Abstract
Sphingosine 1-phosphate (S1P) is a bioactive phosphorylated product of sphingosine catalyzed by sphingosine kinase (SphK) and implicated in diverse cellular functions including vesicular trafficking. In the present study we have shown the importance of one of the subtypes of SphK, SphK2, in the regulation of cargo content in exosomes released from human myeloid leukemia K562 cells. First, SphK2 has been shown to localize with N-Rh-PE-positive late endosomes in the cells. Next, siRNA-mediated knockdown of Sphk2 but not SphK1 resulted in a reduction of cargo content in purified exosomes. The involvement of SphK2 in this phenomenon was further investigated by pharmacological approaches. When cells were treated with N,N-dimethylsphingosine (DMS), one of the most frequently used inhibitors for SphK, cargo contents in purified exosomes were enhanced unexpectedly. Finally, it has been shown that DMS has a potency to stimulate SphK2 activity depending on the substrate sphingosine- and the inhibitor-doses as estimated by in vitro assay systems using a purified SphK2. These findings suggest that SphK2/S1P signaling plays an important role in the regulation of cargo content in exosomes in K562 cells.
Keywords: sphingosine kinase 2; N,N-dimethylsphingosine; Sphingosine 1-phosphate; exosomes; multivesicular endosomes
INTRODUCTION
Exosomes are membrane-bound vesicles with a size of 50–100 nm that are released from many types of cells into the extracellular space. Exosomes are considered important means of cell-to-cell communication by transferring internal cargo molecules such as proteins, soluble factors, microRNA, and mRNA to recipient cells, thereby playing a key role in intercellular communications such as antigen presentation (1), cancer progression (2), and spreading of neurodegenerative diseases (3).
Exosomes are generated by the fusion of multivesicular endosomes (MVEs) with the plasma membranes. Although mechanism underlying cargo sorting into intralumenal vesicles (ILVs) of MVEs destined for degradation by the fusion with lysosomes is well known, i.e., the one using endosomal sorting complex required for transport (ESCRT) machinery, it remained unclear as to how selective cargo molecules were sorted into exosomal ILVs. Recently an important discovery was made to show the importance of ceramide in the generation of exosomal ILVs. Trajkovic et al. showed through mass spectrometric analysis that secreted proteolipid protein 1 (PLP1)–containing exosomes purified from cell culture medium are enriched in ceramide and that neutral sphingomyelinase inhibitor causes a reduction of exosome secretion (4). They also showed that addition of a bacterial sphingomyelinase to giant unilamellar vesicles (GUVs) containing domains with different degrees of fluidity resulted in inward budding formation specifically from the “raft”-like lipid phase. However the mechanism of cargo sorting into exosomal ILVs remains unclear. More recently, studies in our laboratory have revealed that sustained activation of sphingosine 1-phosphate (S1P) receptor on CD63-positive MVEs in an intracrine manner is essential for cargo sorting into exosomal ILVs (5).
S1P functions as a specific ligand for a family of GTP-binding protein (G-protein)-coupled receptor, termed S1P1–5 receptors and triggers diverse cellular processes, including cell angiogenesis, cardiac development, immunity, cell motility, neurotransmitter release and endosome maturation (5–8). S1P is a phosphorylated product of sphingosine catalyzed by sphingosine kinase (SphK). Two isoforms of mammalian SphK (SphK1 and SphK2) have been cloned and characterized (9, 10). Although both SphK1 and SphK2 are structurally related, they display distinct subcellular and tissue distribution, showing unique and specific functions. Two inhibitors for SphKs, N,N-dimethylsphingosine (DMS) and 2-(p-Hydroxyanilino)-4-(p-chlorophenyl) thiazole (HACPT) are widely used to study cellular function of S1P (11). In the present study we have found that these SphK inhibitors showed opposite effects on cargo sorting into exosomal ILVs of MVEs. Molecular mechanisms underlying the discrepancy of the inhibitor actions are shown herein.
MATERIALS AND METHODS
Materials
S1P was purchased from Enzo Life Sciences; dimethylsphingosine from Calbiochem; sphingosine and N-Rh-PE from Sigma Aldrich. Anti-EEA1 antibody (catalog number 610456) was purchased from BD Biosciences; anti-TfR1 (catalog number HM2134) from Hycult Biotech; anti-HSP70 (catalog number SPA-815) from Stressgen Biotechnologies; anti-flotillin 2 (catalog number sc-48398) from Santa Cruz Biotechnology. Purified SphK2 was obtained from Carna Biosciences (Kobe, Japan).
Plasmid DNA
GFP-SphK1 and HA-SphK1 were constructed as described previously (12). SphK2 and HA-SphK2 were constructed as described previously (13). Site-directed mutagenesis was performed using QuikChange protocol for siRNA-resistant SphK2 silent mutant (5'-CTCTGAAGCTGGGCTCAGCTTCAACCTCATCC-3' and its reverse complement) and kinase-negative SphK2(G248D) mutant (5'-CGGTCTCGGGAGACGACCTGCTCCATGAGG-3' and its reverse complement).
siRNA
For RNA interference, the following oligonucleotides (Japan Bio Services, Saitama, Japan) were used: sense 5′-GGGCAAGGCCUUGCAGCUCdTdT-3′ and antisense 5′-GAGCUGCAAGGCCUUGCCCdTdT-3′ for human SphK1; sense 5'-GCUGGGCUGUCCUUCAACCUdTdT-3' and antisense 5'-AGGUUGAAGGACAGCCCAGCdTdT-3' for human SphK2; sense 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and antisense 5′-ACGUGACACGUUCGGAGAAdTdT-3′ for control. K562 cells were transfected with the siRNAs using Lipofectamine RNAiMAX according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA).
Cell culture
K562 cells were grown in RPMI 1640 medium (Wako Pure Chemical Industries) containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in 5% CO2. HEK293 cells were maintained in DMEM medium (Wako Pure Chemical Industries) containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C in 5% CO2.
Stable transformant of K562 cells
To establish stable K562 cell lines, cells were transfected with either SphK1 or SphK2 in pEGFP-C1 or pEGFP-N1 plasmid DNA (Clontech), respectively, and cultured in the presence of 0.8 mg/ml G418 (Nacalai tesque, Japan). The stable clones expressing either GFP-SphK1 or SphK2-GFP were selected by limiting dilution method using 96 well plate.
Cellular localization of SphK1 and SphK2
For N-Rh-PE labeling, K562 cells were treated with 1 μM N-Rh-PE at 37 °C for 3 hr before fixation. For LysoTracker staining, cells were treated with 50 nM LysoTracker Red at 37 °C for 30 min. K562 cells were fixed with 4% paraformaldehyde, permeabilized by 0.3% Triton X-100/PBS and treated with 1% BSA/PBS for 10 min. Cells were then incubated with anti-EEA1 monoclonal antibody (diluted 1:200) for 1 hr. After washing in PBS, cells were incubated with Alexa-Fluor 594 goat anti-mouse IgG (diluted 1:1,000) for 30 min. After washing with PBS, the fluorescence was observed under a confocal laser scanning microscope (LSM 510 META, Carl Zeiss).
Exosome preparation
Exosomes were prepared from K562 cell culture media essentially as described previously (5). Briefly, after overnight culture of cells with an 'exosome-depleted' medium where exosomes in the culture medium were removed by overnight centrifugation at 100,000 ×g, the culture media were collected and centrifuged for 10 min at 500 × g and for 20 min at 12,000 × g, then the supernatants were filtered on 0.22 μm pore filters (EMD Millipore, Billerica MA, USA), followed by ultracentrifugation for 70 min at 100,000 × g. The exosomes were then resuspended in PBS.
In vitro SphK assay
SphK activity was determined in the reaction mixture (50 μl) containing 20 mM Tris-HCl pH 7.4, 1 mM EDTA, 15 mM NaF, 1 mM 4-deoxypyridoxine, 80 mM β-glycerophosphate, 1 mM 2-mercaptoethanol, 100 μM or 0.2 μM sphingosine, 1 mM [γ-32P]ATP (37 kBq, PerkinElmer) and 10 mM MgCl2 using 60 ng of purified recombinant SphK2 protein for 10 min at 37 °C. After termination of the reactions and lipid extraction, [32P]S1P was separated by TLC on Silica Gel 60 (Merck) with 1-butaol/acetic acid/water (3:1:1) and quantified using a BAS 2500 (Fuji Photo Film) as described previously (14).
RESULTS
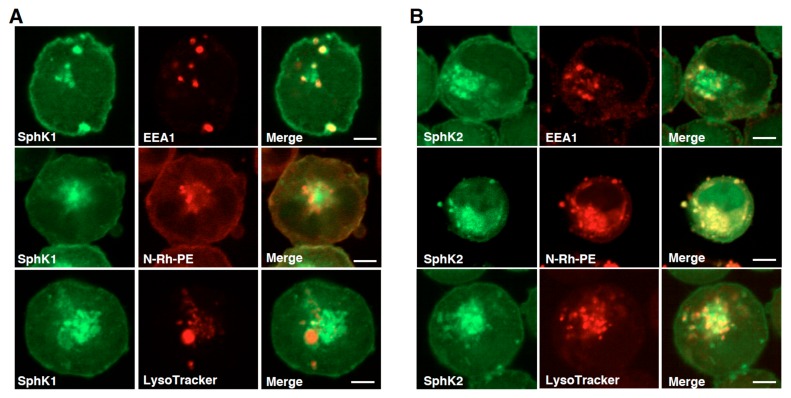
Due to the low transfection efficiency of DNA plasmids in human myeloid leukemia K562 cells, cellular distribution of SphK isozymes were characterized using the cells stably expressing each isozyme. SphK1 was colocalized with some of cellular vesicles, which was stained with an early endosomal marker, early endosomal antigen 1 (EEA1) (Fig. 1A) as previously reported (12). On the other hand, SphK1 showed poor colocalization with late endosomal markers N-Rh-PE and LysoTracker. The other isozyme SphK2 was colocalized with exosomal MVE marker N-Rh-PE (Fig. 1B). The high degree of colocalization did not come from the leakage of fluorescence signals of SphK2-GFP because SphK1-GFP showed a quite distinct distribution from N-Rh-PE signals (Fig. 1A).
Figure 1. Cellular distribution of Sphk1 and SphK2 in K562 cells.
K562 cells stably expressing either SphK1-GFP (A) or SphK2-GFP (B) were prelabeled with N-Rh-PE or LysoTracker, then fixed and analyzed for each fluorescence localization by confocal microscopy. In some experiments cells stably expressing these fluoro-proteins were fixed, permeabilized and stained with anti-EEA1 antibody and analyzed as above. Scale bars, 5 μm.
These results are consistent with our previous observations that SphK2 is associated with exosomal MVEs and constantly supplying S1P, which is a prerequisite for exosomal cargo sorting and MVE maturation in HeLa cells (5).
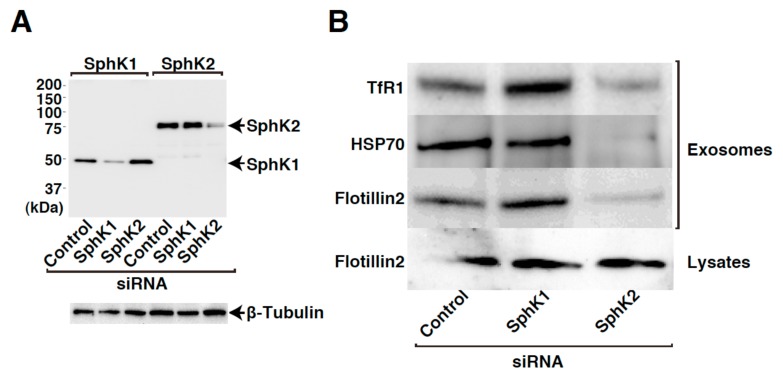
SphK1- and SphK2-siRNAs were validated for their ability to inhibit the expression of HA-hSphK1 and HA-hSphK2 expressed in HEK293 cells. Each SphK isozyme-specific siRNA specifically inhibit the expression of the respective isozyme, while having a minimal effect on the expression of the other counter part (Fig. 2A), assuring that the siRNA system works properly. When cells were treated with the siRNAs, SphK2-siRNA caused a robust reduction of exosomal cargo contents compared with control siRNA treatment (Fig. 2B). SphK1-siRNA had little or no effect on the cargo content.
Figure 2. siRNA-mediated silencing of SphK2 expression in K562 cells results in reduced cargo contents of exosomes released from the cells.
HEK293 cells transiently transfected with a vector encoding either HA-SphK1 or HA-SphK2 together with control, SphK1-siRNA or SphK2-siRNA were cultured for 72 hr. After cell lysis, the lysates were subjected to SDS-PAGE followed by immunoblot analysis using anti-HA and anti-β-tubulin antibodies (A). K562 cells transfected with control, SphK1-siRNA or SphK2-siRNA were cultured for 48 hr. Cell medium was then changed to exosome-depleted one and cultured for 12 hr. Exosomes were purified from the media and analyzed for cargo contents by immunoblot analysis using anti-TfR1, anti-HSP70, and anti-flotillin 2 antibodies. Cell lysates were also subjected to immunoblot analysis with anti-flotillin 2 antibody (B).
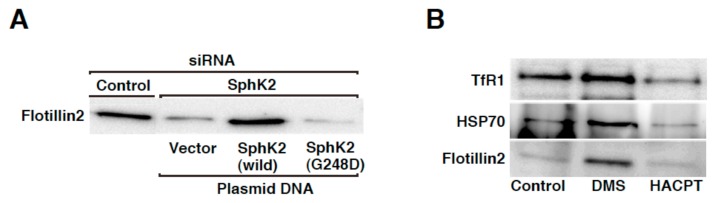
To confirm that SphK2 exerts its role in the regulation of cargo contents in the exosomes in an activity-dependent manner, the ability of an activity-negative mutant SphK2(G248D) to rescue the phenotype seen in SphK2-knockdown cells was tested and compared to the wild-type counterpart. SphK2-siRNA caused a strong reduction of flotillin 2 contents in exosomes compared with control siRNA treatment (Fig. 3A). Importantly, expression of siRNA-resistant wild-type SphK2 almost completely rescued the phenotype, however, expression of activity-negative mutant, SphK2(G248D), could not rescue it. These results strongly suggest that kinase activity of SphK2 is important and that S1P signal plays an important role in the cargo contents in exosomes. To strengthen the importance of kinase activity of SphK2 in this phenomenon, further pharmacological experiments were conducted. As inhibitors of SphK, DMS and HACPT (also called, SKI-II) were widely used to modulate SphK1 and SphK2 activities (10, 15). When K562 cells were treated with HACPT, the cargo contents in exosomes was strongly reduced (Fig. 3B) in consistence with gene-silencing results (Fig. 2B). Unexpectedly, DMS treatment increased the cargo contents (Fig. 3B). So far, we have presented evidence to show that SphK2 is required for the regulation of the cargo contents in exosomes (Figs. 1B, 2B, 3A, (5)). In this context, DMS action may elicit up-regulation of S1P signal on MVEs but not its inhibition.
Figure 3. SphK2 plays a role in cargo sorting into exosomal vesicles in an activity-dependent manner.
K562 cells transiently transfected with a vector encoding either siRNA-resistant HA-SphK2 or HA-SphK2(G248D) together with control or SphK2-siRNA were cultured for 48 hr. Exosomes were prepared as in Fig. 2B and analyzed for cargo contents by immunoblot analysis using anti-flotillin 2 antibody (A). K562 cells were cultured in exosome-depleted medium for 12 hr in the absence or presence of either 10 μM DMS or 50 μM HACPT. Exosomes were purified from the media and analyzed for cargo contents by immunoblot analysis using anti-TfR1, anti-HSP70, and anti-flotillin 2 antibodies (B).
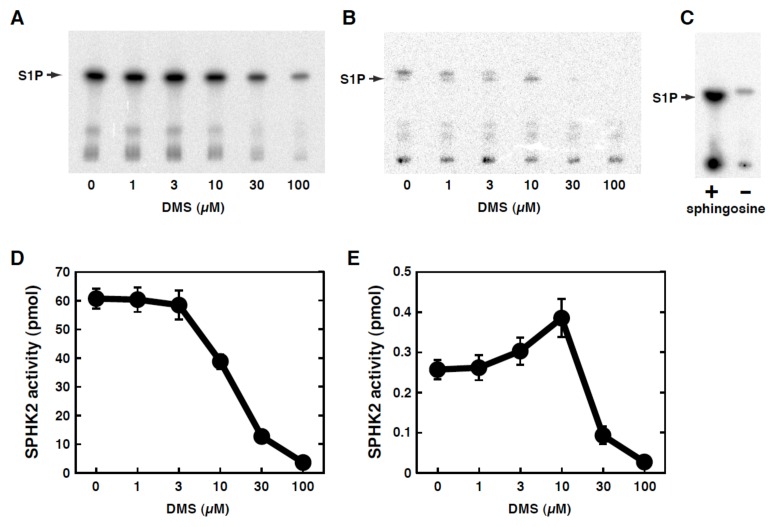
To examine this possibility, the effects of DMS on the kinase activity of SphK2 were studied in a purified in vitro system. DMS inhibited the SphK2 activity in a dose-dependent manner when the substrate sphingosine was at 100 μM (Fig. 4A, D), which are consistent with a previous report (10). Since the cellular concentrations of sphingosine were estimated to be around 100 nM (16), SphK2 activity was also measured with the lower concentrations of sphingosine (0.2 μM). Under these conditions there were doublet bands in the absence of DMS (Fig. 4B). The upper band existed in the absence of sphingosine (Fig. 4C) and was therefore not studied further in the present study. The lower band could be detected only in the presence of sphingosine and corresponded to the position of authentic S1P. Surprisingly, under these lower substrate concentrations DMS caused stimulation of the activity (1.5-fold increase) in the DMS concentrations up to 10 μM, then caused inhibition over the higher concentrations (Fig. 4B, E). These results suggest that DMS treatment of cells may cause SphK2-selective activation in certain circumstances, which may account for the phenomenon (Fig. 3B) that seems contradictory.
Figure 4. Effect of DMS on in vitro SphK2 activity.
Affinity-purified recombinant Sphk2 expressed in insect cells was assayed for its ability to phosphorylate sphingosine (100 μM (A, C, D), 0.2 μM (B, E) or absence (C) ) using [γ-32P]ATP in the presence of various concentrations of DMS as specified. After termination of the reaction, radio-labeled S1P was separated by thin-layer chromatography followed by autoradiography. One of the representative data from three independent experiments is shown (A). The bands corresponding to S1P were quantitated using a Fujix Bio-Imaging Analyzer. Data are means ± s.e.m. from 3 independent experiments (B).
DISCUSSION
In the present study we have characterized cellular distribution of SphKs using GFP-fused proteins. We have previously shown that GFP fusion to these enzymes itself did not cause any apparent changes in cellular distribution as compared with non-fused SphK1 (8) or SphK2 (17). We have also shown that SphK2 is involved in the regulation of cargo content in exosomes in K562 cells as demonstrated by the observation that gene-targeted knockdown of SphK2 resulted in decreased exosomal cargoes such as transferrin receptor, HSP70 and flotillin 2 (Fig. 2). This SphK2-downregulation-induced reduction of the cargo content was rescued by the expression of siRNA-resistant wild-type SphK2 but not by the kinase activity-negative mutant (Fig. 3), indicating the necessity of catalytic activity of SphK2 in this phenomenon. The present results that SphK2 knockdown resulted in the reduced cargo contents in exosomes (Fig. 2B) suggest two possibilities, i.e., inhibition of cargo sorting into exosomal vesicle of MVEs or inhibition of exosome formation. Judging from our previous studies that knockdown of SphK2 in HeLa cells caused a reduction of cargo contents in exosomes without changing the number of exosomes as analyzed by protein-lipid double labeling assay (5), it may be likely that SphK2 knockdown causes inhibition of cargo sorting into exosomal vesicles in K562 cells.
DMS has originally been shown that it is endogenously synthesized in A431 cells as a catabolite of ceramide (18), having dual actions, i.e., inhibition of protein kinase C and activation of src-kinase (19). Subsequently, it has been demonstrated that DMS was a potent inhibitor of SphK using both intact blood platelets and the lysates (20). More recently it has been shown that DMS inhibits SphK1 but activates SphK2 obtained from heart (21). These authors used gel filtration chromatography for the separation of two SphK activities from crude cytosolic fractions of the rat hearts, the first peak being SphK2 and the second peak corresponding to SphK1. In the present study we used purified recombinant SphK2 expressed in insect cells and confirmed that DMS indeed stimulated SphK2 with a low concentrations (physiological) of sphingosine (Fig. 4B, E). Collectively, our present results strongly suggest that SphK2 (through receptor-mediated S1P signaling) plays an important role in exosomal MVE maturation.
It has previously been demonstrated that DMS 1-phosphate (DMS-P) is produced upon stimulation of human platelets by thrombin or by 12-O-tetradecanoylphor 13-acetate (22). Further studies are necessary to study physiological role of DMS as well as pharmacological action of this sphingolipid metabolite.
ACKNOWLEDGEMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (C), a Grant-in-Aid for challenging Exploratory Research, a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan
REFERENCES
- 1.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 5.Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 8.Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol. 2007;27:3429–3440. doi: 10.1128/MCB.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275:19513–19520. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 11.Blom T, Bergelin N, Slotte JP, Tornquist K. Sphingosine kinase regulates voltage operated calcium channels in GH4C1 rat pituitary cells. Cell Signal. 2006;18:1366–1375. doi: 10.1016/j.cellsig.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi S, Okada T, Igarashi N, Fujita T, Jahangeer S, Nakamura S. Identification and characterization of RPK118, a novel sphingosine kinase-1-binding protein. J Biol Chem. 2002;277:33319–33324. doi: 10.1074/jbc.M201442200. [DOI] [PubMed] [Google Scholar]
- 13.Okada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S, Nakamura S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J Biol Chem. 2005;280:36318–36325. doi: 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Okada T, Hayashi S, Jahangeer S, Miwa N, Nakamura S. Delta-catenin/NPRAP (neural plakophilin-related armadillo repeat protein) interacts with and activates sphingosine kinase 1. Biochem J. 2004;382:717–723. doi: 10.1042/BJ20040141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Kim M, Kim N, D'Agati VD, Emala CWS, Lee HT. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 16.Mano N, Oda Y, Yamada K, Asakawa N, Katayama K. Simultaneous quantitative determination method for sphingolipid metabolites by liquid chromatography/ionspray ionization tandem mass spectrometry. Anal Biochem. 1997;244:291–300. doi: 10.1006/abio.1996.9891. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi Y, Kitamura K, Toyokuni T, Dean B, Fenderson B, Ogawass T, Hakomori S. A specific enhancing effect of N, N-dimethylsphingosine on epidermal growth factor receptor autophosphorylation. Demonstration of its endogenous occurrence (and the virtual absence of unsubstituted sphingosine) in human epidermoid carcinoma A431 cells. J Biol Chem. 1990;265:5385–5389. [PubMed] [Google Scholar]
- 19.Igarashi Y, Hakomori S, Toyokuni T, Dean B, Fujita S, Sugimoto M, Ogawa T, el-Ghendy K, Racker E. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry. 1989;28:6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 20.Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. 1996;35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- 21.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 22.Yatomi Y, Ozaki Y, Satoh K, Kume S, Ruan F, Igarashi Y. N, N-dimethylsphingosine phosphorylation in human platelets. Biochem Biophys Res Commun. 1997;231:848–851. doi: 10.1006/bbrc.1997.6207. [DOI] [PubMed] [Google Scholar]