Abstract
In the tumor microenvironment, extracellular nucleotides are released and accumulate, and can activate the P2Y2 receptor (P2Y2R), which regulates various responses in tumor cells, resulting in tumor progression and metastasis. Moreover, the inflammasome has recently been reported to be associated with tumor progression. However, the role of P2Y2R in inflammasome activation in breast cancer cells is not yet well defined. Therefore, in this study, we investigated the role of P2Y2R in inflammasome-mediated tumor progression in breast cancer using breast cancer cells and radiotherapy-resistant (RT-R) breast cancer cells. We established RT-R-breast cancer cells (RT-R-MDA-MB-231, RT-R-MCF-7, and RT-R-T47D cells) by repeated irradiation (2 Gy each, 25 times) in a previous study. In this study, we found that the RT-R breast cancer cells exhibited an increased release of adenosine triphosphate (ATP) and P2Y2R activity. In particular, the RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 cells, exhibited a markedly increased ATP release, which was potentiated by tumor necrosis factor (TNF)-α. The MDA-MB-231 cells exhibited inflammasome activation, as measured by caspase-1 activity and interleukin (IL)-1β secretion following treatment with TNF-α and ATP; these effects were enhanced in the RT-R-MDA-MB-231 cells. However, the increased caspase-1 activities and IL-1β secretion levels induced in response to treatment with TNF-α or ATP were significantly reduced by P2Y2R knockdown or the presence of apyrase in both the MDA-MB-231 and RT-R-MDA-MB-231 cells, suggesting the involvement of ATP-activated P2Y2R in inflammasome activation. In addition, TNF-α and ATP increased the invasive and colony-forming ability of the MDA-MB-231 and RT-R-MDA-MB-231 cells, and these effects were caspase-1-dependent. Moreover, matrix metalloproteinase (MMP)-9 activity was modulated by caspase-1, in a P2Y2R-dependent manner in the MDA-MB-231 and RT-R-MDA-MB-231 cells. Finally, nude mice injected with the RT-R-MDA-MB-231-EV cells (transfected with the empty vector) exhibited increased tumor growth, and higher levels of MMP-9 in their tumors and IL-1β levels in their serum compared with the mice injected with the RT-R-MDA-MB-231- P2Y2R shRNA cells (transfected with P2Y2R shRNA). On the whole, the findings of this study suggest that extracellular ATP promotes tumor progression in RT-R-breast cancer cells and breast cancer cells by modulating invasion and associated molecules through the P2Y2R-inflammasome activation pathway.
Keywords: adenosine triphosphate, interleukin-1β, inflammasome, P2Y2 receptor, radiotherapy-resistant breast cancer cells, tumor progression
Introduction
Inflammation is a double-edged sword in cancer. Inflammation was initially believed to be a host response against tumors, resulting in tumor suppression and a favorable prognosis (1). However, it has been reported that inflammation, particularly chronic inflammation, is associated with an unfavorable clinical prognosis of cancer patients (2,3), and inflammation is now suggested as the seventh hallmark for cancer establishment and progression (4). There are abundant data suggesting that inflammation and hypoxia in the tumor microenvironment are critical components that are necessary for tumor progression and the metastatic cascade (5). Indeed, such an environment is more permissive for tumor cell proliferation and motility than are normal conditions. Moreover, several studies have indicated that tumor cell signaling and extracellular signaling affect cancer cell migration and therefore, metastasis in vivo and in vitro (reviewed in ref. 6). However, the innate pathways or mechanisms controlling the inflammatory response in the tumor microenvironment are not yet fully understood.
Pro-inflammatory cytokines, such as interleukin (IL)-1β and IL-18, are detected at high levels in cancer patients, and are suggested to promote an immune-suppressive tumor microenvironment (4,7, 8). The inflammasome is an important innate immune pathway responsible for the production of mature IL-1β. Inflammasome sensors are classified according to their structural features into nucleotide-binding domain-like receptors (NLRs), absent in melanoma 2-like receptors (ALRs), and the recently identified pyrin. These receptors can assemble the inflammasome and activate the cysteine protease, caspase-1. Active caspase-1 cleaves the precursor pro-inflammatory cytokines, pro-IL-1β and pro-IL-18, into their mature secreted forms, and these cytokines can ultimately be released (9). In particular, IL-1β is abundant in tumor tissue and enhances tumor growth, invasion, carcinogenesis and host-tumor interactions (10,11), and increased concentrations of IL-1β in tumor tissues are associated with a poor prognosis in cancer patients (12-14), suggesting that IL-1β is one of the essential components that mediate inflammation-associated tumor progression.
Of note, the inflammasome has been reported to be activated by adenosine triphosphate (ATP) (15). Various cellular stimuli trigger the secretion of ATP (16,17) and subsequently induce the activation of purinergic receptors present on the cell surface and/or on adjacent cells. Under pathological conditions, ATP is released passively from damaged cells at high levels, acts as a pro-inflammatory danger signal, and activates the NLRP3 inflammasome through bonding to the P2 purinergic receptor, P2Y purinergic receptor 2 (P2X7R) (15). Recent studies have reported that ATP is released from both damaged cells and tumor cells and accumulates in the tumor microenvironment, which can be related to tumor progression (18,19). Among the purinergic receptors that are activated by ATP, P2Y2R is expressed (or overexpressed) in cancer cells or solid tumors and performs various functions; it regulates proliferation in various tumors, such as lung, bladder, and prostate cancer and melanoma (20-23). In our previous studies, we reported that highly metastatic MDA-MB-231 breast cancer cells released higher levels of ATP and exhibited a higher P2Y2R activity than the MCF7 breast cancer cells with a low metastatic potential (24). In addition, ATP-activated P2Y2R played an important role in tumor progression, particularly in invasion and metastasis, by regulating hypoxia-inducible factor-1α (HIF-1α) (24,25).
In general, cancer patients are treated based on a combinatorial approach that consists of surgery, chemotherapy and radiotherapy. However, each therapy has inherent limitations that lead to therapeutic resistance and disease recurrence, ultimately resulting in therapeutic failure. Radiotherapy is a crucial treatment option in modern cancer therapy in addition to surgery and systemic therapy; currently, >60% of all cancer patients receive radiotherapy. Radiotherapy has been shown to improve overall survival (26-28), to help avoid surgical amputation and to preserve bodily beauty, and it can be used in palliative settings (29,30). Although benefits are achievable with radiotherapy, tumor recurrence following radiotherapy is common; particularly for ductal carcinoma and early invasive cancer, advanced invasive tumors can exhibit radiotherapy resistance, and the related molecular mechanisms are poorly understood. Thus, in this study, we established radiotherapy- resistant (RT-R)-breast cancer cells and investigated the association between P2Y2R and the inflammasome in RT-R- breast cancer cell progression and invasiveness.
Materials and methods
Cell culture
The human breast cancer cell lines, MDA-MB-231, MCF7 and T47D, were obtained from the Korea Cell Line Bank and grown in RPMI-1640 supplemented with 10% FBS (HyClone, Logan, UT, USA), 100 U/ml penicillin and 10 µg/ml streptomycin (HyClone). The human umbilical endothelial cell line, EA.hy926, was obtained from ATCC (Manassas, VA, USA) and grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin and 10 µg/ml streptomycin.
Establishment of RT-R MDA-MB-231 MCF7 and T47D cells (termed RT-R-MDA-MB-231, RT-R-MCF7 and RT-R- T47D cells, respectively)
Isogenic models of radiotherapy resistance can be generated by the exposure of cancer cells to various schedules with the total concentrations within a 40-60 Gy range (31,32). Moreover, the fractionated irradiation is clinically universal. Clinical total body irradiation is generally fractionated with smaller doses delivered in several sessions, rather than delivering the entire doses at once, due to lower toxicity and better outcomes (33). Thus, in this study, RT-R-MDA-MB-231, RT-R-MCF7 and RT-R-T47D cells were generated by treating the cells with fractionated X-ray irradiation until a final concentration of 50 Gy was attained. In detail, the cells that had grown to 70% confluence in a cell culture flask were irradiated with 2 Gy (radiation dose rate, 1.0 Gy/min) using a 6-MV photon beam produced by a linear accelerator (Clinac 21EX; Varian Medical Systems, Palo Alto, CA, USA). Following irradiation, the cells were incubated with fresh complete medium immediately. When the cell confluence reached approximately 90%, the cells were trypsinized and subcultured into new flasks. The cells were irradiated again when they grew to approximately 70% confluence. Until the total irradiation dose attained 50 Gy, the fractionated irradiations were continued. The parental cells were subjected to identical trypsinization, subculture and incubation conditions, but were not subjected to irradiation. The RT-R-MDA-MB-231, RT-R-MCF7 and RT-R-T47D cells were used through 5 passages. The radiation output was regularly checked by medical physicists in the Department of Radiation Oncology using an ionization detector.
Extracellular ATP release measurements
The extracellular release of ATP was measured according to previously described methods (24). Briefly, the cells were incubated with HEPES buffer containing adenosine-5′-O-(α,β-methylene)- diphosphonate (AOPCP), an ectonucleotidase inhibitor (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at 37°C. The cells were treated with 10 ng/ml tumor necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN, USA) or PBS as a vehicle for an additional 5 min, and the supernatants were then collected. ATP release was measured with the ENLITEN ATP assay system kit (Promega, Madison, WI, USA), and the ATP levels were calculated based on an ATP standard curve.
Measurement of intracellular calcium ion concentration ([Ca2+]i)
[Ca2+]i measurements were made according to previously described methods (34). Briefly, the cells were seeded on a coverslip mounted onto a self-designed perfusion chamber, and then incubated for 45 min with 5 µM fluo-3-AM (Invitrogen, Carlsbad, CA, USA) in culture medium. The stained cells were washed with a physiological solution and then treated with ATP. Fluorescent images were scanned every 5 sec using a confocal laser scanning microscope (IX70 Fluoview, Olympus, Tokyo, Japan). Every scanned image was processed to analyze changes in [Ca2+]i. The basal fluorescence intensity (F0), fluorescence intensity (F), and the maximum level of fluorescence intensity (Fmax) were recorded.
Matrigel invasion assay
For invasion assays, the upper chambers of inserts were coated with 100 µl of Matrigel (1 mg/ml; BD Biosciences, Franklin Lakes, NJ, USA), and endothelial cells (2×105 cells) were added to the Matrigel-coated insert wells. The breast cancer cells were pre-treated with 20 µM Ac-YVAD-CMK (Sigma-Aldrich), a selective and irreversible inhibitor of caspase-1, for 1 h, and then stimulated with 10 ng/ml TNF-α or 10 µM ATP. After 6 h, the cells were harvested, and 2×105 cells per insert were added to the upper chambers in serum-free medium, and 500 µl of RPMI medium was added to the lower chambers. The invasion chambers were incubated for 24 h in a 37°C cell culture incubator. The non-invaded cells that remained on the upper surface of the insert membranes were removed by scrubbing. The cells that had invaded across the insert well membrane were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI, Sigma-Aldrich), and the cells were counted under a fluorescence microscope (Eclipse Ti-U, Nikon, Tokyo, Japan).
Caspase-1 activity assay
Caspase-1 activity was measured using Caspase-1/ICE Colorimetric Assay kit (R&D Systems) following the manufacturer’s instructions. Briefly, the cells were treated with 10 ng/ml TNF-α or 10 µM ATP for indicated times. As shown in Fig. 3, the cells were transfected with indicated siRNA (100 nM) as described below, and then pre-treated with 10 U/ml apyrase (Sigma-Aldrich), an enzyme that rapidly hydrolyzes extracellular nucleotides. After 1 h, the cells were stimulated with 10 ng/ml TNF-α or 10 µM ATP for 3 h, and the total proteins were then extracted from the cells using lysis buffer. A volume of 50 µl of protein sample from cells was added to 50 µl of 2X caspase-1 reaction buffer containing 10 mM dithiothreitol (DTT) in a 96-well plate. Five microliters of 4 mM caspase-1 colorimetric substrate (YVAD-pNA) were added to each sample and then incubated at 37°C for 1-2 h. The colorimetric intensity was measured at a wavelength of 405 nm using a microplate reader (Tecan, Männedorf, Switzerland).
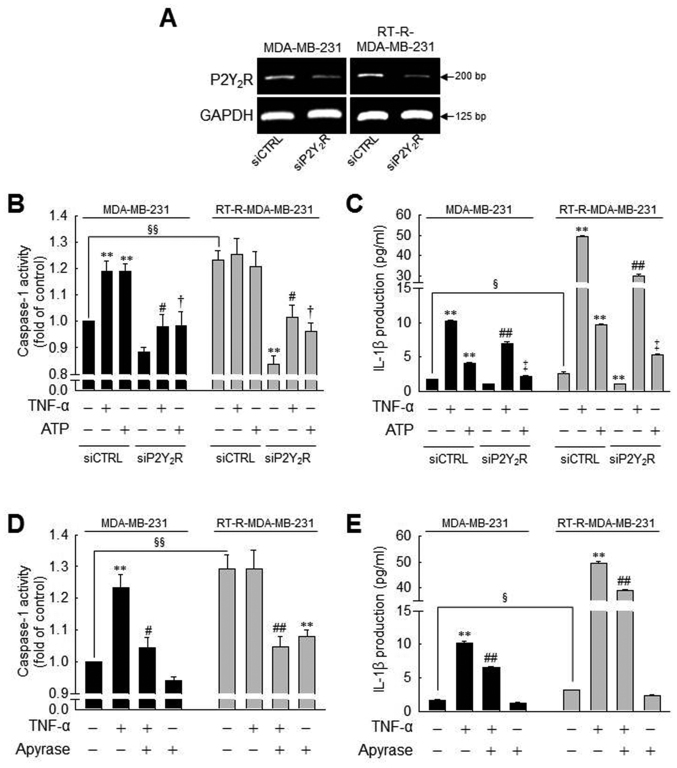
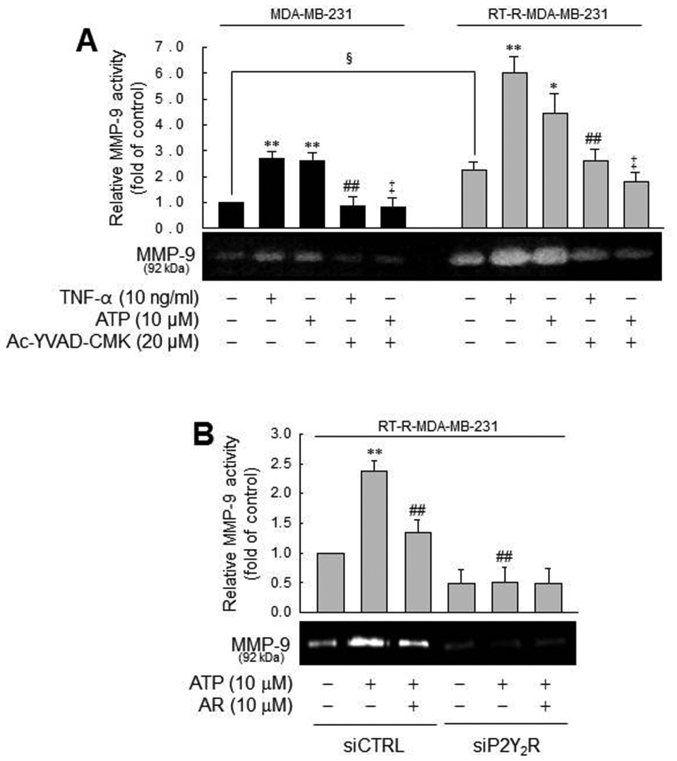
Figure 3.
Caspase-1 activity or IL-1β secretion induced by TNF-α or ATP is significantly suppressed by P2Y2R knockdown or apyrase (an enzyme that rapidly hydrolyzes extracellular nucleotides) in MDA-MB-231 or RT-R-MDA-MB-231 cells. (A) P2Y2R mRNA levels were analyzed by RT-PCR to confirm the efficiency of the knockdown in control siRNA (siCTRL)- or P2Y2R siRNA (siP2Y2R)-transfected cells. (B and C) siCTRL or siP2Y2R-transfected cells were stimulated with TNF-α (10 ng/ml) or ATP (10 µM). (B) Caspase-1 activity and (C) IL-1β secretion were measured 3 h and 24 h after treatment, respectively, as described in the Materials and methods. (D and E) The cells were pre-treated with 10 U/ml apyrase for 1 h and stimulated with TNF-α (10 ng/ml). (D) Caspase-1 activity and (E) IL-1β secretion were measured as described in (A). The values represent the means ± SEM of 3 independent experiments. **P<0.01, compared to the control (CTRL) of each cells; #P<0.05 and ##P<0.05, compared to the TNF-α treatment of each of the cells; †P<0.05 and ‡P<0.01, compared to the ATP treatment of each of the cells; §P<0.05 and §§P<0.01, significance between the MDA-MB-231 and RT-R-MDA-MB-231 cells. ATP, adenosine triphosphate; RT-R, radiotherapy-resistant; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
Quantification of IL-1β secretion
To quantify the amounts of secreted cytokines, cell culture supernatants or animal serum samples were assayed using the Human IL-1β/IL-1F2 Quantikine ELISA kit (R&D Systems), according to the manufacturer’s instructions. Briefly, the cells were treated as described above in the ‘Caspase-1 activity assay’. Following stimulation with 10 ng/ml TNF-α or 10 µM ATP for 24 h, cell culture supernatants were collected. Mouse serum was obtained by heart puncture before sacrifice and centrifugation was used for the measurement of the IL-1β levels. A volume of 200 µl of sample was added to a microplate and sequentially mixed with Conjugate, Substrate Solution and Stop Solution. The optical density of each well was measured at a wavelength of 450 nm using a microplate reader (Tecan).
Gene silencing with siRNA
Breast cancer cells and RT-R breast cancer cells were transfected with 100 nM negative control siRNA (siCTRL) or P2Y2R siRNA (siP2Y2R) (Bioneer, Daejeon, Korea) in serum-containing medium using Turbofect® (Thermo Fisher Scientific, Waltham, MA, USA). The sequences of the siRNAs were as follows: siCTRL forward, 5′-CCUACGCCACCAAUUUCGU-3′ and reverse, 5′-ACGAA AUUGGUGGCGUAGG-3′; siP2Y2R forward, 5′-GAGGAAGGUGGCUUACCAA-3′ and reverse, 5′-UUGGUAAGCCACCUUCCUC-3′. Following 24 h of incubation at 37°C, the transfection medium was replaced with fresh serum-free medium for starvation. Following serum starvation for 16 h, the cells were treated with the indicated reagents. Gene silencing efficiency was determined by reverse transcription-polymerase chain reaction (RT-PCR).
RT-PCR
Total RNA was extracted from the cells using TRIzol reagent (Thermo Fisher Scientific), and RT-PCR was performed using TOPscript One-step RT PCR Drymix (Enzynomics, Daejeon, Korea), according to the manufacturer’s instructions. The primer sets used were as follows: hP2Y2R forward, 5′-GTG CTC TAC TTC CTG GCT-3′ and reverse, 5′-CTG AAG TGT TCT GCT CCT AC-3; ′ and hGAPDH forward, 5′- TCA ACA GCG ACA CCC ACT CC-3′ and reverse, 5′-TGA GGT CCA CCA CCC TGT TG-3′. Thirty cycles of amplification were performed under the following conditions: Melting at 95°C for 30 sec, annealing at 56°C for 30 sec and extension at 72°C for 30 sec.
Colony formation assay
The MDA-MB-231 or RT-R- MDA-MB-231 cells (1×103) were seeded in 6-well plates. Following serum starvation for 16 h, the cells were pre-treated with 20 µM Ac-YVAD-CMK for 1 h, and then stimulated with 10 ng/ml TNF-α or 10 µM ATP at 37°C. The culture medium was discarded following treatment, and changed with complete medium every 2-3 days. After 10 days, the medium was discarded and each well was carefully washed with PBS. The colonies were fixed in methanol for 10 min at room temperature and then stained with 0.1% Giemsa staining solution, and the number of visible colonies was counted.
Gelatin zymography
The cells which were transfected with the indicated siRNA (100 nM or the untransfected cells were pre-treated with 20 µM Ac-YVAD-CMK or 10 µM AR-C 118925XX (Tocris Bioscience, Bristol, UK), a specific P2Y2R antagonist. After 1 h, the cells were stimulated with 10 ng/ml TNF-α or 10 µM ATP for 6 h, and the same volume of each conditioned medium was then concentrated 20-fold using protein concentrators (9K MWCO; Thermo Fisher Scientific) at a fixed angle (35 degrees) and centrifugation at 6,000 × g for 25 min at 4°C. The concentrated samples were mixed with 2X loading dye, and the proteins were separated on 8% SDS-polyacrylamide gels containing 1 mg/ml gelatin. Following electrophoresis, the gels were washed in 2.5% Triton X-100 twice for 30 min to remove the remaining SDS. The gels were then incubated in developing buffer (50 mM Tris, 20 mM NaCl, 5 mM CaCl2, 0.02% Brij35) at 37°C overnight. Following incubation, the gels were stained with coomassie blue solution (0.2% coomassie brilliant blue R, 50% methanol, 10% acetic acid) for 30 min at room temperature, and destained with destaining buffer (50% methanol, 10% acetic acid). Enzyme-digested regions which represent matrix metalloproteinase (MMP)-9 activity were identified as white bands on a blue background.
Animal experiments
RT-R-MDA-MB-231 cells were transfected with the P2Y2R shRNA plasmid (Santa Cruz Biotechnology Inc., Dallas, TX, USA) which contains a puromycin resistance gene for the selection of cells stably expressing targeted shRNA in serum-free medium using Lipofectamine 2000 (Thermo Fisher Scientific). Following 4 h of incubation at 37°C, the transfection medium was replaced with fresh medium containing 5 µg/ml puromycin (Sigma-Aldrich). The culture medium containing puromycin was changed every 2-3 days. At 30 days following transfection, the stably treasfected subclone was designated RT-R-MDA-MB-231-P2Y2R shRNA. This subclone and a CTRL subclone transfected with an empty vector (designated as MDA-MB-231-EV) were grown in serum-containing culture medium until the cell density was ~70-80%. The cells were then trypsinized, and the pellets were resuspended in serum-free RPMI at 5-6×106 cells/100 µl of cell suspension. A total of 10 female NU-Foxn1nu athymic nude mice at 7-8 weeks of age (weighing 20-22 g) were purchased from OrientBio (Gyeonggi-do, Korea). The animals were maintained under the following environmental conditions: 22-26°C; 40-60% of humidity, 12 h-light/dark cycle; with free access to sterilized feed and water. The mice were injected subcutaneously with RT-R-MDA-MB-231-P2Y2R shRNA or RT-R-MDA-MB-231-EV. Body weights and tumor volumes were measured every 3 days, starting at 7 days after the injection. At the end of 60 days, the mice were sacrificed, and the tumor tissues were fixed in 4% formaldehyde at room temperature, followed by paraffin infiltration and embedding. Sections of 5 µm thickness were mounted onto ProbeOn Plus microscope slides (Thermo Fisher Scientific). Immunohistochemical analysis was performed using a Lab Vision™ UltraVision™ LP Detection System: HRP Polymer/DAB Plus Chromogen and an anti-MMP-9 antibody (ab38898, Abcam, Cambridge, UK). Briefly, the tissue sections were deparaffinized and rehydrated, and incubated in Hydrogen Peroxide Block solution for 10 min and then incubated in Ultra V Block solution for 5 min at room temperature to reduce non-specific background staining. After the washing step, the slides were incubated with MMP-9 primary antibody (1:100) for 1 h at room temperature, and then sequentially applied with Primary Antibody Enhancer (10 min), HRP Polymer (15 min), and DAB Plus Chromogen and DAB Plus Substrate mixture (5 min) at room temperature. Following DAB staining, the sections were counterstained with Mayer’s Hematoxylin solution (Sigma-Aldrich) for 3 min at room temperature. Immunohistochemical analysis was performed under a light microscope (CKX41, Olympus). Mouse serum was obtained by heart puncture before sacrifice and centrifugation, and the IL-1β levels were measured from the serum of mice using Quantikine ELISA kits for IL-1β as described above. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee at Gyeongsang National University (approval number: GLA-120208-M004), and all experiments were performed in compliance with the institutional guidelines set.
Statistical analysis
All statistical analysis was carried out using SigmaPlot (version 7.0 for windows, SPSS Inc.). The data are represented as the means ± standard error of the mean (SEM) of the results obtained from the number of replicate treatments. Treatment groups were compared using one-way analysis of variance (ANOVA) with the Newman-Keuls post-hoc test. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells exhibit much higher levels of released ATP, P2Y2R activity and invasiveness than other RT-R breast cancer cells
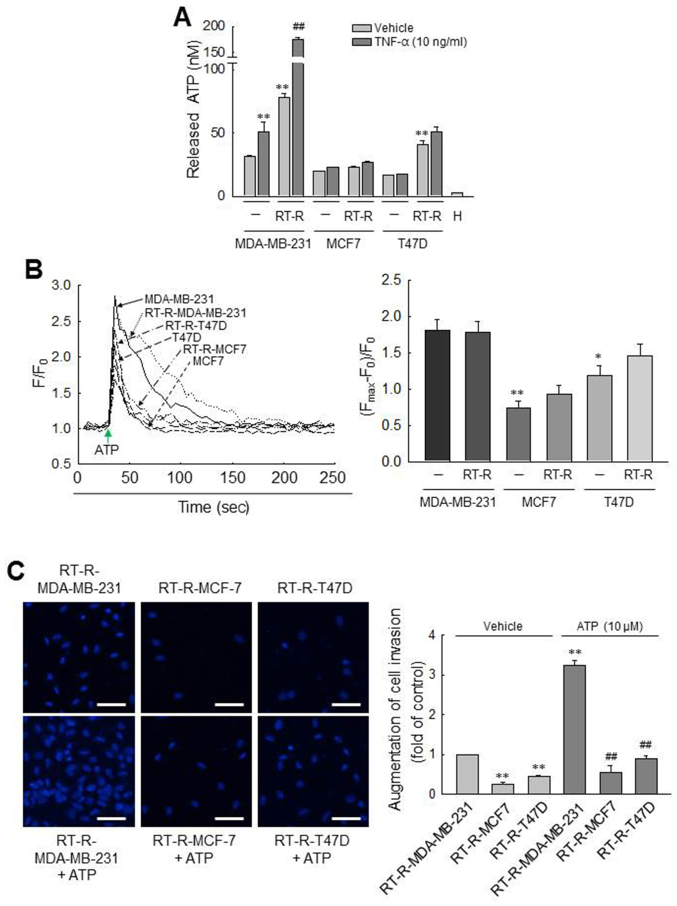
First, we observed the amount of released ATP from various breast cancer cells (MDA-MB-231, MCF7 and T47D cells) and RT-R breast cancer cells (RT-R-MDA-MB-231, RT-R-MCF7 and RT-R-T47D cells). As previously reported (24), we confirmed that the amounts of ATP released into the extra- cellular medium of MDA-MB-231 cells were higher than those released by MCF7 and T47D cells. Of note, in this study, we found that the RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells released much higher levels of ATP than did the MDA-MB-231 and other RT-R breast cancer cells, and that effect was enhanced by TNF-α (Fig. 1A), an essential factor in tumor progression and metastasis and released highly in tumor microenvironment (35,36). To further compare the P2Y2R activity between the breast cancer cells and RT-R breast cancer cells, [Ca2+]i was measured in response to ATP, an agonist of P2Y2R. Although 10 µM ATP evoked a rapid and prompt augmentation in [Ca2+]i in all the breast cancer cells, the activities of P2Y2R in the MCF7/RT-R-MCF7 and the T47D/RT-R-T47D cells were significantly lower than those observed in the MDA-MB-231 or RT-R-MDA-MB231 cells (Fig. 1B). Moreover, the RT-R-MDA-MB-231 cells also exhibited a higher invasiveness than the other RT-R breast cancer cells and markedly increased invasiveness following treatment with ATP (Fig. 1C). These results suggest that RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells exhibit much higher levels of released ATP, P2Y2R activity and invasiveness than other RT-R breast cancer cells derived from breast cancer cells with low metastatic potential. From these results, we considered it more suitable to investigate the association between P2Y2R and the inflammasome in MDA-MB-231 and RT-R-MDA-MB-231 cells which exhibited high levels of released ATP, P2Y2R activity and invasiveness, than the MCF7 and T47D cells, breast cancer cells with low metastatic potential. Thus, the following experiments were performed with the MDA-MB-231 and RT-R-MDA-MB-231 cells.
Figure 1.
Comparisons of ATP release, P2Y2R activity and invasiveness between breast cancer cells and RT-R breast cancer cells. (A) ATP released into the extracellular medium was measured using the ENLITEN ATP assay system kit, as described in the Materials and methods. The values represent the means ± SEM of 3 independent experiments (H, HEPES buffer only). **P<0.01, compared to the control (CTRL) of each parent breast cancer cell; ##P<0.01, compared to the CTRL of each RT-R breast cancer cells. (B) [Ca2+]i levels were determined in breast cancer cells and RT-R breast cancer cells to measure P2Y2R activities. Arrows indicate the points at which ATP (10 µM) was added. The values represent the means ± SEM from 3 independent determinations. *P<0.05 and **P<0.01, compared to the CTRL of MDA-MB-231 cells. (C) RT-R-breast cancer cells were treated with ATP for 6 h, and Matrigel invasion assay was performed as described in the Materials and methods. The values represent the means ± SEM of 3 independent experiments. **P<0.01, compared to the CTRL of RT-R-MDA-MB-231 cells; ##P<0.01, compared to ATP-treated RT-R-MDA-MB-231 cells. Scale bar, 50 µm. ATP, adenosine triphosphate; RT-R, radiotherapy-resistant.
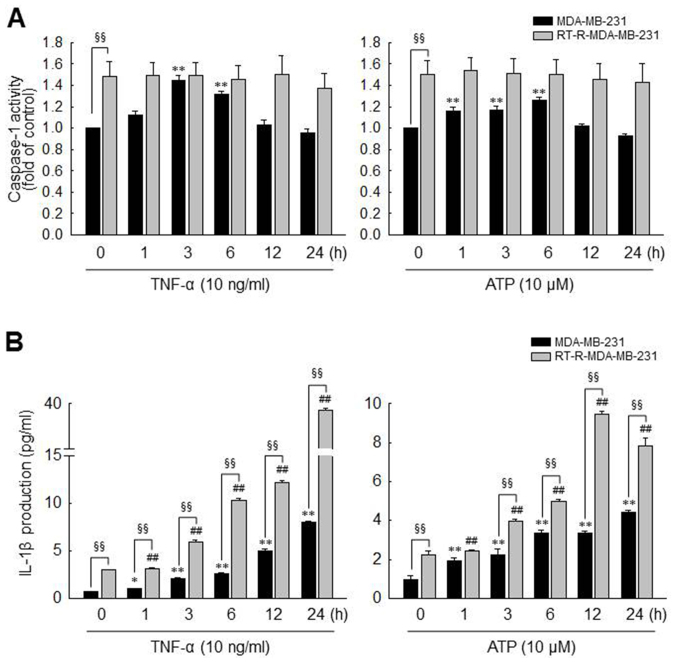
MDA-MB-231 cells exhibit an increased caspase-1 activity and IL-1β secretion induced by TNF-α and ATP treatment, and these effects are enhanced in RT-R-MDA-MB-231, through P2Y2R activation
As described above in the Introduction, the inflammasome, a multiprotein complex, regulates the activation of caspase-1, which promotes the secretion of the pro-inflammatory cytokine, IL-1β (37,38), and IL-1β is abundant in tumor tissue and enhances tumor growth and invasion (10). Therefore, in this study, we investigated whether TNF-α- or ATP-mediated P2Y2R activation increases inflammasome activity by examining the activities of caspase-1 and the levels of IL-1β production in the MDA-MB-231 and RT-R-MDA-MB-231 cells. As previously mentioned (35,36), TNF-α is highly released in the tumor microenvironment and as shown in Fig. 1A, the release of ATP from breast cancer cells was promoted in response to TNF-α treatment. Thus, we experimented with ATP, as well as TNF-α. As shown in Fig. 2A, caspase-1 activity was significantly increased and reached a maximum level at 3 or 6 h in response to TNF-α (10 ng/ml) or ATP (10 µM), respectively, in the MDA-MB-231 cells. By contrast, caspase-1 activity was not altered by TNF-α or ATP in the RT-R-MDA-MB-231 cells. However, the basal activity of caspase-1 in the RT-R-MDA-MB-231 cells was higher than that observed in the MDA-MB-231 cells, and the maximum activity level of caspase-1 induced by TNF-α or ATP in the MDA-MB-231 cells did not exceed the basal activity in the RT-R-MDA-MB-231 cells. Of note, the IL-1β production levels were markedly increased following stimulation with TNF-α or ATP in both types of cells, and the RT-R-MDA-MB-231 cells produced higher levels of IL-1β than the MDA-MB-231 cells, not only in terms of basal levels but also following stimulation (Fig. 2B).
Figure 2.
MDA-MB-231 cells exhibit increased caspase-1 activity and IL-1β secretion after TNF-α and ATP treatment, which is enhanced in RT-R-MDA-MB-231 cells. (A and B) MDA-MB-231 and RT-R-MDA-MB-231 cells were treated with TNF-α or ATP with the indicated dose and times, and (A) caspase-1 activity and (B) IL-1β secretion levels were then measured as described in the Materials and methods. The values represent the means ± SEM of 3 independent experiments. *P<0.05 and **P<0.01, compared to the control (CTRL) of MDA-MB-231 cells; ##P<0.01, compared to the CTRL of RT-R-MDA-MB-231 cells; §§P<0.01, comparison between MDA-MB-231 and RT-R-MDA-MB-231 cells at each time point. ATP, adenosine triphosphate; RT-R, radiotherapy-resistant; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
Moreover, to clarify whether ATP-activated P2Y2R is involved in inflammasome activation in the MDA-MB-231 and RT-R-MDA-MB-231 cells, we examined caspase-1 activity and IL-1β production using P2Y2R siRNA or apyrase, an enzyme that rapidly hydrolyzes extracellular nucleotides. First, we confirmed the efficiency of the P2Y2R siRNA by determining the mRNA levels in the MDA-MB-231 and RT-R-MDA-MB-231 cells (Fig. 3A). The increased caspase-1 activity and IL-1β secretion induced by TNF-α or ATP were significantly reduced by P2Y2R knockdown (Fig. 3B and C) or in the presence of apyrase (Fig. 3D andE) in both the MDA-MB-231 and RT-R-MDA-MB-231 cells. Although treatment with TNF-α or ATP did not alter the activity of caspase-1 in the RT-R-MDA-MB-231, the knockdown of P2Y2R or the hydrolyzation of ATP significantly reduced the activity of caspase-1 in these (Fig. 3B and D). Furthermore, apyrase treatment alone decreased caspase-1 activity in RT-R-MDA-MB-231 cells, and apyrase also suppressed the TNF-α-induced increase in caspase-1 activity and IL-1β secretion in both the MDA-MB-231 and RT-R-MDA-MB-231 cells. These results suggest that P2Y2R activation by ATP released from RT-R-MDA-MB-231 cells and MDA-MB-231 cells may regulate inflammasome activation.
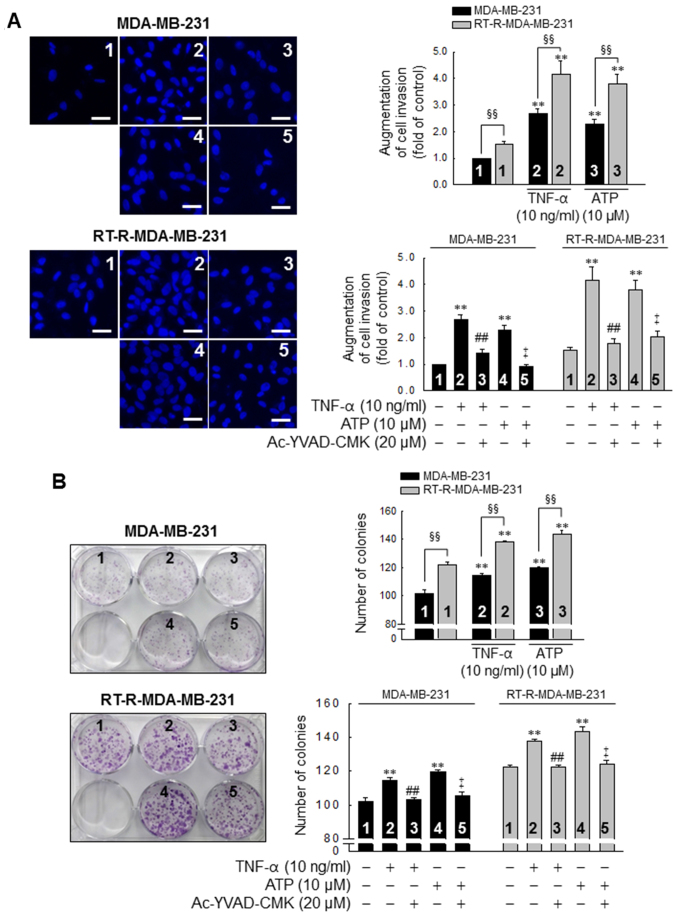
TNF-α and ATP increase the invasive and colony-forming ability of MDA-MB-31 cells, with enhanced effects in the RT-R-MDA-MB-231 cells, in a caspase-1-dependent manner
Subsequently, we investigated whether the P2Y2R- mediated activation of the inflammasome is linked to the invasive and colony-forming ability of the MDA-MB-231 and RT-R-MDA-MB-231 cells. The invasive ability of the MDA-MB-231 and RT-R-MDA-MB-231 cells was increased by treatment with TNF-α (10 ng/ml) or ATP (10 µM), and this induction was abolished by inhibiting the activity of the inflammasome with 20 µM Ac-YVAD-CMK, a selective and irreversible inhibitor of caspase-1 (Fig. 4A). Moreover, the colony-forming ability of the cells was also promoted following stimulation with TNF-α or ATP and abolished by the suppression of caspase-1 activity in both the MDA-MB-231 and RT-R-MDA-MB-231 cells (Fig. 4B). Of note, the invasive and colony-forming ability of the RT-R-MDA-MB-231 cells was significantly enhanced compared to that of the MDA-MB-231 cells following TNF-α- or ATP treatment and in non-stimulated conditions (Fig. 4).
Figure 4.
TNF-α and ATP increase the invasive and colony-forming ability of MDA-MB-231 cells, with an enhanced effect in the RT-R-MDA-MB-231 cells, in a caspase-1-dependent manner. (A) Cells were pre-treated with Ac-YVAD-CMK, an irreversible caspase-1 inhibitor and stimulated with TNF-α or ATP for 6 h. Matrigel invasion assay was then performed as described in the Materials and methods. The values represent the means ± SEM of 3 independent experiments. **P<0.01, compared to the control (CTRL) of each of the cells; ##P<0.01, compared to the TNF-α treatment of each of the cells; ‡P<0.01, compared to the ATP treatment of each of the cells; §§P<0.01, significance between the MDA-MB-231 and RT-R-MDA-MB-231 cells. Scale bar, 100 µm. (B) Cells (1,000 cells/well) were seeded in 6-well plates. The cells were pre-treated with Ac-YVAD-CMK and stimulated with TNF-α or ATP for 6 h. Following treatment, colony formation assay was performed as described in the Materials and methods, and quantified by counting the colonies. The values represent the means ± SEM of 3 independent experiments. **P<0.01, compared to the CTRL of each of the cells; ##P<0.01, compared to the TNF-α treatment of each of the cells; ‡P<0.01, compared to the ATP treatment of each of the cells; §§P<0.01, between the MDA-MB-231 and RT-R-MDA-MB-231 cells. ATP, adenosine triphosphate; RT-R, radiotherapy-resistant; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
MMP-9 activity is modulated by caspase-1 in a P2Y2R-dependent manner, in the MDA-MB-231 or RT-R-MDA-MB-231 cells
In addition, we examined the effects of the inflammasome and P2Y2R activation on MMP activity, which is involved in tumor invasion and metastasis (39). Treatment with TNF-α or ATP increased MMP-1 activity in the MDA-MB-231 cells, which was diminished by a caspase-1 inhibitor. These phenomena were more prominent in the RT-R-MDA-MB-231 cells (Fig. 5A). The prominent induction of MMP-9 activity by ATP in the RT-R-MDA-MB-231 cells was diminished by a P2Y2R antagonist and P2Y2R siRNA, suggesting that TNF-α- or ATP-mediated MMP-9 activity is dependent on caspase-1 and P2Y2R. Even though MMP-9 activity in the MDA-MB-231 and RT-R-MDA-MB-231 cells exhibited a similar tendency, the real difference between the MDA-MB-231 and RT-R-MDA-MB-231 cells was that the RT-R-MDA-MB-231 cells exhibited relatively higher MMP-9 activity than the MDA-MB-231 cells in the control level and much increased MMP-9 activity following treatment with TNF-α or ATP. Coherent with this finding, the RT-R-MDA-MB-231 cells exhibited higher MMP-9 activity than the MDA-MB-231 cells, as shown in the colony formation assay.
Figure 5.
MMP-9 activity is modulated by caspase-1 in a P2Y2R-dependent manner, in MDA-MB-231 or RT-R-MDA-MB-231 cells. (A) Cells were pre-treated with Ac-YVAD-CMK and then stimulated with TNF-α or ATP for 6 h. MMP-9 gelatinase activity was determined as described in the Materials and methods and quantified. The values represent the means ± SEM of 3 independent experiments. *P<0.05 and **P<0.01, compared to the control (CTRL) of each of the cells; ##P<0.05, compared to the TNF-α treatment of each of the cells; ‡P<0.05, compared to the ATP treatment of each of the cells; §P<0.05, comparison between the MDA-MB-231 and RT-R-MDA-MB-231 cells. (B) siCTRL- or siP2Y2R-transfected RT-R-MDA-MB-231 cells were pre-treated with AR-C 118925XX (AR), a specific P2Y2R antagonist or not. The cells were then stimulated with ATP, and MMP-9 gelatinase activity was determined as described in the Materials and methods. The values represent the means ± SEM of 3 independent experiments. **P<0.05, compared to the CTRL; ##P<0.01, compared to ATP treatment. ATP, adenosine triphosphate; RT-R, radiotherapy-resistant; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; MMP-9, matrix metalloproteinase-9.
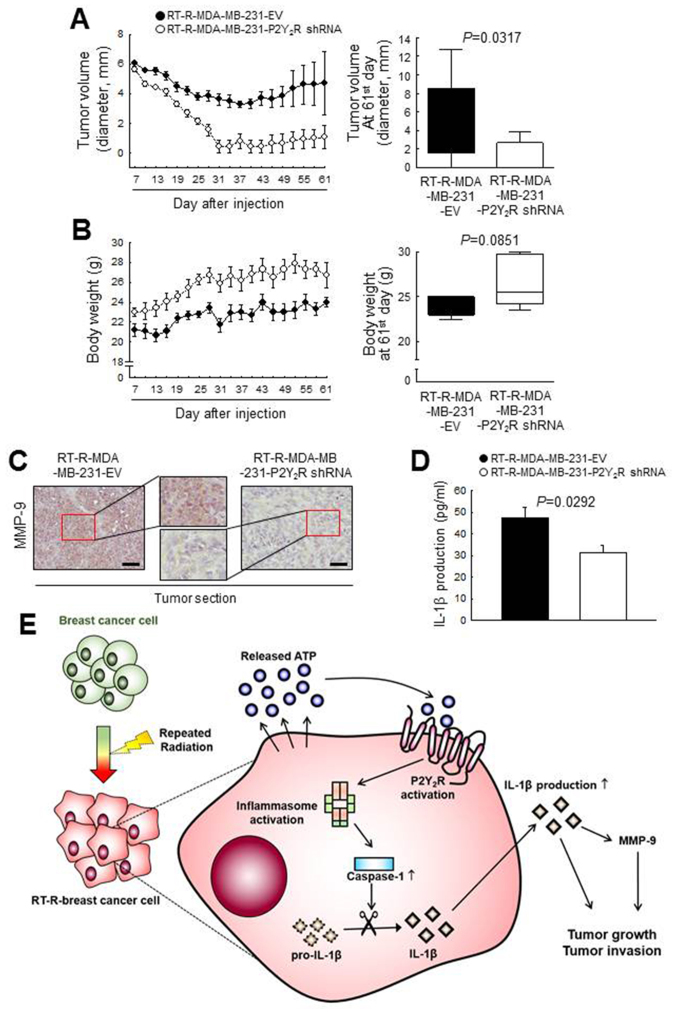
P2Y2R is related to the tumor growth and invasion of RT-R- breast cancer cells in an in vivo mouse model by regulating MMP-9 and inflammasome activation Finally, to confirm the involvement of the P2Y2R in RT-R-MDA-MB-231 cell progression in vivo, athymic nude mice were injected subcutaneously with control-shRNA-transfected RT-R-MDA-MB-231 cells (RT-R-MDA-MB-231-EV) or P2Y2R shRNA-transfected RT-R-MDA-MB-231 cells (RT-R-MDA-MB-231-P2Y2R shRNA). The tumor sizes and body weights of the mice were measured every 3 days from 7 days until 60 days after the injection. The tumor volume in the mice injected with RT-R-MDA-MB-231-P2Y2R shRNA cells was markedly decreased and the body weight was slightly augmented compared with that of the RT-R-MDA-MB-231-EV cell- injected mice (Fig. 6A and B). Furthermore, a higher MMP-9 expression was detected in the tumor sections from the mice injected with RT-R-MDA-MB-231-EV cells than in the sections from mice injected with the RT-R-MDA-MB-231- P2Y2R shRNA cells (Fig. 6C). In addition, we observed that the IL-1β levels in the serum were significantly lower in the mice injected with RT-R-MDA-MB-231-P2Y2R shRNA cells than in the mice injected with RT-R-MDA-MB-231-EV cells (Fig. 6D). These results suggest that P2Y2R plays an important role in RT-R-breast tumor progression via inflammasome regulation, as shown by the schematic representation in Fig. 6E.
Figure 6.
Suppression of P2Y2R reduced RT-R-breast cancer cell growth by regulating MMP-9 expression in an in vivo mouse model. Athymic nude mice were divided into 2 groups and injected subcutaneously with empty vector-transfected RT-R-MDA-MB-231 cells (RT-R-MDA-MB-231-EV; n=5) or P2Y2R-shRNA- transfected RT-R-MDA-MB-231 cells (RT-R-MDA-MB-231-P2Y2R-shRNA; n=5) (5×106 cells/100 µl of serum-free medium). RT-R-MDA-MB-231-EV-injected or RT-R-MDA-MB-231-P2Y2R shRNA-injected animals were sacrificed on day 61. (A) Tumor volumes and (B) body weights were measured every 3 days during tumor development, and the bar graphs were presented at the end of 61st day. (C) Tumor tissue sections were stained with anti-MMP-9 antibody (scale bar, 100 µm), and the sections were counterstained with Mayer’s hematoxylin solution. (D) IL-1β levels in the serum were analyzed as described in the Materials and methods (n=3). (E) Schematic representation of the proposed role of P2Y2R in RT-R-breast cancer cell progression and invasiveness through interaction with the inflammasome.RT-R, radiotherapy-resistant; P2Y2R, P2Y purinergic receptor 2.
Discussion
Radiation is an essential component of breast cancer therapy for the majority of breast cancer patients at all stages of disease following lumpectomy (40). However, it has been reported that a small population of breast cancer cells that exhibits resistance against radiotherapy promotes tumor recurrence and metastasis and leads to a poor prognosis (41,42). Our previous study demonstrated that RT-R breast cancer cells were established by repeated irradiation and exhibited enhanced invasiveness (43). Moreover, we have previously reported that breast cancer growth and metastasis are involved in the activation of P2Y2R by ATP released from breast cancer cells (24,25). Therefore, we hypothesized that P2Y2R may also play a role in the enhanced invasiveness of RT-R breast cancer cells.
Recent studies have reported that inflammasome signaling is closely associated with several human cancers. However, the function of the inflammasome in tumor growth and metastasis remains controversial. The activation of the inflammasome is beneficial in colitis-associated colorectal cancer, mostly due to the positive epithelial effects of the IL-18 signaling pathway, the control of cellular proliferation, the maturation and maintenance of a healthy gut microbiota and cell death (44,45). In addition, inflammasome-modulated IL-1 signaling induces the suppression of anticancer immune responses in natural killer (NK) cells and T cells, which is disadvantageous for the progression and development of fibrocarcinoma, gastric cancer, melanoma and breast cancer (46-49). Thus, in this study, we wished to determine whether RT-R breast cancer cells release higher levels of ATP than breast cancer cells, and whether P2Y2R activation caused by ATP released from RT-R breast cancer cells enhances invasiveness through inflammasome activation. We demonstrated that RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells released much higher levels of ATP, and a exhibited stronger P2Y2R activity and invasiveness than MDA-MB-231 cells and other RT-R breast cancer cells. In addition, RT-R-MDA-MB-231 cells exhibited an increased caspase-1 activity and IL-1β secretion in response to TNF-α and ATP treatment through P2Y2R activation, resulting in enhanced invasiveness and colony forming ability.
The extracellular concentration of ATP is known to be higher in the tumor microenvironment than in normal conditions (50,51), and extracellular ATP can activate purinergic receptors, especially P2Y2R (52). In this study, the released ATP concentration of RT-R breast cancer cells was higher than that of breast cancer cells; in particular, the RT-R-MDA-MB-231 cells exhibited the highest levels of ATP released. Although the activity of P2Y2R induced by the same dose of ATP did not differ between the MDA-MB-231 and RT-R-MDA-MB-231 cells, the higher ATP levels released from the RT-R-MDA-MB-231 cells compared to the MDA-MB-231 cells may mediate P2Y2R activation and the P2Y2R-mediated signaling cascade. Actually, the concentration of ATP in the extracellular space and tumor microenvironment is the net amount of release and degradation. Thus, the actual ATP concentration released from cancer cells may be higher than the extracellular concentration of ATP observed in the tumor microenvironment as previously reported by Jin et al (24). Moreover, we previously demonstrated that ATP and UTP increased the cancer cell proliferation, adhesion molecules expression, and MMP activity at very low concentration (0.1-1 µM) through the activation of P2Y2R (24). Based on these reports, it is suggested that the concentration of ATP in the tumor microenvironment is sufficient to activate P2Y2R in breast cancer cells.
Inflammasomes are key signaling platforms that detect pathogenic microorganisms and sterile stressors. The sentinel receptor of the inflammasome recognizes these pathogens and is then activated via assembling apoptosis-associated speck-like protein containing a carboxy-terminal CARD (ASC) and caspase-1, which cleaves immature, pro-IL-1β into its mature secreted form (53). Through these sequential processes, mature released IL-1β performs diverse roles in tumor progression (10-14). Of note, in this study, we found that TNF-α and ATP significantly increased caspase-1 activity in the MDA-MB-231 cells, but not in the RT-R-MDA-MB-231 cells. The basal caspase-1 activity in the RT-R-MDA-MB-231 cells was much higher level than the basal levels, and TNF-α- or ATP-stimulated levels in MDA-MB-231 cells. This phenomenon may be caused by the high level of ATP released from RT-R-MDA-MB-231 cells in the basal state, which is supported by the finding that the basal level of caspase-1 in RT-R-MDA-MB-231 cells was significantly decreased by the knockdown of P2Y2R and in the presence of apyrase, an enzyme that rapidly hydrolyzes extracellular nucleotides. In addition, we found that the production of IL-1β in response to stimulation with TNF-α or ATP exhibited a steady increase until 24 h, while caspase-1 activity peaked at 3-6 h following stimulation and then decreased. The synthesis and secretion of IL-1β are stimulated by pathogen-associated molecular pattern molecules (PAMPs) or damage-associated molecular pattern molecules (DAMPs) and involve several steps (37,54). IL-1β is first synthesized as inactive pro-IL-1β and then processed into active IL-1β by caspase-1 and sequentially secreted into the extracellular space (55). According to previous reports, extracellular nucleotides also induce pro-IL-1β production by activating nuclear factor-κB (NF-κB) or mitogen-activated protein kinase (MAPK) (56-58). In addition, released mature IL-1β can promote the production of pro-IL-1β by binding to the IL-1 receptor, which is known to be expressed in various breast cancer cells including MDA-MB-231 cells (13). Based on these studies, we considered that IL-1β production would increase constantly until late time points after stimulation with extracellular nucleotides and autocrine mechanism of IL-1β, despite caspase-1 activity was peaked at a relatively early phase. Moreover, it was determined that the inflammasome activation induced by TNF-α or ATP, as determined by caspase-1 activity and the resulting IL-1β production, was regulated via the activation of P2Y2R, which was proven by knocking down P2Y2R or hydrolyzing ATP. This finding suggests that among the purinergic receptors, not only P2X7R but also P2Y2R, a G protein-coupled receptor, are involved in the activation of the inflammasome. Interestingly, some evidence indicated that extracellular ATP released from cells is finally converted to adenosine which also activates inflammasome through binding to adenosine receptors (59,60). Therefore, it is possible that adenosine, as well as ATP could increase of IL-1β production until late time through adenosine receptor activation. Accordingly, we plan to further study the role of adenosine and adenosine receptor on cancer cell progression and involvement with inflammasome activation. In addition, several studies have indicated that microRNAs, particularly, micro-RNA-144 regulates breast cancer cell proliferation, invasion and migration (61,62). Moreover, Yu et al (63) described the role of micro-RNA-144 in the regulation of radiotherapy sensitivity, migration and invasion of breast cancer cells. Thus, it is expected that micro-RNA-144 may be sufficient to affect P2Y2R-mediated inflammasome activation in RT-R breast cancer cells, even though it has not been examined in this study.
Breast cancer metastasis is a complex process determined by a number of factors and pathways. Metastasis begins with the local invasion of the surrounding host tissue by tumor cells that are located in the primary tumor and continues until the tumor cells invade and intravasate into the blood or lymphatic vessels (64,65). The tumor cells are spread through the blood stream or lymphatic vessels to distant organs and then undergo cell cycle arrest and adhere to capillary beds within the target organ. Consequently, the tumor cells extravasate into the organ parenchyma and proliferate within the organ (64). Our previous study demonstrated that RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells have more aggressive properties in invasion and adhesion to endothelial cells due to upregulated the epithelial-mesenchymal transition (EMT)- or adhesion-involved proteins (43). In this study, we determined that RT-R-MDA-MB-231 cells derived from highly metastatic MDA-MB-231 breast cancer cells exhibited an increased invasiveness and colony-forming ability compared to MDA-MB-231 or other breast cancer cells and RT-R breast cancer cells. Furthermore, the activation of P2Y2R by ATP enhanced the invasive and colony-forming ability of the RT-R-MDA-MB-231 cells, which was reduced by an inflammasome inhibitor.
The IL-1β stimulation of tumor cells activates multiple signaling pathways involving protein kinase B, MAPK and NF-κB (65). The activation of these signaling molecules is required for IL-1β-mediated production of MMP-9, a matrix degrading enzyme that is regarded as a critical regulator for IL-1β-induced tumor invasion (66-68). Recently, it was reported that MMP-9 overexpression in the serum is associated with poor patient prognosis in breast cancer (69). It has also been demonstrated that HIF-1α response elements are present in the human and mouse IL-1β promoter (60,70), and this finding led us to hypothesize that the activation of HIF-1α may be an important step in increasing pro-IL-1β production. In addition, IL-1β activates the hypoxia-angiogenesis program by upregulating HIF-1α, the pivotal mediator of cellular responses to hypoxia (71). HIF-1α expression in cancers is associated with clinical aggressiveness and poor outcomes (72). HIF-1α rapidly accumulates and transactivates hundreds of genes under hypoxic conditions, including angiogenic and growth factors and receptors and extracellular proteases, such as MMPs (73,74). In our results, the expression levels of HIF-1α were notably increased in the RT-R-MDA-MB-231 cells following stimulation with P2Y2R with ATP, and this effect was markedly decreased in the cells treated with P2Y2R antagonist or subjected to P2Y2R knockdown (data not shown).
Finally, we aimed to confirm whether P2Y2R plays important roles in radiotherapy-resistant tumor progression, including tumor growth and invasion in an in vivo animal model; Mice injected with RT-R-MDA-MB-231-EV cells exhibited a marked increase in tumor size. Of note, the tumors in both mice injected with RT-R-MDA-MB-231-EV or RT-R-MDA-MB-231-P2Y2R shRNA cells exhibited a similar increase in size until the 7th day (data not shown), but seemed to decline during the early phase. The tumors in the mice then exhibited growth again and the tumors in the mice injected with RT-R-MDA-MB-231-P2Y2R shRNA cells grew more rapidly than those in the mice injected with RT-R-MDA-MB-231-EV cells. In this study, we used athymic nude mice (absent of T cell function, high functional activity of macrophages and natural killer cells) which are acceptable for use in xenograft and allograft transplantation experiments. Thus, we hypothesized that NK cells may be functional to the xenograft tumor cells during in the early phase when tumor cells could not compose the tumor microenvironment yet. In addition, in tumor tissue sections from mice injected with RT-R-MDA-MB-231- P2Y2R shRNA cells, we observed higher levels of MMP-9 compared with the levels observed in the mice injected with RT-R-MDA-MB-231-EV cells. The concentration of IL-1β was slightly reduced in serum from mice injected with RT-R-MDA-MB-231-P2Y2R shRNA cells. Thus, we hypothesized that P2Y2R is related to the tumor growth and invasion of RT-R breast cancer cells in vivo, and the regulation of this purinergic receptor in RT-R-tumor cells may be helpful for controlling tumor progression in patients.
In conclusion, in this study, we demonstrate that RT-R breast cancer cells, particularly RT-R-MDA-MB-231 cells, release higher levels of ATP than do breast cancer cells, and extracellular ATP promotes invasion and tumor growth through the activation of P2Y2R. Moreover, inflammasome activation is more prominent in RT-R breast cancer cells and is P2Y2R dependent, ultimately resulting in increased tumor invasion and progression. Our results suggest the involvement of the P2Y2 purinergic receptor in inflammasome activation in breast cancer cells and RT-R breast cancer cells for the first time, at least to the best of our knowledge and highlight the importance of controlling P2Y2R activity to achieve a good prognosis in patients with RT-R tumors (Fig. 6E).
Funding
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1A1A3A04001029) and by the Ministry of Science, ICT and Future Planning (NRF-2015R1A5A2008833).
Availability of data and materials
All the data generated and analyzed during the study are available from the corresponding author on reasonable request.
Author’s contributions
HJ performed the experiments and wrote the manuscript. YSK performed data analysis. HJK conceived the hypothesis, directed the project and wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee at Gyeongsang National University (approval number: GLA-120208-M004), and all experiments were performed in compliance with the institutional guidelines set.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Abbreviations
- ALR
absent in melanoma 2-like receptors
- ASC
apoptosis-associated speck-like protein containing a carboxy-terminal CARD
- ATP
adenosine triphosphate
- EMT
epithelial-mesenchymal transition
- IL-1β
interleukin-1β
- MAPK
mitogen-activated protein kinase
- MMP-9
matrix metalloproteinase-9
- NF-κB
nuclear factor-κB
- NLRs
nucleotide-binding domain-like receptors
- P2X7R
P2X purinergic receptor 7
- P2Y2R
P2Y purinergic receptor 2
- RT-R
radiotherapy-resistant
- TNF-α
tumor necrosis factor-α
References
- 1.Jinushi M. The role of innate immune signals in antitumor immunity. OncoImmunology. 2012;1:189–194. doi: 10.4161/onci.1.2.18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baniyash M, Sade-Feldman M, Kanterman J. Chronic inflammation and cancer: Suppressing the suppressors. Cancer Immunol Immunother. 2014;63:11–20. doi: 10.1007/s00262-013-1468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–351. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 8.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25:439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 10.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor- host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 11.Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur J Cancer. 2006;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al. Expression of interleukin- 1beta in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(SICI)1097-0142(19970801)80:3<421::AID-CNCR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: Implications for tumor progression. Int J Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- 14.Perrier S, Caldefie-Chézet F, Vasson MP. IL-1 family in breast cancer: Potential interplay with leptin and other adipocytokines. FEBS Lett. 2009;583:259–265. doi: 10.1016/j.febslet.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/A:1012388618693. [DOI] [PubMed] [Google Scholar]
- 18.Vénéreau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immuno l. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L376–L385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- 21.Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int. 2008;101:106–112. doi: 10.1111/j.1464-410X.2007.07286.x. [DOI] [PubMed] [Google Scholar]
- 22.Janssens R, Boeynaems JM. Effects of extracellular nucleotides and nucleosides on prostate carcinoma cells. Br J Pharmacol. 2001;132:536–546. doi: 10.1038/sj.bjp.0703833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White N, Butler PE, Burnstock G. Human melanomas express functional P2 X(7) receptors. Cell Tissue Res. 2005;321:411–418. doi: 10.1007/s00441-005-1149-x. [DOI] [PubMed] [Google Scholar]
- 24.Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim HJ. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014;16:R77. doi: 10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell MDA-MB-231 contributes to pre- metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget. 2014;5:9322–9334. doi: 10.18632/oncotarget.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 27.Veronesi U, Boyle P, Goldhirsch A, Orecchia R, Viale G. Breast cancer. Lancet. 2005;365:1727–1741. doi: 10.1016/S0140-6736(05)66546-4. [DOI] [PubMed] [Google Scholar]
- 28.Gebski V, Lagleva M, Keech A, Simes J, Langlands AO. Survival effects of postmastectomy adjuvant radiation therapy using biologically equivalent doses: A clinical perspective. J Natl Cancer Inst. 2006;98:26–38. doi: 10.1093/jnci/djj002. [DOI] [PubMed] [Google Scholar]
- 29.Ringborg U, Bergqvist D, Brorsson B, Cavallin-Ståhl E, Ceberg J, Einhorn N, Frödin JE, Järhult J, Lamnevik G, Lindholm C, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001 - summary and conclusions. Acta Oncol. 2003;42:357–365. doi: 10.1080/02841860310010826. [DOI] [PubMed] [Google Scholar]
- 30.Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda K, Sakakura C, Miyagawa K, Kuriu Y, Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y, et al. Differential gene expression profiles of radioresistant oesophageal cancer cell lines established by continuous fractionated irradiation. Br J Cancer. 2004;91:1543–1550. doi: 10.1038/sj.bjc.6602187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henness S, Davey MW, Harvie RM, Banyer J, Wasinger V, Corthals G, Davey RA. Changes in gene expression associated with stable drug and radiation resistance in small cell lung cancer cells are similar to those caused by a single X-ray dose. Radiat Res. 2004;161:495–503. doi: 10.1667/RR3165. [DOI] [PubMed] [Google Scholar]
- 33.Thomas ED, Clift RA, Hersman J, Sanders JE, Stewart P, Buckner CD, Fefer A, McGuffin R, Smith JW, Storb R. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8:817–821. doi: 10.1016/0360-3016(82)90083-9. [DOI] [PubMed] [Google Scholar]
- 34.Jin H, Ham SA, Kim MY, Woo IS, Kang ES, Hwang JS, Lee KW, Kim HJ, Roh GS, Lim DS, et al. Activation of peroxisome proliferator-activated receptor-δ attenuates glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. J Neurosci Res. 2012;90:1646–1653. doi: 10.1002/jnr.23053. [DOI] [PubMed] [Google Scholar]
- 35.Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha- deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- 36.Egberts JH, Cloosters V, Noack A, Schniewind B, Thon L, Klose S, Kettler B, von Forstner C, Kneitz C, Tepel J, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68:1443–1450. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 37.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 38.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson RW, McCormick B. Update: NCCN breast cancer clinical practice guidelines. J Natl Compr Canc Netw. 2005;3(Suppl 1):S7–S11. [PubMed] [Google Scholar]
- 41.Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Essen CF. Radiation enhancement of metastasis: A review. Clin Exp Metastasis. 1991;9:77–104. doi: 10.1007/BF01756381. [DOI] [PubMed] [Google Scholar]
- 43.Ko YS, Jin H, Joo Y, Lee JS, Park SW, Chang KC, Kang KM, Jeong BK, Kim HJ. Radio-resistant breast cancer cells derived from highly metastatic breast cancer cells exhibit increased resistance to chemotherapy, enhanced invasive properties and premetastatic niche formation due to cancer stem cells. Oncol Rep. doi: 10.3892/or.2018.6714. In press. [DOI] [PubMed] [Google Scholar]
- 44.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Chow MT, Sceneay J, Paget C, Wong CS, Duret H, Tschopp J, Möller A, Smyth MJ. NLRP3 suppresses NK cell-mediated responses to carcinogen-induced tumors and metastases. Cancer Res. 2012;72:5721–5732. doi: 10.1158/0008-5472.CAN-12-0509. [DOI] [PubMed] [Google Scholar]
- 47.Bhagat Tu, Cui GS, Takaishi G, Kurt-Jones S, Rickman EA, Betz B, Penz-Oesterreicher KS, Bjorkdahl M, Fox OJG, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Luo Y, Dunn JH, Norris DA, Dinarello CA, Fujita M. Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Invest Dermatol. 2013;133:518–527. doi: 10.1038/jid.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolb R, Phan L, Borcherding N, Liu Y, Yuan F, Janowski AM, Xie Q, Markan KR, Li W, Potthoff MJ, et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat Commun. 2016;7:13007. doi: 10.1038/ncomms13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corriden R, Insel PA. Basal release of ATP: An autocrine- paracrine mechanism for cell regulation. Sci Signal. 2010;3:re1. doi: 10.1126/scisignal.3104re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazarowski E. Regulated release of nucleotides and UDP sugars from astrocytoma cells. Novartis Found Symp. 2006;276:73–84. discussion 84–90, 107–112, 275–281. [PubMed] [Google Scholar]
- 52.Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5:75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 55.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 56.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J Bone Miner Res. 2004;19:642–651. doi: 10.1359/JBMR.040108. [DOI] [PubMed] [Google Scholar]
- 57.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J Cell Biol. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]
- 59.Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F, et al. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis. 2015;6:e1629. doi: 10.1038/cddis.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, Cronstein BN, Mehal WZ. Adenosine is required for sustained inflammasome activation via the A2A receptor and the HIF-1α pathway. Nat Commun. 2013;4:2909. doi: 10.1038/ncomms3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin Y, Cai J, Meng F, Sui C, Jiang Y. MiR-144 suppresses proliferation, invasion, and migration of breast cancer cells through inhibiting CEP55. Cancer Biol Ther. 2018;19:306–315. doi: 10.1080/15384047.2017.1416934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Y, Zhang J, Fu H, Shen L. miR-144 functions as a tumor suppressor in breast cancer through inhibiting ZEB1/2-mediated epithelial mesenchymal transition process. OncoTargets Ther. 2016;9:6247–6255. doi: 10.2147/OTT.S103650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L, Yang Y, Hou J, Zhai C, Song Y, Zhang Z, Qiu L, Jia X. MicroRNA-144 affects radiotherapy sensitivity by promoting proliferation, migration and invasion of breast cancer cells. Oncol Rep. 2015;34:1845–1852. doi: 10.3892/or.2015.4173. [DOI] [PubMed] [Google Scholar]
- 64.Hunter KW, Crawford NP, Alsarraj J. Mechanisms of metastasis. Breast Cancer Res. 2008;10(Suppl 1):S2. doi: 10.1186/bcr1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 67.Ruhul Amin AR, Senga T, Oo ML, Thant AA, Hamaguchi M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1beta: A role for the dual signalling pathways, Akt and Erk. Genes Cells. 2003;8:515–523. doi: 10.1046/j.1365-2443.2003.00652.x. [DOI] [PubMed] [Google Scholar]
- 68.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 18252012:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, Li Z, Wei K, Chen G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLoS One. 2015;10:e0135544. doi: 10.1371/journal.pone.0135544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Petrovic JM, Callaghan D, Jones A, Cui H, Howlett C, Stanimirovic D. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta- mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 72.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semenza GL. Surviving ischemia: Adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000;106:809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(Suppl 4):S62–S67. doi: 10.1016/S1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated and analyzed during the study are available from the corresponding author on reasonable request.