Scientific, Inc. was used to harvest the proteins and separate the nuclear protein from the cytoplasmic protein.
Flow cytometry for the analysis of apoptosis
The DU-145 and PC-3 cells (40,000 cells/flask) were cultured in a T25 cell culture flasks. The flasks were treated with either ICA-1 or ACPD at a dose 10 µM, with an untreated control set for each. After 24 h, the media were removed and dead cells were collected by centrifugation using 350 × g for 4 min at 4°C. The cells were washed twice with ice-cold DPBS (1X) prior to lifting with trypsin and trypsin was neutralized with an equal volume of media. The cells were then combined with the collected dead cells and washed twice with ice- cold DPBS (1X). A PE Annexin V apoptosis detection kit (BD Biosciences, Pharmingen, San Jose, CA, USA) was used to detect the apoptosis according to the detailed protocol provided by BD Pharmingen. The BD Accuri™ C6 Plus personal flow cytometer instrument was used to analyze the samples. Three replicate experiments were performed.
Statistical analysis
All values are presented as the mean ± standard deviation (n=3, where n is a single trial and each trial contained 9-24 replicates). Statistical analysis was performed with Student’s t-test or one-way analysis of variance followed by Tukey’s multiple comparison tests using the ‘VassarStats’ web tool (vassarstats.net) for statistical analysis. P≤0.05 was considered to indicate a statistically significant difference.
Results
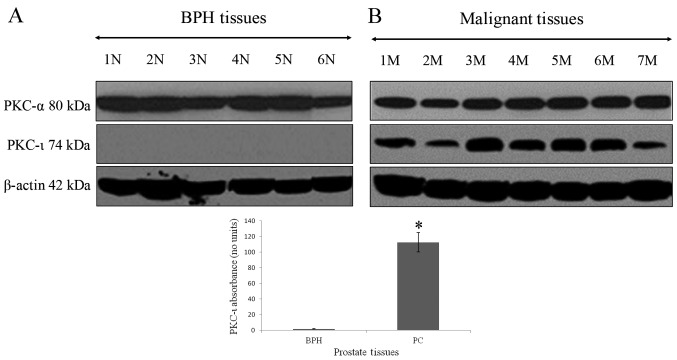
The association between the absence of PKC-ι in BPH tissue and its robust presence in PC is shown in Fig. 1. PKC-ι was abundant in all PC tissues. PKC-α (positive control PKC) and PKC-ι were identified in western blot analysis by bands with molecular weights of 80 and 74 kDa, respectively, which corresponded to the immunoreactive signal obtained from U-373MG glioma cells, which contain PKC-ι and PKC-α (data not shown). The western blot controls for PKC-α did not show a pattern of expression specific to BPH, or malignant prostate tumors. β-actin was used as the internal control to ensure equal quantities of protein loaded in to each well of the SDS-PAGE gel. A 100-fold increase in PKC-ι immunoreactivity was detected in PC tissue when compared with BPH tissue (Fig. 1A and B). The level of PKC-ι in the BPH tissue differed significantly from that in the PC tissue (P=0.00048). This demonstrated that PKC-ι was significantly overexpressed in PC tissues compared with BPH tissues.
Figure 1.
Expression of PKC-α and PKC-ι in prostate tissues. Tissue extracts (100 µg) of (A) BPH and (B) PC were immunoblotted for PKC-α and PKC‑ι. All patient tissues were maintained in liquid nitrogen and processed the same day of collection. BPH tissues showed minimal to no expression of PKC‑ι. PC tissues showed overexpression of PKC-ι compared to BPH tissues (*P=0.00048). Western blot analysis for β-actin demonstrated equal loading of each sample. Three trials were performed in triplicate. BPH, benign prostate hyperplasia; PC, prostate cancer; PKC, protein kinase C; N, normal; M, malignant.
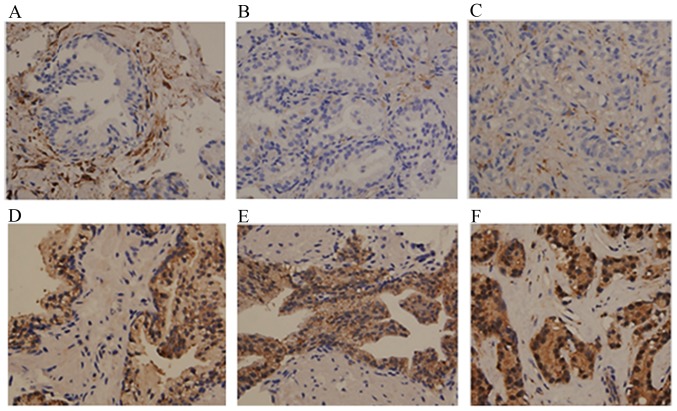
Subsequently, IHC was performed to investigate comparatively the tissue distribution and intracellular localization of PKC-ι and PKC-α. The IHC data showed that PKC-α was expressed in the stromal cells but showed minimal to no expression in the majority of the BPH (Fig. 2A), HGPIN (Fig. 2B) or PC (Fig. 2C) glands. In only one case did the PC glands show moderate expression of PKC-α. In that case the PC glands expressing PKC-α were Gleason pattern 4 (Table I), which may explain the increase of PKC-α.
Figure 2.
Immunohistochemical staining of PKC-α and PKC-ι in BPH, HGPIN and PC. Tissue was stained with PKC-α antibody as a control for PKC-ι staining. Results show PKC-ι staining in (A) BPH, (B) HGPIN, and (C) PC tissue. PKC-α staining is shown in (D) BPH glands, (E) glands with HGPIN, and (F) PC glands. Tissues examined comprised BPH (n=9), HGPIN (n=8) and PC tissues (n=10). Magnification for all micrographs was x400. Three experiments were performed in triplicate. BPH, benign prostate hyperplasia; HGPIN, high grade prostate intraepithelial neoplasma; PC, prostate adenocarcinoma; PKC, protein kinase C.
Table I.
PKC-α and PKC-ι staining of glands and stroma of patients with BPH, HGPIN and prostate adenocarcinoma.
| Total cases | PKC-α | Staining (No. of cases)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8+ | 7+ | 6+ | 5+ | 4+ | 3+ | 2+ | 1+ | 0 | ||
| BPH | Glands | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 4 |
| 9 | Stroma | 0 | 1 | 2 | 5 | 1 | 0 | 0 | 0 | 0 |
| HGPIN | Glands | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 5 |
| 8 | Stroma | 0 | 1 | 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| Adenocarcinoma | Glands | 0 | 0 | 1 | 0 | 1 | 0 | 4 | 0 | 4 |
| 10 | Stroma | 0 | 1 | 4 | 2 | 1 | 0 | 2 | 0 | 0 |
|
| ||||||||||
| Total cases | PKC-ι | Staining (No. of cases)a
|
||||||||
| 8+ | 7+ | 6+ | 5+ | 4+ | 3+ | 2+ | 1+ | 0 | ||
|
| ||||||||||
| BPH | Glands | 0 | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | Stroma | 0 | 0 | 0 | 2 | 4 | 2 | 1 | 0 | 0 |
| HGPIN | Glands | 1 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| 8 | Stroma | 0 | 0 | 0 | 0 | 3 | 4 | 1 | 0 | 0 |
| Adenocarcinoma | Glands | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Stroma | 0 | 0 | 0 | 3 | 5 | 2 | 0 | 0 | 0 |
Staining based on the Allred score (26). PKC, protein kinase C; BPH, benign prostate hyperplasia; HGPIN, high grade prostate intraepithelial neoplasma; PC, prostate adenocarcinoma.
By contrast, the expression of PKC-ι was noted in all glands, with a proportion score of 5 in the majority of cases (Table I and Fig. 2D-F), however, the intensity of staining was more marked in the PC glands with Allred scores of +8 and +7 (Table I and Fig. 2F), compared with that in the BPH glands (Fig. 2D) and glands with HGPIN (Table I and Fig. 2E). The BPH glands showed similar weak staining in samples obtained from patients with and without prostatic adenocarcinoma. The stromal cells showed weak to moderate staining in samples with and without adenocarcinoma. PKC-ι was present at low levels in a comparative number of the BPH samples, however, the intensity was markedly increased in the adenocarcinoma samples, resulting in higher composite scores. Overall, the IHC data from the PNB specimens confirmed the higher expression of PKC-ι in PC detected in the western blot analysis from fresh frozen excisional clinical prostate tissue specimens.
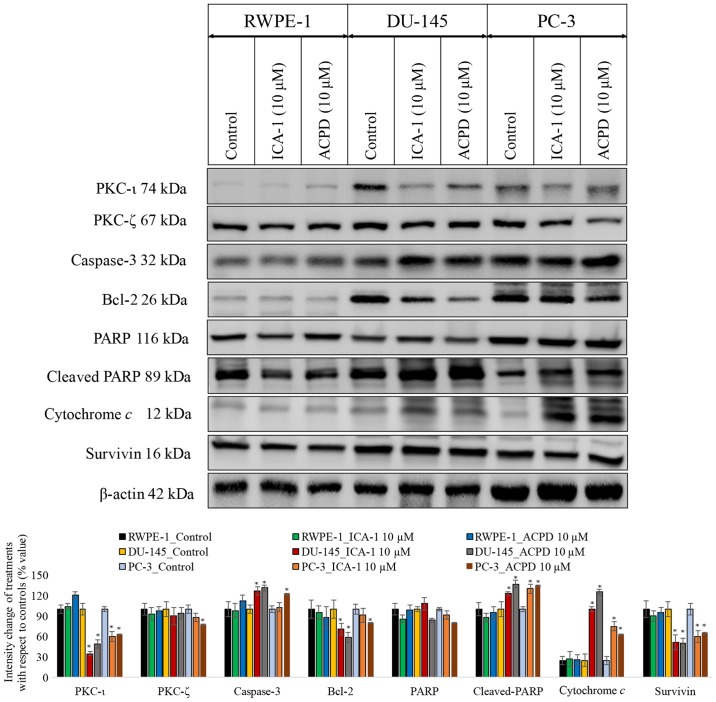
To establish whether ACPD or ICA-1 affected PKC-ι and PKC-ζ, their protein levels were determined by western blot analysis. In the malignant cells (DU-145 and PC-3), there was an abundance of PKC-ι (Fig. 3) compared with the normal cells (RWPE-1). There was a marginal difference in the RWPE cells, however, neither drug reduced the levels of PKC-ι significantly (P=0.077). The effects were more marked in the DU-145 cells. ICA-1 inhibited PKC-ι and, following 3 days of treatment, there was a decrease of 64% (P=0.01) in PKC-ι. Following 3 days of treatment with ACPD, which inhibits PKC-ι and PKC-ζ, there was an average decrease of 51% (P=0.018) in PKC-ι. In the PC-3 cells, there was a 40% (P=0.028) decrease in PKC-ι following treatment with ICA-1 and a 37% (P=0.036) decrease in PKC-ι following treatment with ACPD. ICA-1 had no significant effect on the levels of PKC-ζ in any of the cell lines, whereas ACPD had a significant effect, reducing the levels of PKC-ζ by 23% (P≤0.05), in the PC-3 cells only.
Figure 3.
Effects on PKC-ι regulation and apoptosis. Effects on PKC-ι and apoptosis were examined following inhibition of PKC-ι with either ICA-1 (10 µM) or ACPD (10 µM) for 72 h with respect to their controls. Protein expression of PKC-ι, PKC-ζ, caspase 3, Bcl-2, PARP, cytochrome c, and survivin in the RWPE-1 cell line and the DU-145 and PC-3 malignant cell lines. A total of 35 µg protein was loaded into each well and results were normalized by β-actin. Three trials were performed in triplicate. Densitometry bar graphs show the percentage change of the treated sample with respect to their controls (mean ± standard devia- tion). *P≤0.05, vs. control. PKC, protein kinase C; Bcl-2, B-cell lymphoma 2; PARP, poly (ADP-ribose); ACPD, polymerase; 2-acetyl-1,3-cyclopentanedione; ICA-1, 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide.
To determine whether ICA-1 or ACPD induced apoptosis, the apoptotic markers caspase-3, Bcl-2, PARP, cleaved PARP, survivin and cytochrome c were used to detect ongoing apoptosis in the cell lines (Fig. 3). In all three cell lines treated with ICA-1 and ACPD, there was a measurable increase in apoptosis. Following ICA-1 treatment for 72 h, the RWPE-1 control line showed no significant change in either cytochrome c or survivin. Following treatment with ACPD for 72 h, the RWPE-1 cells showed no significant change in cytochrome c or in survivin. By contrast, the DU-145 cell line showed a more substantial change, with an almost 3-fold increase in cytochrome c and a reduction in survivin by 50% following treatment of ICA-1. Following treatment with ACPD for 72, the DU-145 cells showed ~400% (P<0.001) increase in cytochrome c and a 50% (P=0.015) decrease in survivin. In the PC-3 cells treated with ICA-1, there was a 2-fold increase in cytochrome c (P=0.011) and a 40% decrease in survivin (P=0.028). The PC-3 cells treated with ACPD showed a 150% (P=0.013) increase in cytochrome c and a 35% decrease in survivin (P=0.042). The levels of caspase-3 and cleaved-PARP were significantly increased and those of Bcl-2 and total PARP were significantly reduced upon inhibitor treatment in the PC cell lines. All cell lines were also probed with β-actin to ensure equal loading between lanes.
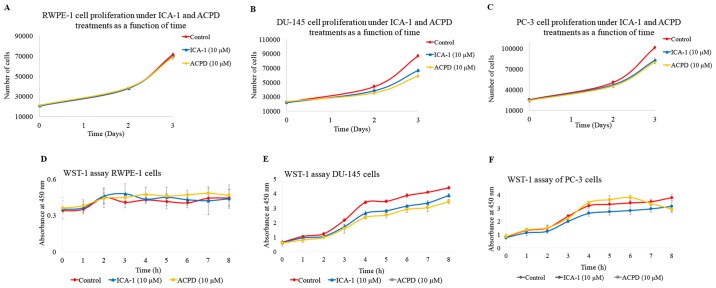
The effects of ICA-1 (10 µM) and ACPD (10 µM) on cell proliferation were also determined (Fig. 4A-C). There was a noticeable but statistically insignificant effect of the inhibitors ICA-1 and ACPD on the RWPE-1 cell line. Both inhibitors retained >90% of the cell population present in the untreated RWPE-1 cells (Fig. 4A). By contrast, there was a substantial decrease in the population of DU-145 cells treated with the inhibitors ICA-1 (P≤0.01) and ACPD (P≤0.01) (Fig. 4B). The PC-3 cells showed a similar trend of population decline following treatment with ICA-1 (P=0.01) and ACPD (P=0.0052) (Fig. 4C).
Figure 4.
Effects of ICA-1 or ACPD on cell populations in a 72-h period. Populations of cells under normal conditions (red) and under treatment with either 10 µM ICA-1 (blue) or 10 µM ACPD (yellow) in (A) RWPE-1, (B) DU-145, and (C) PC-3 cell lines. Effects of ICA-1 and ACPD on in vitro cytotoxicity over 8 h were measured by absorbance in nm under normal conditions (red) and under the effects of either 10 µM ICA-1 (blue) or 10 µM ACPD (yellow), in (D) RWPE-1, (E) DU-145, and (F) PC-3 cell lines. There were 24 replicates per sample performed over three trials. ACPD, polymerase; 2-acetyl-1,3-cyclopentanedione; ICA-1, 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide.
To determine the in vitro cytotoxicity of ICA-1 and ACPD on normal and malignant cell lines, a WST-1 assay was performed. ICA-1 and ACPD demonstrated no significant cytotoxicity towards the normal prostate epithelial RWPE-1 cell line (Fig. 4D). As shown in Fig. 4E and F, ICA-1 exhibited significant cytotoxicity (P≤0.05) on the two metastatic prostate cancer cell lines (DU-145 and PC-3). These results indicated that ICA-1 and ACPD appeared to be cytostatic more than cytotoxic in the time range assessed, thereby retarding PC cell growth and proliferation.
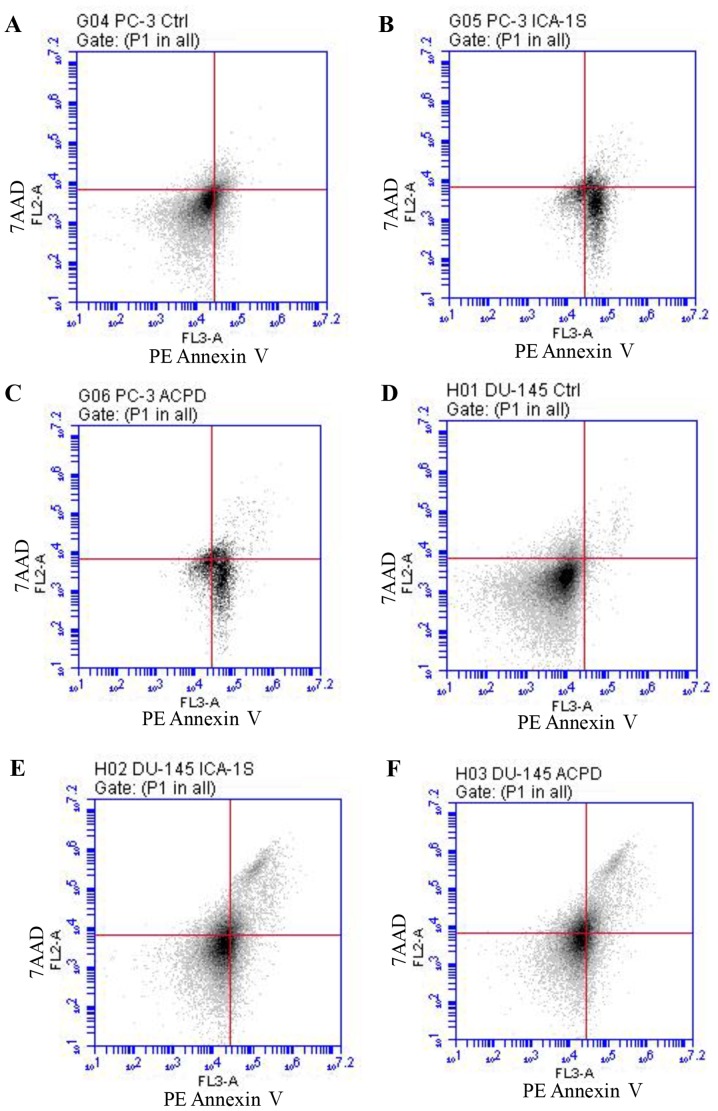
As shown in Fig. 5, flow cytometric analysis of apoptosis showed that PC-3 viability decreased from 91.8% (Fig. 5A) to 60.8% following ICA-1 treatment (Fig. 5B) and 60.9% following ACPD treatment (Fig. 5C). Early apoptosis increased from 6.8% (Fig. 5A) to 37.3 and 36.3% following ICA1 and ACPD treatments, respectively. By contrast, the DU-145 cells showed a marginally lower response, in which viability decreased from 97.9% (Fig. 5D) to 89% following ICA-1 treatment (Fig. 5E) and 85.5% following ACPD treatment (Fig. 5F). Early apoptosis increased from 0.9% (Fig. 5D) to 3.4 and 2.6% following ICA-1 and ACPD treatments, respectively (Fig. 5E and F). The late stage of apoptosis significantly increased from 1.2% (Fig. 5D) to 7.6 and 12% for ICA-1 and ACPD treatments, respectively, in the DU-145 cell line (Fig. 5E and F).
Figure 5.
Flow cytometric analysis of PE Annexin V staining for prostate cancer cells. Graphs show fluorescent emission of PC-3 cells in the (A) control, (B), ICA-1- and (C) ACPD-treated groups, and DU-145 cells in the (D) control, (E) ICA-1- and (F) ACPD-treated groups. Cells were incubated with PE Annexin V (x-axis) against 7AAD (y-axis). Cells were treated with ICA-1 and ACPD with respective IC50 concentrations for 24 h and 50,000 events were analyzed and recorded to obtain FL3-A (PE-Annexin V), vs. FL-2A (7-AAD). Three experiments were performed with 24 replicates and representative plots are shown. ACPD, polymerase; 2-acetyl-1,3-cyclopentanedione; ICA-1, 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole- 4-carboxamide; 7AAD, 7-amino-actinomycin; Ctrl, control.
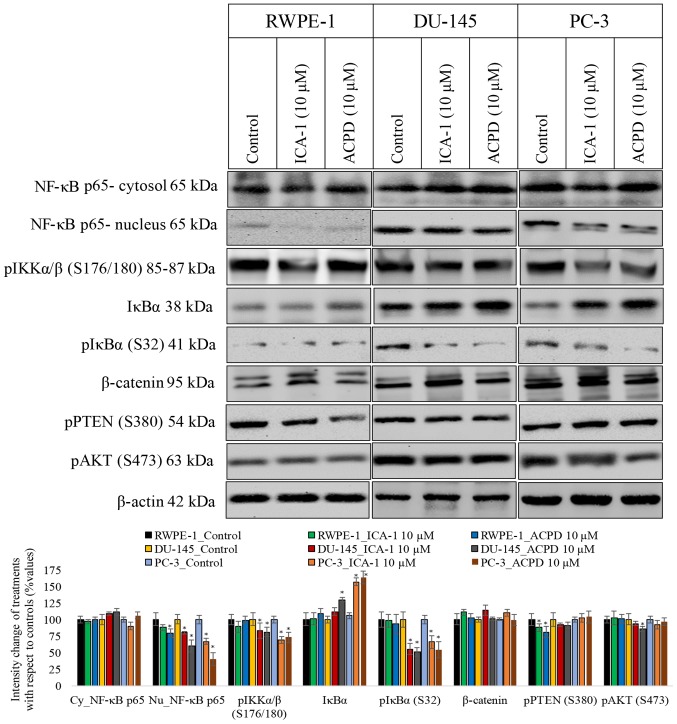
Subsequently, the present study determined whether ICA-1 or ACPD affected cell survival by inhibiting the translocation of NF-κB from the cytoplasm to the nucleus (Fig. 6). Following exposure to TNF-α, the protein levels of NF-κB were measured in the nucleus and the cytosol of DU-145 and PC-3 cells. Following treatment with ICA-1, there was a 5-fold decrease in nucleic NF-κB in the DU-145 cells (P=0.02) and a 3-fold decrease of nucleic NF-κB in the PC-3 cells (P=0.025), compared with the untreated samples. With ACPD, there was a 1.5-fold decrease in nucleic NF-κB (P=0.046) in the DU-145 cells and a 40% decrease in NF-κB (P=0.028) in the PC-3 cells, compared with the untreated samples (Fig. 6). In addition, the phosphorylation of IKKαβ (S176/180) and IκBα (S32) were examined, both of which reduced significantly (P≤0.05) following inhibitor treatments in the two PC cell lines, which confirmed a reduction of NF-κB translocation to the nuclei. The phosphorylation of PTEN (S380) and AKT (S473) also decreased significantly (P≤0.05) in the two PC cell lines, indicating that the AKT pathway also slowed down upon inhibitor treatment.
Figure 6.
Effects of 10 µM ICA-1 and 10 µM ACPD on NF-κB translocation in RWPE-1, DU-145, and PC-3 cells. Protein expression of NF-κB p65 is shown in nuclear and cytoplasmic cell fractions following 72 h of treatment. Cells were exposed to 10 nM of TNF-α 30 min prior to harvest to induce NF-κB transloca- tion. Other targets in the proposed pathway and targets known to be affected by PKC-ι activation were assessed, including pIKKα/β, IKKα/β, pIκBα, IκBα, β-catenin, pPTEN, and pAKT. A total of 50 µg protein was loaded into each well and the results were normalized by β-actin. Three trials were performed in triplicate. Densitometry bar graphs are shown as the percentage change of the treated sample with respect to their controls (mean ± standard deviation). *P≤0.05, vs. control. NF-κB, nuclear factor-κB; IκBα, inhibitor of NF-κBα; IKK, IκBα kinase; PTEN, phosphatase and tensin homolog; p, phosphorylated; ACPD, polymerase; 2-acetyl-1,3-cyclopentanedione; ICA-1, 5-amino-1-(1R,2S,3S,4R)-2,3-dihydroxy-4-methylcyclopentyl)-1H-imidazole-4-carboxamide.
Discussion
There has been increased interest in the status of aPKC-ι, which does not contain a Ca2+-binding region, has one zinc finger-like motif, and is the human homolog of the mouse PKC-λ (31). Eder et al (32) provided evidence for the role of PKC-ι in cell proliferation by demonstrating that increased protein levels of PKC-ι were associated with increased protein expression of cyclin E and proliferation in ovarian cancer. Eder et al (32) demonstrated in non-serous ovarian cancer that increased protein levels of PKC-ι markedly decreased overall survival rate. PKC-ι is critical for non-small cell lung cancer proliferation in vivo via activation of the Ras-related C3 botulinum toxin substrate 1 (Rac1)/Rac1-activated kinase/mitogen-activated protein kinase kinase 1,2/extracellular signal-regulated kinase 1,2 signaling pathway, which has been implicated in tumor cell proliferation, indicating PKC-ι as an oncogene in human non- small cell lung cancer (20,23). Additionally, the importance of PKC-ι is further recognized due to its exclusive association with transformed phenotype of human melanomas in vivo and in vitro (33), its overexpression in human non-small lung cancer cell lines and cholangiocarcinoma (34), and its presence in the transformed growth of the human lung adenocarcinoma A549 cell line in vitro and tumorigenicity in vivo (20). Our previous study also highlighted the exclusive association of PKC-ι with the transformed phenotype of glioma and meningioma (35). However, the linkage between the PKC family of proteins and PC remains to be fully elucidated.
The results of the western blot analysis demonstrated that the PKC-ι protein was overexpressed in all PC tissues, but not in BPH tissues (P=0.00048). Furthermore, the IHC results demonstrated increased PKC-ι staining intensity in PC glands compared with BPH glands and glands with HGPIN. The BHP glands showed weak staining of PKC-ι, similar to samples obtained from patients with and without prostatic adenocarcinoma. The stromal cells showed weak to moderate staining of PKC-ι in samples with and without adenocarcinoma. It is possible that the reduced categorical expression of PKC-ι observed in the comparison between malignant and BPH tissues may be associated with the antigen retrieval methodology of the IHC process in formalin-fixed, archival specimens. The observation of marked and consistent expression of PKC-ι in all PC specimens and certain HGPIN specimens is significant, as it may allow the prediction of the proportion of HGPIN patients, estimated to be up to 50%, who progress to clinical PC. The fact that benign prostatic acini in patients with PC do not significantly express PKC-ι further underscores the histopathological dichotomy of the BPH and PC pathways. Finally, if PKC-ι is demonstrated to have be significant in prostate carcinogenesis, it may offer an opportunity to target PKC-ι in HGPIN patients for the prevention of PC. The results of the present study suggested that PC showed a higher dependency on PKC-ι compared with non-cancerous prostate tissue. The increase in the expression of PKC-ι may be due to gene amplification or genetic duplication of PKC-ι. Previous studies have shown that chromosome 3q26, where PKC-ι is located, is an amplicon and that gene amplification of PKC-ι is associated with non-small cell lung cancer and esophageal squamous cell carcinoma (23,36). Studies have also shown that PKC-ι is co-amplified with neighboring gene SRY-box 2 (37). These findings have clinical ramifications as the increased expression of PKC-ι has been shown to correlate with poor prognosis in other types of cancer (32,34). The results of the present study suggest that prostate cancer behaves similarly and that increased expression of PKC-ι indicates a less favorable prognosis.
Although PKC-ι has been previously shown to be involved in the survival of the normal RWPE-1 cell line, these results were observed following serum starvation (38). As PKC-ι is activated under stress, for example, following the addition of TNF-α or serum starvation, and the results from the tissues were so different, the present study examined the reliance of cultured cell lines on PKC-ι without serum starvation. It was found that PKC-ι had a significant effect in PC cells, but had minimal effect in normal cells. This provided evidence of the dependence of PC on PKC-ι and assists in explaining its overexpression in PC cells. As PKC-ι can be a predictor for PC (37) and is also an anti-apoptotic factor (39), resistance to apoptosis is decreased by inhibiting PKC-ι. The results of the present study showed that inhibitors, including ICA-1, may not be directly toxic to cancer cells; however, by inhibiting PKC-ι, carcinoma cells that overexpress and rely on PKC-ι are profoundly affected. The WST-1 results demonstrated that the inhibitors had a cytostatic effect on the PC cells thereby inhibiting their growth, differentiation and proliferation prior to the induction of apoptosis.
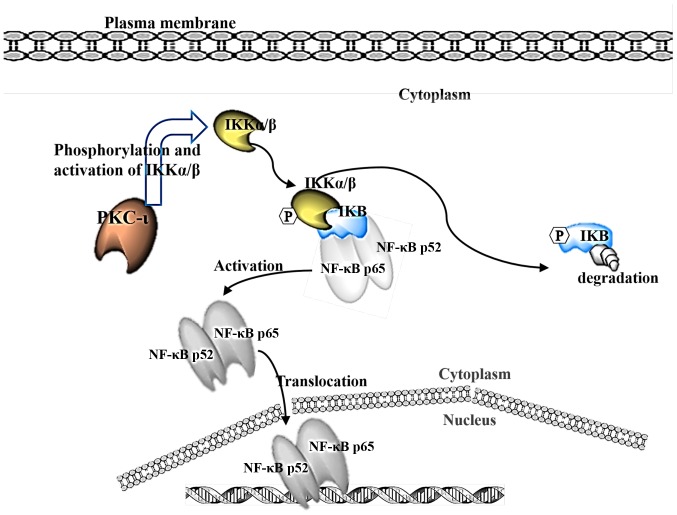
It is suggested that PKC-ι phosphorylates IKK, which in turn releases the inhibitor IκBα from NF-κB, allowing NF-κB to translocate to the nucleus as a transcription factor known to propagate cancer (Fig. 7). A previous study linked the activation of IKK through the action of PKC (38). The present study showed that the phosphorylation of IKKα/β decreased upon inhibition of PKC-ι, which decreased the phosphorylation of IκBα and prevented its degradation by the release of activated NF-κB, allowing it to translocate into the nuclei. These data suggest that, by inhibiting PKC-ι, the transcription factor NF-κB remained inhibited, and the chain of events was not set in motion. NF-κB is generally released during periods of stress and is not the only pro-survival factor affecting the cell. However, this pathway inhibition may explain the decrease in cell growth rates for the two cell lines treated with PKC-ι inhibitors. In neuroblastoma cells, ICA-1 has been shown to be a specific inhibitor to PKC-ι, and its inhibition has been shown to induce apoptosis (24). In the present study, the DU-145 cells also showed signs of increased apoptotic markers and retarded growth rate. The PC-3 cells showed a similar decrease in growth rate and were subject to apoptotic factors despite their aggressive nature. One limitation of the present study was the sample size of patient tissues used. A larger sample combined with the in vivo assessment of the inhibitors is recommended.
Figure 7.
Details the proposed pathway where PKC-ι acts on IKK α/β to release NF-κB, causing IKK to be degraded and allowing NF-κB to translocate to the nucleus. PKC, protein kinase C: NF-κB, nuclear factor-κB; IκBα, inhibitor of NF-κBα; IKK, IκBα kinase.
In conclusion, the results in the present study suggested that aPKCs are major components responsible for inducing cell growth, differentiation and survival in human PC cells. In addition, PKC-ι was found to be involved in the activation and nuclear translocation of NF-κB. The results also suggested that ICA-and ACPD were effective aPKC inhibitors in PC cells and did not affect normal prostate epithelial cells at the optimal working concentrations for PC cells used. These inhibitors reduced cell proliferation and induced the apoptosis of PC cells. Taken together, these results indicate that the detection of PKC-ι may be used as a predictor of prostate carcinogenesis and suggest that patients with PC may benefit from anti-PKC-ι therapy.
Acknowledgments
The authors would like to thank Dr Hercules Apostolatos and Ms. Roberta Blanchard of Tampa Bay Technology Incubator at USF for their assistance with equipment and cell culturing, and Ms. Gina Bladuell and Ms. Kayla Batista of USF College of Medicine and Mr. Sloan Breedy of USF for their assistance with editing.
Funding
This study was funded by the generous support of The Leo and Anne Albert Charitable Trust (USFF 42-1042), The Sapphire Foundation for Prostate Cancer (USFF 42-0044), The Daniel Tanner Foundation (USFF 42-044) and The Frederick H. Leonhardt Foundation (USFF 42-0044).
Availability of data and materials
All data generated or analyzed during this study are included within the manuscript.
Authors’ contributions
AHA and MAD conceived and planned the experiments. Cell culturing was performed by AHA, WSR, HWP, CAA and TS. Prostate tissue collection and analysis was performed by RS and HWP. Immunohistochemical staining and analysis was performed by LK and HWP. Western blot analysis was performed by AHA, WSR and HWP, and TS carried out the experiments. Cell proliferation assays were performed by AHA, WSR and CAA. Cell subfractionation was performed by AHA and WSR. Flow cytometry was performed by RH and WSR. AHA, MAD, RH, RS, WSR, HWP and LK contributed to the interpretation of the results. AHA took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Ethics approval and consent to participate
All patients provided informed consent as part of a clinical protocol approved by the Institutional Review Board (IRB no. 103023) of the University of South Florida.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.American Cancer Society Key statistics for prostate cancer. Prostate Cancer Facts. https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html
- 2.Kume H, Kawai T, Nagata M, Azuma T, Miyazaki H, Suzuki M, Fujimura T, Nakagawa T, Fukuhara H, Homma Y. Intermittent docetaxel chemotherapy is feasible for castration-resistant prostate cancer. Mol Clin Oncol. 2015;3:303–307. doi: 10.3892/mco.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, Azimi A, Hultenby K, Zubarev R, Ullén A, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740–21754. doi: 10.18632/oncotarget.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newton AC. Protein kinase C: Poised to signal. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 6.Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 7.Hirai T, Niino YS, Chida K. PKC zeta II, a small molecule of protein kinase C zeta, specifically expressed in the mouse brain. Neurosci Lett. 2003;348:151–154. doi: 10.1016/S0304-3940(03)00780-8. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi A, Seki N, Hattori A, Kozuma S, Saito T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim Biophys Acta. 1999;1450:99–106. doi: 10.1016/S0167-4889(99)00040-3. [DOI] [PubMed] [Google Scholar]
- 9.Persons DA, Wilkison WO, Bell RM, Finn OJ. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988;52:447–458. doi: 10.1016/S0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- 10.Housey GM, Johnson MD, Hsiao WL, O’Brian CA, Murphy JP, Kirschmeier P, Weinstein IB. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988;52:343–354. doi: 10.1016/S0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamata T, Sullivan NF, Wooten MW. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987;1:37–46. [PubMed] [Google Scholar]
- 12.Weyman CM, Taparowsky EJ, Wolfson M, Ashendel CL. Partial down-regulation of protein kinase C in C3H 10T 1/2 mouse fibroblasts transfected with the human Ha-ras oncogene. Cancer Res. 1988;48:6535–6541. [PubMed] [Google Scholar]
- 13.Mizuguchi J, Nakabayashi H, Yoshida Y, Huang KP, Uchida T, Sasaki T, Ohno S, Suzuki K. Increased degradation of protein kinase C without diminution of mRNA level after treatment of WEHI-231 B lymphoma cells with phorbol esters. Biochem Biophys Res Commun. 1988;155:1311–1317. doi: 10.1016/S0006-291X(88)81284-1. [DOI] [PubMed] [Google Scholar]
- 14.Hudes GR. Signaling inhibitors in the treatment of prostate cancer. Invest New Drugs. 2002;20:159–172. doi: 10.1023/A:1015678427111. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Thakore CU, Wescott GG, McCubrey JA, Terrian DM. Integrin signaling links protein kinase Cepsilon to the protein kinase B/Akt survival pathway in recurrent prostate cancer cells. Oncogene. 2004;23:8659–8672. doi: 10.1038/sj.onc.1207900. [DOI] [PubMed] [Google Scholar]
- 16.Rusnak JM, Lazo JS. Downregulation of protein kinase C suppresses induction of apoptosis in human prostatic carcinoma cells. Exp Cell Res. 1996;224:189–199. doi: 10.1006/excr.1996.0127. [DOI] [PubMed] [Google Scholar]
- 17.Jaggi M, Rao PS, Smith DJ, Hemstreet GP, Balaji KC. Protein kinase C mu is down-regulated in androgen-independent prostate cancer. Biochem Biophys Res Commun. 2003;307:254–260. doi: 10.1016/S0006-291X(03)01161-6. [DOI] [PubMed] [Google Scholar]
- 18.Rao PS, Jaggi M, Smith DJ, Hemstreet GP, Balaji KC. Metallothionein 2A interacts with the kinase domain of PKCmu in prostate cancer. Biochem Biophys Res Commun. 2003;310:1032–1038. doi: 10.1016/j.bbrc.2003.09.118. [DOI] [PubMed] [Google Scholar]
- 19.Inoue T, Yoshida T, Shimizu Y, Kobayashi T, Yamasaki T, Toda Y, Segawa T, Kamoto T, Nakamura E, Ogawa O. Requirement of androgen-dependent activation of protein kinase Czeta for androgen-dependent cell proliferation in LNCaP Cells and its roles in transition to androgen-independent cells. Mol Endocrinol. 2006;20:3053–3069. doi: 10.1210/me.2006-0033. [DOI] [PubMed] [Google Scholar]
- 20.Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280:31109–31115. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- 21.Weichert W, Gekeler V, Denkert C, Dietel M, Hauptmann S. Protein kinase C isoform expression in ovarian carcinoma correlates with indicators of poor prognosis. Int J Oncol. 2003;23:633–639. [PubMed] [Google Scholar]
- 22.Win HY, Acevedo-Duncan M. Atypical protein kinase C phosphorylates IKKalphabeta in transformed non-malignant and malignant prostate cell survival. Cancer Lett. 2008;270:302–311. doi: 10.1016/j.canlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- 24.Desai S, Pillai P, Win-Piazza H, Acevedo-Duncan M. PKC-ι promotes glioblastoma cell survival by phosphorylating and inhibiting BAD through a phosphatidylinositol 3-kinase pathway. Biochim Biophys Acta. 18132011:1190–1197. doi: 10.1016/j.bbamcr.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Pillai P, Desai S, Patel R, Sajan M, Farese R, Ostrov D, Acevedo-Duncan M. A novel PKC-ι inhibitor abrogates cell proliferation and induces apoptosis in neuroblastoma. Int J Biochem Cell Biol. 2011;43:784–794. doi: 10.1016/j.biocel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Biotechnology. 1979;1992;24:145–149. [PubMed] [Google Scholar]
- 29.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 30.Ratnayake WS, Apostolatos AH, Ostrov DA, Acevedo- Duncan M. Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis. Int J Oncol. 2017;51:1370–1382. doi: 10.3892/ijo.2017.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Meco MT, Municio MM, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype lambda/iota and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/MCB.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, Kango-Singh M, Lu KH, Warneke CL, Atkinson EN, et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selzer E, Okamoto I, Lucas T, Kodym R, Pehamberger H, Jansen B. Protein kinase C isoforms in normal and transformed cells of the melanocytic lineage. Melanoma Res. 2002;12:201–209. doi: 10.1097/00008390-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Wang J-M, Liu C, Xiao B-L, Lu J-X, Zou S-Q. Correlation of aPKC-iota and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2008;7:70–75. [PubMed] [Google Scholar]
- 35.Patel R, Win H, Desai S, Patel K, Matthews JA, Acevedo- Duncan M. Involvement of PKC-iota in glioma proliferation. Cell Prolif. 2008;41:122–135. doi: 10.1111/j.1365-2184.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y-L, Chu J-Y, Luo M-L, Wu YP, Zhang Y, Feng YB, Shi ZZ, Xu X, Han YL, Cai Y, et al. Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosomes Cancer. 2008;47:127–136. doi: 10.1002/gcc.20514. [DOI] [PubMed] [Google Scholar]
- 37.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S-W, Schifano M, Oleksyn D, Jordan CT, Ryan D, Insel R, Zhao J, Chen L. Protein kinase C-associated kinase regulates NF-κB activation through inducing IKK activation. Int J Oncol. 2014;45:1707–1714. doi: 10.3892/ijo.2014.2578. [DOI] [PubMed] [Google Scholar]
- 39.Xie J, Guo Q, Zhu H, Wooten MW, Mattson MP. Protein kinase C iota protects neural cells against apoptosis induced by amyloid beta-peptide. Brain Res Mol Brain Res. 2000;82:107–113. doi: 10.1016/S0169-328X(00)00187-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within the manuscript.