Abstract
Prolongation of the QT interval can predispose to a potentially fatal polymorphic ventricular tachycardia called torsades de pointes (TdP). Although usually self-limited, TdP may degenerate into ventricular fibrillation and cause sudden death. Some medications that cause QT prolongation and possible TdP are commonly used in general practice. This paper presents a case of sudden death that is likely from drug-induced TdP. It reviews the mechanisms, risk factors, offending agents, and management of drug-induced torsades de pointes.
Case Report
A 27-year-old female was at home resting when she had a witnessed episode of sudden generalized loss of consciousness. No jerking or seizure-like movements were noted. Her husband called 911 and performed CPR. On arrival, paramedics determined that the patient was in ventricular fibrillation and successfully defibrillated her. Lidocaine IV bolus was given, after which brief episodes of self-terminating polymorphic ventricular tachycardia were noted (See Figure 1). EKG revealed sinus rhythm with no evidence of pre-excitation, no changes consistent with infarction, injury or ischemia, and a QTc of 459 msec (See Figure 2). Cardiovascular examination revealed normal heart sounds with no murmurs or gallops. Serum potassium was 3.1 mEq/L, while ionized calcium and magnesium levels were normal. An echocardiogram showed normal left and right ventricular systolic function and normal ventricular size. Serum and urine toxicology screens only showed elevated levels of benzodiazepine, which was given during transit to control severe myoclonic jerking.
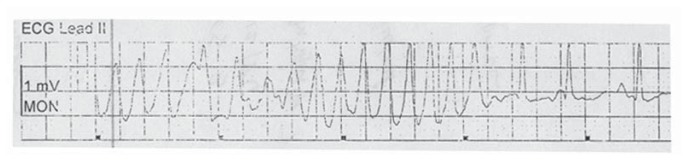
Figure 1.
Brief episodes of self-terminating polymorphic ventricular tachycardia were noted after Lidocaine IV bolus was given.
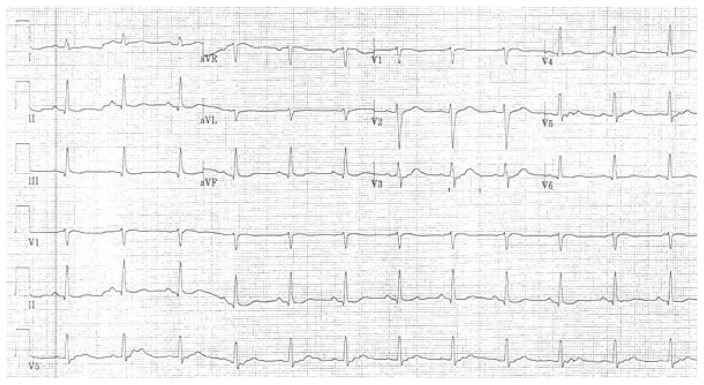
Figure 2.
EKG revealed sinus rhythm with no evidence of pre-excitation, no changes consistent with infarction, injury or ischemia, and a QTc of 459 msec.
Of note, the patient has a history of fibromyalgia, depression, anxiety, and chronic low back pain. There was no family history of sudden death. Her medications included bupropion, trazodone, zolpidem, tizanidine, fexofenadine, tramadol PRN, ibuprofen PRN, sumatriptan PRN. Due to an upper respiratory tract infection, she was being concomitantly treated with azithromycin.
She became comatose due to anoxic brain injury. After 72 hours, a thorough evaluation confirmed that she had sustained severe, irreversible brain injury. Comfort care measures were initiated and her organs were harvested for donation upon declaration of brain death. Her cardiac arrest was likely from ventricular fibrillation or hemodynamically unstable torsades de pointes. She was concomitantly taking two medications that potentially cause TdP: azithromycin and tizanidine. Lastly, the patient possibly had an undiagnosed congenital long QT which predisposed her to TdP.
Introduction: The Importance of Drug-induced prolonged QT and Torsades de Pointes
Prolongation of the QT interval on an ECG can predispose to a potentially fatal ventricular arrhythmia called torsades de pointes (TdP), a polymorphic ventricular tachycardia where QRS complexes ‘twist’ around an isoelectric line in a sinusoidal fashion. Torsades de pointes is usually self-limited, but may degenerate into VF and cause sudden cardiac death. Symptoms of TdP include palpitations, syncope, and seizure-like activity. Drug-induced prolongation of the QT interval and ventricular fibrillation were first reported by Selzer and Wray (1964) in a patient taking quinidine.1 Several other medications have been found to cause TdP. This paper reviews the mechanisms, risk factors, offending agents, and management of drug-induced torsades de pointes.
Mechanisms of QT Prolongation and TdP
Delayed rectifier K+ current (Ikr)
The efflux of potassium ions is the primary driver of myocardial repolarization. This activity is mediated by two subtypes of channels of the delayed rectifier K+ current: Ikr (rapid) and Iks (slow). All known drugs that prolong QT preferentially block Ikr, causing a delay in phase 3 rapid repolarization of the action potential.2 The increase in duration of the action potential is manifested in the EKG by QT prolongation. De Bruin et al (2005) demonstrated strong correlation between a drug’s ability to block Ikr and its potential to cause ventricular arrhythmias and sudden death.3
Early After Depolarizations (EADs) and Extrasystoles
Prolonged repolarizatrion may activate inward depolarizing currents thereby causing early afterdepolarizations (EADs). This is likely mediated by L-type calcium channels or sodium-calcium exchange current (INCX).4 EADs appear as depolarizing oscillations in membrane voltage during phases 2 and 3 of the action potential. EADs that reach the threshold voltage cause ventricular extrasystoles. Heterogeneity in ventricular repolarization (dispersion of refractoriness) can create zones of unidirectional block. Repetitive extrasystoles, unidirectional block, and zones of slow conduction can lead to reentry and ultimately, TdP.
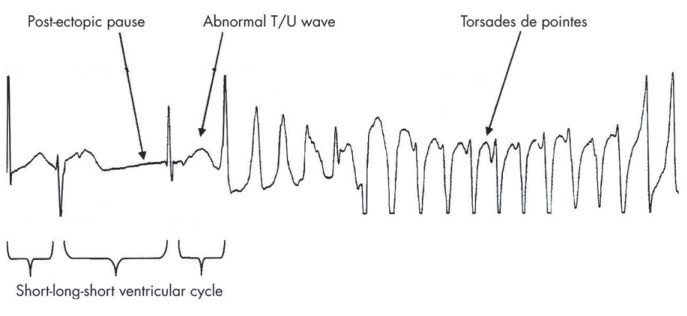
Genesis of TdP: Short-Long-Short ECG Sequence
Drug-induced TdP is usually preceded by a short-long-short ECG sequence (See Figure 3).5 It is initiated by one or more premature ventricular complexes (PVCs) followed by a compensatory pause. A sinus beat follows, which generally has a prolonged QT interval and/or deformities of T or U waves. There is a vulnerable period near the T wave peak during which premature stimuli may induce ventricular arrhythmias. When another PVC occurs during this vulnerable period, torsades de pointes may occur.
Figure 3.
Drug-induced TdP is usually preceded by a short-long-short ECG sequence.
Other ECG Variables: QT Dispersion, T Wave Alternans
Other ECG variables have been investigated as predictors of TdP. QT dispersion is defined as the difference between the maximum and minimum QT intervals. QT dispersion is hypothesized to be a more direct measure of spatial heterogeneity of repolarization. QT dispersion was found to be mostly dependent on T wave morphology and does not accurately predict drug-induced TdP.6 Manifest T wave alternans, defined as the beat to beat alternation in amplitude or polarity, is considered a marker of electrical instability in congenital long QT syndrome (LQTS). However, T wave alternans is rare in acquired LQTS and may not have the same implications.7 Microvolt T wave alternans is a variant of T wave alternans that detects visually inapparent beat-to-beat oscillations on the surface ECG and measures signals that are as small as one-millionth of a volt. Some studies have been shown that this technology can predict susceptibility to ventricular arrhythmias.8 Its use in predicting drug-induced TdP remains unclear. In the absence of other reliable predictors, QT prolongation remains the most useful clinical variable to predict risk of TdP despite its lack of specificity.
QT Measurement
The QT interval is measured from QRS complex onset to T wave termination. V3 or V4 appear most reliable for assessing QT prolongation.9 There may be significant intra-and interobserver variability in determining QT intervals due to variation caused by electrolyte imbalance, autonomic and diurnal fluctuations, and ECG acquisition technique.10 QT intervals normally shorten with tachycardia and lengthen with bradycardia. In 1920, Bazett proposed a formula dividing the longest QT interval by the square root of the R-R interval to calculate the rate-corrected QT interval (QTc). Although there is no formal consensus on the best QTc method, Bazett’s formula remains the gold standard. QTc intervals <440 milliseconds are clearly normal. QTc intervals of 440 to 460 milliseconds in men and 440 to 470 milliseconds in women are considered borderline.11 In the absence of a regular R-R interval, such as in atrial fibrillation, Bazett’s formula may not apply. One method of obtaining the QTc is to measure the QT over 10 consecutive beats and average the values. Alternatively, the QT interval between the shortest and longest R-R intervals may be averaged.12
Risk Factors for TdP and Sudden Cardiac Death: Multiple Hit Hypothesis
Clinical Risk Factors
Only a minority of patients taking medications that prolong the QT develop TdP. Zeltser et al (2003) reviewed 249 cases of TdP due to noncardiac medications.13 Female gender was the most common risk factor for developing TdP and was present in 71% (consistent with the fact that women have longer QT intervals than men do). Common risk factors identified were structural heart disease (myocardial infarction, heart failure, valvular disease, or cardiomyopathy), electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia), multiple QT prolonging drugs or agents interfering with their metabolism, higher-than-average drug dosage, prolonged baseline QTc (≥450 milliseconds), family history of congenital LQTS, and prior drug-induced TdP.13
Genetic Risk Factors
Patients with medication-induced QT prolongation and ventricular arrhythmias have been found to have subclinical mutations in genes that cause congenital long QT syndrome (LQTS).14 As an example, patients with congenital LQTS 6 (mutations in the gene encoding the β subunit of Ikr) generally do not have arrhythmias in the absence of an agent that prolongs the QT. Yang et al (2002) found that 10% to 15% of patients with drug-associated LQTS had mutations or polymorphisms in one of the genes encoding for ancillary units of the pore-forming channel proteins.15 One or more mutations (‘multiple hits’) may need to be present before TdP occurs. The concept of repolarization reserve (variable redundancy of repolarizing currents) may explain why some patients with mutant genes do not develop QT prolongation or TdP until additional insults further limit repolarization.16
Medications That Prolong the QT Interval
Prolongation of the QT interval and the occurrence of TdP occurs in a dose-dependent manner for some medications. In others, TdP may be precipitated at any dose, usually resulting from potassium channel blockade. Table 1 lists the commonly used medications that prolong QTc and cause TdP. It is also very important to be familiar with drugs that inhibit the cytochrome P450 3A4 (CYP3A4) system, as most of the medications associated with TdP are metabolized by this system.
Table 1.
Drugs With Substantial Risk of Torsades de pointes
Antiaarrhythmics
|
Psychotropic Medications
|
Antimicrobials
|
| Promotility Medications |
| Antihistamines |
Miscellaneous Medications and Supplements
|
No longer available in the U.S.
Antiarrhythmics
Antiarrhythmic medications are the most common culprits for drug-induced TdP. Patients on these commonly have heart disease and are also often on diuretics that cause hypokalemia and hypomagnesemia. Class IA drugs (quinidine, disopyramide, procainamide) cause TdP in a dose-independent manner. They block outward K+ currents and inward Na+ currents. TdP frequently occurs at low serum potassium levels.5 The risk of TdP is highest with quinidine, with an incidence of approximately 1.5%.17 Procainamide is less likely to cause TdP, but patients with impaired renal function develop high N-acetylprocainamide levels causing Ikr blockade, QT prolongation and possible TdP.17
Unlike class IA agents, class III antiarrhythmics (potent Ikr blockers) prolonging the QT in a dose-dependent manner. Dofetilide, ibutilide, and sotalol pose the highest risk, progressively prolong QT as serum levels increase.5,18 The risk of TdP increases with bradycardia, due to a phenomenon known as reverse use dependence (i.e. increased channel binding at lower heart rates). For sotalol, TdP risk ranges from 0.8% to 3.8%.18 For dofetilide, the rates are 0 to 10.5%.5 Intravenous ibutilide, which is commonly used in pharmacologic cardioversion of atrial fibrillation and flutter causes TdP in up to 8.3% of patients.5 Despite significant QT prolongation, amiodarone is unlikely to cause TdP, with an incidence of <1%.5
Antipsychotics
Typical and atypical antipsychotic medications cause a dose-dependent prolongation in the QT interval. In a review of 495 patients on psychotropic drug therapy, 8% had QT prolongation. Thioridazine and droperidol seem to pose the highest risk in this cohort.17 Phenothiazines (thioridazine, chlorpromazine, and mesoridazine) and butyrophenones (droperidol and haloperidol) have been associated with TdP.17 In a large retrospective cohort analysis of more than 280,000 Medicaid enrollees in Tennessee, current use of typical and atypical antipsychotics was associated with a dose-related increase in sudden cardiac death.19
Antibiotics
The macrolide antibiotics erythromycin and clarithromycin have been implicated in TdP. Proarrhythmia may be precipitated by Ikr blockade. In addition, these drugs are metabolized by CYP3A4 and are especially dangerous for patients receiving another CYP3A4 inhibitor or a QT prolonging medication metabolized by CYP3A4 (see Table 2). In a retrospective cohort analysis of more than 1.2 million Medicaid patients in Tennessee, current use of erythromycin increased the risk of sudden cardiac death two-fold. Concomitant use of a CYP3A inhibitor increased the adjusted rate of sudden death five-fold.20 Polymorphic VT has been reported with the use of azithromycin even in patients with normal QT interval.21 Its proarrhythmic potential is well below that of erythromycin or clarithromycin in an experimental model.22
Table 2.
Inhibitors of CYP3A4
|
Macrolide Antibiotics
|
Azole Antifungals
|
Antiarrhythmics
|
Antineoplastic Medications
|
Endocrine Medications
|
Miscellaneous Medications
|
Food
|
Fluoroquinolones as a class prolong the QT interval by blocking Ikr, thus causing a proarrhythmic potential. The overall incidence of torsades de pointes associated with fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin, gemifloxacin, gatifloxacin, and moxifloxacin) is very low.23 According to a review of available case reports and clinical studies, moxifloxacin carries the greatest risk of QT prolongation and highest rates of TdP, while ciprofloxacin has the lowest risk.23 In TdP cases reported, at least one predisposing factor for TdP was identified, such as an electrolyte abnormality (hypokalemia, hypocalcemia, hypomagnesemia) or concomitant use of a CYP3A inhibitor. Sparfloxacin causes excessive prolongation of the QT interval and has been taken out of the US market.17
Pentamidine, an antiprotozoal drug used to treat parasitic infections and Pneumocystis carinii pneumonia, has been shown to cause QT prolongation and TdP.17 There are rare case reports of systemic antifungals (fluconazone, itraconazole, ketoconazole, and voriconazole) causing TdP, but the risk is low without preexisting risk factors.17
Miscellaneous Drugs and Supplements
Methadone, a long-acting synthetic opiate considered safe and effective for heroin addiction and chronic pain, blocks Ikr and prolongs QT in a dose-dependent fashion. Fifty-nine cases of methadone-induced LQTS or TdP were reported to the Food and Drug Administration between 1969 and 2002, a majority of whom had an additional risk factor for TdP.17
Some herbal and nutritional supplements have also been linked to torsades de pointes. Licorice and zhigancao (a chinese herb prepared from licorice used to treat dyspepsia and peptic ulcer disease), cause hypokalemia and have been associated with TdP.17 Some dietary items inhibit CYP3A4 and may interfere with the metabolism of QT prolonging medications (e.g. grapefruit juice, olive leaves, licorice extract and red grape skins, see Table 2).
Treatment
Intravenous magnesium sulfate (2 g bolus followed by an infusion of 2–4 mg/minute) is the initial therapeutic agent of choice regardless of serum magnesium level and is continued until TdP has resolved.17 In the presence of hemodynamic instability, immediate non-synchronized defibrillation is indicated. Serum potassium should be monitored and maintained in the 4.5 – 5.0 mmol/L range. All QT prolonging agents and drugs that interfere with their metabolism must be immediately discontinued.
Overdrive transvenous ventricular pacing (at rates of 90–110 bpm) may also prove to be useful, particularly if TdP is precipitated by a pause or bradycardia. Overdrive pacing shortens the QT interval and is highly effective in preventing recurrence. In patients with sick sinus syndrome or atrioventricular block and bradycardia or pause-dependent drug-induced TdP, permanent pacing with programmable pause prevention algorithms may be indicated.24 Experience with permanent pacemakers suggests rates >70 beats/min protect against drug-induced TdP.25
Long-term management involves avoidance of QT prolonging medications and drugs interfering with their metabolism. Conditions that predispose to electrolyte imbalance must also be corrected.
Conclusions and Recommendations
Despite several limitations, treatment with QT prolonging medications is frequently necessary. Physicians have the responsibility to ensure that the potential benefits are clinically important and that the risks for developing TdP are minimized. Preexisting risk factors should be identified, and correctable conditions must be addressed, prior to drug initiation. Although routine EKGs are not indicated, checking a baseline QT interval in patients who have one or more risk factors is probably a prudent step prior to starting a medication associated with TdP.
Information about the proaarhythmic risk should be available to both the patient and the physician to help them come to an informed decision. In cases where there is paucity of information or where the risk is substantial, alternative treatments should be considered.
An important resource for patients and physicians is the database of drugs associated with TdP maintained by the University of Arizona Center for Education and Research in Therapeutics (ArizonaCERT). The list is available online (www.torsades.org, www.qtdrugs.org, www.longqt.org, www.sads.org).
Biography
Marc E. Del Rosario, MD, Cardiovascular Medicine Fellow, Richard Weachter, MD, MSMA member since 2004, Associate Professor, and Greg C. Flaker, MD, are at the University of Missouri Hospital and Clinics, Division of Cardiovascular Medicine.
Contact: flakerg@health.missouri.edu

Footnotes
Disclosure
None reported.
References
- 1.Selzer A, Wray HW. Quinidine syncope. Paroxysmal ventricular fibrillation occurring during treatment of chronic atrial arrhythmias. Circulation. 1964;30:17–26. doi: 10.1161/01.cir.30.1.17. [DOI] [PubMed] [Google Scholar]
- 2.Mitcheson JS, Chen J, Lin M, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci U S A. 2000;97:12329–33. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bruin ML, Pettersson M, Meyboom RH, et al. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–7. doi: 10.1093/eurheartj/ehi092. [DOI] [PubMed] [Google Scholar]
- 4.Roden DM, Lazzara R, Rosen M, et al. Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps, and future directions. The SADS Foundation Task Force on LQTS. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- 5.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–72. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah RR. Drug-induced QT dispersion: does it predict the risk of torsade de pointes? J Electrocardiol. 2005;38:10–8. doi: 10.1016/j.jelectrocard.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Trohman RG, Sahu J. Drug-induced torsade de pointes. Circulation. 1999;99:E7. doi: 10.1161/01.cir.99.16.e7. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum DS, Jackson LE, Smith JM, et al. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–41. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 9.Sadanaga T, Sadanaga F, Yao H, et al. An evaluation of ECG leads used to assess QT prolongation. Cardiology. 2006;105:149–54. doi: 10.1159/000091227. [DOI] [PubMed] [Google Scholar]
- 10.Morganroth J, Brozovich FV, McDonald JT, et al. Variability of the QT measurement in healthy men, with implications for selection of an abnormal QT value to predict drug toxicity and proarrhythmia. Am J Cardiol. 1991;67:774–6. doi: 10.1016/0002-9149(91)90541-r. [DOI] [PubMed] [Google Scholar]
- 11.Surawicz B, Knilans T, Chou TC. Chou’s electrocardiography in clinical practice. Philadelphia, PA: W.B. Saunders Company; 2001. [Google Scholar]
- 12.Al-Khatib SM, LaPointe NM, Kramer JM. What clinicians should know about the QT inter val. JAMA. 2003;289:2120–7. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 13.Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82:282–90. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano C, Schwartz PJ, Brown AM, et al. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol. 2000;11:691–6. doi: 10.1111/j.1540-8167.2000.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–8. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 16.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–34. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current conceprs in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–9. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Lazzara R. Antiarrhythmic drugs and torsade de pointes. Eur Heart J. 1993;14:88–92. doi: 10.1093/eurheartj/14.suppl_h.88. [DOI] [PubMed] [Google Scholar]
- 19.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–35. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray WA, Murray KT, Meredith S, et al. Oral er ythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–96. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Berkowitz C, Trohman RG. Polymorphic ventricular tachycardia with a normal QT inter val following azithromycin. Pacing Clin Electrophysiol. 2005;28:1221–2. doi: 10.1111/j.1540-8159.2005.50146.x. [DOI] [PubMed] [Google Scholar]
- 22.Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther. 2002;303:218–25. doi: 10.1124/jpet.102.037911. [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Rafailidis PI, Rosmarakis ES. Arrhythmias associated with fluoroquinolone therapy. Int J Antimicrob Agents. 2007 Apr;29(4):374–9. doi: 10.1016/j.ijantimicag.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Viskin S. Cardiac pacing in the long QT syndrome: review of available data and practical recommendations. J Cardiovasc Electrophysiol. 2000;11:593–600. doi: 10.1111/j.1540-8167.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinski SL, Eguia LE, Trohman RG. What is the minimal pacing rate that prevents torsades de pointes? Insights from patients with permanent pacemakers. Pacing Clin Electrophysiol. 2002;25:1612–5. doi: 10.1046/j.1460-9592.2002.01612.x. [DOI] [PubMed] [Google Scholar]