Abstract
Traditional Chinese medicines, including Radix Salvia miltiorrhiza (SM) and Lignum Dalbergia odorifera (DO) extracts, have historically been used to treat myocardial ischemia and other cardiovascular diseases. The volatile oil of DO (DOO) is one of the main components of DO. The aim of the present study was to assess the cardioprotective effects and possible underlying mechanisms of SM-DOO in pigs with ameroid constriction-induced chronic myocardial ischemia. An ameroid constrictor was placed around the left anterior descending coronary artery of pigs to induce chronic myocardial ischemia. At weeks 2, 6 and 8, myocardial injury markers and blood gas levels were detected. At week 8, coronary angiography, echocardiography and hemodynamics analysis were performed to evaluate myocardial function. Following sacrifice, myocardial tissue was collected and subjected to morphological, histopathological and apoptosis assays. Western blotting was used to detect the protein expression of Bcl-2 associated X (Bax), Bcl-2, Akt, phosphorylated (p)-Akt, glycogen synthase kinase (GSK)-3β and p-GSK-3β. It was revealed that SM-DOO treatment following chronic myocardial ischemia significantly downregulated the expression of myocardial injury markers, ameliorated myocardial oxygen consumption, increased collateralization, reduced regional cardiac dysfunction and limited the extent of myocardial damage. Furthermore, the results of an apoptosis assay revealed that the apoptosis rate was decreased, the expression of Bax decreased and Bcl-2 increased, and the ratio of Bcl-2/Bax was increased. Further experiments indicated that treatment with SM-DOO increased the phosphorylation of Akt and GSK-3β. These findings suggest that SM-DOO treatment ameliorates myocardial injury in a chronic myocardial ischemia model, and that the underlying mechanisms responsible may be associated with the activation of the Akt/GSK-3β signal pathway. Thus, experimental evidence that SM-DOO may be an effective drug for the prevention and treatment of chronic myocardial ischemia in clinical applications has been provided.
Keywords: chronic myocardial ischemia, Radix Salvia miltiorrhiza, Lignum Dalbergia odorifera, cardioprotection, apoptosis, Akt, glycogen synthase kinase-3β
Introduction
Myocardial ischemia, which is caused by an imbalance between the myocardial oxygen supply and myocardial oxygen requirement, can induce arrhythmia, cardiac dysfunction, myocardial infarction and sudden death (1). Although there are a number of novel drugs available for the treatment and prevention of myocardial ischemia, the mortality and morbidity continue to increase (2). It was previously reported that apoptosis may be an important step in the pathogenesis of myocardial injury, and exploring anti-apoptotic drugs may provide novel therapeutic opportunities for the treatment of myocardial ischemia (3).
Traditional Chinese medicine (TCM) has been used to treat cardiovascular diseases for more than 1,000 years with significant efficacy and minimal side effects (4). Among the herbs used in TCM, Radix Salvia miltiorrhiza (SM) and Lignum Dalbergia odorifera (DO) have been widely used in clinical and basic laboratory studies for the prevention and treatment of cardiovascular diseases, including in China (5-10). SM, known as Danshen in Chinese, has been used to treat cardiovascular diseases, including coronary heart disease, hyperlipidemia and cerebrovascular diseases (11). DO, known as Jiangxiang in Chinese, has been used to treat ischemia, necrosis, swelling, blood disorders and rheumatic pain (12). A number well-known Chinese medicines for treating cardiovascular disease contain SM and DO, including Guanxin II, Guanxin Danshen capsules, Xiangdan injection and Qishen Yiqi pills. The effect of the combination of SM-DO has been shown to be efficacious in clinical use (13,14); however, there are few studies regarding their action and the underlying mechanisms responsible. In our previous experiment, the cardioprotective effects of SM and DO were evaluated in a rat myocardial ischemia-reperfusion model. The results indicated that combined treatment with SM and DO had significant cardioprotective effects, and that the combination of SM with DO volatile oil (DOO) (SM 5 g/kg/day, DOO 0.5 ml/kg/day) was significantly more effective than SM, DO or DOO alone (15). However, the effects of SM-DOO in chronic myocardial ischemia have yet to be reported.
The aim of the present study was to investigate the cardio-protective effect and potential mechanisms of SM-DOO in a pig model of chronic myocardial ischemia. The major chemical components of SM and DOO were also determined using high-performance liquid chromatography (HPLC) and gas chromatography coupled with mass spectrometry (GC-MS), respectively.
Materials and methods
Materials and reagents
SM extract was purchased from Xi'an Honson Biotechnology Co., Ltd. (Xi'an, China; batch no. 161025). The major components were hydrophilic salvanolic acid and hydrophobic tanshinones, as evaluated by HPLC analysis. DOO was purchased from Jishui Jinhai Natural Perfume Oil Technology Co., Ltd. (Jiangxi, China; batch no. XC20160918) and the major components were evaluated by GC-MS. Standards for danshensu, rosmarinic acid, salvianolic acid B, salvianolic acid A, protocatechuic acid, protocatechuic aldehyde, dihydrotanshinone I, cryptotanshinone, tanshinone IIA and tanshinon IIA silate sodium were purchased from Shanghai ANPEL Laboratory Technologies, Inc. (Shanghai, China; batch no. Q2880010). Acetonitrile was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). All other reagents were analysis grade.
Deionized water was prepared using a Milli-Q water purification system (EMD Millipore, Bedford, MA, USA). 2,3,5-triphenyltetrazolium chloride (TTC) was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Pig creatine kinase-MB (CK-MB), cardiac troponin I (cTnI) and myoglobin (Myo) ELISA kits were bought from Xitang Biology Technology Company (Shanghai, China). Lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) test kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All primary antibodies, including Bcl-2 associated X (Bax; cat. no. 2772), Bcl-2 (cat. no. 2870), Akt (cat. no. 9272), phosphorylated (p)-Akt (cat. no. 9271), glycogen synthase kinase (GSK)-3β (cat. no. 9315), p-GSK-3β (cat. no. 9336) and β-actin (cat. no. 4970), were purchased from Cell Signaling Technologies, Inc. (Danvers, MA, USA). The secondary antibody (anti-rabbit IgG-B; cat. no. sc-53804) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Herbal extraction
SM was dried at 50°C and ground into powder (<1 mm). SM (150 g) was soaked in 8-fold the volume of water for 30 min at room temperature and extracted for 3 h (1.5 h/reflux), followed by reflux extraction with 75% ethanol. The filtrates were combined, concentrated under reduced pressure and freeze-dried. DOO was isolated by steam distillation for 5 h, with a yield of 0.5% DOO, which was stored at 4°C.
Preparation of standard solutions
Primary standard solutions of the 10 components were prepared by dissolving the solid standards in methanol. Working mixed standard solutions (40 μg/ml) were prepared by mixing and diluting the stock solution with methanol. The mixed standard solution was filtered through a 0.22-μm membrane before HPLC analysis.
Preparation of herb solutions
SM was dried at a temperature of 50°C and ground into powder. Subsequently, 0.25 g SM was soaked in 8-fold the volume of water for 30 min at room temperature and extracted for 4 h, followed by ultrasound-assisted extraction for 30 min with 80% methanol. The suspension was filtered, concentrated and diluted to a 50-ml volume with methanol. The solution was filtered through a 0.22-μm membrane prior to HPLC analysis.
An aliquot of 200 μl DOO was diluted in 20 ml of ethyl acetate and dried over anhydrous sodium sulfate. The supernatant was filtered through a 0.22-μm membrane prior to GC-MS analysis.
Chromatographic conditions
HPLC analysis was performed using an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA) and chromatographic separation was accomplished using an Agilent TC-C18 column (4.6×250 mm, 5 μm). The column temperature was set at 30°C with a 20-μl injection volume. The mobile phase consisted of 0.2% formic acid (v/v) in water (A) and acetonitrile (B). The gradient program used was as follows: 10% B to 30% B (0-15 min), 30% B to 32% B (15-40 min), 32% B to 55% B (40-55 min), 55% B to 67% B (55-65 min), 67% B to 72% B (65-75 min), 72% B to 85% B (75-85 min) and 100% B (85-100 min). The flow rate was set at 0.8 ml/min throughout the analysis, and UV detection was set at 270 nm.
The components of DOO were analyzed using an Agilent 7890A gas chromatograph coupled with an Agilent 5975C mass selective detector (Agilent Technologies). Chromatographic separation was performed using an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm film thickness, Agilent Technologies). The split ratio was 15:1, and the injection volume was set to 2 μl. The inlet and ion source temperatures were set to 260°C and 230°C, respectively. The helium flow rate was set to 1.0 ml/min. The oven temperature was initially 60°C, followed by a 1.5°C/min increase to 105°C for 10 min, then a 1°C/min increase to 115°C for 10 min, a 2.5°C/min increase to 160°C for 5 min and finally to 260°C at a heating rate of 20°C/min. MS was used in full scan mode with the acquisition range set to 30-800 m/z. GC-MS post-run analysis was performed, and the main components were identified using the library of the National Institute of Standards and Technology (NIST) (16). The peak area normalization method was used to establish the relative content of each substance in the sample.
Animals and experimental protocol
A total of 18 male Yorkshire pigs (25-30 kg) were purchased from the Institute of Experiment Animals at the Fuwai Hospital Experimental Animal Center (Beijing, China) and kept in an animal room at 25±1°C with 55±5% humidity. The Animal Welfare and Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences approved all experiments [approval no. 0072-1-27-HX (F)]. After one week of acclimation, the pigs were randomized into 3 groups as follows: i) Sham group (n=6), oral administration of regular diet and sham-operated without ameroid constrictor implantation; ii) Model group (n=6), oral administration of regular diet; iii) SM-DOO group (n=6), oral administration of regular diet with 1 g/kg/day SM and 0.1 ml/kg/day DOO. The dosages of SM and DOO were determined based on our previous studies, and adjusted for pigs according to a previously published method (15,17). Pigs were provided free access to water and were fed twice a day. All animals were observed during feeding to ensure the entire portion of food was consumed.
At 4 weeks after the dietary modification, pentobarbital sodium (25 mg/kg) was administered intramuscularly (i.m.) to induce anesthesia. Animals were intubated, ventilated with oxygen at 1.5-2 l/min and anesthetized with 3% isoflurane. The pigs were right lateral decubitus, and a small thoracotomy was performed in intercostal segments 3 and 4 by sterile techniques. An ameroid constrictor (Research Instruments NW, Inc., Lebanon, OR, USA) with a 2.5-mm internal diameter was implanted around the left anterior descending artery (LAD). The ameroid constrictor was designed to achieve myocardial ischemia by inducing progressive coronary artery stenosis with a progression similar to that observed in humans. The pericardium was closed using a 6-0 polypropylene suture (Ethicon, Inc., Somerville, NJ, USA) and the chest was sutured closed. After the surgery, penicillin (6,400,000 U/day, i.m.) was administered to all pigs to prevent infection.
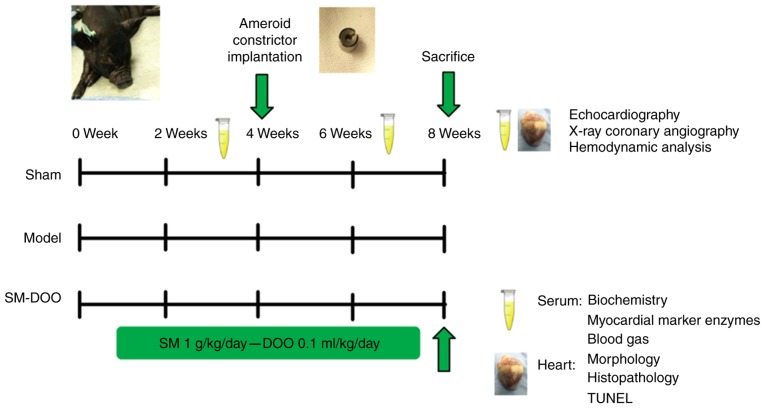
After 8 weeks, echocardiography, X-ray coronary angiography and hemodynamics analysis were performed. All surgical protocols were designed to minimize the suffering of the pigs. A schematic presentation of the study protocol is shown in Fig. 1.
Figure 1.
Schematic presentation of the study protocol. Pigs were randomly divided into 3 groups: Sham, Model and SM-DOO (n=6). The SM-DOO group received SM (1 g/kg/day) and DOO (0.1 ml/kg/day) orally for 8 weeks. At 4 weeks, an ameroid constrictor was placed around the left anterior descending coronary artery in the Model and SM-DOO groups. At weeks 2, 4 and 8, biochemical markers, myocardial marker enzymes and blood gas constituents were measured in the serum. After 8 weeks, echocardiography, X-ray coronary angiography and hemodynamic analysis were performed. Myocardial tissue underwent morphological, histopathological, apoptosis and western blot analysis to evaluate the cardioprotective effects and underlying mechanisms of SM-DOO. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling.
Measurement of myocardial injury markers
Blood samples were collected in heparinized tubes, allowed to clot for 30 min and centrifuged at 2,285 × g at 4°C for 15 min. Myocardial injury markers, including CK-MB, LDH, ALT, AST, cTnI and Myo, were measured using assay kits or pig-specific ELISA kits according to the manufacturers' protocols.
Blood gas analysis
At weeks 2, 6 and 8, pH, PO2 and PCO2 were measured in arterial blood samples taken from the femoral artery (500-700 μl) using pre-heparinized syringes (1,000 IU/ml) and analyzed using an i-STAT 1 blood gas system (Abbott Laboratories, Chicago, IL, USA).
Echocardiography
Echocardiography was performed at the end of the study using a Vivid 9 machine (GE Vingmed Ultrasound AS, Horten, Norway). Left ventricular end-diastolic diameter (LVEDd), left ventricular end-systolic diameter (LVEDs), end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction (EF) and fractional shortening (FS) were recorded to assess cardiac function. The FS value was calculated based on the following equation: FS (%) = [(LVEDd-LVEDs)/LVEDd] × 100%. Images were stored and analyzed using Echopac software (GE Vingmed Ultrasound AS).
X-ray coronary angiography
X-ray coronary angiography (GE Medical Systems, Milwaukee, WI, USA) with iopromide (Juste SAQF, Madrid, Spain) was performed on all experimental animals to verify the occlusion of the LAD and assess collateral vessel filling at the end of the experiment. The grade of collateral filling was scored using a standard 4-point scale as follows (18): 0 = no collateral filling; 1 = minimal collateral filling; 2 = moderate collateral filling, but less than the contralateral or ipsilateral non-infarct coronary artery; 3 = normal collateral filling compared with the contralateral or ipsilateral non-infarct coronary artery.
Hemodynamic monitoring
The heart rate (HR), mean arterial blood pressure (MAP), left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), left ventricular maximum upstroke velocity (+dp/dtmax) and left ventricular maximum descent velocity (−dp/dtmax) were recorded using a pressure transducer (Millar Instruments, Houston, TX, USA) connected to an anesthesia monitor (Philips, Eindhoven, Netherlands).
Morphometric and histopathological examination
Animals were sacrificed by intravenous injection with potassium chloride after the imaging study, and their hearts were explanted. Hearts were serially sliced and incubated with 2% TTC for 20 min at 37°C. An Image-Pro Plus image system (Media Cybernetics, Bethesda, MD, USA) was used to analyze the infarct (white) and non-infarct (red) areas. The percentage of myocardial infarct was calculated as infarct area divided by total area.
The left ventricle was harvested, fixed in 4% paraformaldehyde, embedded in paraffin and sectioned. Sections were stained with hematoxylin and eosin (H&E) for histological examination. The pathology of heart sections was investigated using a light microscope, and photomicrographs were captured.
Measurement of myocardial apoptosis
Myocardial apoptosis was measured using an In-Situ Cell Death Detection kit (Wanlei Bio, Shenyang, China) according to the manufacturer's protocol. Myocardial slices were subjected to terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining combined with a DAPI (Sigma-Aldrich; Merck KGaA) counterstain. Images were obtained under a fluorescence microscope at ×400 magnification (Nikon Corporation, Tokyo, Japan), to confirm the successful labeling of apoptotic cells and nuclei. The percentage of TUNEL-positive cells was calculated as the number of apoptotic cells divided by the total number of nuclei × 100.
Western blotting analysis
Myocardial tissues were homogenized in RIPA buffer containing 1% PMSF and 1% protease inhibitor cocktail (Nanjing Jiancheng Bioengineering Institute), and the supernatant was harvested by concentration (18,447 x g at 4°C for 10 min). The protein concentration was determined based on the BCA method using a Protein Quantitative Analysis kit (Nanjing Jiancheng Bioengineering Institute). The lysates were separated by 10% SDS-PAGE. Following separation, the proteins were transferred onto polyvinyl difluoride membranes, blocked for 1 h at 37°C with 5% non-fat dried milk, washed 3 times with Tween in Tris-buffered saline, and incubated with primary antibodies against Bax, Bcl-2, Akt, p-Akt, GSK-3β, p-GSK-3β and β-actin (dilution, 1:1,000) overnight at 4°C. Subsequent to washing 3 times as described above, the membranes were incubated with secondary antibodies (dilution, 1:5,000) at 37°C for 1 h. Immunoreactive bands were detected using the enhanced chemiluminescence method and semi-quantified using ImageJ Software version 1.47 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was conducted using GraphPad Prism version 6.02 (GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as the mean ± standard deviation. One-way analysis of variance with Tukey's multiple comparison post-hoc test was performed to analyze the data. P<0.05 was considered to represent a statistically significant difference.
Results
Evaluation of the chronic myocardial ischemia model and effect of SM-DOO on collateral vessel filling
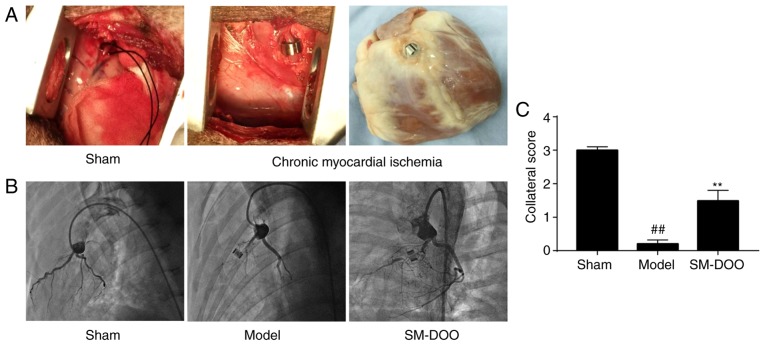
The ameroid constrictor was used to induce coronary closure. Coronary angiography indicated that the rate of coronary stenosis reached 98-100% 4 weeks after ameroid constrictor implantation, indicating that chronic myocardial ischemia was stably established. As shown in Fig. 2, coronary angiography revealed LAD stenosis with no evidence of collateral vessel filling in the Model group (0.20±0.11); however, collateral filling was observed in the SM-DOO group (1.49±0.31). Collateral scores were significantly increased by the administration of SM-DOO compared with the Model group (P<0.01).
Figure 2.
At 4 weeks after ameroid constrictor implantation, coronary angiography was performed. (A) The Sham group underwent the surgical procedure without ameroid constrictor implantation, whereas in the Model and SM-DOO groups a 2.5-mm ameroid constrictor was implanted around the LAD to produce chronic myocardial ischemia. (B) Representative coronary angiographies from each group. No coronary stenosis was observed in the Sham group. No collateral vessel filling was observed in the Model group. In the SM-DOO group, collateral vessel filling was observed with distal filling of the LAD. (C) Assessment of the myocardial collateral grade revealed increased collateral vessel filling in the SM-DOO group. All values are presented as the mean ± standard deviation. ##P<0.01 vs. Sham group; **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil; LAD, left anterior descending artery.
Effect of SM-DOO on myocardial injury markers
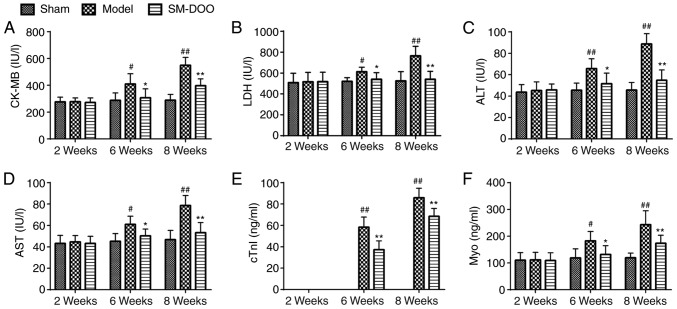
As presented in Fig. 3, no differences were observed in CK-MB, LDH, ALT, AST, cTnI and Myo between the 3 groups at week 2. CK-MB, LDH, ALT, AST, cTnI and Myo levels in the Model group were higher compared with the Sham group at weeks 6 and 8. In the Model group, at week 8, the expression of myocardial injury markers was higher compared with at week 6. Conversely, CK-MB, LDH, ALT, AST, cTnI and Myo were significantly decreased in the SM-DOO group compared with the Model group (P<0.05 or P<0.01), demonstrating that SM-DOO ameliorated injury in chronic myocardial ischemia.
Figure 3.
Effects of SM-DOO on myocardial injury markers at weeks 2, 6 and 8 in pigs with chronic myocardial ischemia, including (A) CK-MB, (B) LDH, (C) ALT, (D) AST, (E) cTnI and (F) Myo. All values are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. Sham group; *P<0.05 and **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergiae odoriferae volatile oil; CK-MB, Pig creatine kinase-MB; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; cTnI, cardiac troponin I; Myo, myoglobin.
Effect of SM-DOO on myocardial oxygen consumption
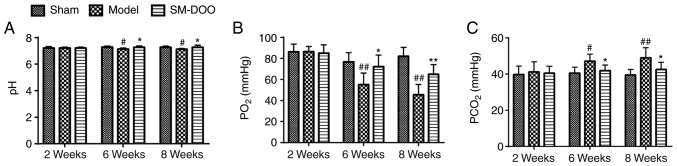
To investigate whether the cardioprotective effects of SM-DOO were mediated by affecting myocardial oxygen consumption, blood gas was quantified. As presented in Fig. 4, pH, PO2 and PCO2 were the same in all 3 groups prior to the induction of chronic myocardial ischemia. By weeks 6 and 8, the arterial pH and PO2 were markedly lower, while the PCO2 was higher in the Model group than that in the Sham group (47.13±3.91 vs. 40.57±3.29 mmHg at week 6; 49.05±5.56 vs. 39.46±3.10 mmHg at week 8). Thus, compared with the Model group, SM-DOO markedly ameliorated the effects on pH, PO2 and PCO2 induced by myocardial ischemia.
Figure 4.
Effects of SM-DOO on myocardial oxygen consumption. At weeks 2, 6 and 8 the (A) pH, (B) PO2 and (C) PCO2 levels in the blood were measured. All values are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. Sham group; *P<0.05 and **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil.
Effects of SM-DOO on left ventricular function
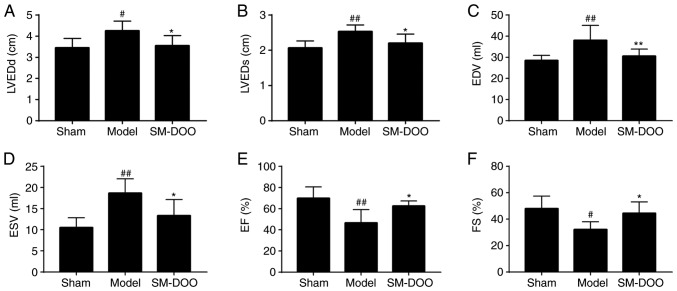
To assess the cardioprotective effects of SM-DOO, left ventricular function was examined. Compared with the Sham group, the mitral and apical levels in the Model group were reduced, the papillary muscle was thinned, and the left ventricular anterior wall moments were weakened (Table I). Additionally, in the Model group, the echocardiography parameters of LVEDd, LVEDs, EDV and ESV were significantly increased, while EF and FS were markedly reduced (P<0.01 or P<0.05). The results indicated that the left ventricular function in the Model group was markedly altered. Conversely, the effects on the left ventricular anterior wall thickness and moments were ameliorated in the SM-DOO group; the levels of LVEDd, LVEDs, EDV and ESV were significantly reduced, whereas EF and FS were increased following treatment with SM-DOO (62.5±4.848% for EF, P<0.05; 44.5±8.526% for FS; P<0.01; Fig. 5).
Table I.
Movement of the left ventricular anterior wall at the mitral level, papillary muscle level and apical level.
| Group | Mitral level
|
Papillary muscle level
|
Apical level
|
|||
|---|---|---|---|---|---|---|
| End-systolic | End-diastolic | End-systolic | End-diastolic | End-systolic | End-diastolic | |
| Sham | 1.067±0.121 | 0.773±0.103 | 1.100±0.141 | 0.750±0.084 | 1.100±0.126 | 0.700±0.063 |
| Model | 0.800±0.063b | 0.550±0.055b | 0.850±0.138a | 0.583±0.075b | 0.767±0.103b | 0.533±0.052b |
| SM-DOO | 0.950±0.105c | 0.683±0.075c | 1.067±0.105c | 0.717±0.075c | 0.950±0.085c | 0.667±0.103c |
All values are presented as the mean ± standard deviation.
P<0.05,
P<0.01 vs. Sham group,
P<0.05 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil.
Figure 5.
Effects of SM-DOO on left ventricular function after chronic myocardial ischemia, including (A) LVEDd, (B) LVEDs, (C) EDV, (D) ESV, (E) EF and (F) FS. All values are presented as the mean ± standard deviation. #P<0.05 and ##P<0.01 vs. Sham group; *P<0.05 and **P<0.01 vs. Model group. LVEDd, left ventricular end-diastolic diameter; LVEDs, left ventricular end-systolic diameter; EDV, end-diastolic volume; ESV, end-systolic volume; EF, ejection fraction; FS, fractional shortening.
Effects of SM-DOO on hemodynamic factors
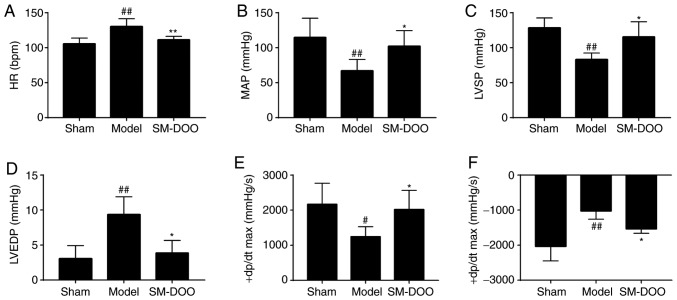
As shown in Fig. 6, the hemodynamic parameters indicated that the left ventricular function of the Model group was significantly decreased compared with the Sham group. In the SM-DOO group, HR, LVEDP and −dp/dtmax were markedly reduced (P<0.05 or 0.01), whereas MAP, LVSP and +dp/dtmax were significantly increased, compared with the Model group (102.176±22.203 vs. 67.079±16.378 mmHg for MAP; 115.522±21.528 vs. 83.206±9.189 mmHg for LVSP; and 2,017.917±548.228 vs. 1,244.587±284.314 mmHg/sec for +dp/dtmax; P<0.05).
Figure 6.
Effects of SM-DOO on hemodynamics. The (A) HR, (B) MAP, (C) LVSP, (D) LVEDP, (E) +dp/dtmax and (F) −dp/dtmax levels in each group were determined. All values are presented as the mean ± SD. #P<0.05 and ##P<0.01 vs. Sham group, *P<0.05 and **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil; HR, heart rate; MAP, mean arterial blood pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; +dp/dtmax, left ventricular maximum upstroke velocity; −dp/dtmax, left ventricular maximum descent velocity.
Effects of SM-DOO on myocardial damage
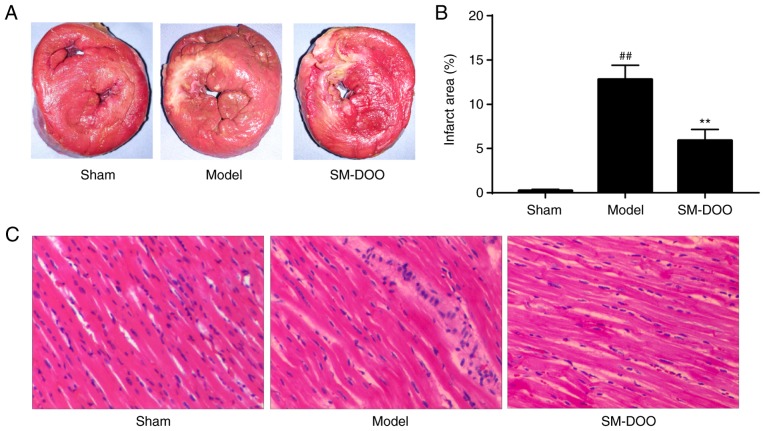
After the pigs were sacrificed, hearts were collected for morphometric and histopathological analysis. Cardiac sections were stained with TTC to compare the infarct size between the 3 groups. As shown in Fig. 7A, the mean infarct size in the Model group was larger than that in the SM-DOO group. The infarct size was significantly decreased in the SM-DOO group (5.91±1.25%) compared with the Model group (12.93±1.68%; P<0.01; Fig. 7B). Thus, SM-DOO treatment reduced the myocardial infarct size after chronic myocardial ischemia.
Figure 7.
Effects of SM-DOO on myocardial infarct size after myocardial injury induced by ameroid constrictor implantation. (A) The infarct area was stained white, while the non-infarct area was stained red. (B) Infarct area (%) relative to the total area. (C) Representative photomicrographs of left ventricles of the pigs with hematoxylin and eosin staining (magnification, ×200) following chronic myocardial ischemia. All values are presented as the mean ± standard deviation. ##P<0.01 vs. Sham group; **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil.
As presented in Fig. 7C, the myocardium was H&E stained for histological examination. Myocardial tissues in the Sham group exhibited no abnormalities. As expected, chronic myocardial ischemia induced evident injury to the myocardial structure, including chronic inflammatory cells, edema in the myocardial fibers and ruptured myocardial fibers in the Model group. Myocardial alterations following chronic myocardial ischemia, in particular, disruption to the myocardial fibers, were significantly ameliorated by SM-DOO.
Effects of SM-DOO on myocardial apoptosis and Bax and Bcl-2 expression
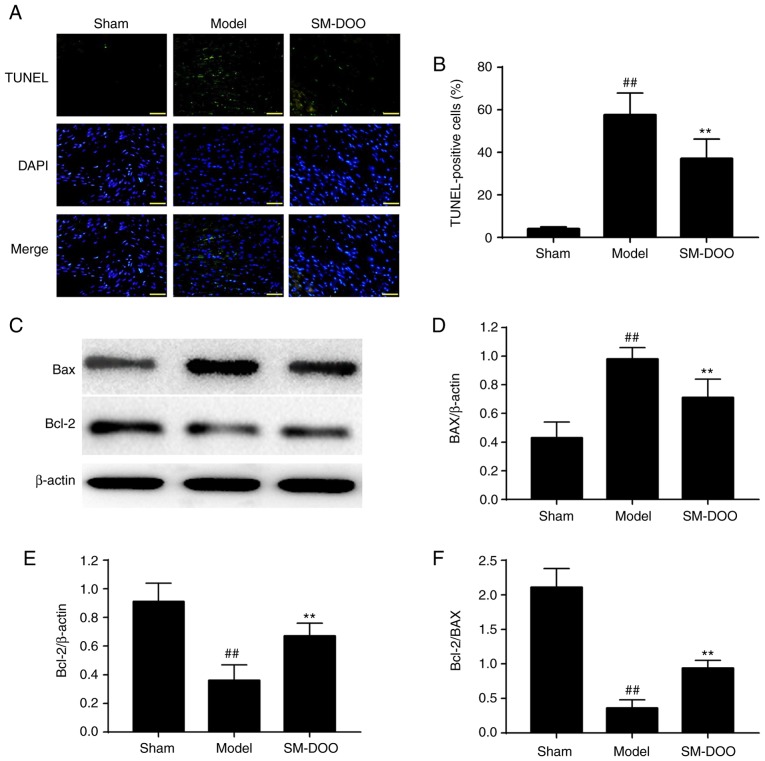
As shown in Fig. 8A, more apoptotic cells were observed in the Model group compared with the Sham group. The percentage of TUNEL-positive cells was significantly lower in the SM-DOO group (19.78±2.51%) compared with the Model group (28.20±5.33%), as shown in Fig. 8B (P<0.01). To further examine the potential mechanisms of the anti-apoptotic effect of SM-DOO, the expression of Bax and Bcl-2 was determined. As shown in Fig. 8C, D and E, the expression of Bax was significantly increased and Bcl-2 was significantly decreased in the Model group. In addition, the Bcl-2/Bax ratio was significantly decreased in the Model group (Fig. 8F; P<0.01). Thus, treatment with SM-DOO ameliorated the effects of chronic myocardial ischemia on apoptosis.
Figure 8.
Effects of SM-DOO on the number of apoptotic cells following chronic myocardial ischemia. (A) Representative images of TUNEL staining in the sham, model and SM-DOO groups (magnification, ×400). Apoptotic cells were detected by TUNEL staining (green). DAPI staining (blue) indicates the total number of nuclei. (B) The percentage of TUNEL-positive cell were expressed as (apoptotic cells/total nuclei) × 100. (C) Representative western blots of Bax and Bcl-2. Relative density of (D) Bax and (E) Bcl-2 normalized to β-actin. (F) Analysis of the Bcl-2/Bax ratio. All values are presented as the mean ± standard deviation. ##P<0.01 vs. Sham group; **P<0.01 vs. Model group. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling; Bax, Bcl-2-associated X.
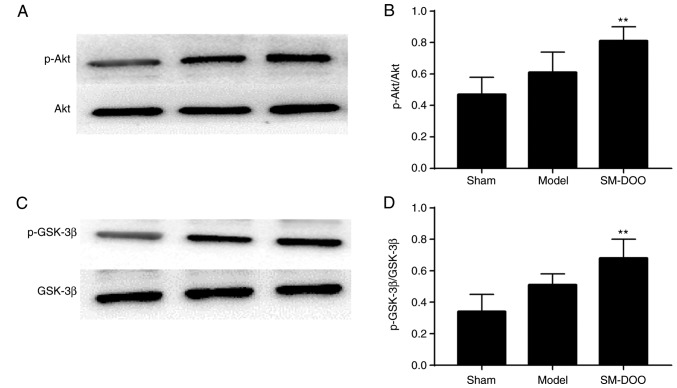
Effect of SM-DOO on Akt and GSK-3β activation
To investigate the signaling pathways involved in the cardioprotective effects of SM-DOO, the expression of Akt, p-Akt, GSK-3β and p-GSK-3β was determined. As shown in Fig. 9, compared with the Sham group, the expression of p-Akt and p-GSK-3β was slightly increased, while Akt and GSK-3β levels were not altered in the Model group. Compared with the Model group, the SM-DOO group had significantly higher levels of p-Akt/Akt and p-GSK-3β/ GSK-3β (P<0.01), indicating that SM-DOO administration markedly increased p-Akt and p-GSK-3β levels.
Figure 9.
Akt, p-Akt, GSK-3β and p-GSK-3β protein levels in the myocardial tissue of the Sham, Model and SM-DOO groups were measured using western blotting. (A) Representative western blotting results for Akt and p-Akt. (B) Analysis of the p-Akt/Akt ratio. (C) Representative western blotting results for GSK-3β and p-GSK-3β. (D) Analysis of the p-GSK-3β/GSK-3β ratio. All values are presented as the mean ± standard deviation. **P<0.01 vs. Model group. p-, phosphorylated-; GSK, glycogen synthase kinase; SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil.
Detection of SM and DOO components by chromatography
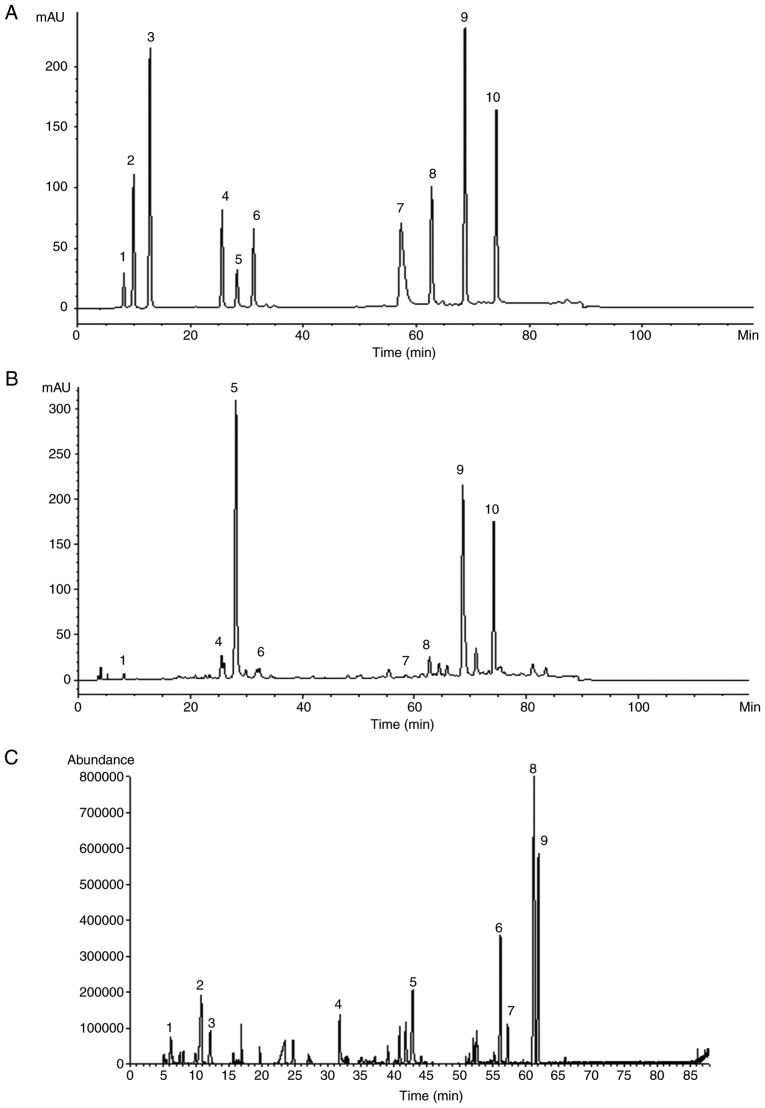
By comparing the retention times of mixed standard solutions with SM (Fig. 10A), 8 components were identified as danshensu, rosmarinic acid, salvianolic acid B, salvianolic acid A, tanshinone IIA silate sodium, dihydrotanshinone I, cryptotanshinone and tanshinone IIA by chromatography (Fig. 10B). The main components of SM were salvianolic acid B (18.67%), crypto-tanshinone (11.83%) and tanshinone IIA (10.55%). A total of 9 chemical components of DOO were detected by GC-MS and identified by NIST Database retrieval, as shown in Table II. The major components of DOO were nerolidol (22.52%), caryophyllene oxide (12.70%), α-santalol (9.01%) and β-farnesene (5.16%). The total ion current (TIC) of DOO is given in Fig. 10C.
Figure 10.
Representative chromatograms of the identified components in (A) mixed standard solutions of SM and (B) the SM extract, including 1. danshensu, 2. protocatechuic acid, 3. protocatechuic aldehyde, 4. rosmarinic acid, 5. salvianolic acid B, 6. salvianolic acid A, 7. tanshinone IIA silate sodium, 8. dihydrotanshinone I, 9. cryptotanshinone and 10. tanshinone IIA. (C) Total ion chromatogram of DOO, as determined by gas chromatography coupled with mass spectrometry. SM, Radix Salvia miltiorrhiza; DOO, Lignum Dalbergia odorifera volatile oil.
Table II.
Components of Lignum Dalbergia odorifera volatile oil as determined by gas chromatography coupled with mass spectrometry.
| No. | Compound name | Fomula | Retention time (min) | Relative mass fraction (%) |
|---|---|---|---|---|
| 1 | Pinene | C10H16 | 6.216 | 1.89 |
| 2 | Terpinolene | C10H16 | 10.841 | 4.71 |
| 3 | Eucalyptol | C10H18O | 12.216 | 2.38 |
| 4 | Eugenol | C10H12O2 | 31.883 | 3.49 |
| 5 | β-Farnesene C | 15H24 | 42.945 | 5.16 |
| 6 | α-santalol C | 15H24O | 56.260 | 9.01 |
| 7 | α-Farnesene C | 15H24 | 57.368 | 2.63 |
| 8 | Nerolidol | C15H26O | 61.348 | 22.52 |
| 9 | Caryophyllene oxide | C15H24O | 62.001 | 12.70 |
Discussion
Myocardial ischemia has become one of the most common cardiovascular diseases, and it is difficult to treat in the clinic. The beneficial effects of SM-DOO in the prevention and treatment of heart disease have been demonstrated. However, little has been reported regarding the potential cardioprotective effects of SM-DOO in chronic myocardial ischemia. To address this problem, a clinically relevant animal model was used in the present study to evaluate the cardioprotective effects of SM-DOO in chronic myocardial ischemia.
Small animal models have been extensively used in cardiovascular research (19-21). However, they bear only a partial resemblance to humans, which may not translate to clinical trials (22). Pig models are widely used to research cardiovascular disease because of the similarities between pig and human hearts, such as cardiac anatomy and function, hemodynamics, coronary circulation, drug dosing, pharmacokinetics and a lack of preexisting collateral circulation (23). An ameroid constrictor is a stainless steel jacket with a casein bar in its central lumen. Dehydrated casein has a tendency to absorb fluid and swell, thus gradually leading to the chronic myocardial ischemia by obstructing the coronary artery (24-27). In the present study, an X-ray coronary angiography demonstrated that the chronic myocardial ischemia model was stably established.
CK-MB, LDH, Myo and cTnI levels are considered to be important indicators of myocardial damage, and are significantly increased by blockage of the LAD (28-32). In addition, AST and ALT have previously been used to diagnose coronary heart disease (33,34). The elimination and release of myocardial injury markers occurred at different times, suggesting that the combined analysis of myocardial injury markers may provide more precise information to influence therapeutic decisions (35). Thus, sensitive myocardial injury markers, including CK-MB, LDH, ALT, AST, cTnI and Myo, were measured to determine whether SM-DOO alleviated the degree of myocardial ischemia. As expected, SM-DOO significantly reduced changes in these markers, demonstrating an amelioration of myocardial injury. Furthermore, the echocardiographic (LVEDd, LVEDs, EDV, ESV, EF and FS) and hemodynamic (MAP, HR, LVSP, LVEDP, +dp/dtmax and −dp/dtmax) parameters suggested that SM-DOO had a beneficial effect on preventing the impairment of cardiac function. In addition, LAD occlusion leads to myocardial injury and metabolic acidosis, decreasing the levels of PO2 and increasing PCO2 (29,36). In the present study, SM-DOO significantly prevented these changes, ameliorating myocardial oxygen consumption. Severe myocardial ischemia is associated with microstructural abnormalities in the myocardium (37). Pre-treatment with SM-DOO decreased the infarct size and reduced the extent of myocardial damage, supporting that conclusion that SM-DOO provided cardioprotective effects.
The results of the present study have determined that SM-DOO has a cardioprotective effect against chronic myocardial ischemia. Next, the potential mechanisms of these effects were explored. Apoptosis plays an important role in cardiac injury and has become a focus of research (38). The expression of several apoptosis-related genes is altered in ischemic tissue, including Bax and Bcl-2, which play important roles in apoptosis (39). The Bcl-2 family members are key regulators of physiological and pathological apoptosis, including cell death promoters such as Bax and Bcl-2 associated agonist of cell death, and cell death inhibitors such as Bcl-2, Bcl-X and Mcl-1 (40). Changes in the Bcl-2/Bax ratio leads to the induction of apoptotic cell death (41). In the present study, the anti-apoptotic effects of SM-DOO may be mediated by inhibiting the upregulation of Bax and downregulation of Bcl-2. This finding is consistent with the results of the TUNEL assay. These results suggest that SM-DOO may exert its cardioprotective effect against myocardial injury following chronic myocardial ischemia by preventing apoptosis.
The PI3K/Akt pathway is particularly important in mediating myocardial cell survival. Studies have shown that activation of the PI3K/Akt signaling pathway is essential for anti-apoptotic and cardioprotective effects (42,43). GSK-3 is a serine/threonine kinase with versatile cellular functions in the heart, including in gene expression, hypertrophy and apoptosis (44). GSK-3β is active in cardiomyocytes unstimulated by ischemia and ischemia/reperfusion, and sensitizes cells to death-promoting insults, which may be an underlying mechanism of cardioprotection (45). Akt phosphorylates and subsequently inactivates GSK-3β, which contributes to the attenuation of myocardial injury (46). To investigate the involvement of the PI3K/Akt signaling pathway on the cardio-protective effects of SM-DOO treatment, the expression of Akt and GSK-3β was determined using western blotting. As shown in the results, pretreatment with SM-DOO significantly increased the relative p-Akt and p-GSK-3β levels compared with the Model group. These findings suggest that the PI3K/Akt/GSK-3β signaling pathway may be involved in the cardioprotective effects of SM-DOO in a chronic myocardial ischemia model. However, further studies utilizing an inhibitor of PI3K/Akt are required to validate the involvement of this pathway.
The chemical composition of SM includes water-soluble and lipid-soluble components (47,48). Using HPLC analysis, 8 components of SM were identified by comparing its retention time with the standards. The main components of SM were salvianolic acid B, cryptotanshinone and tanshinone IIA. DOO was also analyzed using the established GC-MS method. The majority of the components, including pinene, terpinolene, eucalyptol, eugenol, β-farnesene, α-santalol, α-farnesene, nerolidol and caryophyllene oxide, were identified by performing a similarity match with the NIST Database. Studies have demonstrated that various chemical components of SM, such as danshensu, salvianolic acid B, salvianolic acid A, rosmarinic acid and tanshinone IIA, have anti-apoptotic activities (49-52). In addition, recent research indicates that tanshinone IIA, dihydrotanshinone I, danshensu, salvianolic acid B and salvianolic acid A play a protective role by regulating GSK-3β (53-56). However, there are few studies regarding whether the chemical components of DOO achieve a protective effect by regulating GSK-3β. We plan to explore the effects of active chemical constituents of SM-DOO against chronic myocardial ischemia in the future.
In conclusion, pretreatment with SM-DOO may ameliorate cardiac injury resulting from chronic myocardial ischemia. Myocardial injury markers (CK-MB, LDH, ALT, AST, cTnI and Myo), markers of myocardial oxygen consumption (pH, PO2 and PCO2), metrics of left ventricular function (LVEDd, LVEDs, EDV, ESV, EF, FS, MAP, HR, LVSP, LVEDP, +dp/dtmax and −dp/dtmax) and indicators of myocardial tissue damage (TTC, H&E, TUNEL and western blotting) revealed the effects of SM-DOO against chronic myocardial ischemia. Furthermore, the cardioprotective effects of SM-DOO may be partly mediated via activation of the PI3K/Akt/GSK-3β signaling pathway. The results of the present study may help to improve our understanding of the molecular mechanisms underlying the cardioprotective effects of SM-DOO, and provide a basis for the use of SM-DOO as an effective drug for the prophylaxis and treatment of chronic myocardial ischemia.
Acknowledgments
The authors would like to thank Beijing Key Laboratory of Pre-clinical Research and Evaluation for Cardiovascular Implant Materials (Fuwai Hospital, Chinese Academy of Medical Sciences) for providing the experimental animals and platform.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81470174 and 31771265).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
The present study was performed with collaboration between all authors. MX, YY and AW defined the research theme and revised the manuscript critically. RL, JD and FM designed the methods and experiments, performed the laboratory experiments and wrote the paper. HB, MNZ, MZ and YL collected and analyzed the data, and interpreted the results. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Animal Welfare and Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences approved all experiments [approval no. 0072-1-27-HX (F)].
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shimokawa H, Yasuda S. Myocardial ischemia: Current concepts and future perspectives. J Cardiol. 2008;52:67–78. doi: 10.1016/j.jjcc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Lv Y, Liu X, Yan S, Liang X, Yang Y, Dai W, Zhang W. Metabolomic study of myocardial ischemia and intervention effects of Compound Danshen Tablets in rats using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2010;52:129–135. doi: 10.1016/j.jpba.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Xu T, Wu X, Chen Q, Zhu S, Liu Y, Pan D, Chen X, Li D. The anti-apoptotic and cardioprotective effects of salvianolic acid a on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of the ERK1/2/JNK pathway. PLoS One. 2014;9:e102292. doi: 10.1371/journal.pone.0102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.JianXin C, Xue X, ZhongFeng L, Kuo G, FeiLong Z, ZhiHong L, Xian W, HongCai S. Qishen Yiqi Drop Pill improves cardiac function after myocardial ischemia. Sci Rep. 2016;6:24383. doi: 10.1038/srep24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Shi P, Yao H, Shao Q, Fan X. Metabolite profiling and pharmacokinetics of herbal compounds following oral administration of a cardiovascular multi-herb medicine (Qishen yiqi pills) in rats. Curr Drug Metab. 2012;13:510–523. doi: 10.2174/1389200211209050510. [DOI] [PubMed] [Google Scholar]
- 6.Wu SD, Wang J, Chen SC, Xu JB, Zheng Q, Yan YF, Wen TM, Tang YR. Effect of Xiangdan Injection on mRNA expression of endothelial vasoactive factors of patients with coronary heart disease and blood stasis. Zhong Xi Yi Jie He Xue Bao. 2004;2:94–96. doi: 10.3736/jcim20040205. In Chinese. [DOI] [PubMed] [Google Scholar]
- 7.Wang SX, Luo K, Liang J, Fan F, Li H, Zheng JB, Zheng XH. Metabolomics study on the synergistic interaction between Salvia miltiorrhiza and Lignum dalbergiae odoriferae used as 'Jun-Shi' herbs in a S. miltiorrhiza recipe. Med Chem Res. 2011;20:16–22. doi: 10.1007/s00044-009-9275-8. [DOI] [Google Scholar]
- 8.Sugiyama A, Zhu BM, Takahara A, Satoh Y, Hashimoto K. Cardiac effects of salvia miltiorrhiza/dalbergia odorifer a mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ J. 2002;66:182–184. doi: 10.1253/circj.66.182. [DOI] [PubMed] [Google Scholar]
- 9.Mu F, Duan J, Bian H, Zhai X, Shang P, Lin R, Zhao M, Hu D, Yin Y, Wen A, et al. Metabonomic strategy for the evaluation of Chinese medicine salvia miltiorrhiza and dalbergia odorifera interfering with myocardial ischemia/reperfusion injury in rats. Rejuvenation Res. 2017;20:263–277. doi: 10.1089/rej.2016.1884. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X, Zhao X, Wang S, Luo K, Wei Y, Zheng J. Co-administration of Dalbergia odorifera increased bioavailability of Salvia miltiorrhiza e in rabbits. Am J Chin Med. 2007;35:831–840. doi: 10.1142/S0192415X07005302. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 12.Liu R, Ye M, Guo H, Bi K, Guo DA. Liquid chromatography/electrospray ionization mass spectrometry for the characterization of twenty-three flavonoids in the extract of Dalbergia odorifera. Rapid Commun Mass Spectrom. 2005;19:1557–1565. doi: 10.1002/rcm.1936. [DOI] [PubMed] [Google Scholar]
- 13.Tu J, Song K. Curative effect observation on Xiangdan injection in the treatment of coronary artery disease. Chin Med Pharm. 2011;22:110–111. In Chinese. [Google Scholar]
- 14.Qin F, Huang X. Guanxin II for the management of coronary heart disease. Chin J Integr Med. 2009;15:472–476. doi: 10.1007/s11655-009-0472-6. [DOI] [PubMed] [Google Scholar]
- 15.Mu F, Duan J, Bian H, Yin Y, Zhu Y, Wei G, Guan Y, Wang Y, Guo C, Wen A, et al. Cardioprotective effects and mechanism of Radix Salviae miltiorrhizae and Lignum Dalbergiae odoriferae on rat myocardial ischemia/reperfusion injury. Mol Med Rep. 2017;16:1759–1770. doi: 10.3892/mmr.2017.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnamoorthy K, Subramaniam P. Phytochemical profiling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) Gandhi using GC-MS. Int Sch Res Notices. 2014;2014:567409. doi: 10.1155/2014/567409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang JH, Huang XZ, Chen ZY, Zheng QS, Sun RY. Dose conversion among different animals and healthy volunteers in pharmacological study. Chin J Clin Pharmacol Therap. 2004;9:1069–1072. [Google Scholar]
- 18.Henriques JP, Zijlstra F, van 't Hof AW, de Boer MJ, Dambrink JH, Gosselink M, Hoorntje JC, Suryapranata H. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107:2115–2119. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Chen JM, Huang H, Kuznicki M, Zheng S, Sun W, Quan N, Wang L, Yang H, Guo HM, et al. The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway. Metabolism. 2016;65:122–130. doi: 10.1016/j.metabol.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito N. Letter by Saito regarding article, 'Collateral donor artery physiology and the influence of a chronic total occlusion on fractional flow reserve'. Circ Cardiovasc Interv. 2015;8:e002794. doi: 10.1161/CIRCINTERVENTIONS.115.002794. [DOI] [PubMed] [Google Scholar]
- 21.Zhao HW, Qin F, Liu YX, Huang X, Ren P. Antiapoptotic mechanisms of Chinese medicine formula, Guan-Xin-Er-Hao, in the rat ischemic heart. Tohoku J Exp Med. 2008;216:309–316. doi: 10.1620/tjem.216.309. [DOI] [PubMed] [Google Scholar]
- 22.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmadhun NY, Sabe AA, Robich MP, Chu LM, Lassaletta AD, Sellke FW. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann NY Acad Sci. 2013;1290:130–135. doi: 10.1111/nyas.12216. [DOI] [PubMed] [Google Scholar]
- 24.Ikonen TS, Pätilä T, Virtanen K, Lommi J, Lappalainen K, Kankuri E, Krogerus L, Harjula A. Ligation of ameroid-stenosed coronary artery leads to reproducible myocardial infarction-a pilot study in a porcine model. J Surg Res. 2007;142:195–201. doi: 10.1016/j.jss.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Caillaud D, Calderon J, Réant P, Lafitte S, Dos Santos P, Couffinhal T, Roques X, Barandon L. Echocardiographic analysis with a two-dimensional strain of chronic myocardial ischemia induced with ameroid constrictor in the pig. Interact Cardiovasc Thorac Surg. 2010;10:689–693. doi: 10.1510/icvts.2010.232819. [DOI] [PubMed] [Google Scholar]
- 26.Tuzun E, Oliveira E, Narin C, Khalil H, Jimenez-Quevedo P, Perin E, Silva G. Correlation of ischemic area and coronary flow with ameroid size in a porcine model. J Surg Res. 2010;164:38–42. doi: 10.1016/j.jss.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 27.von Degenfeld G, Raake P, Kupatt C, Lebherz C, Hinkel R, Gildehaus FJ, Münzing W, Kranz A, Waltenberger J, Simoes M, et al. Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia. J Am Coll Cardiol. 2003;42:1120–1128. doi: 10.1016/S0735-1097(03)00915-X. [DOI] [PubMed] [Google Scholar]
- 28.Eisenman A. Troponin assays for the diagnosis of myocardial infarction and acute coronary syndrome: Where do we stand. Expert Rev Cardiovasc Ther. 2006;4:509–514. doi: 10.1586/14779072.4.4.509. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Shao D, Wang G, Jiang T, Wu H, Gu B, Cao K, Zhang J, Qi L, Chen Y. Effects of different LAD-blocked sites on the development of acute myocardial infarction and malignant arrhythmia in a swine model. J Thorac Dis. 2014;6:1271–1277. doi: 10.3978/j.issn.2072-1439.2014.07.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsaroucha A, Chondrogiannis C, Mani A, Staikou C. Myocardial involvement during ischemia-induced acute liver failure in the pig. J Invest Surg. 2013;26:99–104. doi: 10.3109/08941939.2012.705953. [DOI] [PubMed] [Google Scholar]
- 31.Arya DS, Bansal P, Ojha SK, Nandave M, Mohanty I, Gupta SK. Pyruvate provides cardioprotection in the experimental model of myocardial ischemic reperfusion injury. Life Sci. 2006;79:38–44. doi: 10.1016/j.lfs.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Doganci S, Yildirim V, Bolcal C, Korkusuz P, Gumusel B, Demirkilic U, Aydin A. Sodium nitrite and cardioprotective effect in pig regional myocardial ischemia-reperfusion injury model. Adv Clin Exp Med. 2012;21:713–726. [PubMed] [Google Scholar]
- 33.Elizondo-Montemayor L, Ugalde-Casas PA, Lam-Franco L, Bustamante-Careaga H, Serrano-González M, Gutiérrez NG, Martínez U. Association of ALT and the metabolic syndrome among Mexican children. Obes Res Clin Pract. 2014;8:e79-e87. doi: 10.1016/j.orcp.2012.08.191. [DOI] [PubMed] [Google Scholar]
- 34.Xu Q, Higgins T, Cembrowski GS. Limiting the testing of AST: A diagnostically nonspecific enzyme. Am J Clin Pathol. 2015;144:423–426. doi: 10.1309/AJCPO47VAWYRIDHG. [DOI] [PubMed] [Google Scholar]
- 35.Solymoss BC, Bourassa MG, Fortier A, Théroux P. Evaluation and risk stratification of acute coronary syndromes using a low cut-off level of cardiac troponin T, combined with CK-MB mass determination. Clin Biochem. 2004;37:286–292. doi: 10.1016/j.clinbiochem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Dong W, Lu S, Yu Y, Liu H, Li G, Qiang W, Wang L, Lou J. Study on blood-gas changes in the coronary artery of myocardial ischemia reperfusion injury of rabbit. J Naval Med College. 1996;1:9–12. In Chinese. [Google Scholar]
- 37.Feng YJ, Chen C, Fallon JT, Lai T, Chen L, Knibbs DR, Waters DD, Wu AH. Comparison of cardiac troponin I, creatine kinase-MB, and myoglobin for detection of acute ischemic myocardial injury in a swine model. Am J Clin Pathol. 1998;110:70–77. doi: 10.1093/ajcp/110.1.70. [DOI] [PubMed] [Google Scholar]
- 38.Ishihara Y, Shimamoto N. Sulfaphenazole attenuates myocardial cell apoptosis accompanied with cardiac ischemia-reperfusion by suppressing the expression of BimEL and Noxa. J Pharmacol Sci. 2012;119:251–259. doi: 10.1254/jphs.12079FP. [DOI] [PubMed] [Google Scholar]
- 39.Qu D, Han J, Ren H, Yang W, Zhang X, Zheng Q, Wang D. Cardioprotective effects of Astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid Med Cell Longev. 2016;2016:8194690. doi: 10.1155/2016/8194690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan H, Li D, Fang F, Chen D, Qi L, Zhang R, Xu T, Sun H. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacolm. 2011;58:535–542. doi: 10.1097/FJC.0b013e31822de355. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Wang G, Yuan W, Wu J, Wang M, Li C. The effects of phosphodiesterase-5 inhibitor sildenafil against post-resuscitation myocardial and intestinal microcirculatory dysfunction by attenuating apoptosis and regulating microRNAs expression: Essential role of nitric oxide syntheses signaling. J Transl Med. 2015;13:177. doi: 10.1186/s12967-015-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L, Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al. bFGF regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the PI3K/Akt/mTOR pathway. Sci Rep. 2015;5:9287. doi: 10.1038/srep09287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Ai Q, Feng K, Li YB, Liu X. The cardioprotective effect of dihydromyricetin prevents ischemia-reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1α signaling pathways. Apoptosis. 2016;21:1366–1385. doi: 10.1007/s10495-016-1306-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhai P, Sciarretta S, Galeotti J, Volpe M, Sadoshima J. Differential roles of GSK-3β during myocardial ischemia and ischemia_reperfusion. Circ Res. 2011;109:502–511. doi: 10.1161/CIRCRESAHA.111.249532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duan J, Guan Y, Mu F, Guo C, Zhang E, Yin Y, Wei G, Zhu Y, Cui J, Cao J, et al. Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: Involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci Rep. 2017;7:41491. doi: 10.1038/srep41491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagaoka K, Matoba T, Mao Y, Nakano Y, Ikeda G, Egusa S, Tokutome M, Nagahama R, Nakano K, Sunagawa K, et al. A new therapeutic modality for acute myocardial infarction: Nanoparticle-mediated delivery of Pitavastatin induces cardio-protection from ischemia-reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat model. PLoS One. 2015;10:e0132451. doi: 10.1371/journal.pone.0132451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu ZJ, Shi ZL, Tu C, Zhang HZ, Gao D, Li CY, He Q, Li RS, Guo YM, Niu M, et al. An activity-calibrated chemical standardization approach for quality evaluation of Salvia miltiorrhiza Bge. RSC Adv. 2017;7:5331–5339. doi: 10.1039/C6RA26281C. [DOI] [Google Scholar]
- 48.Zhou GJ, Wang W, Xie XM, Qin MJ, Kuai BK, Zhou TS. Post-harvest induced production of salvianolic acids and significant promotion of antioxidant properties in roots of Salvia miltiorrhiza (Danshen) Molecules. 2014;19:7207–7222. doi: 10.3390/molecules19067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan G, Yu J, Asare PF, Wang L, Zhang H, Zhang B, Zhu Y, Gao X. Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. J Cell Mol Med. 2016;20:1908–1919. doi: 10.1111/jcmm.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia LQ, Yang GL, Ren L, Chen WN, Feng JY, Cao Y, Zhang L, Li XT, Lei P. Tanshinone IIA reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA.hy926 cells. J Ethnopharmacol. 2012;143:100–108. doi: 10.1016/j.jep.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M, Zhang J, Liu Y, Yang X, Isaiah AO, Lin Y, et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One. 2012;7:e35636. doi: 10.1371/journal.pone.0035636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li XL, Liu JX, Li P, Zheng YQ. Protective effect of rosmarinic acid on hypoxia/reoxygenation injury in cardiomyocytes. Zhongguo Zhong Yao Za Zhi. 2014;39:1897–1901. In Chinese. [PubMed] [Google Scholar]
- 53.Jung SH, Seol HJ, Jeon SJ, Son KH, Lee JR. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine. 2009;16:327–335. doi: 10.1016/j.phymed.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 54.Guo C, Yin Y, Duan J, Zhu Y, Yan J, Wei G, Guan Y, Wu X, Wang Y, Xi M, Wen A. Neuroprotective effect and underlying mechanism of sodium danshensu [3-(3,4-dihydroxyphenyl) lactic acid from Radix and Rhizoma Salviae miltiorrhizae = Danshen] against cerebral ischemia and reperfusion injury in rats. Phytomedicine. 2015;22:283–289. doi: 10.1016/j.phymed.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q, Hu X, Jiang XJ, Guo MJ, Ji H, Wang Y, Fan YC. Cardiomyocyte-like cells differentiation from non β-catenin expression mesenchymal stem cells. Cytotechnology. 2014;66:575–584. doi: 10.1007/s10616-013-9605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XL, Fan JP, Liu JX, Liang LN. Salvianolic acid A protects neonatal cardiomyocytes against hypoxia/reoxygenation-induced injury by preserving mitochondrial function and activating Akt/GSK-3beta signals. Chin J Integr Med. 2017:1–8. doi: 10.1007/s11655-016-2747-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.