Abstract
We reported a term newborn case of early onset sepsis caused by nontypeable Haemophilus Influenzae (NTHi) with massive bacterial invasion in the placenta. Based on the consistent results of maternal placental pathology and neonatal bacterial culture, we diagnosed this as vertical transmission of NTHi via vaginal delivery. In general, NTHi infections occur in preterm infants, and our term infant case is very unusual. In conclusion, clinicians should consider NTHi as a cause of neonatal sepsis, even in term infants.
Keywords: Haemophilus influenzae, nontypeable Hi, Early-onset sepsis, Newborn, term infant
INTRODUCTION
Early-onset sepsis (EOS) is the main cause of neonatal death and neurodevelopmental abnormalities among survivors (1). The incidence of EOS caused by Group B streptococcus infection declines after introduction of intrapartum antibiotic prophylaxis. However, this prevention strategy is not fully effective against other organisms, especially antimicrobial-resistant pathogens (2).
Haemophilus influenzae (Hi) is a gram-negative coccobacillus that can cause severe invasive disease in humans, and can be divided into either encapsulated (typeable) or unencapsulated (nontypeable) (3). Hi serotype b (Hib) is the most virulent and was responsible for more than 80% of all invasive Hi infection before the introduction of routine vaccination (3). In 2013, routine vaccination against Hib started for children in Japan, resulting in a marked decrease in its incidence (4). Since then, nontypeable Hi (NTHi) has become a more common colonizer. A Finnish study showed that neonates had a greater risk of invasive NTHi disease, suggesting vertical transmission from mothers (5). In Israel, where the routine vaccination with Hib conjugate vaccines was introduced in 1994 (6), a cluster of EOS and pneumonia caused by NTHi was reported after the introduction of Hib vaccination (7). Because maternal infection is associated with extremely premature birth (3), most EOS cases caused by NTHi were in preterm infants, and only one case of term infant have been reported from nationwide survey of England and Wales over a 5-year period (8). We describe a case of EOS caused by NTHi in a term infant born from the mother without positive vaginal culture or premature rupture of the membranes (PROM).
CLINICAL CASE
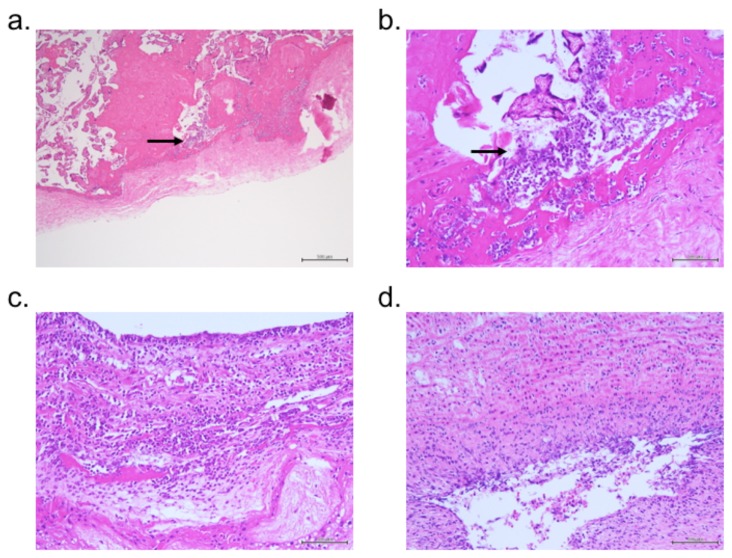
A 25-year-old woman with a history of spontaneous abortion, gravid 3, para 2, delivered a male infant at 37 and 2/7 weeks of gestation. The pregnant course was uncomplicated, except for low-grade fever (37.2°C) with fetal tachycardia (170–180bpm) at the day of delivery. There was no evidence of positive vaginal culture at 34 weeks of gestation or PROM. The male newborn was born by spontaneous vaginal delivery with a birthweight of 3.1 kg and Apgar scores of 9 at 1 min and 9 at 5 min. Amniotic fluid was stained with meconium. His mother had a fever and elevated C-reactive protein (CRP) level (>9 mg/dl) postpartum. During skin-to-skin contact, he developed dyspnea and cyanosis, and oxygen supplementation was started. He then gradually developed tachypnea and grunting. Laboratory data showed a mild increase in white blood cells (WBC, 10,500/μl) and CRP level (0.68 mg/dl). Five hours after birth, an apnea attack was observed and he was diagnosed with bacterial infection, and ampicillin (150mg/dose) was started. On 1 day age, his clinical conditions improved. However, growth of Hi like Gram-negative rods from an initial culture of blood, the nasal cavity, and the external auditory canal was observed, as well as an increase in WBC (19,800/μl) and CRP level (3.52 mg/dl). Therefore, the patient was transferred to our hospital for treatment of neonatal sepsis. On admission, he showed low-grade fever (37.6°C) and mild irritability. Laboratory findings showed elevated WBC (25,100/μl, neutrophils: 81.9%) and CRP level (2.71 mg/dl), but normal cerebrospinal fluid (CSF, 5 WBCs/mm3). Based on the respiratory symptoms and temperature instability, we speculated that the patient was complicated with pneumonia, despite nonspecific findings in the chest x-ray. We started cefotaxime (200mg/kg/day) and amikacin (15mg/kg/day) to cover Hi for 3 days until obtaining the report of identification and antimicrobial susceptibility testing of NTHi (APBC; Resistant, [R], CCL; R, CTX; Susceptible [S], CDTR; S, CFPN; R, A/S; R, MEPM; S, CAM; S, LVFX; S) from the previous hospital, then only cefotaxime was continued for 10 days. And the laboratory data gradually improved. NTHi was only grown from the external auditory canal, not other samples taken at our hospital (blood, throat, skin, CSF). A culture of a maternal vaginal swab taken 1 month before delivery was not positive for NTHi, but massive bacterial invasion was observed in the placenta with positive findings of chorioamnionitis and funisitis (Figure). He recovered completely without complication and was discharged on day 12.
Figure.
Histological view of the placenta using hematoxylin and eosin stain. A bacterial body (arrows; could not identified) had invaded the chorionic membrane (a, ×40; b, ×200). Inflammatory cells were found in the chorion (c, ×200) and umbilical artery (d, ×200).
DISCUSSION
NTHi is one of main causes of noninvasive upper respiratory tract infections in children, but it can also cause invasive disease in pregnant women. Moreover, although mortality of pregnant women due to NTHi is not high, NTHi infection during pregnancy is strongly associated with miscarriage or extremely premature birth (3). Importantly, Rusin and coworkers reported a stillbirth at 39 gestational weeks due to maternal Hi infection (9). NTHi usually colonizes the upper respiratory tract, but it can also colonize the female reproductive tract. Importantly, carriage studies showed that only 0.18% of pregnant women carry NTHi in their genital tract, but its prevalence in women with PROM is much higher (7.3%). This suggests a high case to carrier ratio, which is in contrast to group B Streptococci, which has a lower case to carrier ratio (3).
In this case, we could not detect NTHi from routine screening of maternal vaginal culture. And there is a possibility of hematogenous NTHi transmission to placenta from upper respiratory tract infection, though the mother did not show any respiratory symptoms (10, 11). However, it is reported that NTHi was identified only when present in moderate to large quantities (9). Thus, we assume that the quantities of NTHi which colonized maternal vagina in our case was not enough to be culture-positive. This small quantity might contribute to the mild initial symptoms of both mother and infant. We could diagnose transplacental vertical transmission of NTHi based on the placental pathology findings that revealed massive bacterial invasion into chorionic membrane. It must be emphasized that pathologic examination of the placenta can be a useful tool to confirm vertical infection of invasive NTHi.
Notably, our case was a term infant without PROM and his mother only had mild symptoms of a common cold at delivery. Though ascending infection from vagina is not popular in cases without RPOM, Kaneko et al. reported 10 NTHi sepsis in preterm infants whose mothers did not have the history of PROM from their literature reviews (12). Towers et al. reported that risk of EOS delivered from mothers with intrapartum fever or a diagnosis of clinical chorioamnionitis is low at 0.24% (13). Linder et al. reported that no cases of neonatal infection or mortality was detected among the 159 singleton full term neonates born from women with intrapartum fever in their retrospective study (14). On the other hands, the current recommendation regarding the management of a term newborn delivered from mother with intrapartum fever supported baseline laboratory test along with blood cultures, in addition to universal antibiotic treatment (15, 16).
Initial symptoms of this infant were dyspnea followed by an apnea attack with mild elevation of WBC and CRP levels, however, these clinical signs can occur to normal term newborns during their transitional period after birth (17, 18). On the other hands, most reported cases of neonatal NTHi sepsis are in preterm infants, and the manifestations of preterm sepsis are more severe and fatal (2, 7, 9, 19). Therefore, initial characteristics of EOS caused by NTHi in term infants might be mild or subclinical, and difficult to diagnose. Thus, we believe that conventional sepsis workup in neonates delivered from febrile mothers is important for early detection of EOS in term newborns.
The limitation of this study is that we could not identify the pathogen from our pathological examination, although a bacterial body that invaded the chorionic membrane was detected. Placental culture should be considered to detect transplacental infections in case of clinically suspected chorioamnionitis.
In conclusion, clinicians should consider NTHi as a cause of neonatal sepsis, even in term infants. Pathological examination of the placenta and sepsis workup in term neonates delivered from mother with intrapartum fever plays an important role for the prevention and treatment of NTHi EOS.
ACKNOWLEDGEMENTS
This work was partially supported by grants for Scientific Research from Shinryokukai (KF), the Mother and Child Health Foundation (KF), and the JSPS KAKENHI (Research Activity start-up grant number: 16H06971, KF and Scientific Research (B) grant number: 17H04341, IM). The authors gratefully acknowledge the staff of our institutions for the patient’s care.
REFERENCES
- 1.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 2.Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins S, Ramsay M, Slack MP, Campbell H, Flynn S, Litt D, et al. Risk of invasive Haemophilus influenzae infection during pregnancy and association with adverse fetal outcomes. JAMA. 2014;311:1125–1132. doi: 10.1001/jama.2014.1878. [DOI] [PubMed] [Google Scholar]
- 4.Shinjoh M, Yamaguchi Y, Iwata S. Pediatric bacterial meningitis in Japan, 2013–2015 - 3–5 years after the wide use of Haemophilus influenzae type b and Streptococcus pneumoniae conjugated vaccines. J Infect Chemother. 2017;23:427–438. doi: 10.1016/j.jiac.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Takala AK, Pekkanen E, Eskola J. Neonatal Haemophilus influenzae infections. Arch Dis Child. 1991;66:437–440. doi: 10.1136/adc.66.4_spec_no.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamberger EE, Ben-Shimol S, Abu Raya B, Katz A, Givon-Lavi N, Dagan R, et al. Pediatric invasive Haemophilus influenzae infections in Israel in the era of Haemophilus influenzae type b vaccine: a nationwide prospective study. Pediatr Infect Dis J. 2014;33:477–481. doi: 10.1097/INF.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 7.Hershckowitz S, Elisha MB, Fleisher-Sheffer V, Barak M, Kudinsky R, Weintraub Z. A cluster of early neonatal sepsis and pneumonia caused by nontypable Haemophilus influenzae. Pediatr Infect Dis J. 2004;23:1061–1062. doi: 10.1097/01.inf.0000143650.52692.42. [DOI] [PubMed] [Google Scholar]
- 8.Collins S, Litt DJ, Flynn S, Ramsay ME, Slack MP, Ladhani SN. Neonatal invasive Haemophilus influenzae disease in England and Wales: epidemiology, clinical characteristics, and outcome. Clin Infect Dis. 2015;60:1786–1792. doi: 10.1093/cid/civ194. [DOI] [PubMed] [Google Scholar]
- 9.Rusin P, Adam RD, Peterson EA, Ryan KJ, Sinclair NA, Weinstein L. Haemophilus influenzae: an important cause of maternal and neonatal infections. Obstet Gynecol. 1991;77:92–96. [PubMed] [Google Scholar]
- 10.Johnston JW, Apicella MA. Haemophilus Influenzae. In: Schaechter M, editor. Encyclopedia of Microbiology. Third ed. Amsterdam: Elsevier Inc; 2009. pp. 153–162. [Google Scholar]
- 11.Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis. 2014;14:1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko M, Yamashita R, Suzuki T, Kodama Y, Sameshima H, Ikenoue T. Early Onset Nontypable Haemophilus influenzae Sepsis in a Preterm Newborn. J Clin Case Rep. 2014;4:1000392. [Google Scholar]
- 13.Towers CV, Yates A, Zite N, Smith C, Chernicky L, Howard B. Incidence of fever in labor and risk of neonatal sepsis. Am J Obstet Gynecol. 2017;216:596 e591–596 e595. doi: 10.1016/j.ajog.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Linder N, Fridman E, Makhoul A, Lubin D, Klinger G, Laron-Kenet T, et al. Management of term newborns following maternal intrapartum fever. J Matern Fetal Neonatal Med. 2013;26:207–210. doi: 10.3109/14767058.2012.722727. [DOI] [PubMed] [Google Scholar]
- 15.American College of O, Gynecologists Committee on Obstetric P. ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2011;117:1019–1027. doi: 10.1097/AOG.0b013e318219229b. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Infectious D, Committee on F, Newborn. Baker CJ, Byington CL, Polin RA. Policy statement-Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–616. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 17.Waite SP, Thoman EB. Periodic apnea in the full-term infant: individual consistency, sex differences, and state specificity. Pediatrics. 1982;70:79–86. [PubMed] [Google Scholar]
- 18.Kelly DH, Shannon DC. Treatment of apnea and excessive periodic breathing in the full-term infant. Pediatrics. 1981;68:183–186. [PubMed] [Google Scholar]
- 19.Porter M, Charles AK, Nathan EA, French NP, Dickinson JE, Darragh H, et al. Haemophilus influenzae: a potent perinatal pathogen disproportionately isolated from Indigenous women and their neonates. Aust N Z J Obstet Gynaecol. 2016;56:75–81. doi: 10.1111/ajo.12413. [DOI] [PubMed] [Google Scholar]