Abstract
Aim
This study aimed to explore novel metabolite biomarker candidates for screening oral squamous cell carcinoma (OSCC).
Patients & Methods
We collected plasma samples from 48 patients with OSCC and 29 with an oral disease and conducted a plasma metabolomics analysis of patients with OSCC using gas chromatography mass spectrometry. Then, we used the cross-validation procedure to ensure the accuracy of biomarker candidates.
Results
We selected four biomarker candidates against OSCC. Their sensitivity was more than 90%, and the AUC was over 0.9 according to the receiver operating characteristic curve analysis.
Conclusions
The findings of this study suggest four potential metabolites as biomarkers for OSCC screening.
Keywords: Biomarker, Oral squamous cell carcinoma, Metabolomics, Plasma
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the leading cancer of the oral cavity, and the number of patients with OSCC has apparently increased in recent years due to growth of an aging society. Despite the advancements in diagnostic and therapeutic technology, the 5-year survival rate of OSCC remains at approximately 50% (1,2). However, if OSCC is detected at an early stage, its 5-year survival rate is approximately 80%–90%. Therefore, detecting OSCC at an earlier stage is imperative for patients’ survival and sustenance of their quality of life postoperatively. The examination of OSCC is primarily conducted by inspection; however, it is difficult to distinguish a potentially malignant disorder in the oral cavity, thereby increasing the risk of transition to malignant tumor. In addition, early detection might be affected by the clinical experience of a diagnostician. Although SCC antigen is common in the OSCC screening, its sensitivity is as low as 30% (3), which is not useful for screening. Therefore, the development of a simple, objective, and effective screening method for OSCC is a necessity.
In recent years, metabolomics analysis, a technique to comprehensively analyze metabolites, has gained widespread attention for assessing metabolite profiles facilitating the evaluation of genotype–phenotype relationships owing to its proximity to physiological functions and pathological characteristics (4). Therefore, metabolomics analysis has found utility in the research of disease-specific biomarkers, such as gastroenterological cancer and lung cancer, (5,6) among others. In this analysis, blood, urine, saliva, and tissue are often used as sample types. Of these, blood samples are highly reliable because these can be collected in a well-controlled state. A blood sample can be further categorized into serum and plasma samples. Perhaps, the difference in the metabolite profiles of serum and plasma samples correlates with the difference in the sample collection process. In the process of serum separation, differences are reported in the characteristics of the metabolites level in the plasma because of the inclusion of metabolites derived from blood cells released from the coagulation cascade (7,8). Yu et al. reported (7) that the reproducibility of both plasma and serum metabolite data was good; however, plasma samples displayed better reproducibility than serum samples. The aim of this study is to identify metabolite biomarker candidates to facilitate the OSCC screening method. Thus, we performed a plasma metabolomics analysis of patients with OSCC by using gas chromatography mass spectrometry (GC/MS).
MATERIALS AND METHODS
Participants
In this study, we enrolled 48 patients with OSCC. Of note, we excluded patients with a history of a malignant tumor, metabolic disease, or endocrine disease from this study. The control plasma samples were collected from 29 patients who underwent surgery for oral diseases (OD), and the patients with a history of a malignant tumor, metabolic disease, or endocrine disease were not included in the OD group. The OD group included bone cyst (N = 13), periodontitis (N = 5), bone fracture (N = 5), osteomyelitis of jaw (N = 3), and dental implant (N = 3). The plasma samples of patients with OSCC and an OD were collected at Kobe University Hospital from 2015 to 2017. In consideration of the postoperative effects of surgery under general anesthesia, we considered that the patients returns to the normal states at one week after surgery, so one week after surgery was set as the postoperative blood sampling timing. In the case of observing the postoperative complications, the blood sampling at 1 week after surgery was not performed, and the postoperative blood collection was carried out after healing. This study was approved by the Ethics Committee of Kobe University Graduate School of Medicine (Hyogo, Japan; No. 1781). We obtained written informed consent from all participants, and human samples were used in accordance with the guidelines of Kobe University Hospital.
Sample collection and preparation
We collected blood samples in the morning after fasting, which were then transferred into blood-collecting bottles containing EDTA-2Na as an anticoagulant. In the same way, we also collected blood samples in the morning after fasting at one week after surgery. After gentle mixing, the collected blood samples were stored at 4°C for 10 min, followed by centrifugation within 6 h after blood collection. The plasma was obtained by centrifugation at 2,270 × g for 15 min at 4°C, and all plasma samples were stored at −80°C. Then, we performed the extraction and derivatization of low molecular weight metabolites, as described our previously (8). First, we mixed 50 μL of plasma with 250 μL of methanol. We used 10 μL of 0.5 mg/mL 2-isopropylmalic acid dissolved in distilled water as an internal standard. Then, we shook the mixture at 1,200 rpm for 30 min at 37°C, before centrifuging at 19,300 × g for 5 min at 4°C. After that, we transferred 225 μL of the supernatant into a new 1.5-mL tube and freeze-dried it overnight. For derivatization, we added 80 μL of methoxyamine (20 mg/mL pyridine) into freeze-dried samples. The mixture was incubated at 1,200 rpm for 90 min at 30°C, and added 40 μL of MSTFA later. After that, we incubated the mixture at 1,200 rpm for 30 min at 37°C, before centrifuging at 19,300 × g for 5 min at 20°C. Finally, we collected the supernatant in a GCMS vial.
GC/MS analysis
According to our previously described method (4), GC/MS analysis was performed using a GCMS-QP2010 Ultra (Shimadzu Co., Kyoto, Japan) with a fused silica capillary column (CP-SIL 8 CB low bleed/MS; inner diameter, 30 mm×0.25 mm; film thickness, 0.25μm; Agilent Co., Palo Alto, CA, USA). The front inlet temperature was 230°C, and the flow rate of helium gas through the column was 39.0 cm/s. The column temperature was maintained at 80°C for 2 min, which was then increased by 15°C/min to 330°C and held for 6 min. The transfer line and ion-source temperatures were 250°C and 200°C, respectively. We recorded 20 scans/s over the mass range 85–500 m/z using the Advanced Scanning Speed Protocol (Shimadzu Co).
Data processing
We performed the data processing as described previously (4). The MS data were exported in the netCDF format, and the peak detection and alignment were performed using the MetAlign software (Wageningen UR, the Netherlands). The resulting data were exported into the CSV format and analyzed with in-house analytical software (AIoutput), which enables peak identification and semi-quantification using an in-house metabolite library. For semi-quantification, we calculated the peak height of each ion and normalized to that of 2-isopropylmalic acid as an internal standard (4).
Statistical analysis
In this study, the P values for age and BMI were calculated using the Student’s t-test, and sex distribution was calculated using the χ2 test. The statistical difference of the metabolites level was performed by the Student’s t-test for comparisons between patients with OSCC and patients with OD, or by the Wilcoxon signed-rank test for comparisons between OSCC patients’ pre- and postoperative plasma samples. We considered P < 0.05 as statistically significant. Ratios of the level of a particular metabolite in patients with OSCC to that in patients with OD are highlighted as the fold induction value.
We used two approaches in an attempt to validate the performance of biomarker candidates. First, we extracted metabolites with remarkable fold changes and statistically significant difference between patients with OSCC and patients with OD by using the volcano plot. Metabolites with fold induction above ±1.5 (log2(±1.5) = ±0.58) on the x-axis and P < 0.05 (−log10(0.05) = 1.3) on the y-axis were selected as biomarker candidates with significant differences between the OSCC and OD groups. Then, we split the whole data randomly into the training set (50%) and validation set (50%) in each OSCC and OD group. The training set comprised 24 patients with OSCC and 15 patients with OD. In addition, we used the receiver operating characteristic (ROC) curve analysis to evaluate the diagnostic performance of biomarker candidates on the AUC, sensitivity, and specificity. We used the training set to develop the robust and optimal biomarker candidate using the five-fold cross-validation technique. Among metabolites with the AUC of 0.9–1.0 were considered having a high predictive ability (10), and metabolites with the sensitivity of >90% were considered as final biomarker candidates. As OSCC is a rare cancer by accounting for only about 1% of all cancer types, from the idea of developing a screening method of OSCC, the sensitivity was considered a priority index than the specificity in this study. Furthermore, the ability of biomarker candidates was tested in the validation set by using the ROC curve analysis. These analyses were performed by using JMP 13.2 (SAS Institute Inc.).
RESULTS
Participant characteristics
We collected plasma samples from 48 and 29 patients with OSCC and OD, respectively. Notably, patients with OSCC were only those who were diagnosed histopathologically with SCC. Patients with OD comprised those who underwent general anesthesia surgery for bone cyst (N = 13), periodontitis (N = 5), bone fracture (N = 5), osteomyelitis of jaw (N = 3), and dental implant (N = 3). We classified patients with OSCC using the TNM classification of the Union for International Cancer Control. Table I summarizes the clinical characteristics of the study population. Patients with OSCC comprised clinical stage I (N = 9), II (N = 10), III (N = 11), and IV (N = 18), implying that 19 patients were in early stage. Comparing the characteristics of patients with OSCC and OD, no significant difference was observed in age and sex. The OSCC group tended to have a lower BMI compared to the OD group, implying the advanced stage of patients with OSCC who often suffered from eating disorder because of cancer pain and often exhibited weight loss. We selected patients with OD as the controls because their nutritional conditions and surgical invasion are similar to those of patients with OSCC, and moreover we can more precisely determine the relevance of cancer presence and absence for metabolite alterations.
Table I.
| Characteristics | OSCC | OD | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 23 | 14 | ||
| Male | 25 | 15 | 0.1976 | |
| Age | Mean | 66.3 | 60.3 | 0.0976 |
| BMI | Mean | 21.8 | 24.1 | 0.0196 |
| Primary site | ||||
| Tongue | 21 | |||
| Gingiva | 19 | |||
| Buccal mucosa | 8 | |||
| Stage*** | ||||
| I | 9 | |||
| II | 10 | |||
| III | 11 | |||
| IV | 18 | |||
OSCC: Oral squamous cell carcinoma
OD: Oral disease
According to the TNM Classification of Malignant Tumors
No difference (P > 0.05) in sex and age between patients with OSCC and OD. The BMI score tends to be lower in patients with OSCC. P values for age and BMI were calculated using the Student’s t-test, and sex distribution was calculated using the χ2 test.
Exploring biomarker candidates for OSCC by GC/MS
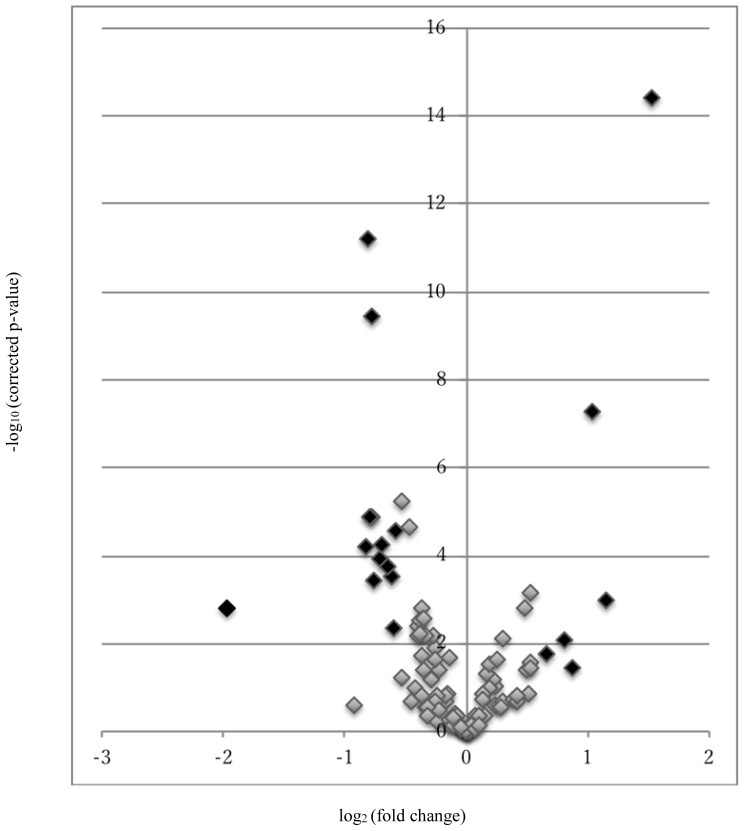
In our GC/MS-based metabolite analysis system, which primarily targeted water-soluble metabolites, we detected 100 metabolites patients’ plasma (data not shown). Among 100 detected metabolites, we used 2-isoproplylmalic acid as an internal standard. The plasma level of 44 of 100 metabolites was significantly different when between patients with OSCC and OD (P < 0.05; Table II). In this study, we plotted the volcano plot of metabolites to detect metabolites that were highly contributing to the distinction between patients with OSCC and OD (Fig. 1). Based on the volcano plot, we selected the following metabolites with fold induction above ±1.5 and P < 0.05 as biomarker candidates: oxalate, sarcosine, 3-hydroxyisovaleric acid, benzoic acid, β-alanine, creatinine, threo-β-hydroxyaspartic acid, phthalic acid, 5-dehydroquinic acid, glucose, sebacic acid, galactose, galactosamine, glucarate, urea, nonanoic acid, hypoxanthine, and cysteine + cystine. In addition, we used the cross-validation technique to determine the robust and optimal biomarker candidates and elucidate how accurately a predictive model will perform in practice.
Table II.
A list of metabolites that were significantly different (P < 0.05) between patients with OSCC and OD
| Compound Name | Fold induction | p-value |
|---|---|---|
| Pyruvate+Oxalacetic acid | 0.76 | 0.0039 |
| Lactic acid | 0.69 | <0.0001 |
| Glycolic acid | 0.84 | 0.0120 |
| Alanine | 0.77 | 0.0016 |
| Oxalate | 0.65 | 0.0003 |
| Sarcosine | 0.56 | 0.0001 |
| 2-Aminoisobutyrate | 1.39 | 0.0015 |
| 3-Hydroxyisovaleric acid | 1.74 | 0.0085 |
| Urea | 0.25 | 0.0016 |
| Benzoic acid | 0.58 | <0.0001 |
| Oxamic acid | 1.12 | 0.0496 |
| Glycerol | 1.40 | 0.0388 |
| Phosphate | 0.90 | 0.0205 |
| Nonanoic acid | 2.88 | <0.0001 |
| β-Alanine | 0.61 | 0.0001 |
| Methionine | 1.45 | 0.0254 |
| Creatinine | 1.83 | 0.0369 |
| Phenylalanine | 1.14 | 0.0289 |
| Threo-β-Hydroxyaspartic acid | 0.66 | 0.0044 |
| Lauric acid | 1.44 | 0.0007 |
| Ribose | 0.78 | 0.0193 |
| Phthalic acid | 0.62 | 0.0001 |
| Methoxy-4-Hydroxyphenylacetate | 0.86 | 0.0400 |
| O-Phosphoethanolamine | 0.77 | 0.0029 |
| Glycyl-Glycine | 0.72 | <0.0001 |
| Citric acid+Isocitric acid | 1.23 | 0.0075 |
| Ornithine | 0.78 | 0.0026 |
| Hypoxanthine | 2.22 | 0.0010 |
| Tagatose | 0.78 | 0.0408 |
| Mannose | 1.19 | 0.0246 |
| 5-Dehydroquinic acid | 0.64 | 0.0002 |
| Glucose | 0.59 | <0.0001 |
| Sebacic acid | 0.58 | <0.0001 |
| Galactose | 0.57 | <0.0001 |
| Lysine | 0.82 | 0.0065 |
| Galactosamine | 0.59 | 0.0004 |
| Glucuronate | 0.80 | 0.0069 |
| Ascorbic acid | 0.76 | 0.0068 |
| Cysteic acid | 0.84 | 0.0233 |
| 1-Hexadecanol | 1.59 | 0.0178 |
| Glucarate | 0.67 | <0.0001 |
| Dopa | 0.76 | 0.0063 |
| Elaidic acid | 1.44 | 0.0352 |
| Cysteine+Cystine | 2.06 | <0.0001 |
The values are shown as fold induction values (the peak intensity of patients with OSCC divided by that of patients with OD). If the fold induction shows lower than 1, metabolite level is lower in patients with OSCC than patients with OD. P values were calculated with the Student’s t-test.
Figure 1.
The Volcano plot of metabolites.
The volcano plot of log2(fold induction) (X-axis) versus −log10(corrected P) (Y-axis). Metabolites with fold induction above ±1.5 (log2(±1.5)= ±0.58) on the X-axis and P < 0.05 (−log10(0.05)=1.3) on the Y-axis were shown in black plot.
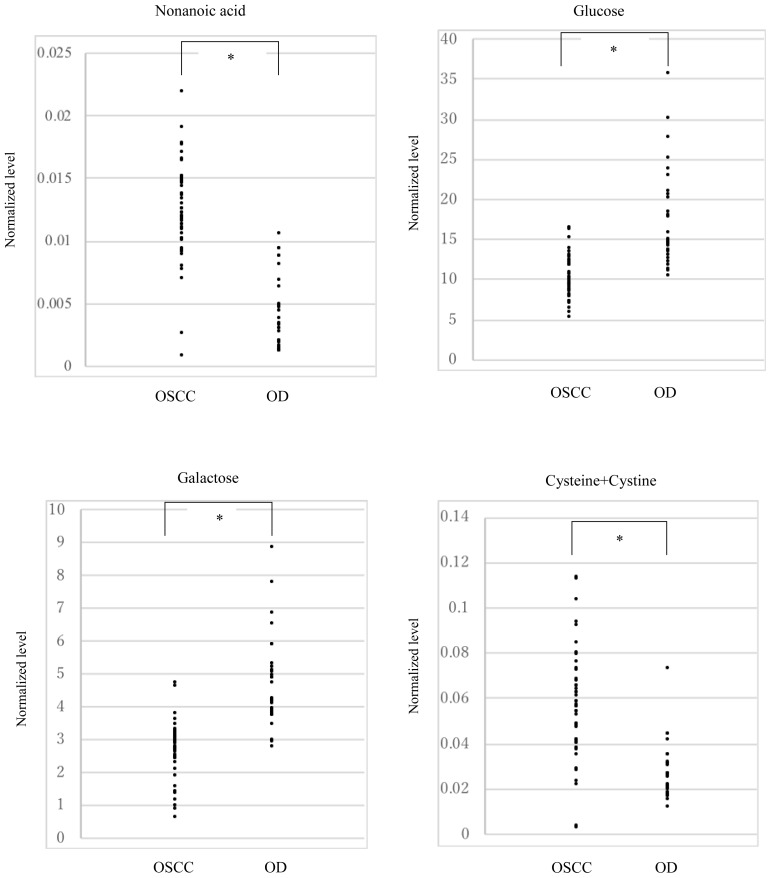
We calculated the AUC as well as the sensitivity/specificity in the training set. Table III presents the results of five-fold cross-validation method and suggests that four metabolites, nonanoic acid, glucose, galactose, and cysteine + cystine, demonstrated high predictivity and sensitivity (AUC, 0.9–1.0; sensitivity, >90%). Then, we tested the biomarker candidates from the training set in the validation set using the ROC curve analysis (Table IV). Figure 2 also showed the distribution of the levels of each 4 biomarker candidate in patients with OSCC and OD. The performance of the training and validation sets exhibited almost similar tendency with AUC (≥0.85, which was a high value), except cysteine; however, despite being somewhat low as 0.77, cysteine demonstrated moderate accuracy as a biomarker candidate. Each metabolite in patients with OSCC and OD was compared between the pre- and postoperative plasma using the Wilcoxon signed-rank test (Table V). Each biomarker candidate exhibited significant differences between pre- and postoperation in patients with OSCC (P < 0.05; Table IV). While the metabolite levels of glucose and galactose increased, those of nonanoic acid and cysteine + cystine decreased compared to that preoperatively. To determine whether four biomarker candidates were also effective in the early stage of OSCC, 19 patients with early-stage OSCC (stages I and II) among 48 patients with OSCC were compared to 29 patients with OD (Table VI). While the sensitivity for glucose, galactose, and cysteine + cystine was ≥85%, that for nonanoic acid was 79%.
Table III.
Performance of OSCC biomarker candidates in the training set and result of five-fold cross-validation
| Training set | |||
|---|---|---|---|
|
| |||
| AUC | Sensitivity | Specificity | |
| Oxalate | 0.783 | 0.750 | 0.733 |
| Sarcosine | 0.678 | 0.958 | 0.333 |
| 3-Hydroxyisovaleric acid | 0.778 | 0.792 | 0.800 |
| Urea | 0.728 | 0.792 | 0.667 |
| Benzoic acid | 0.819 | 0.833 | 0.800 |
| Nonanoic acid | 0.978 | 0.917 | 0.933 |
| β-Alanine | 0.750 | 0.583 | 0.867 |
| Creatinine | 0.631 | 0.500 | 0.867 |
| Threo-β-Hydroxyaspartic acid | 0.803 | 0.708 | 0.933 |
| Phthalic acid | 0.783 | 0.750 | 0.733 |
| Hypoxanthine | 0.800 | 0.675 | 0.583 |
| 5-Dehydroquinic acid | 0.739 | 0.667 | 0.733 |
| Glucose | 0.925 | 0.917 | 0.867 |
| Sebacic acid | 0.747 | 0.747 | 0.933 |
| Galactose | 0.914 | 0.917 | 0.867 |
| Galactosamine | 0.808 | 0.958 | 0.600 |
| Glucarate | 0.744 | 0.917 | 0.533 |
| Cysteine+Cystine | 0.947 | 0.917 | 1.000 |
| 5-fold cross validation | |||
|---|---|---|---|
|
| |||
| AUC | Sensitivity | Specificity | |
| Oxalate | 0.780 | 0.863 | 0.633 |
| Sarcosine | 0.644 | 0.642 | 0.650 |
| 3-Hydroxyisovaleric acid | 0.775 | 0.747 | 0.850 |
| Urea | 0.723 | 0.800 | 0.650 |
| Benzoic acid | 0.823 | 0.842 | 0.800 |
| Nonanoic acid | 0.976 | 0.914 | 0.933 |
| β-Alanine | 0.740 | 0.695 | 0.783 |
| Creatinine | 0.644 | 0.568 | 0.850 |
| Threo-β-Hydroxyaspartic acid | 0.804 | 0.726 | 0.933 |
| Phthalic acid | 0.780 | 0.864 | 0.617 |
| Hypoxanthine | 0.654 | 0.589 | 0.783 |
| 5-Dehydroquinic acid | 0.729 | 0.674 | 0.733 |
| Glucose | 0.922 | 0.916 | 0.850 |
| Sebacic acid | 0.766 | 0.621 | 0.933 |
| Galactose | 0.914 | 0.926 | 0.850 |
| Galactosamine | 0.804 | 0.958 | 0.617 |
| Glucarate | 0.725 | 0.916 | 0.533 |
| Cysteine+Cystine | 0.946 | 0.916 | 0.983 |
Four metabolites (nonanoic acid, cysteine + cystine, glucose, and galactose) shows high potential as biomarker candidate (over 0.9 in AUC, over 90% in sensitivity). Result of five-fold cross-validation shows similar tendency with the training set.
Table IV.
Performance of OSCC biomarker candidates in the validation set
| Validation set | |||
|---|---|---|---|
|
| |||
| AUC | Sensitivity | Specificity | |
| Nonanoic acid | 0.909 | 0.840 | 0.929 |
| Glucose | 0.889 | 0.640 | 1.000 |
| Galactose | 0.946 | 0.920 | 0.929 |
| Cysteine+Cystine | 0.774 | 0.840 | 0.714 |
Biomarker candidates revealed a high value of ≥0.85 except cysteine in the AUC, but it was somewhat low as 0.77 for cysteine, but it still showed moderate accuracy as a biomarker candidate. In sensitivity, biomarker candidates showed over 0.85 except glucose, which was slightly lower, 0.64.
Figure 2.
Distribution of plasma levels of 4 biomarker candidates in the OSCC patients and OD patients.
The plasma levels of 4 biomarker candidates in the OSCC patients and OD patients are shown as closed circles. The Y-axis means the levels normalized by the internal standard. Asterisks means the significant differences between the OSCC patients and OD patients, and the P-values were calculated using the Student’s t-test.
Table V.
The four metabolites showed significant difference between the OSCC patients’ per- and postoperative plasma samples using the Wilcoxon signed-rank test (P < 0.05)
| OSCC | Post-operation/Pre-operation | p-value |
|---|---|---|
| Nonanoic acid | 0.66 | 0.0008 |
| Glucose | 1.59 | 0.0008 |
| Galactose | 1.39 | <0.0001 |
| Cysteine+Cystine | 0.61 | <0.0001 |
| OD | Post-operation/Pre-operation | p-value |
|---|---|---|
| Nonanoic acid | 0.84 | 0.2554 |
| Glucose | 1.46 | <0.0001 |
| Galactose | 1.09 | 0.2179 |
| Cysteine+Cystine | 1.12 | 0.1000 |
The selected four biomarker candidates were also significantly different between the OSCC patients’ per- and postoperative plasma sample using the Wilcoxon signed-rank test (P < 0.05).
Table VI.
Performance of OSCC biomarker candidates in the early-stage OSCC group
| Compound Name | Sensitivity |
|---|---|
| Nonanoic acid | 0.790 |
| Glucose | 0.947 |
| Galactose | 0.947 |
| Cysteine+Cystine | 0.842 |
Sensitivity for glucose, galactose, and cysteine + cystine was ≥85% and that for nonanoic acid was 79%.
DISCUSSION
The incidence of OSCC is increasing due to an increasing aging society. Of note, the 5-year survival rate of OSCC is approximately 80%–90% when detected in the early stage, but it is 30% or less when detected in the advanced stage (11). Therefore, detection of OSCC at an early stage is imperative. Typically, screening for OSCC is performed by visual inspection. The sensitivity and specificity of screening by visual inspection are reported to be 0.85 and 0.97, respectively (11). Although screening by visual inspection performed by a skilled diagnostician is simple and effective, standardization of the quality of diagnosis based on the experience of a diagnostician might pose a problem.
This study investigated whether alterations in plasma metabolite levels can detect the occurrence of OSCC. As shown in Table II, 44 metabolites were significantly different between the OSCC and OD groups. Of these, the following four metabolites were finally selected as biomarker candidates by cross-validation (Table III): nonanoic acid, glucose, galactose, and cysteine + cystine. In addition, a comparison of plasma metabolites in pre- and postoperative plasma samples revealed that the four biomarker candidates in the OSCC group were significantly different. In the OD group, however, no significant difference was observed in the three metabolites (nonanoic acid, galactose, and cysteine + cystine) when compared between pre- and postoperative plasma samples, and only glucose significantly increased postoperatively. In both OSCC and OD groups, only glucose significantly increased after surgery compared to that before surgery, suggesting the involvement of hyperglycemia due to surgical stress. Surgery stress might increase the levels of hormones, such as glucagon, cortisol, and growth hormone, and enhance the sympathetic nerve. Consequently, postoperative hyperglycemia has been observed previously (12). In the four biomarker candidates, levels of glucose and galactose, which are monosaccharides, are altered significantly. Glucose is the primary source of energy metabolism in vivo and plays an essential role in energy metabolism for cancer cell proliferation. Conversely, galactose has not been reported as an energy source in most cancer cells. The phenomenon that cancer cells perform energy metabolism depending on glycolysis rather than oxidative phosphorylation even under an aerobic environment is called the Warburg effect (13). In this study, a comparison between the plasma of OSCC and OD revealed decreasing glucose levels in OSCC, suggesting that active energy metabolism using glucose is performed because of the cell proliferation in cancer cells. Another study reported the alteration in the OSCC patients’ serum or plasma levels of metabolites associated with the glycolytic pathway, especially about glucose (14,15). The biomarker candidate contains galactose as a saccharide in addition to glucose. The primary pathway of galactose metabolism is the Leloir pathway, in which UDP-galactose and glucose-1-phosphate are produced as intermediate metabolites. In this study, galactose tended to be in low level in the OSCC group compared to the OD group. In pancreatic cancer cell lines, the UDP-glucose and UDP-galactose decline is reported at the time of glucose starvation (16), suggesting a correlation between the glucose and galactose reduction in the plasma of OSCC in our study. These considerations support that glucose and galactose might be a biomarker for OSCC. In contrast, nonanoic acid was high in the OSCC group compared to patients with OD, and also pre- and postoperative comparisons of OSCC revealed a high value in nonanoic acid. While nonanoic acid has not been reported as a biomarker in oral cancer, the elevation of nonanoic acid in lung cancer has been reported as a biomarker (17). In this study as well, a comparison between the OSCC and OD before surgery revealed a significant increase in nonanoic acid in the OSCC group, consistent with Scachez et al., (17) who investigated lung cancer. Scachez et al.’s (17) subject of analysis was breath, which is different from plasma samples in this study; however, the tissue type of patients with lung cancer contained SCC that we covered in this study and was similar to our results. Although this study targeted lung cancer, and the mechanism by which nonanoic acid levels increase remains unclear, nonanoic acid might be a biomarker for detecting OSCC. In addition, the biomarker candidates include one of the amino acids, cysteine + cystine; the changes in cysteine have been reported in SCC of the human head skin (18). In this study, cysteine + cystine levels were high in the OSCC group compared to the OD group, and pre- and postoperative comparisons of OSCC revealed a high value of cysteine + cystine. Along with glutamic acid and glycine, cysteine is a component of glutathione, which is known as an antioxidant. Cancer cells take up cystine from the blood and maintain high levels of intracellular glutathione and, thus, their proliferative capacity. Reportedly, it inhibits the proliferation of cancer cells because of depleting glutathione by inducing the cystine deficiency, which is a target for drug discovery (19). In this study, a significant cysteine increase was also observed when compared to OD, suggesting that the active glutathione metabolism in the OSCC group forms the background. In addition, from the perspective of energy metabolism of cancer, glucose depletion is reportedly caused by active glycolysis metabolism in cancer cells, resulting in a change of the amino acid level in cancer cells (14,18,20). These results suggest that changes in glucose and cysteine, which is one of the nonessential amino acids, in our study reflect the result of energy metabolism of cancer cells.
This study has some limitations. First, patients with advanced stage are included in targeted samples. In this study, metabolomics analysis was conducted on patients regardless of early stage or advanced stage disease. Our OSCC biomarker candidates demonstrated significant differences between the OSCC and OD groups. Second, the sample size was small. Although the number of samples was small (N = 19), four biomarker candidates were higher than the sensitivity of the existing oral cancer biomarker SCC antigen (30% sensitivity). However, further examination of the effectiveness as a biomarker in early-stage OSCC is warranted by increasing the number of samples including higher number patients with early-stage OSCC.
CONCLUSION
The findings from our plasma metabolomics analysis for patients with OSCC suggest four metabolites as biomarker candidates of OSCC for screening. In this study, plasma samples were collected regardless of early cancer and advanced cancer. While oral cancer is easy to diagnose by visual inspection at the advanced stage, it is difficult to diagnose early-stage cancer from an oral potentially malignant disorder. Hence, by conducting metabolomics analysis focusing on the early stage, there is a possibility that it can contribute to the discovery of early stage that is hard to diagnose by screening at visual inspection. Ultimately, it contributed to the early detection and early treatment of OSCC, leading to an increase in the survival rate of patients and an improvement in quality of life.
ACKNOWLEDGEMENTS
This study was supported in part by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science [M.Y.] and the AMED-CREST program run by the Japan Agency for Medical Research and Development [S.N., M.Y.].
REFERENCES
- 1.Warnakulasuriya S. Global epidemiology of oral and oro- pharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2002;68:617–21. [PubMed] [Google Scholar]
- 3.Krimmel M, Hoffmann J, Krimmel C, Cornelius CP, Schwenzer N. Relevance of SCC-Ag, CEA, CA 19.9 and CA 125 for diagnosis and follow-up in oral cancer. Journal of Cranio-Maxillofacial Surgery. 1998;26:243–48. doi: 10.1016/s1010-5182(98)80020-6. [DOI] [PubMed] [Google Scholar]
- 4.Kimoto A, Nishiumi S, Kobayashi T, et al. A novel gas chromateography mass spectrometry-based serum screening method for oral squamous cell carcinoma. Head Neck Oncol. 2013;5:40. [Google Scholar]
- 5.Nishiumi S, Suzuki M, Kobayashi T, Matsubara A, Azuma T, Yoshida M. Metabolomics for Biomarker Discovery in Gastroenterological Cancer. Metabolites. 2014;4:547–71. doi: 10.3390/metabo4030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar N, Shahjaman MNH, Islam SMS, Hoque MA. Serum and Plasma Metabolomic Biomarkers for Lung Cancer. Bioinformation. 2017;13:202–8. doi: 10.6026/97320630013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Kastenmüller G, He Y, et al. Differences between human plasma and serum metabolites profile. PLos One. 2011;6:e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishiumi S, Suzuki M, Kobayashi T, Yoshida M. Differences in metabolite profile caused by pre-analytical blood processing procedures. J Biosci Bioeng. 2017 doi: 10.1016/j.jbiosc.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Nishiumi S, Kobayashi T, Kawana S, et al. Investigations in the possibility of earyl detection of colorectal cancer by gas chromateography/triple-quadrupole mass spectrometry. Oncotarget. 2017;8:17115–126. doi: 10.18632/oncotarget.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akobeng AK. Understanding diagnostic tests 3: Receiver operation characteristic curves. Acta Paediatr. 2007;96:644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 11.Downer MC, Moles DR, et al. A systemic review of test performance in screening for oral cancer and precancer. Oral Oncol. 2004;40:264–73. doi: 10.1016/j.oraloncology.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Davis G, Fayfman M, et al. Stress hyperglycemia in general surgery: Why should we care? Journal of Diabetes and Its Complications. 2017 doi: 10.1016/j.jdiacomp.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Sience. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Yonezawa K, Nishiumi S, Kitamoto J, et al. Cancer Genomics & Proteomics. Cancer genomics & Proteomics. 2013;10:233–38. [PubMed] [Google Scholar]
- 15.Tiziani S, Lopes V, Gunther UL. Early stage diagnosis of oral canser using H NMR-based metabolomics. Neoplasia. 2009;11:269–76. doi: 10.1593/neo.81396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima K, Kitazume S, et al. Simultaneous determination of nucleotide sugars with ion-pair reversed-phase HPLC. Glycobiology. 2010;20:865–71. doi: 10.1093/glycob/cwq044. [DOI] [PubMed] [Google Scholar]
- 17.Callol-Sanchez L, Munoz-Lucas MA, et al. Observation of nonaoic acid and aldehydes in exhaled breath of patients with lung cancer. J Breath Res. 2017;25:026004. doi: 10.1088/1752-7163/aa6485. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto T, Nishiumi S, Fujiwara S, Yoshida M, Nishigori C. Novel serum metabolomics-based approach by gas chromatography/triple quadrupole mass spectrometry for detection of humanskin cancers: Candicate biomarkers. Journal of Dermatology. 2017:1–8. doi: 10.1111/1346-8138.13921. [DOI] [PubMed] [Google Scholar]
- 19.Lewerenz J, Hewett SJ, Huang Y, et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18:522–55. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tumas J, Kvederaviciute K, Petrulionis M, et al. Metabolomics in pancreatic cancer biomarkers research. Med Oncol. 2016;33:133. doi: 10.1007/s12032-016-0853-6. [DOI] [PubMed] [Google Scholar]