Abstract
Objective
Endobronchial ultrasonography and guide sheath (EBUS-GS) technique has high diagnostic yield in lung nodules. Virtual bronchoscopic navigation (VBN) can lead bronchoscope to the target bronchi. The aim of this prospective study was to compare the diagnostic yield of two bronchoscopic procedures: bronchoscopy under EBUS-GS and VBN with or without x-ray fluoroscopy in small peripheral pulmonary lesions (PPLs, ≤30mm) with apparent CT-bronchus sign.
Methods
31 patients with PPLs which had apparent CT-bronchus sign were randomly assigned to the X-ray or the non-X-ray groups (18 with and 13 without fluoroscopy) between September 1, 2012, and September 30, 2015. A bronchoscope was introduced into the target bronchus using the VBN system. Sites of specimen sampling were verified using EBUS-GS with or without fluoroscopy.
Results
The overall diagnostic yield was 83.3% in the X-ray and 69.2% in the non-X-ray group. The diagnostic yield of malignancy was 88.2% and 81.8%, respectively. The duration of the examination and time elapsed until the first EBUS visualization were similar in the X-ray and the non-X-ray group (9.0 (5.8–20.) min vs 11.0 (5.3–17.3) min, and 2.5 (1.3–14.2) min vs 4.1 (1.4–8.1) min, respectively). The fluoroscopy exposure time was 3.7 (2.9–10.56) min. The only adverse event was mild pneumothorax in a patient from the non-X-ray group, who had consequent TBB under fluoroscopy.
Conclusions
There was a possibility that VBN-guided EBUS-transbronchial diagnosis without fluoroscopy might be equivalent to that under fluoroscopy. Further multi-center randomized study may be desired. (UMIN000008592)
Keywords: Endobronchial ultrasonography with a guide sheath (EBUS-GS), Virtual bronchoscopic navigation (VBN), Fluoroscopy, Lung cancer
INTRODUCTION
Broncoscopy is one of the diagnostic methods for small peripheral pulmonary lesions (PPLs), but the sensitivity of it was not satisfactory for small PPLs as it shows only 36% – 86% depending on the size of the lesion (1–3). Recent modifications of bronchoscopy with adjunct techniques, such as the use of endobronchial ultrasonography and guide sheath (EBUS-GS) technique and virtual bronchoscopic navigation (VBN), have increased the diagnostic yield of bronchoscopy.
VBN is reconstructed from CT data and is one of the methods to navigate a bronchoscope to the correct path to the target lesion (4). The bronshoscopic diagnostic yield with VBN was 63.3–81.6% (5–9). Radial-type (R) -EBUS is useful for identifying the location of the lesion in real time during the bronchoscopy of PPLs (10). On meta-analysis, the pooled sensitivity of R-EBUS for the detection of lung cancer in PPLs were 73% (95% CI 70–76%) (11). Furthermore, the combination of EBUS technique with a guide sheath (GS) was introduced by Kurimoto et al. The advantages of the EBUS-GS technique are that it provides access to bronchial lesions for repeated sampling via a GS placed in the lesion (12).The diagnostic yield with a combination of VBN and EBUS-GS was found to be between 77.9 and 84.4% for small PPLs (13–16).
Herth FJ et al reported EBUS-guided TBB is a safe and very effective method for PPLs that cannot be visualized by fluoroscopy (16). And we also demonstrated EBUS-GS–guided bronchoscopy without x-ray fluoroscopy is effective for diagnosing PPLs and the diameter, location, CT scan appearance of the PPLs, and the identification of the bronchus leading to the PPLs were valuable as factors related to a higher diagnostic sensitivity with this procedure (17). Eberhardt R et al showed their experience with another VBN system (Lung point®, Broncus Medical Inc., Mountain View, Calif., USA) without fluoroscopy at 80% diagnostic yield (18).
Thus, the present prospective study examines the value of VBN assisted EBUS-GS for diagnosing small solid PPLs with CT bronchus sign in the absence of x-ray fluoroscopy.
MATERIALS AND METHODS
This prospective study was approved by the institutional review board of Kobe University Graduate School of Medicine (# 240033) on August 20, 2012. This clinical trial was registered in UMIN(#000008592) on August 20, 2012. The protocol of bronchoscopic procedure was in accordance with the principles outlined in the Declaration of Helsinki of the World Medical Association. Written informed consent was obtained from all the patients prior to performing the procedure.
Patients
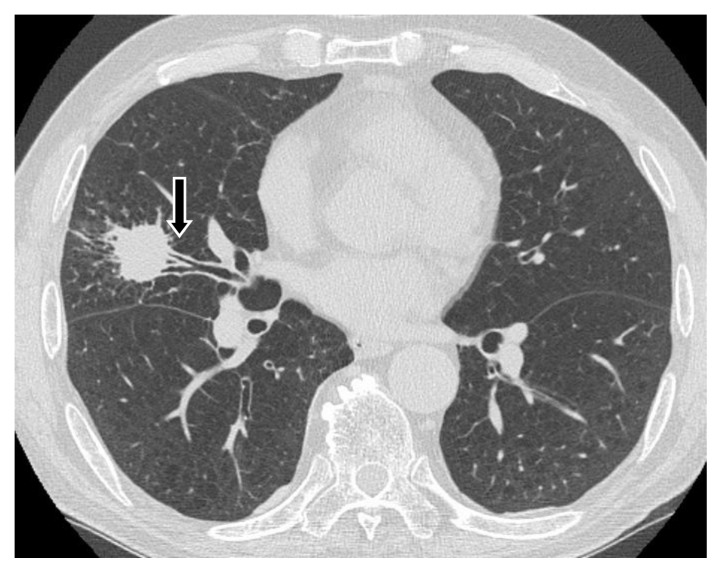
We enrolled consecutive patients who were referred to our hospital between September 2012 and September 2015 with solid PPLs (longest diameter ≤3 cm calculated from CT images with apparent CT bronchus sign) suspected to be cancer (Fig. 1). Peripheral pulmonary lesions were defined as those that are surrounded by normal lung parenchyma and thus unlikely to be visualized by bronchoscopy. Eligible patients were men and women ≥20 years old who could tolerate bronchoscopy. The exclusion criteria comprised percutaneous oxygen saturation <90%, a range of known severe co-morbid conditions (unstable angina, acute myocardial infarction within the past 3 months, severe asthma or uncontrolled pulmonary infection), pregnancy and unable to proceed without anticoagulant or antiplatelet medications. The lesions with ground-glass opacity confirmed by CT were excluded from the study.
Figure 1. CT bronchus sign.
Arrow shows CT bronchus sign.
Methods
Eligible patients were randomly assigned to X-ray or non-X-ray fluoroscopy groups. Randomization was based on lesion size (mean diameter <2 cm or 2–3 cm) used a randomized block design to ensure that this factors were balanced in the study arms. Independent, blinded, trial staff randomly assigned the patients before bronchoscopy. Scan data from multidetector chest CT (64-row; slice width, 0.5–1.0 mm) were acquired from all patients before bronchoscopy. Digital data of CT were transferred to workstation (Bf-NAVI®, Olympus Medical Systems, Tokyo, Japan) which VBN software automatically created virtual bronchoscopic images. VBN system was positioned beside the bronchoscopic screen in the endoscopy suite. Each patient was premedicated using 7.5 to 15 mg of pentazocine hydrochloride. All patients were locally anaesthetized with lidocaine and examined using a thin video-bronchoscope (type P260F; outer diameter, 4.0 mm; Olympus Medical Systems, Tokyo, Japan). Bronchoscopic insertion was assisted by the VBN system. Bronchoscopic insertion was assisted by the VBN system and fluoroscopy in the X-ray group. In the non-X-ray group, the bronchoscope was introduced without fluoroscopy and with the only VBN system. Lesions were visualized in both groups by inserting a 20 MHz mechanical radial-type EBUS probe (external diameter, 1.4 mm; UM-S20–17S; Olympus Medical Systems) with a guide sheath (K-201; Olympus Medical Systems) via a working channel. The GS-covered EBUS probe was introduced via the working channel of the bronchoscope and advanced to the PPL to obtain an EBUS image. Once a typical EBUS image could be seen, the probe was withdrawn from the sheath and the GS was left in place. A biopsy forceps or a bronchial brush was introduced through the GS to obtain pathologic specimens. These procedures were performed without fluoroscopy in the non-X-ray group. When an EBUS image could not be obtained, we terminated the protocol study and made the shift to examination under fluoroscopy.
The primary end point was bronchoscopic diagnostic yield and the secondary end point was total examination time, the interval until EBUS first visualization, duration of fluoroscopy and safety.
Statistical analysis
Sample size was calculated based on the primary end point. The estimated diagnostic yields in the X-ray and the non-X-ray group based on published records were 75% and 65%, respectively. Thus, at 80% power andα=0.05, we calculated that 65 patients would be required in each group. We planned to enroll 140 patients to account for incomplete data or undiagnosed patients. We analyzed the diagnostic yield and safety of the entire intent-to-treat population. Primary and secondary variables were analyzed using the Pearsonχ2 test and the Manne Whitney U test. Results are shown as median (range). For all statistical analyses, a p-value of <0.05 was considered to indicate a significant difference.
RESULTS
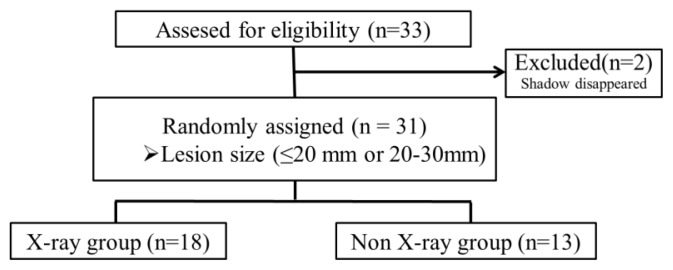
Estimated sample size was 140, but we couldn’t accumulate appropriate cases in the planned periods. We finished this study in the planned duration. 31 patients with small peripheral pulmonary lesions were randomly assigned to the X-ray or the non-X-ray groups (18 with and 13 without fluoroscopy) (Fig. 2).
The baseline patient characteristics are shown in Table I. Age, sex, lesion size and bronchial generation estimated by VBN in patients at baseline were similar between the groups. The proportions of lung cancer and non-malignant diseases were similar between the groups. Non-malignant diseases comprised one non-tuberculous mycobacterial infection and two inflammatory nodules. Bronchoscopically undiagnosed patients underwent re-bronchoscopy, CT-guided FNA, video-assisted thoracoscopy, or follow-up. If the nodule didn’t grow larger in two years, we judged it inflammatory nodule. Images of VB accorded well with actual bronchoscopic images in both groups.
Table I.
Baseline characteristics and final diagnoses
| Characteristics | X-ray group (n=18) | non-X-ray group (n=13) |
|---|---|---|
| Gender male/female | 11/7 | 8/5 |
| Age (years, median; range) | 73 (60–85) | 71 (60–82) |
| Lesion size(mm, median; range) | 22 (15–30) | 19 (12–30) |
| ≤20 mm, n (%) | 6 (33.3) | 7 (53.8) |
| ›20 to ≤30 mm, n (%) | 12 (66.7) | 6 (46.2) |
| Bronchial generation(n, median; range) | 5 (3–6) | 5 (3–6) |
| Final diagnosis | ||
| Lung cancer, n (%) | 17 (94.4) | 11 (84.6) |
| Benign lesion, n (%) | 1 (5.6) | 2 (15.4) |
The diagnostic yield was 83.3% (15 of 18 PPLs) in the X-ray group and 69.2% (9 of 13PPLs) in the non-X-ray group (Table II). Among lung cancer, the diagnostic yield was 88.2% (15 of 17 PPLs) and 81.8% (9 of 11PPLs), respectively. No significant differences were found in the diagnostic rate between the two groups.
Table II.
Bronchoscopic outcomes
| X-ray group | non-X-ray group | P value | |
|---|---|---|---|
| Over all diagnostic yield (n, %) | 15 (83.3) | 9 (69.2) | 0.31 |
| ≤20 mm, n (%) | 4/6 (66.7) | 5/7 (71.4) | |
| >20 to ≤30 mm, n (%) | 11/12 (91.7) | 4/6 (66.7) | |
| Diagnostic yield of malignant lesion (n, %) | 15/17 (88.2) | 9/11 (81.8) | 0.52 |
| EBUS image (n, %) | – | ||
| within | 15 (83.3) | 7 (53.8) | |
| adjacent to | 2 (11.1) | 4 (30.8) | |
| invisible | 1 (5.6) | 2 (15.4) | |
| Sampling by biopsy, (n, median, range) | 3 (3–5) | 3 (0–5) | 0.82 |
| Sampling by brushing (n, median, range) | 2 (2) | 2 (2) | 0.81 |
| Total examination (min, median, range) | 9.0(5.8–20.0) | 11.0(5.3–17.3) | 0.76 |
| Duration to the first EBUS visualization (min, median, range) | 2.5 (1.3–14.2) | 4.1 (1.4–8.1) | 0.66 |
| X-ray fluoroscopy exposure (min, median, range) | 3.7 (2.9–10.6) | 0 | – |
The rate of visualization by EBUS was 17/18 (94.4%) in the X-ray vs 11/13 (84.6%) in the non-X-ray group. Diagnostic yield did not differ according to lesion size between the two groups. Numbers of biopsies and brushings did not differ significantly between the groups. Total examination duration 9.0 (5.8–20.0) vs 11.0 (5.3–17.3) min; p=0.76) and the duration to the first EBUS visualization 2.5 (1.3–14.2) vs 4.1 (1.4–8.1) min; p=0.66) was slightly shorter in the X-ray than in the non-X-ray group, but there was no significant difference. The duration of fluoroscopy exposure was 3.7 (2.9–10.6) min in the X-ray group. The only adverse event was mild pneumothorax that did not require chest drainage in a patient from the non-X-ray group, who had consequent TBB under fluoroscopy. It was not certain that when pneumothorax was occurred, but we confirmed that there was no evidence that pneumothorax after sampling in absence of fluoroscopy.
DISCUSSION
This is the first prospective, randomized study to try to attest to the usefulness of combined techniques with VBN and EBUS-GS without x-ray fluoroscopy for small PPLs. But, with sincere regret, we couldn’t achieve this study. Even though it was a small sample size, the findings showed that there was a possibility that VBN-guided EBUS-transbronchial diagnosis without fluoroscopy may be feasible method. And it might be equivalent to that under fluoroscopy and was performed safely under EBUS-GS guidance without fluoroscopy.
This present study showed the overall diagnostic yield was 83.3% in the X-ray group and 69.2% in the non-X-ray group. Inconclusive histologic results, such as nonspecific inflammation, were analyzed as nondiagnostic in the present study. So, the prevalence of inflammatory lesions influences the overall diagnostic yield in our small sample study. Among the malignancy, the diagnostic yield was 88.2% and 81.8%, respectively. The diagnostic yield seemed to be equivalent in malignant lesions, but it was easier to gain EBUS “within” image in the X-ray group than that in non-X-ray group with no significant difference. Previous reports have indicated the probe position was an important factor in predicting the diagnostic yield of EBUS-GS-guided TBB (19, 20). The “within” PPL probe position was associated with a successful diagnosis using radial EBUS. If we gain EBUS “adjacent to the lesion” image, we try to acquire “within” image. To do so, we might need to confirm the positional relation of EBUS probe and lesion by fluoroscopy.
On the other hand, fluoroscopy is known to be unreliable in the identification of small lesions. The lesion size (≤20 mm or ≥20 mm) may be one of factors affecting PPL visualization by fluoroscopy. In the present study, a lesion size of >20 mm were found to associated with the higher diagnostic yield, especially in X-ray group. In non-X-ray group, there was no difference between the sizes of the lesion.
We examined the duration of total examination time and the interval to the first EBUS visualization. In the non-X-ray group, they were elevated by 2 min. Since only reliable method in the identification of PPLs was EBUS in the non-X-ray group, it was fact that we became more careful in all the procedure. To shorten examination time as much as possible is thought to be significant in terms of patient comfort under local anaesthesia.
Some reports reported that radiation exposure from fluoroscopy used together with EBUS does not pose a clinical problem (21, 22). However, we found that radiation exposure accounted for 40% of the total duration of the examination. It can do no better than reduce fluoroscopic exposure. EBUS-GS guided TBB has a potential limitation that it is not “real-time” guidance, because the ultrasonic probe must be removed from the bronchoscope to introduce biopsy forceps after the target lesion is localized. We couldn’t see specimen sampling to confirm proper device use, such as forceps opening and cutting, as well as brushing at adequate sites. The only affirmation method is thought to be using fluoroscopy. In fact, we could not help having extreme stress to perform bronchoscopy without fluoroscopy.
The current study has several limitations. The most major limitations was small sample size. The reason why we couldn’t accumulate planned cases was, 1) it was performed in a single center, 2) we selected patients which would have EBUS “within” image with care.
In conclusion, VBN-guided EBUS-transbronchial diagnosis without fluoroscopy was feasible method. There was a possibility that it might be equivalent to that under fluoroscopy, without the accompanying radiation exposure. To prove this concept clearly, further multi-center randomized study may be desired.
Figure 2. CONSORT flow diagram.
The flow of patients enrolled in the study. During the study period, 33 patients were assessed for eligibility. Two patients were excluded and finally 31 patients were included in this study.
ACKNOWLEDGEMENTS
We thank all the physicians and broncoscopists (Daisuke Hazama, Ryota Dokuni, Kanoko Umezawa, Nanako Miwa, Tomomi Terashita, Kei Kunimasa, Yoshitaka Kawa, Yumiko Ishikawa and Suya Hori) and endoscopy suite personnel involved in this trial for their cooperation and recruitment of patients.
REFERENCES
- 1.Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123:115S–128S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 3.Yung RC. Tissue diagnosis of suspected lung cancer: selecting between bronchoscopy, transthoracic needle aspiration, and resectional biopsy. Respir Care Clin N Am. 2003;9:51–76. doi: 10.1016/s1078-5337(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 4.Asano F, Matsuno Y, Matsushita T, Seto A. Transbronchial diagnosis of a pulmonary peripheral small lesion using an ultrathin bronchoscope with virtual bronchoscopic navigation. J Bronchol. 2002;9:108–111. [Google Scholar]
- 5.Asano F, Matsuno Y, Shinagawa N, Yamazaki K, Suzuki T, Ishida T, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest. 2006;130:559–66. doi: 10.1378/chest.130.2.559. [DOI] [PubMed] [Google Scholar]
- 6.Shinagawa N, Yamazaki K, Onodera Y, Asano F, Ishida T, et al. Virtual bronchoscopic navigation system shortens the examination time – feasibility study of virtual bronchoscopic navigation system. Lung Cancer. 2007;56:201–206. doi: 10.1016/j.lungcan.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Tachihara M, Ishida T, Kanazawa K, Sugawara A, Watanabe K, et al. A virtual bronchoscopic navigation system under X ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer. 2007;57:322–327. doi: 10.1016/j.lungcan.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Omiya H, Kikuyama A, Kubo A, Okishio K, Kawaguchi T, et al. A feasibility and efficacy study on bronchoscopy with a virtual navigation system. J Bronchology Interv Pulmonol. 2010;17:11–18. doi: 10.1097/LBR.0b013e3181cc3c86. [DOI] [PubMed] [Google Scholar]
- 9.Asano F, Shinagawa N, Ishida T, Shindoh J, Anzai M, et al. Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med. 2013;188:327–333. doi: 10.1164/rccm.201211-2104OC. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–974. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 11.Steinfort DP, Khor YH, Manser RL, Irving LB. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37:902–910. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 12.Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126:959–965. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 13.Asano F, Matsuno Y, Tsuzuku A, Anzai M, Shinagawa N, et al. Diagnosis of peripheral pulmonary lesions using a bronchoscope insertion guidance system combined with endobronchial ultrasonography with a guide sheath. Lung Cancer. 2008;60:366–373. doi: 10.1016/j.lungcan.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66:1072–1077. doi: 10.1136/thx.2010.145490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oshige M, Shirakawa T, Nakamura M, Mineshita M, Kurimoto N, et al. Clinical application of virtual bronchoscopic navigation system for peripheral lung lesions. J Bronchology Interv Pulmonol. 2011;18:196–202. doi: 10.1097/LBR.0b013e3182198f24. [DOI] [PubMed] [Google Scholar]
- 16.Herth FJ, Eberhardt R, Becker HD, Ernst A. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest. 2006;129:147–150. doi: 10.1378/chest.129.1.147. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa M, Sukoh N, Yamazaki K, Kanazawa K, Fukumoto S, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest. 2007;131:1788–1793. doi: 10.1378/chest.06-2506. [DOI] [PubMed] [Google Scholar]
- 18.Eberhardt R, Kahn N, Gompelmann D, Schumann M, Heussel CP, Herth FJ. Lung Point -new approach to peripheral lesions. J Thorac Oncol. 2010;5:1559–1563. doi: 10.1097/JTO.0b013e3181e8b308. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya M, Okamoto N, Sasada S, Shiroyama T, Morishita N, et al. Diagnostic yield of combined bronchoscopy and endobronchial ultrasonography, under LungPoint guidance for small peripheral pulmonary lesions. Respirology. 2013;18:834–839. doi: 10.1111/resp.12095. [DOI] [PubMed] [Google Scholar]
- 20.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2017;132:603–608. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 21.Steinfort DP, Einsiedel P, Irving LB. Radiation dose to patients and clinicians during fluoroscopically-guided biopsy of peripheral pulmonary lesions. Respir Care. 2010;55:1469–1474. [PubMed] [Google Scholar]
- 22.Katsurada M, Izumo T, Nagai Y, Chavez C, Kitagawa M, et al. The dose and risk factors for radiation exposure to medical staff during endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions under X-ray fluoroscopy. Jpn J Clin Oncol. 2014;44:257–262. doi: 10.1093/jjco/hyt224. [DOI] [PubMed] [Google Scholar]