Abstract
Humans and wildlife are exposed to an intractably large number of different combinations of chemicals via food, water, air, consumer products, and other media and sources. This raises concerns about their impact on public and environmental health. The risk assessment of chemicals for regulatory purposes mainly relies on the assessment of individual chemicals. If exposure to multiple chemicals is considered in a legislative framework, it is usually limited to chemicals falling within this framework and co-exposure to chemicals that are covered by a different regulatory framework is often neglected. Methodologies and guidance for assessing risks from combined exposure to multiple chemicals have been developed for different regulatory sectors, however, a harmonised, consistent approach for performing mixture risk assessments and management across different regulatory sectors is lacking. At the time of this publication, several EU research projects are running, funded by the current European Research and Innovation Programme Horizon 2020 or the Seventh Framework Programme. They aim at addressing knowledge gaps and developing methodologies to better assess chemical mixtures, by generating and making available internal and external exposure data, developing models for exposure assessment, developing tools for in silico and in vitro effect assessment to be applied in a tiered framework and for grouping of chemicals, as well as developing joint epidemiological-toxicological approaches for mixture risk assessment and for prioritising mixtures of concern. The projects EDC-MixRisk, EuroMix, EUToxRisk, HBM4EU and SOLUTIONS have started an exchange between the consortia, European Commission Services and EU Agencies, in order to identify where new methodologies have become available and where remaining gaps need to be further addressed. This paper maps how the different projects contribute to the data needs and assessment methodologies and identifies remaining challenges to be further addressed for the assessment of chemical mixtures.
Abbreviations: AO, adverse outcome; AOP, adverse outcome pathway; BMD, benchmark dose modelling; BQE, biological quality element; CA, concentration addition; CAG, cumulative assessment group; CMEP, chemical monitoring and emerging pollutants; CRA, cumulative risk assessment; DART, developmental and reproductive toxicity; DEB, dynamic energy budget; EBT, effect-based tools; EDC, endocrine disrupting chemical; EQS, environmental quality standard; HBM, human biomonitoring; IA, independent action; IATA, integrated approach to testing and assessment; IPRA, integrated probabilistic risk assessment; iPSC, induced pluripotent stem cells; LOE, lines of evidence; MCR, maximum cumulative ratio; MCRA, Monte Carlo risk assessment tool; MEC, measured exposure concentration; MoA, mode of action; MRA, mixture risk assessment; MSFD, Marine Strategy Framework Directive; NAM, new approach methodology; PBTK, physiologically based toxicokinetic (model); PEC, predicted exposure concentration; PNEC, predicted no effect concentration; QSAR, quantitative structure activity relationship; RDT, repeated dose systemic toxicity; TK, toxicokinetic; SMRI, similar mixture risk indicator; SYRINA, systematic review and integrated assessment; TTC, Threshold of Toxicological Concern; WFD, Water Framework Directive
Highlights
-
•
Mapping EU funded research projects to different aspects of mixture risk assessment.
-
•
Overview of current status and methodological developments
-
•
Need to further address data and knowledge gaps overarching different chemical sectors
1. Introduction
Humans and wildlife are exposed to an intractably large number of different combinations of chemicals via food, water, air, consumer products, materials and goods. The possible combinations of mixtures are increased by use of inter alia pharmaceuticals, drugs, tobacco and occupational exposures. Taken together, this raises significant concerns about the impacts on public and environmental health. The risk assessment of chemicals for regulatory purposes does only in rare cases take into account the “real life” exposure to multiple chemicals, but mainly relies on the assessment of individual chemicals. If exposure to multiple chemicals is considered in a legislative framework, this is usually limited to chemicals falling within this framework and neglects co-exposure to chemicals that are covered by a different piece of legislation (Evans et al., 2016). A detailed overview of the different legislative requirements for assessing mixtures in EU legislation can be found in Kienzler et al., 2014, Kienzler et al., 2016.
Guidance documents are available within specific regulatory sectors and international frameworks have been proposed (Kienzler et al., 2014, Kienzler et al., 2016). However, a harmonised, consistent approach for performing mixture risk assessments and management across different regulatory sectors is lacking. As outlined in the Commission Communication on the combination effects of chemicals - Chemical mixtures (EC, 2012), there are several open issues to address, such as a lack of understanding of real co-exposures, lack of information on combined toxicity, interactions, chemicals' modes of action and criteria for grouping chemicals.
Several EU research projects are presently underway, funded by the current European Research and Innovation Programme Horizon 2020 (EC, 2013; Karjalainen et al., 2017) or the Seventh Framework Programme (FP7; EC, 2006). They aim at addressing research gaps, by e.g. generating and making available internal and external exposure data, developing models for exposure assessment, developing tools for in silico and in vitro effect assessment to be used in a tiered framework and for grouping of chemicals, as well as developing joint epidemiological-toxicological approaches for mixture risk assessment and for prioritising mixtures of concern.
The research projects and several European Commission services and EU agencies have joined forces to link these projects, map the achievements and identify remaining gaps. These aspects were also discussed in a workshop entitled ‘Advancing the Assessment of Chemical Mixtures and their Risks for Human Health and the Environment’, on 29–30 May 2018, at the Joint Research Centre in Ispra, Italy. The main features of these projects are presented in this publication, as well as how the projects link to specific aspects of mixture risk assessment. However, the list of projects presented below is not exhaustive, as it focuses on ongoing projects funded by EU research and innovation programmes and related activities within EU institutions. Nevertheless, considering the listed projects we expect to cover the current main areas of mixture research and development, in order to draw the conclusions presented at the end of this document.
2. Main concepts and terminology in the assessment of mixtures
2.1. Terminology
Many different terms are used in the context of chemical mixtures. This publication follows the terminology proposed by WHO/IPCS and published in Meek et al. (2011). It is important to distinguish exposure to the same chemical from multiple sources and/or by multiple pathways, which is termed “aggregate exposure”, while exposure to multiple chemicals via single or multiple sources and/or pathways is termed “combined exposure to multiple chemicals”. Chemicals grouped together for evaluation of combined exposure are referred to as an “assessment group”. The term “chemical mixture” refers to a combined exposure to multiple chemicals, and is defined as any set of multiple chemicals, regardless of their source, that may or may not be identifiable and that may contribute to joint toxicity in a target population (ATSDR, 2004). Manufactured products, such as pesticide formulations or cosmetic products are considered “intentional mixtures”, whereas coincidentally formed and variable mixtures originating from one or several sources, such as surface water contaminations or pesticide residues in food, are considered unintentional mixtures. In order to facilitate the readability of the document, we generally refer to Mixture Risk Assessment (MRA) as representing the assessment of risks from combined exposures to multiple chemicals. Only in the field of plant protection products, the EU legislative framework uses the terms “cumulative risk assessment” (CRA) and “cumulative assessment groups” (CAGs), which we therefore use in that context.
In the context of this paper, risk assessment is referred to as defined by WHO/IPCS (2004): “A process intended to calculate or estimate the risk to a given target organism, system, or (sub)population, including the identification of attendant uncertainties, following exposure to a particular agent, taking into account the inherent characteristics of the agent of concern as well as the characteristics of the specific target system. The risk assessment process includes four steps: hazard identification, hazard characterization (related term: Dose–response assessment), exposure assessment, and risk characterization.” MRA therefore applies this definition in the context of combined exposure to multiple chemicals.
2.2. Concepts for mixture risk assessment
2.2.1. Mixtures in regulatory toxicology
The risk from exposure to chemical mixtures can be assessed as a whole (whole-mixture approach), or based on the individual components of the mixture (components-based approach).
Whole mixture effects can be assessed by testing a mixture itself, but can also be based on data generated with a mixture of similar composition (i.e. similar in composition regarding components and proportions). In this case, a quantitative MRA can be carried out directly using toxicity data on the whole mixture. Whole mixture testing can be performed for intentional mixtures, e.g. pesticide formulations assuming direct exposure of an operator, but also for unintentional mixtures and indirect exposures such as mixtures of pollutants in river water. This approach allows consideration of any unidentified chemicals in the mixtures and any interactions among mixture components. If the mixture is further characterised using e.g. effect-directed analyses by fractionating the samples and testing the fractions, relevant chemical groups or chemicals driving the mixture effect can be further characterised (Brack et al., 2016).
The problem in applying whole-mixture approaches is, however, the nearly infinite number of possible combinations of chemicals in mixtures, which cannot all be subjected to (eco)toxicological testing. The majority of whole-mixture studies so far have mainly concentrated on either environmental, dietary or consumer product mixtures, while whole sources in real life are much broader and more variable.
Another approach, which is generally used when the components of the mixture are known, is to mathematically predict the combined action of the components. The choice of the mathematical approach to use depends mainly on considerations whether the mixture components act by the same mode of action (MoA) or whether they are acting independently (Groten et al., 2001). The optimal use of component-based approaches is therefore dependent on the knowledge of the composition of the mixture and the corresponding MoA of the individual components, or on the information regarding their association with groups of chemicals demonstrating similar or identical MoA (assessment groups). Such information may be based on chemical structures and structure-activity relationships (either qualitative or quantitative), molecular modelling, structural alerts or on toxicological responses or effects (SCHER, SCCS, SCENIHR, 2012).
Within component-based approaches, three basic types of action are usually considered: (i) dose or concentration addition (CA), applied to chemicals with a similar MoA; (ii) independent action (IA) or response addition, applied to chemicals with a dissimilar MoA; and (iii) interactions between chemicals in the mixture. The term interaction includes all forms of joint action that deviate from the above additivity concepts. Hence, the combined effect of two or more chemicals is either greater (synergistic, potentiating) or less (antagonistic) than that predicted on the basis of dose or response addition. Both CA and IA are based on the assumption that chemicals do not influence each other's toxicity by interacting at the biological target site. They have been suggested as default approaches in regulatory risk assessment of chemical mixtures, although chemical mixtures are rarely composed of either only similarly or of only dissimilarly acting chemicals (SCHER, SCCS, SCENIHR, 2012). For further information on the underlying concepts please refer to, e.g., Kortenkamp et al. (2009), SCHER, SCCS, SCENIHR (2012) or Kienzler et al. (2014).
Overall, evidence in the literature supports the application of concentration addition as a first, protective approach. It is therefore also the default approach to start from in several international recommendations and frameworks, independent of components' similar or dissimilar mode of action. However, once a detailed risk assessment for a mixture is performed, chemical grouping should be considered and based on common target organs and/or a common MoA. Considering large numbers of chemicals in a group might lead to overly conservative assessments. Therefore, carefully designed refinements tailored to the assessment needs have to be found. The choice of the approach depends strongly on the context of the risk assessment as well as on the information on which to base the grouping of components. Irrespective of the starting point for grouping, it is recommended to use all available information on the mixture and its components: physico-chemical properties, structural alerts, (Q)SAR and read-across information, evidence from omics, in vitro (high throughput screening or other) or in vivo experimental data, depending on availability. The overall body of evidence needs to be considered to decide whether it is sufficient to draw conclusions or additional information must be gathered or generated.
It should be noted that the concepts above have been developed considering the exposure to and effects of mixtures on individuals. However, the environmental protection goals are established at population level. That means that effects of single compounds on individual environmental organisms might be acceptable as long as the population is not impacted. In the context of mixtures, this means that such slight effects on individuals by single chemicals might translate to population level effects when exposure to chemical mixtures occurs. The concepts for assessing mixtures are applicable in environmental risk assessment (ERA), when all individuals in the population are expected to be exposed to the same mixture and level. However, different individuals in the same population may be exposed to different chemicals and different mixtures over time, introducing an additional level of complexity. This can be dealt with by linking population models with landscape assessment (Topping et al., 2015).
2.2.2. Mixtures in environmental epidemiology
In environmental epidemiology, exposure indicators are used to describe the exposure of concern in relation to human health and disease. Where mixtures are concerned, a variety of indicators can be used, depending on the type of mixture of interest. Complex mixtures of chemicals are sometimes represented by one or a few markers. For instance for childhood exposure to environmental tobacco smoke (ETS), the number of cigarettes smoked at home may be the exposure indicator to capture the mixture of thousands of chemicals present in tobacco smoke. Alternatively, one may measure cotinine, a metabolite of nicotine in sputum, as a proxy to ETS exposure. One may further analyse exposures to the individual chemicals in ETS e.g. carbon monoxide, benzene and other aromatics, polycyclic aromatic hydrocarbons, tobacco additives, etcetera. However, this typically does not substantially improve the quality of the exposure indicator, while substantially increasing the (analytical) costs. In a similar fashion, proximity to traffic or agriculture plots may capture the exposure of traffic related pollutants or multiple use of pesticides in cost effective ways. A substantial fraction of epidemiological research indeed involves the validation of such (crude) exposure indicators with detailed studies of underlying multiple chemical and physical characteristics, e.g. measurement of particulate matter and nitrogen dioxide as markers for the total mixture of traffic-related exposure, ozone as marker for the mixtures of summer smog, benzo(a)pyrene as marker for multiple polycyclic aromatic hydrocarbons. Increasingly, this involves the use human biomonitoring to characterise body burden/internal exposure to multiple chemicals. Existing human biomonitoring (HBM) studies have measured multiple chemicals in different (sub)populations, allowing to draw conclusions on the most likely combined chemical exposure patterns and thereby guiding further research on mixtures. In epidemiological research into the etiology of disease, complex mixture exposures characterised in molecular epidemiology and exposome studies can now be directly linked to human health effects and to specific diseases. In many cases, these complex exposures can be associated to (patho)physiological changes or to biomarkers of effects that constitute risk factors for future disease, e.g. pulmonary function decrements, decreased kidney function, increased blood pressure, high cholesterol levels, or biomarkers such as epigenetic changes. Linking these effect markers to complex exposures contributes to establishing causal relationships between these exposure and various diseases. Such exercises rely on modern statistical techniques and computation power to estimate the joint effect of relevant components of a mixture through supervised or unsupervised selection of exposure (variables) of concern. Modern environmental epidemiology can also contribute to the study of interactions between multiple exposures under real life conditions.
2.3. Methodological issues and hurdles hampering the risk assessment of chemical mixtures
When having a closer look into existing case studies dealing with MRA, some methodological issues are recurrent (Bopp et al., 2016). The data sources used are variable in quality and the data sets often not complete, having a direct impact on the quality of the RA and the related uncertainties. Exposure data are usually modelled, from (bio)monitoring or published data from surveys on exposure, and the reliability of exposure data directly depends on the (bio)monitoring practice (Dewalque et al., 2014; Malaj et al., 2014) and on the quantity of available data. The exposure assessment of persistent and bioaccumulating chemicals is even more challenging. It requires consideration of the chemical's kinetics at realistic environmental exposure levels (e.g. Tarazona et al., 2015), the body burden as well as the exposure history, rather than the daily intake, as a starting point for the RA, as the exposure patterns might change over time.
Toxicological data stem mostly from published databases, including regulatory assessments. In case of missing data, methods like the Threshold of Toxicological Concern (TTC) or in silico methods are used. In fact, data gaps seem to be the major issue when it comes to RA of chemical mixtures. Those data gaps are numerous, both regarding hazard and exposure data, for compounds such as pharmaceuticals (Backhaus and Karlsson, 2014), pesticides (Junghans et al., 2006; Kennedy et al., 2015; Nowell et al., 2014), cosmetics, etc., and imply the use of extrapolations (e.g. acute to chronic), which increase the uncertainties of the MRA. Models for estimating aggregate exposure of consumers to chemicals that occur in personal care products are being developed (Delmaar et al., 2015), but sufficiently elaborated data on the frequency of use of those products are still lacking (Gosens et al., 2013). The integration of existing HBM data is rare so far, but could help in addressing combined and aggregate exposure of humans more realistically.
As a result, MRA requires a considerable amount of assumptions. Their choice can have a large impact on the outcome and should be carefully documented and justified (Boon et al., 2015; Kennedy et al., 2015). This is also the case for single chemical assessments; however, for MRA it is of particular importance since the uncertainties around single chemical assessments are compounded when combined risks are assessed.
Moreover, in the case where different models are combined and used in the same RA (i.e. for dietary and non-dietary exposure), care must be taken when interpreting the result to recognize possible differences in the degree of conservatism between dietary and non-dietary exposure models. Furthermore, the assessment of combined effects for chemicals with common effects or common MoA implies that reference values for the specific effect under consideration should be used. However, toxicity values reported are often those driving the risk of the single chemical, i.e. the lowest reference value might be for a different effect than the one relevant for the mixture assessment. Using these reference values in lower tiers can be a first conservative estimate, but might lead to large overestimations of the combined effects. In addition, interactions of the organism (human or wildlife) with these varying mixtures may lead to stimulation or suppression of different toxicity pathways and thus to other MoA. In effect, this may lead to other adverse outcomes (AOs) and diseases than those established in toxicological MRA and over- or underestimation of the actual health effects in the human population or ecosystem.
3. Overview of ongoing EU research projects on chemical mixtures
3.1. European research projects with relevance to mixture assessment
3.1.1. EDC-MixRisk1
Integrating Epidemiology and Experimental Biology to Improve Risk Assessment of Exposure to Mixtures of Endocrine Disruptive Compounds (EDC-MixRisk) aims to meet the societal need for improved decision-making regarding risks from human exposure to mixtures of endocrine disrupting chemicals (EDCs). EDCs are chemicals that interfere with hormonal signalling by different mechanisms already at low doses. EDCs from different sources (e.g., pesticides, plastic softeners, surfactants, etc.) can disrupt the same hormonal pathways, thus adding to each others effects (Kortenkamp, 2014). EDC-MixRisk determines risks for multiple adverse health outcomes based on molecular mechanisms involved after early life exposure to EDC mixtures by an interdisciplinary cooperation between experts in epidemiology, experimental toxicology and molecular biology, and risk assessment. It has three main aims: i) Identification of EDC mixtures that are associated with adverse health outcomes in epidemiology; ii) Identification of molecular mechanisms and pathways underlying the associations between exposure and adverse health outcomes; and iii) Development of a transparent and systematic framework for integrating epidemiological and experimental research to facilitate the assessment of risk and societal impact, thus promoting better risk management of EDCs and their mixtures.
Since the start in 2015, two sets of mixtures have been established for metabolism and growth (G), neurodevelopment (N) and sexual development (S), based on exposure data for 20 (mixtures 0) or 45 chemicals (mixtures 1) with known or suspected endocrine disrupting properties. The mixtures are based on data from the Swedish mother-child pregnancy cohort SELMA including chemical analyses from mother's urine and serum at pregnancy week 10 and the following health outcomes of their children: birth weight (growth and metabolism), language delay at age 2.5 (neurodevelopment), and anogenital distance (AGD) in boys (sexual development). All of these outcomes are early signs for adversity in the respective domains. Using these data and a novel biostatistical method, we identified so-called bad actors, chemicals that contribute to the association between exposure and adverse health outcome. These bad actors were mixed in ratios corresponding to the mean exposure of SELMA mothers and are tested in animal and cell models including mice, tadpoles, zebrafish, and cell models. Our results show that mixtures 0 induce negative effects on the molecular, cellular, and organismal level at concentrations corresponding to the actual levels of the SELMA mothers. The mixtures disrupted common signalling pathways in cell and in animal models, in particular thyroid hormone signalling. The molecular effects could be linked to adverse outcomes such as increased adipose tissue, behavioural changes, and disruption of sexual organ development (Birgersson et al., 2017). Selected single chemicals were also tested and their effects compared to the mixtures. In most cases, the single compounds did not have an effect at concentrations comparable to the mixtures. Some of the molecular signatures affected by the mixtures will now be analysed in the SELMA samples and associations with exposure and health outcomes in the children investigated. An important part of the project is the improvement and development of methods for regulatory risk assessment of mixtures. One of them is the Similar Mixture Approach (SMACH) described below (Section 4.4).
3.1.2. EuroMix2
A tiered strategy for the risk assessment of mixtures of multiple chemicals is developed in the EuroMix project. Risk assessors have to deal with data gaps, uncertainties and lack of models hampering realistic risk assessment of combined exposure to multiple chemicals (‘combined exposure’) via multiple exposure routes (‘aggregated exposure’). Therefore, EuroMix aims to develop bioassays and models to perform future risk assessment with a tiered strategy for chemical mixtures with focus on (1) reducing uncertainties and generating more refined hazard data by testing several chemicals and mixtures thereof using cost-effective in vitro assays; (2) priority setting for testing chemicals based on hazard (using in silico tools) and/or exposure considerations; (3) exploring how these in vitro assays can be used as reliable alternatives for animal experiments; (4) developing specific and general physiological based-toxicokinetic (PB-TK) or in vitro to in vivo extrapolation (IVIVE) models to use in vitro test results in mixture risk assessment; and (5) developing hazard and exposure models for risk assessment and to apply these models on the newly generated data.
To explore concepts, methodologies and models which address these goals, three adverse outcome pathways (AOPs), for fatty changes in liver, decreased anogenital distance and cranio-facial malformation, were selected. Prioritisation of chemicals for in vitro testing is based on in silico models (quantitative) structure activity relationship ((Q)SAR) and molecular docking, the concept of Threshold of Toxicological Concern (TTC) and exposure models for identifying mixtures of concern. About 1600 chemicals from 10 different chemical classes were screened in silico. The results can be used for priority setting of test chemicals and/or lower tier input data for mixture risk assessment.
In vitro assays aligning the three AOPs are used to measure the potency of chemicals in a more refined manner. In addition, they are used to investigate the appropriateness of the default assumption of dose addition using chemicals having a similar and dissimilar mode of action. Results from in-vitro testing will be verified against in-vivo experiments.
Although in vitro assays will allow generating new hazard data for yet untested chemicals in a cost-effective manner, their results need to be extrapolated from internal exposure concentrations to external doses before being used in mixture risk assessment. For this, nine specific and one generic PB-TK (or IVIVE) models were developed.
The EuroMix toolbox will result in data and models allowing 1) classification into cumulative assessment groups (CAGs) based on AOP-wise testing, 2) use of in silico and in vitro data in mixture risk assessment (MRA), 3) performing MRA overarching regulatory sectors and 4) integrating hazard and exposure data into a Margin of Exposure in line with a tiered assessment as described in international guidance. EuroMix aims at an openly available toolbox. Therefore, the data obtained from the in silico models and the in vitro assays, together with new models for PB-TK, and hazard and exposure (combined and aggregated) assessment will be embedded in a web-based EuroMix data and model toolbox. Case studies for combined and aggregated exposure assessment using this toolbox have been performed. A case study addressing combined exposure of pesticides, additives and contaminants, as an example of mixture risk assessment overarching regulatory sectors, is ongoing.
Access to the tools will be facilitated by training. Practical guidance on how to use the tests and models in line with international developments will be delivered. Dissemination and harmonisation of the approach will be achieved by involving key-experts and EFSA, WHO and US-EPA and through four harmonisation workshops.
3.1.3. HBM4EU3
The European Human Biomonitoring Initiative (HBM4EU) is a joint effort of 28 countries, 109 partners including the European Environment Agency. HBM4EU has designed its research programme to answer concrete policy relevant questions from EU and national policy makers. The main aim of the initiative is to coordinate and advance human biomonitoring (HBM) in Europe in order to provide better evidence of the actual internal exposure of citizens to chemicals, the aggregate exposure, and its impact on health to support policy making in relevant chemical regulatory domains. Key objectives include: i) Harmonising procedures for HBM across countries, to provide policy makers with comparable data on human internal exposure to chemicals at the EU level; ii) Linking data on aggregate internal exposure to chemicals to external exposure and identifying exposure pathways and upstream sources; iii) Generating scientific evidence on the causal links between human exposure to chemicals and adverse health outcomes; and iv) Adapting chemical risk assessment methodologies to use HBM data to account for the contribution of multiple exposure pathways to the total chemical body burden. A specific work package on mixtures is included with the aim to identify real-life exposure patterns, priority mixtures, drivers of mixture toxicity and to assess potential health risks and impacts of mixtures. To this end, existing HBM mixture data will be analysed, combining data driven approaches, with toxicity weighed grouping based e.g. on adverse outcome pathways (AOPs). New mixture data will be collected in a joint survey in 3–5 countries and three case studies on health effect assessment of mixtures will be developed. A rich set of mixture HBM data across Europe will be analysed jointly and generated de novo, as novel avenues to address associated health risks. HBM4EU also has a work package addressing ‘Emerging chemicals’ through the development and application of “suspect screening” approaches for the identification and monitoring of already known emerging chemicals that are not yet routinely measured, as well as non-targeted profiling approaches for revealing unknown chemicals that are potentially hazardous. This will add further insights into the nature and scope of mixture exposures in the European population. In its first year, the achievements so far are still mainly methodological in nature. For instance, procedures for exchange of human samples from biobanks, and data management protocols for the exchange of data from existing HBM programmes and studies between data owner and data user within the European General Data Protection Regulation (GDPR, Regulation (EU) 2016/679) requirement were developed. Also, Interlaboratory Comparison Investigations (ICI) and External Quality Assurance Scheme (EQUAS) were established for the analysis of the HBM4EU priority chemicals and reference laboratories identified. Stakeholder dialogues are being initiated and procedures for the derivation of HBM health-based guidance values (HBM HBGVs) were established for the general population and for workers and applied to a first HBM4EU priority chemical (di(2-ethylhexyl) phthalate (DEHP)). Protocols for the alignment of existing national studies and programmes are established and data collection through these aligned studies starts in the second half of 2018, with sample collection in different age groups across different geographical units in Europe.
3.1.4. EU-ToxRisk4
The vision of the large-scale project ‘An integrated European ‘flagship’ program driving mechanism-based toxicity testing and risk assessment for the 21st century’ (EU-ToxRisk) is to drive a paradigm shift in toxicology towards an animal-free, mechanism-based integrated approach to chemical safety assessment (Daneshian et al., 2016). The EU-ToxRisk project started in January 2016 and has united all relevant disciplines and stakeholders to establish: i) pragmatic, solid read-across procedures incorporating mechanistic and toxicokinetic knowledge; and ii) ab initio hazard and risk assessment strategies of chemicals with little background information. The project is focused on repeated dose systemic toxicity (RDT) targeting the liver, kidney, lung and nervous system, as well as developmental/reproduction toxicity (DART) (Delp et al., 2018). The consortium brings together a large panel of both in silico and robust in vitro human cell-based assays as well as high throughput technologies (Wink et al., 2018), including targeted transcriptomics (Mav et al., 2018), altogether referred to as new approach methodologies (NAMs). The integration of the various NAMs in defined case studies will allow the assessment of the overall applicability domain of these NAMs in chemical hazard and ultimate risk assessment. The case studies involve integration of both toxicodynamics as well as toxicokinetics information to ultimately derive to an improved hazard and risk assessment strategy. The first 2.5 years of EU-ToxRisk have focussed on a panel of case studies that have addressed the question whether biological information from NAMs can contribute to read across cases. Examples involved steatotic liver injury caused by valproic analogues, pesticides targeting the mitochondrial respiratory chain and onset of neurotoxicity and phenoxycarboxylic acid and peroxisome proliferation. The case studies make advantage of the adverse outcome pathways that have been developed within EU-ToxRisk. In various of our case studies we compare the in vitro prediction of adverse outcomes in the context of available in vivo data, to ensure correct prediction by our in vitro methods. The first case studies that allow such a systematic comparison indicate a good correlation between our in vitro results and prior knowledge on in vivo adverse outcomes for case study compounds. Importantly, the activities in the case studies are supported and guided by both, industry stakeholders as well as regulators, from the cosmetics, (agro)chemical, pharmaceutical sector. The first case studies are in their final stage and will be reported in regulatory mock submission documents that will be shared with the regulatory advisory board of the project. The aim is to provide practical guidance for regulatory read across. The next phase of the project will involve case study that are focussed on ab initio safety assessment. Moreover, we start with joint case studies with industry stakeholders to assess the validity and applicability of our NAMs toolbox for chemical safety prediction. We have also further optimized our in silico and in vitro toolbox methods. Thus, among other tools, we established novel fluorescent reporter in induced pluripotent stem cells, developed a multi-organ on a chip models as well as established and validated diseased liver microspheres. Moreover, we have integrated high throughput transcriptomics based on targeted RNA-sequencing to increase the mechanistic information density. The final goal of EU-ToxRisk is to deliver testing strategies to enable reliable, animal-free hazard and risk assessment of chemicals. Although EU-ToxRisk is not directly addressing mixture effects, the tools and approaches developed will support the hazard assessment of mixtures.
3.1.5. Solutions5
The project ‘Solutions for Present and Future Emerging Pollutants in Land and Water Resources Management’ (SOLUTIONS) (Brack et al., 2015) developed a comprehensive set of tools for holistic monitoring, assessment and prioritisation of complex mixtures of contaminants in European water bodies (Altenburger et al., 2015) together with a user-friendly web-based guidance system for the application of these tools called RiBaTox. A specific focus was given to the development and rigorous evaluation of effect-based tools (Altenburger et al., 2018), non-target chemical screening (Hollender et al., 2017), effect-directed analysis (Brack et al., 2016) and appropriate sampling technologies (Schulze et al., 2017). The toolbox has been extensively demonstrated in large case studies (rivers Danube, Rhine and Ebro) together with additional field sites. An integrated set of models from emission via fate and transport up to risk has been developed (Lindim et al., 2016) and used for spatially and temporally explicit modelling of exposure and risk of more than 5000 chemicals in all European rivers. This approach was helpful to identify chemicals that might pose a risk but have not been included in monitoring yet but also of chemicals that probably, do not pose a risk. Modelling and monitoring were mutually validated in the case studies resulting in agreement for the majority of chemicals within ± one order of magnitude. Chemical footprints characterizing the impact of complex contaminant mixtures as a result of emissions and available water amounts for dilution together with an extensive compilation on abatement options (van Wezel et al., 2017) and their efficacy for specific compounds have been applied to prioritize mitigation measures. Integrated ecological, effect-based and chemical monitoring have been used to record the improvement of water quality and aquatic ecosystems after management measures such as the upgrade of wastewater treatment plants in Switzerland. Recommendations for the enhancement of coherence of different regulations relevant for chemicals and water as well as for the revision of Water Framework Directive to better cope with complex mixtures have been made (Brack et al., 2017).
3.2. Other European activities of relevance to mixtures
3.2.1. The European Commission's Information Platform for Chemical Monitoring (IPCHEM6)
IPCHEM is the European Commission's reference platform for chemical monitoring data collected across various media (environment, food & feed, human matrices, consumer products and indoor air) by the European Commission bodies, Member States, international and national organisations and research communities.
The Platform aims to support a coordinated approach for collecting, storing, accessing and comparing data related to the occurrence of chemicals, their metabolites, and chemical mixtures, in relation to humans and the environment.
IPCHEM has been designed and implemented as a distributed infrastructure, providing remote access to existing chemical monitoring data and information systems. Moreover, it offers hosting facilities to data owners and providers who do not have the resources to publish their data online. It is structured into four modules, according to the chemical monitoring data categorisation: ‘Environmental Monitoring’, ‘Human Biomonitoring’, ‘Food and Feed’, ‘Products and Indoor Air’. The primary objectives of IPCHEM are focused on: (1) Assisting policy makers and scientists to discover and access chemical monitoring data covering a range of matrices and media; (2) Offering safe and secure data storage for data currently not readily accessible; (3) Boosting data harmonisation and comparison, by integrating quality control rules and procedures into the platform; (4) Facilitating exposure and risk assessment practices in support of EU policies.
IPCHEM is progressively collaborating with research projects, such as HBM4EU, EuroMix, SOLUTIONS, to make research data and metadata shareable and accessible at the early stage possible for policy and regulatory purposes.
Furthermore, to best meet the needs of the “community” of users working in the area of MRA, the following upgrades are envisaged: (a) aligning IPCHEM's chemical nomenclature registry with other existing registries for the coherent identification of chemicals which are dealt with by European Commission Services, European Agencies and scientific communities; (b) exploring options for grouping of chemicals, based on different parameters (as explained in 4.3); (c) defining and developing technical solutions to enable interoperability of IPCHEM with tools and information systems performing mixture risk assessments, in particular those built under the H2020 research framework.
3.2.2. European Food Safety Authority (EFSA) mixture projects7
3.2.2.1. EFSA MIXTOX project
In 2013, EFSA reviewed the international frameworks available for human risk assessment of mixtures (EFSA, 2013). From the recommendations of this report, EFSA initiated data collection on mixture toxicity for human and ecological risk assessment and organised a colloquium on “harmonisation of human and ecological risk assessment of multiple chemicals” (EFSA, 2013; EFSA, 2015; Quignot et al., 2015a, Quignot et al., 2015b). In 2016, the EFSA's Scientific Committee started the MixTox project, aiming to develop a guidance document (GD) on harmonised methodologies for human health, animal health and ecological risk assessment using tiering principles and stepwise approaches taking into account international developments in the field (WHO, US-EPA, JRC, OECD etc.) and specific needs for the food and feed safety area. The “Draft guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals” has been published on 26th June 2018 for public consultation.8The GD constitutes an overarching document aimed at supporting the work of EFSA panels and scientific units as well as relevant scientific advisory bodies dealing with chemical risk assessment both within and across regulatory applications and sectors. By the beginning of 2019, a technical report on the public consultation will be published together with the final GD taking into account comments from all stakeholders. In spring 2019, EFSA is also planning an international workshop to discuss and disseminate the GD and further progress in the area of mixture risk assessment with national and international scientific advisory bodies, industry and NGOs.
3.2.2.2. EFSA Landscape Environmental Risk Assessment Project
This EFSA project aims at developing spatially explicit risk assessment methodologies and tools for mapping environmental risk of chemical and biological stressors at the EU level, and is connected to the recommendations from the EFSA's Panel on Plant Protection Products and their Residues (PPR) to include landscape characteristics in the environmental assessment of pesticides. The overall objective is to integrate the calculators, tools and models developed for supporting the guidance on ERA of pesticides in a GIS-based IT platform, allowing the consideration of true environmental (e.g. climatic, geological, etc.), ecological (species distribution, ecosystem services, etc.) and (agri)cultural (soil use patterns, landscape characteristics, connectivity of agricultural and non-agricultural areas) variability in the environmental risk assessment. The project is primarily designed for the assessment of individual pesticides, but as the estimations will be spatially explicit, it will allow for the assessment of combined exposure to pesticides and other agrochemicals. The project is structured in two consecutive phases: a) a testing phase with pilot projects for assessing the feasibility and to “prove the concept”; and b) an implementation phase where the ERA guidance, tools and models are updated and integrated into a GIS compatible platform.
3.2.2.3. EFSA Cumulative Risk Assessment of pesticides
EFSA's PPR Panel has developed a new approach for grouping pesticides that paves the way for the implementation of cumulative risk assessment (CRA) for multiple pesticide residues (EFSA PPR, 2014). The general methodology for classifying pesticides into so-called cumulative assessment groups (CAGs) is based on identifying compounds that exhibit similar toxicological properties in a specific organ or system. A key characteristic of the proposed approach is that the grouping is not based on mechanistic assumptions on the mode of action for chemical classes. Instead, the grouping is based on a detailed evaluation of the effects observed in the toxicological studies, first at organ/organ system level and then based on specific phenomenological effects of toxicological relevance. For example effects on the central nervous system are further discriminated as effects on motor division (e.g. locomotor activity, muscle strength, coordination and equilibrium); effects on sensory division (e.g. including reflex action or sensory-motor responses and neurophysiological assays); effects on autonomic division (e.g. cholinergic modulation); neurochemical effects (e.g. brain or erythrocyte acetylcholinesterase inhibition); and neuropathological effects (mainly axonal and myelin degeneration). As a first step, the Authority's Panel on Plant Protection Products and their Residues (PPR) has applied this methodology to define groups of pesticides which are toxic to the thyroid and central nervous systems. This approach will be gradually introduced in regulating the use of pesticides in the European Union. Further information can be found in the EFSA PPR Panel opinion on relevance of dissimilar mode of action in CRA of pesticides (EFSA PPR, 2013) and its guidance on probabilistic dietary exposure assessment (EFSA PPR, 2012).
This activity is further supported via the EFSA-RIVM (Dutch National Institute for Public Health and the Environment) partnership on CRA of pesticides. The main purpose of this Partnership Agreement is to further develop the suitability of the Monte Carlo Risk Assessment (MCRA) software so that it becomes fully accessible and usable by EFSA and EU Member States organisations competent for the implementation of plant protection products legislation to perform regulatory assessments (van der Voet et al., 2016; Kruisselbrink et al., 2018).
3.2.3. European Commission Joint Research Centre (JRC) mixture projects
3.2.3.1. Toxicity assessment of combined exposures and chemical mixtures
In follow-up to the Commission Communication on the Combined Effects of Chemicals (EC, 2012), JRC started its activities in the area of MRA with a view to developing a harmonised risk assessment methodology and informing regulatory guidance. The activity focuses on the use of existing information, in vitro experiments and computational modelling to characterise and predict the toxicokinetic and toxicodynamic combined effects of chemicals in mixtures. Regulatory requirements, available guidance and approaches were reviewed (Kienzler et al., 2014, Kienzler et al., 2016) and the applicability of novel, non-animal tools in MRA was investigated (Bopp et al., 2015). In order to gain further insight into the current practices and issues linked to MRA, relevant case studies from the peer-reviewed literature were reviewed (Bopp et al., 2016). Currently, JRC is performing several new case studies focusing on endocrine disrupting effects and developmental neurotoxicity. JRC is also actively contributing to a guidance document developed within the OECD project on “Consideration for assessing the risks of combined exposure to multiple chemicals”, to be published in 2018.
3.2.3.2. JRC mixture activity linked to EU Water Framework Directive
In December 2011, JRC organised a workshop “Towards the implementation of existing and innovative bioassays for water quality assessment” to establish a strategic plan for the application of existing and innovative bioassays for assessing water quality. Water quality assessment under the EU Water Framework Directive (WFD, Directive 2000/60/EC) focuses on the effects of single chemicals instead of evaluating the combined action of environmentally relevant mixtures.
Based on the workshop outcome, the first exercise was launched to evaluate the suitability of the current single chemical based assessment of water quality. Combinations of 14 or 19 chemicals of concern for the contamination of surface waters were produced as reference mixtures and tested using bioassays by a consortium of 17 research institutes from eleven EU and associated countries, led by JRC. The mixtures included several classes of chemicals, such as pesticides, pharmaceuticals and different industrial chemicals (Carvalho et al., 2014). Each compound was present at its individual safety concentration limit according to European legislation, the environmental quality standard (EQS; Directive 2008/105/EC). The bioassays covered the most relevant ecotoxicological endpoints and included OECD-validated and non-validated methods. In 2017, JRC launched the second exercise inviting the same research groups to use the Mix14 or 19 as reference mixture to compare to the effects of a real water sample in the routine bioassays. The results are expected in September 2018.
Furthermore since 2012, a subgroup of experts on Chemical Monitoring and Emerging Pollutants (CMEP) of the WG chemicals, chaired by Sweden, Italy and JRC, delivered a technical report on existing and innovative effect-based methodologies (Wernersson et al., 2014). Currently, the CMEP group is investigating the possible implementation of effect-based methods for monitoring and assessment of aquatic surface water-bodies in the context of the WFD and Marine Strategy Framework Directive (MSFD; Directive 2008/56/EC).
4. How do the projects link to specific aspects of Mixture Risk Assessment?
In this section, the contribution of the different projects to various aspects of MRA is discussed in some detail. A summary is provided in Table 1 and Table 2.
Table 1.
Mapping EU research project contributions to elements of Mixture Risk Assessment (MRA).
| Project acronym | EDC-MixRisk | EuroMix | HBM4EU | SOLUTIONS | EUToxRisk |
|---|---|---|---|---|---|
| Project title | Integrating Epidemiology and Experimental Biology to Improve Risk Assessment of Exposure to Mixtures of Endocrine Disruptive Compounds; | A tiered strategy for risk assessment of mixtures of multiple chemicals | Coordinating and advancing human biomonitoring in Europe to provide evidence for chemical policy making | SOLUTIONS for present and future emerging pollutants in land and water resources management | An Integrated European ‘Flagship’ Program Driving Mechanism-based Toxicity Testing and Risk Assessment for the 21st Century |
| Duration | 2015–2019 | 2015–2019 | 2017–2021 | 2013–2018 | 2016–2021 |
| Webpage | http://edcmixrisk.ki.se/ | http://www.euromixproject.eu/ | https://www.hbm4eu.eu/ | http://www.solutions-project.eu/ | http://www.eu-toxrisk.eu/ |
| Human health | x | x | x | x | x |
| Environmental Health | x | ||||
| Specifically addressing mixturesa | x | x | x | x | a |
| Gathering and/or generating exposure data | x | x | x | ||
| - Human biomonitoring | x | x | x | ||
| - Environmental monitoring | x | ||||
| Developing tools for exposure assessment | |||||
| - Aggregate exposure | x | x | |||
| - Combined exposure | x MCRA | Bio- & chemo-analytical tools for environmental monitoring | |||
| Focus on specific chemicals or effects | Endocrine disrupting chemicals; growth + metabolism, neurodevelopment, sexual development | Chemical regulated under different legislation (pesticides, additives and contaminants) and some cosmetic ingredients; liver + developmental toxicity and endocrine effects | Phthalates and Hexamoll® DINCH, bisphenols, per-/poly-fluorinated compounds, flame retardants, cadmium and chromium, PAHs, aniline family, chemical mixtures, and emerging substances | Wide range of common and emerging chemicals, including river basin-specific pollutants for the Danube | Repeated dose systemic toxicity, with lung, kidney, liver and nervous system as examples of potential target organs; and developmental and reproductive toxicity |
| Developing and using new approach methodologies for MRA | Integration of epidemiological data, in vitro and in vivo end points. | In vitro and in silico toolbox; verification against in vivo | Bioanalytical tools | In silico and in vitro toolbox; high throughput transcriptomics; biokinetics; PBPK modelling | |
| Grouping of chemicals in effect based assessment groups | Using epidemiological data to identify chemicals affecting specific human health domains | Using AOP-networks | Data driven and using AOP-networks | Using in vitro MoA screens for whole water sample testing; Using available evidence on MoAs of aquatic pollutants for component-based approaches |
Based on AOP and refined MoA analysis and read across |
| Generating tools for MRA | Bio-statistical tools: sufficient similarity concept | EuroMix model toolbox: MCRA overarching regulatory sector possibilities Mixture prioritisation tools (MCR and SNMU) IPRA (probabilistic link between hazard and exposure) Link between dietary and non-dietary exposure models Generic and specific PBPK models Endpoint specific QSAR model |
Developing a test battery for whole mixture testing of water samples or extracts Developing an advanced tiered framework for the integrated assessment of mixture risks to aquatic organisms and human health from pollution of freshwaters |
In silico and in vitro new approach methodolgies | |
| Identification of priority mixtures | x | x | x | x | |
| Linking to health effects | x | x | x | AOP based | |
| Considering other than chemical stressors | Nutrition, stress, genetic background | – | Depending on study type, potential confounders and effect-modifiers may be used | – | |
| Relevant EU legislation | REACH regulation | Global food safety legislation, Pesticides, Contaminants, Additives, Water Framework Directive, REACH | Chemical regulations | Water Framework Directive; All other pieces of EU chemicals legislation with a relevance for water pollution |
Directive on the protection of animals used for scientific purposes, REACH regulation, cosmetics regulation. |
| Relevant EU strategies or activities | Community Strategy for Endocrine Disruptors, Chemical Mixtures | Chemical Mixtures | Chemical Mixtures | Chemical Mixtures, Common Implementation Strategy for the WFD |
EUToxRisk is dealing with single chemical assessments rather than chemical mixtures. It is however developing many tools for hazard assessment that are of relevance in the future assessment of chemical mixtures and was therefore included in this review.
Table 2.
Mapping other relevant EU mixture activities to elements of Mixture Risk Assessment (MRA).
| Project acronym | EFSA MixRisk | EFSA CRA for pesticides | EFSA Landscape ERA project | IPCHEM | JRC Mixture project | JRC WFD project |
|---|---|---|---|---|---|---|
| Project focus | Harmonisation of human and ecological risk assessment of multiple chemicals | Cumulative risk assessment of pesticides | Developing spatially explicit risk assessment methodologies and tools for mapping environmental risk of chemical and biological stressors at the EU level | The European Commission's Information Platform for Chemical Monitoring | Toxicity assessment of combined exposures and chemical mixtures | JRC mixture activity linked to EU Water Framework Directive |
| Human Health | x | x | x | x | x | |
| Environmental Health | x | x | x | x | x | |
| Combined exposure assessment | ||||||
| Gathering and/or generating exposure data | x | x | ||||
| - Human biomonitoring | x | |||||
| - Environmental monitoring | x | x | ||||
| Developing tools for exposure assessment | ||||||
| - Aggregate exposure | x | |||||
| - Combined exposure | X MCRA | x | ||||
| Combined effect assessment | ||||||
| Focus on specific chemicals or effects | All within EFSA remit | Pesticides | Pesticides | Across legislative sectors | WFD priority pollutants and emerging pollutants (e.g. chemicals from WFD Watch List) | |
| Developing and using new approach methodologies for MRA | x | x | x | |||
| Grouping of chemicals in effect based assessment groups | Grouping based on target organ and phenomenological effects, can be refined based on MoA | Using AOP networks, toxicity similarity matrices | ||||
| Mixture risk assessment | ||||||
| Generating tools for MRA | Guidance | Guidance | x | |||
| Identification of Priority Mixtures | x | |||||
| Linking to health effects | x | |||||
| Considering other than chemical stressors | x | |||||
| Potential contribution to EU policy and actions | ||||||
| Relevant EU legislation | Food safety regulations | Authorisation of pesticides, Maximum Residue Limites for pesticides | Pesticides | Chemical regulations in general | Chemical regulations in general, Directive on the protection of animals used for scientific purposes | WFD regulation |
| Relevant EU strategies or activities | Chemical Mixtures | Chemical Mixtures | Chemical Mixtures | Chemical Mixtures | Chemical Mixtures, Community Strategy on Endocrine Disruptors | Common Implementation Strategy for the WFD, Chemical Mixtures |
4.1. Combined exposure assessments
Reliable and timely available co-occurrence and co-exposure data are essential components of MRA. In order to determine co-exposure of an organism, concentrations present in an exposure medium (occurrence data) are needed in combination with uptake rates from this exposure medium. Especially in a retrospective context, MRA is often triggered by co-exposure information that can originate from information on exposure sources, modelled exposure scenarios, or human and environmental (bio)monitoring data.
4.1.1. Gathering and generating exposure data
In many cases, occurrence in a specific matrix (such as food, water, air) can be modelled and/or monitored. The related uptake or uptake rates of chemicals by an organism from any of these matrices, can be assessed by data on, inter alia, food consumption, cosmetics use or inhalation rates, if the chemicals are properly identified and quantified in these matrices.
As described above, IPCHEM is a platform making occurrence data, measured in different matrices, accessible to researchers, policy and decision makers. Several of the above-described projects are providing data and will contribute to the enhancement of IPCHEM in the near future, either providing occurrence data collections (e.g. HBM4EU, EDC-MixRisk, SOLUTIONS), or offering/sharing tools and capabilities supporting MRA.
In EDC-MixRisk, exposure to multiple EDCs is investigated based on two large European pregnancy cohort studies, including around 1500 mother-child pairs. The exposure assessment is based on measurements of 54 chemicals with endocrine disruptive properties in bio-banked blood and urine samples from mothers in the SELMA cohort (Bornehag and Gennings, 2016).
HBM4EU is gathering existing HBM data and will generate new HBM data according to harmonised protocols, data templates and codebooks. The data will be made available via IPCHEM, as agreed with data controllers and compliant with the data protection regulation. Chemical classes currently focused on are phthalates and Hexamoll®DINCH, bisphenols, per-/polyfluorinated compounds, flame retardants, cadmium and chromium VI, polycyclic aromatic hydrocarbons (PAHs) and anilines. In addition, the initiative also uses HBM data to investigate chemical mixtures and follows non-target approaches to identify emerging chemicals. The combined activities will give a better view on the actual aggregate exposures to multiple chemicals in the European population.
Biomonitoring data for wildlife can equally help identifying co-exposure patterns for environmental organisms. Chemical monitoring of environmental media can often be directly used in estimating exposures, e.g. for aquatic organisms. The SOLUTIONS project has a particular focus on providing exposure information on emerging pollutants in European river basins. This involves extensive data sets from the SOLUTIONS case studies Danube, Rhine and some Spanish river basins Ebro and Llobregat that have been included in the NORMAN database EMPODAT, and will be made available also via IPCHEM. About 100 organic micropollutants have been analysed in large-volume solid phase extracts taken during the Joint Danube Survey 3 in 2014 (Schulze et al., 2015) and linked to in vitro effect data (Neale et al., 2015). Other ongoing and finalised SOLUTIONS studies look into targeted and non-targeted analysis of waste water treatment plant effluents for up to 405 chemicals, including their spatial and temporal dynamics (König et al., 2017; Neale et al., 2017b; Beckers et al., 2018). SOLUTIONS has also provided an interesting dataset on concentrations and risks of pesticide patterns (81 compounds) in sediments of seven major European rivers (Massei et al., 2018).
4.1.2. Developing exposure models
Environmental, food or other monitoring data alone are not sufficient to assess exposures for humans and the environment as, apart from biomonitoring data, they inform only about the concentrations in matrices an organism can be exposed to, but not directly about the uptake by the organism from those matrices. Therefore in most cases additional modelling based on occurrence data is needed and applied. In some cases, modelling based on use or sales information is also performed, if no further monitoring data are available.
The organisms' external or internal exposure can be modelled, when relevant input data are available. Examples of projects developing such modelling tools are e.g. the FP7-funded project ACROPOLIS9 and the EuroMix project. The ACROPOLIS project resulted in an optimized MCRA (Monte Carlo Risk Assessment) software embedded in a web-based environment in order to assess dietary exposure to pesticide residues (van der Voet et al., 2016; Kruisselbrink et al., 2018). The European Commission, EFSA, industry and regulators were trained to use the MCRA software and a manual on how to use the MCRA software for conducting CRA following the EFSA guidance (EFSA, 2012) was provided to the European Commission. EuroMix is further developing lower and higher tier exposure and risk models for multiple chemicals and will integrate these into an open web-based tool addressing chemicals spanning different regulatory sectors, related to dietary exposure and beyond. In addition, exposure tools addressing multiple exposure routes (aggregated exposure) will be embedded in the EuroMix tool. The modelling results will be compared and validated with the SHEDS software on combined exposure to multiple chemicals developed in the USA and results of a human study.
In the context of MRA, it is important to look not only at external co-exposure, but also to investigate internal co-exposure, i.e. which chemicals will be found in the same organs at the same time, as external and internal co-exposure patterns can differ substantially. Recently, EFSA has engaged in a multi-agency-academia collaboration to develop generic toxicokinetic (TK) models and tools as user-friendly, open-source models, coded in R (R Core Team, 2014). The models range from simple TK tools, dynamic energy budget models to physiologically based toxicokinetic (PBTK) models calibrated with physiological data for humans, farm animals, pets and species of ecological relevance (EFSA, 2014; Grech et al., 2017; Baas et al., 2018). Other EFSA open source tools include EFSA's hazard database, Openfoodtox, and a number of QSAR tools. All together, these models are foreseen to provide a platform to support the integration of TK data in risk assessment including (1) determination of internal dose, (2) tissue residues, and (3) analysis of interspecies differences and human variability in toxicokinetic parameters (Dorne et al., 2017; Toropov et al., 2017; Toropova et al., 2018). EFSA is currently involving agencies from EU Member States (i.e. ANSES, ISS), the JRC and other agencies (i.e. US-EPA, FDA) for further development of the platform and case studies for training the current and future generation of risk assessors.
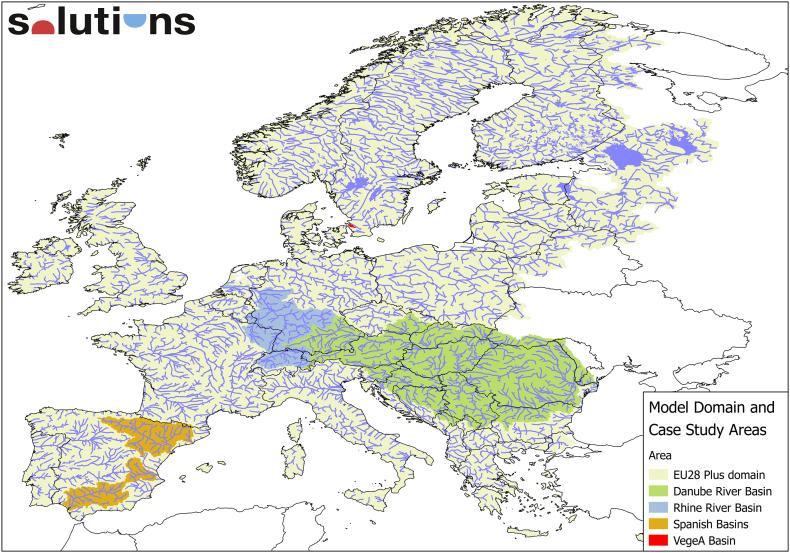
Within the SOLUTIONS project, an integrated sequence of interlinked models has been developed, to simulate the risk of (mixtures of) emerging pollutants to aquatic organisms and to humans exposed via fish consumption and drinking water abstraction. The four major components are: (1) a generic emissions model; (2) a spatially and temporally explicit fate and transport model (STREAM-EU), (3) a sequence of models to estimate chemical properties and (4) models to calculate risk for human health and ecology. The model covers the whole of Europe with a resolution in the order of 10 km (Fig. 1). This “model train” has very limited input requirements: (a) the use volume and use categories of a chemical, and (b) the molecular structure of a chemical. This allows, on the one hand, the application to a large number of chemicals to better approach “real life” exposure, and on the other hand application to new chemicals before chemical-specific laboratory and field data become available. From the exposure side, this approach supplements measured environmental concentrations (MECs) with predicted environmental concentrations (PECs), while covering (many) more chemicals, offering full spatial and temporal coverage, and avoiding issues with analytical quantification limits, analysis errors and natural patchiness. The cost for all this is the limited accuracy of PECs as compared to MECs, which needs to be accounted for in MRA and prioritisation protocols. At present, scientists involved in SOLUTUIONS are evaluating the accuracy of the PECs in Case Studies for the Danube, Rhine and four Spanish River Basins.
Fig. 1.
Europe-wide domain (EU28 plus Norway and Switzerland) of organic chemicals integrated modelling of exposure and risk in SOLUTIONS, as well as and case study basins used for validation.
4.2. Combined effects assessments
Effects of chemical mixtures can be either assessed by testing the mixture as a whole, or by predicting combined effects from the composition of a mixture in terms of its components and their concentrations.
It is practically not feasible to test all possible mixtures experimentally and toxicity data for single chemicals on the relevant endpoints or organisms are not always available. Therefore, smart strategies need to be identified to assess the potential hazards using new tools that rely less on in vivo testing and incorporate alternative experimental and computational tools instead. EuroMix, EDC-MixRisk, EUToxRisk, HBM4EU, and JRC are working towards this goal of exploring new approach methodologies (NAMs) that contribute to deriving more mechanistic knowledge for underpinning MRA, making better use of alternative tools in an integrated way and reducing the need for animal testing.
4.2.1. Developing and using new approach methodologies for MRA
An overview of how NAMs such as in vitro methods, omics techniques, in silico approaches such as quantitative structure activity relationships (QSARs) and read-across, toxicokinetic and dynamic energy budget (DEB) modelling, the AOP concept, and integrated approaches to testing and assessment (IATA) can be used in MRA can be found in Bopp et al. (2015). These approaches allow deriving meaningful information on individual mixture components or whole mixtures, enabling a better understanding of the underlying mechanisms of mixture effects. Their main strengths lie in their integrated use and smart combination to put different aspects regarding the hazard from combined exposure to multiple chemicals into context.
Several activities to gain more confidence in the use of NAM are included in the respective projects. The EuroMix project aims to perform a limited number of animal studies (in vivo experiments) to validate the in vitro experiments for their potentials use for refining assumptions in current MRA. The animal studies will address the mixture effect of a limited number of chemicals with similar and dissimilar modes of action. An important criterion for the selection of the chemicals is their contribution to dietary combined exposure. The validation includes the extrapolation of in vitro findings by developing PB-TK modelling and models for in vitro to in vivo extrapolation (IVIVE) for the chemicals that are tested in the in vivo experiments. EUToxRisk compares in case studies the in vitro prediction of adverse outcomes in the context of available in vivo data, to ensure correct prediction. The first case studies that allow such a systematic comparison indicate a good correlation between EUToxRisk in vitro results and prior knowledge on in vivo adverse outcomes for case study compounds.
4.2.1.1. Activities related to component-based approaches
EuroMix uses the results of in silico testing, such as QSAR and molecular docking, as a starting point to decide whether chemicals other than pesticides, which have already been grouped by EFSA, belong to a CAG (Moretto et al., 2016). Based on in silico testing and the TTC concept, chemicals can be prioritised for in vitro testing. EuroMix does this using AOP networks for three groups, i.e. liver steatosis, skeletal malformation and endocrine disruption. The EuroMix test battery includes test systems covering various key-events of the AOP networks. Mixtures and single chemicals with a similar and dissimilar mode of action will be tested using the in vitro tests and the results will be compared with in vivo experiments. In vitro tests performing well according to this comparison might become candidates for future Integrated Approaches to Testing and Assessment (IATA). The in vitro tests are in line with the goals to promote alternative testing strategies, which is also an aim of EUToxRisk and EDC-MixRisk. The test strategy will serve as showcase for how an AOP-based integrated test strategy can refine worst-case assumptions made e.g. in current CRA of pesticides developed by EFSA based on specific observations of phenomenological effects of toxicological relevance at organ or organ/system level and assuming additivity as default consideration. The EuroMix AOP-based test strategy might confirm or support refining the assumptions made in the EFSA approach and will be an efficient way to generate data aiming at filling data gaps.
JRC is also currently running experimental case studies investigating mixtures of similar and dissimilar compounds in AOP-based testing strategies for developmental neurotoxicants.
EUToxRisk aims to assess the application of NAMs for the identification of hazard and integration of such information into risk assessment scenarios. So even if not directly addressing mixtures, the generated tools and information for single chemicals can support the assessment of chemical mixtures. EUToxRisk involves both in silico approaches and in vitro test systems that cover various target organs like liver, kidney, lung and neuronal systems. This supports also further exploring the strategies for read-across based on NAMs. The in vitro systems range from high throughput systems taking advantage of high content imaging, but also more advanced models such as tissue organoids, organ-on-a-chip, and high throughput transcriptomics. The latter can help unravelling MoAs and support in particular the grouping in mixture assessments.
Both EuroMix and EUToxRisk also address kinetic considerations by including biokinetic measurements and PBTK modelling to translate the in vitro information to an in vivo context. To assess the various test methods in the project and to ensure further integration in IATAs, the EUToxRisk project has established a large panel of case studies that address either repeat dose toxicities (RDT) or developmental and reproductive toxicity (DART). Ultimately, EUToxRisk and EuroMix contribute also to a more quantitative AOP-based evaluation, thus enabling translation of hazard evaluation into risk assessment.
SOLUTIONS is working on a common decision tree and tiered work flow scheme for performing component-based human and ecological MRA for chemical cocktails found in European rivers and lakes, covering micropollutants from different legislative sectors. The proposed approach builds on schemes that have been devised previously to suit different contexts (summarised in Price et al., 2012). The scheme is focused on MRAs for single aquatic species or species groups, including algae, daphnia and fish, and for humans exposed to aquatic pollutants via fish consumption and drinking water abstraction. The proposed scheme starts from measured or modelled concentrations of chemicals co-occurring in water and fish. It builds on the principle of a tiered approach, where the analysis is refined when previous tiers reveal clearly unacceptable exposures, with refinements based on best-case assumptions of minimum expectable risks. The utility of the proposed scheme is tested by using data on the levels of around 300 chemicals that have been measured in the Danube river basin. In addition, SOLUTIONS performs component-based MRAs for aquatic species assemblages by applying the ms-PAF approach (multi-substances potentially affected fraction of species; De Zwart and Posthuma, 2005) to modelled concentrations of aquatic pollutants all over Europe.
4.2.1.2. Activities related to whole mixture approaches
EDC-MixRisk has adopted a whole mixture approach, where mixtures associated with AOs are identified in epidemiological data and subsequently tested, as whole mixtures, in experimental systems for dose-response relationships. Both in vitro and in vivo models are used simultaneously to link molecular, cellular and organismal events following an AOP-driven approach. This integrated approach enables transparent, consistent and systematic assessments of data supporting or disqualifying hypothesis or associations on causality. The results are evaluated from a regulatory perspective to ensure their usefulness for risk assessment. A major goal of the experimental studies is to yield new biomarkers, which in turn will be evaluated in the epidemiological studies and weighted against the defined EDC mixtures. Another aim of the mechanistic studies is to establish causal links between exposure and effect. This exercise will ultimately contribute to knowledge relevant for improving the risk assessment process including better weight of evidence approaches.
HBM4EU seeks to use existing cohort studies and biobanks to address health effects in relation to HBM data, including mixtures. Moreover, biomarkers of effects are being developed for inclusion in future HBM4EU surveys.
Reflecting the enormous complexity of chemical mixtures in water resources, SOLUTIONS puts a strong emphasis on whole mixture approaches to monitor and assess this contamination. To this end, the project developed and rigorously evaluated a modular test battery including in vivo and in vitro assays for effect-based monitoring (Neale et al., 2017a). This battery makes complementary use of short term tests with whole organisms representing the WFD biological quality elements (BQEs) and in vitro assays covering MoAs relevant for chronic effects. The integration of effect-based monitoring has been also suggested for the review of the WFD (Brack et al., 2017). Effect-based trigger values have been suggested for this suite of bioassays supporting the environmental quality standards (EQS) of the WFD (Escher et al., 2018). Effect-based monitoring tools have been validated for whole mixture monitoring after enrichment with in situ large volume solid phase extraction (Neale et al., 2018; Schulze et al., 2017) in several case studies (König et al., 2017; Neale et al., 2015). In cases where effect-based monitoring tools detect effects above the trigger values, SOLUTIONS provides an extensive toolbox for the identification of drivers of these effects (Brack et al., 2016). Their ability to identify so far unknown drivers of toxicity has been demonstrated in several case studies (Muschket et al., 2018; Muz et al., 2017).
4.3. Grouping of chemicals in assessment groups
One important aspect in assessing mixtures is the rationale for grouping chemicals, i.e. the basis for deciding which mixture components need to be considered for addressing combined effects. Chemical mixture assessments are usually initiated because of a concern based on known co-exposure or common effects for a group of chemicals.
The rationale for grouping chemicals in MRA can be based on multiple considerations. It can be co-emission based considering origin from one source, receptor based depending on a receiving compartment, chemical class based, biological effect based, or product/use based.
Grouping will be different depending on the context and regulatory goal. In some cases a group of structurally related chemicals is assessed together (such as phthalates under the REACH regulation, Regulation (EC) No 1907/2006). In the area of plant protection products, EFSA has developed the methodology to assign active substances to CAGs based on similar effects/target organs (EFSA PPR Panel, 2014). Some chemicals such as pesticides, dioxins and PAHs are often considered as a group under various pieces of EU legislation related to unintentional mixtures such as the Water Framework Directive. Legislation around occupational exposures may target chemicals according to their technical function, such as solvents. In food contact materials the chemicals are regulated according to their physico-chemical characteristics. Grouping chemicals based on similar effects allows addressing combined effects using CA based predictions.
As already demonstrated in Section 4.2.1, several of the current projects contribute to ways of effect-based grouping (EuroMix, EUToxRisk, JRC, EFSA for pesticides, EDC-MixRisk, HBM4EU), but also the co-exposure based grouping can be facilitated by the identification of common chemical patterns in human and environmental matrices as supported by SOLUTIONS and HBM4EU.
If an effect-based grouping is envisaged, all EU projects described herein promote an AOP-network based approach as discussed above. The key event level at which grouping should be considered is still under debate. Grouping is often based on common target organ/phenomenological effects at the start, as e.g. for the CAGs developed for pesticides in EFSA, due to a limited availability of mechanistic information. This is in contrast with approaches in other geographical areas, such as in the US approach which uses an approach grouping only pesticides that clearly show a similar mode or mechanism of action. As proposed by most of the involved projects, it is desirable to not only consider chemicals with similar effects within a specific regulatory sector but also across different legislative silos (Evans et al., 2016).
In environmental MRA, MoA based grouping for predicting combined effects plays a different role as the MoA will be different across different species. Furthermore, the endpoints used are often based on more overarching effects, such as mortality or growth. It is often more relevant to stratify the assessment by looking into effects on specific trophic levels or organism groups.
In SOLUTIONS, grouping of chemicals is following two complementary strategies: (1) Grouping of known chemicals produced, used and/or analysed in the environment. Chemicals may be grouped according to MoAs and common AOs towards specific organism groups. This has been based on an extensive literature evaluation of frequently occurring compounds in the aquatic environment that identified more than 100 distinct effect types grouped in 31 mode-of-action categories (Busch et al., 2016); (2) Grouping of chemicals co-occurring in environmental samples following a whole mixture approach and without the claim to be able to appoint and characterise all components of a group. Grouping is done according to effects and sources.
Grouping of environmental chemicals according to effects may be done on the basis of specific MoAs but also more integrative apical endpoints. Effect-based groups are defined by their detectability with effect-based methods in environmental samples. Grouping of environmental chemicals according to common occurrence and sources is based on chemical screening analysis and subsequent multivariate statistics attempting towards a clustering of peaks in environmental samples and validation with source-related fingerprints. The detection of source-related groups of chemicals, for example in surface water, helps to assess the impact of these sources on water quality and thus directly supports management.
4.4. Mixture risk assessments
While the presented projects have their major focus on either exposure or hazard assessment, all of them explore strategies to integrate both to finally address risks from exposure to chemical mixtures.
EuroMix aims at integrating exposure and hazard modelling into an integrated probabilistic risk modelling approach for mixtures, which will become available as a web-based toolbox. EuroMix will include a very conservative risk assessment as a lower tier for data poor situations based on conservative assumptions on CAG membership, aiming to set test priorities. Additionally a higher tier risk assessment will be implanted using more realistic information on CAG membership and on the chemicals' potencies as well as full data on consumption and residues of chemicals in food. These data are currently well-organised in Europe for consumption by EFSA and the EU Member States, for pesticides and various food contaminants for which monitoring is requested by regulation.
The EuroMix test battery will also provide information for hazardous doses, which will be transformed into a point of departure for risk assessment. For this the web-based Benchmark Dose Modelling (BMD) approach will be used. The BMD modelling and the probabilistic exposure modelling will be combined into an integrated probabilistic risk assessment (IPRA) in line with international developments such as RISK21 (Moretto et al., 2016). The outcome of such an IPRA calculation can be plotted using the graphical options of the EuroMix platform and can be compared with a Margin of Exposure at different percentiles of the risk assessment distribution (Crépet et al., 2013; Béchaux et al., 2013).
EDC-MixRisk follows a different strategy, focusing on two main approaches: firstly, building onto existing risk assessment strategies by using the newly proposed framework for the systematic review and integrated assessment (SYRINA) (Vandenberg et al., 2016), and secondly developing novel biostatistical methods to facilitate MRA. The first approach includes applying SYIRNA to the data generated in EDC-MixRisk with the aim to develop this framework further and adjust it to mixtures. The SYRINA method includes seven steps: 1) Formulating the problem; 2) Developing the review protocol; 3) Identifying relevant evidence; 4) Evaluating evidence from individual studies; 5) Summarizing and evaluating each stream of evidence; 6) Integrating evidence across all streams; 7) Drawing conclusions, make recommendations, and evaluate uncertainties. The second, biostatistical approach (Similar Mixture Approach, SMACH) is performed in a four-step process: (1) identification of “bad actors”, EDCs that are associated with sexual development in the children measured in prenatal blood/urine samples from mothers in the SELMA pregnancy cohort; (2) definition and construction of a “typical” mixture consisting of the “bad actors” identified in step 1; (3) experimentally testing this mixture in in vivo and in vitro models to estimate dose response relationships and determine points-of-departure (i.e., reference doses) associated with an adverse health outcome; and (4) using a whole mixture strategy with a statistical measure of sufficient similarity to infer risk assessment from experimental evidence back to the human population and generate a similar mixture risk indicator (SMRI) to compare each individual's total dose concentration to the estimated points of departure from step (3). Linking human observational study results with data from experimental assays of environmental mixtures strengthens the evidence of risk from environmental exposures.
HBM4EU aims to integrate HBM data in risk assessment and to perform health impact assessment for all HBM4EU priority substances, including mixtures and emerging chemicals. This involves the derivation of HBM health-based guidance values for the priority substance groups. In the course of the HBM4EU project new HBM mixture data will be compiled for the European population. Based on experience gained from the initial analysis of already existing HBM mixture data, risk assessment and health impact assessments will be performed on these new mixture data.
Based on the analysis of existing HBM mixture data, alternative approaches to generate aggregated indicators based on toxicity information will be compared. To this end, a reference table will be developed which curates available health-based guidance values, such as the German Human Biomonitoring Values and Biomonitoring Equivalents from different sources, including both workplace and general population values. These will serve as the basis from which a mixture indicator (such as a Hazard Index) may be calculated.
The expectation is that toxic potency information will not be complete for all compounds relevant for HBM4EU, with no information available for some of the compounds. In the case that there are no direct health-based or statistically derived reference values, alternative routes will be explored, such as a QSAR approach and internal dosimetry modelling through PBTK models.
SOLUTIONS performs case studies on MRA for chemical mixtures measured in selected streams and rivers, such as Rhine and Danube, or modelled in freshwater systems all over Europe. The case studies aim to test and to refine novel or improved experimental and computational approaches for assessing chemical mixture risks to and via the aquatic environment, including human health risks from the consumption of fish food and drinking water abstracted from surface waters. The approaches include both, whole mixture testing with effect-based tools (EBTs) and component-based MRAs as described in Section 4.2.
4.5. Identification of priority mixtures
With the large number of chemicals in use, there is an intractably large number of possible combined exposures to multiple chemicals. Therefore, concepts for prioritising chemical combinations of concern are needed, in order to effectively act on them. The three non-food Scientific Committees of the European Commission SCHER/SCENIHR/SCCS (2012) have suggested criteria for identifying priority mixtures based on combinations of exposure and hazard criteria, such as significant environmental exposures close to health based guidance values, multi-constituent products and commercial mixtures containing active substances or substances of concern, likelihood and frequency of exposure and their persistence, etc.
The EuroMix toolbox includes a module for identifying the mixtures of concern based on exposure and hazard considerations. The priority mixture selection will be based on the Maximum Cumulative Ratio (MCR, Price and Han, 2011) and Sparse Non-negative Matrix Underestimation (SNMU). The tools to set priority mixtures are embedded in the EuroMix open data and model platform and the details are described in van der Voet et al. (2017).
EDC-MixRisk has used a novel strategy to identify priority chemical mixtures of concern, namely mixtures that are associated with AOs in humans. Using weighted quantile sum regression, data from the Swedish SELMA pregnancy cohort were analysed to identify EDC mixtures measured in early pregnancy that are associated with health effects in each of three selected health domains for the children. The mixtures were then established using the real-life exposure data from the analyses of mothers' urine and serum at pregnancy week 10. In the first phase, three mixtures (mixtures 0) were established based on analyses of 20 chemicals found in the SELMA mothers. In the second phase, three more complex mixtures (mixtures I) have been established based on analyses of 54 chemicals measured from the samples of SELMA mothers.
The EDC-MixRisk strategy includes the evaluation of chemicals across a wide range of chemical classes. The primary chemicals of concern, the so-called “bad actors”, have been identified by using pharmacokinetic modelling and the application of novel and advanced bio-statistical methods. These bad actors contribute to the association between exposure and adverse health outcome.
HBM4EU work focusses on a first set of priority substances, which by themselves already constitute mixtures. New priority substance groups will be added in the course of the project. Based on grouping of HBM mixture data on toxic potency for common MoA and AOPs, clustering in specific risk groups will be performed, within and across the different countries. Moreover, determinants of these clusters of risk groups will be further assessed.
SOLUTIONS is working on a proposal for an advanced methodological framework for identifying priority pollutants and priority mixtures of chemicals in European freshwaters. The proposal aims to tackle major shortcomings of current prioritisation procedures under the EU WFD, which is mainly focused on single chemicals. The advanced framework is proposed to integrate all available lines of evidence (LOE) on significant mixture risks. This includes evidence from (i) ecological monitoring (field observations on so-called BQEs), (ii) effect-based monitoring (in vitro or in vivo testing in the lab or onsite), (iii) chemical monitoring in combination with component-based mixture risk assessment approaches, and (iv) integrated modelling of co-exposure and resulting mixture risks. Where one or more lines of evidence identify groups of chemicals presenting a significant risk, these should be subject to prioritisation for risk reduction measures. Where appropriate, such groups may be reduced to few mixture components or even one single component which can be demonstrated to explain most of the overall risk, so-called drivers of mixture risks. Wherever conclusive evidence on significant risks and needs for risk reduction cannot be reached because all possible LOEs are somewhere blocked by significant data or knowledge gaps, mixture components of potential concern are not left unnoticed but they are prioritised for further research and testing. Some elements of the advanced methodological framework may be readily applicable under the existing WFD. Full implementation, however, would require changes in the legal text, as detailed in Brack et al. (2017).
4.6. Linking combined exposure assessments to health effects and ecological status
The overall concept underpinning EDC-MixRisk is that early life exposure to EDC mixtures induces changes in the organism that underlie increased susceptibility to diseases during the entire life span. It is essential to understand the molecular mechanisms behind the adverse health effects in order to prove a causal link between exposure and outcome and to develop biomarkers of exposure and risk of disease.
By integrating epidemiological data into experimental research, EDC-MixRisk has developed a multiple-exposure-to-multiple-outcome approach, which mimics the real-life mixture exposure. The first results demonstrate that EDC mixtures associated with adverse health outcomes in population-based epidemiology evoke relevant molecular and physiological effects in experimental systems in cells and animals (Birgersson et al., 2017). This demonstrates the validity of the approach in interacting between epidemiology and experimental toxicology and the need to take mixture effects into account for risk assessment. The results have been obtained in mice, tadpoles, zebrafish, and cell models. Effects have been observed even at the lowest concentrations tested, which correspond to the actual levels of chemicals measured from the SELMA mothers.
In EuroMix, modelling results will be linked to exposure data from biomonitoring studies (e.g. via IPChem and HBM4EU). A human study (n = 140 participants) will serve as a proof of principle.
In HBM4EU, the results of the combination of HBM data with (existing) health registries, cohort studies and biobanks and the application of novel effect biomarkers will generate new insights in the risks of mixtures in the general population.
Whereas it is important to link toxicological risk assessment to health effects, in the environmental assessments it is important to link to environmental health. In the context of the WFD, the chemical and ecological status of European water bodies is assessed. SOLUTIONS case studies examine the linkage between ecological and chemical status of European water bodies. Statistical analyses reveal that chemical pollution makes a significant contribution to a bad ecological status at many sites, in addition to other hydro-morphological and physical stressors. Beyond such general statistical correlations, however, it is often not possible to establish direct causal links between known pollutants or pollutant mixtures and field observations of a bad status of BQEs. This applies in particular to large streams where (i) chemicals are highly diluted; (ii) chemicals occur at relatively constant concentrations with no significant gradients over larger stretches and with no significant differences between seasons; and (iii) appropriate reference conditions for clean waters are difficult to define. However, the situation may be different for local assessments, in particular for small streams with clear seasonal pollution episodes, such as pesticide run-off, or “upstream/downstream situations” where a known pollutant mixture is discharged into a river at a single location. Examples from SOLUTIONS case studies show that under such conditions it may well be possible to attribute observed adverse effects to chemical mixtures and to identify the causative groups of mixture components. However, this requires substantial efforts and the intelligent combination of all available experimental and computational methods, which is not possible for routine water quality monitoring.
4.7. Beyond chemicals: the impact of combined environmental stressors
Apart from chemicals impacting human or environmental health, there are several other possible non-chemicals stressors and risk factors that can contribute to or modulate adverse effects (see e.g. Liess et al., 2016).
HBM4EU seeks to integrate the HBM, toxicological, epidemiological and public health perspectives with novel exposome approaches. The exposome concept aims to integrate all exposures (chemical, physical, biological, psychological, social and economic) over the lifetime. As such, it is an ambitious concept that encompasses the chemical mixture issue but that goes beyond it to a large extent. The time dimension is also important since mixtures could consist in the combination of multiple exposures over time. Furthermore, the exposome is complementary to the genome and in many exposome research studies the contribution of the genome is also explored. The EU exposome project cluster includes three major projects, Exposomics10, Helix11 and Heals12. All three projects link exposures to health outcomes and biomarkers, and develop advanced technologies to delineate such interactions. Exposomics examined different case studies concerning water contamination and air pollution, both corresponding to large mixtures of compounds (Turner et al., 2018). In the case of air pollutants, short term and very long term effects were analysed and in the latter case, epigenetics markers were explored in addition to other omics. The Helix project aims were to combine several birth cohorts in the EU and to study the pregnancy exposome, integrating chemicals and other stressors (Robinson and Vrijheid, 2015). A large number of chemicals were detected and it was possible to delineate co-exposures to combinations of those chemicals. The Heals concept aims at integrating external and internal exposures with health outcomes using a variety of sensors, analytical tools, questionnaires and computational tools (Sarigiannis, 2017). It also integrates epidemiological and toxicological studies of relevant mixtures in order to support causal mechanisms.
Because the exposome projects integrate several environmental stressors in addition to chemicals, they are well suited to identify vulnerable conditions to chemical mixtures effects such as developmental stages, social and economic status, psychological stress, etc. It is intriguing that several of those stressors target similar molecular markers in organisms, for example epigenetic marks (Barouki et al., 2018). This may hint to possible interactions between those different stressors.
EDC-MixRisk will also focus on other than chemical factors such as genetic and epigenetic information, new biomarkers for exposure and health effects, as well as other factors (e.g., stress, nutrition, etc.) that can be of importance for better understanding of the relationship between early life exposure to chemicals and health and development in children. The aim is to test this correlation again in the experimental models. For example, if maternal stress is found to be a significant covariate in the model, the impact of mild stress on the effects produced by the critical EDC mixtures can be studied in the animal models, or the effect of glucocorticoids in the cell models. In order to address the contribution of the individual genetic background, induced pluripotent stem cells (iPSCs) will be generated from individuals with similar exposures but different health outcomes and tested for their susceptibility to the EDC mixtures.
5. Challenges and ways forward in Mixture Risk Assessment
There is a clear need to address chemicals not only as single substances but to assess the actual mixture exposures of humans and the environment across multiple sources and pathways. As shown above, numerous activities have been and are currently ongoing, for developing methodologies and approaches to tackle chemical mixtures. However, there is still a need to fill many knowledge and data gaps and to develop harmonised approaches across different sectors.
5.1. Gaps in data, knowledge and tools
MRA is often hampered by the limited availability of relevant information, starting with gaps in the knowledge of the composition of mixtures humans and wildlife are exposed to, and in knowledge about the effects of those chemicals individually and in combination.
Especially unintentional mixtures are difficult to deal with since MRA is limited by a lack of (co)exposure data and the difficulties in considering exposures from different sources (such as aggregate exposure from dietary combined with non-dietary sources).
In chemical monitoring, there are still several limitations of chemical analysis which are of major importance for assessing mixture compositions. Usually only chemicals suspected to be present are analysed, so non-analysed chemicals or metabolites, which can still contribute to a combined effect or risk, might be overlooked. This can be partly overcome by adding also non-targeted chemical analyses, but this is not possible for routine monitoring programmes. Modelling can help addressing data gaps in monitoring, but is also often hampered by limited availability of input data and models. The understanding of exposure to chemical mixtures could be improved if chemical uses and presence in products would be more consistently declared, which would support both, modelling and monitoring exercises. Several of the presented research projects are generating monitoring data and developing models and toolboxes that support combined exposure assessments.
If chemical monitoring data are generated, another problem is their accessibility. There is a clear need for sharing data, which is facilitated by platforms such as IPCHEM. In order to conclude on real co-exposures it is not sufficient to share aggregated data, but higher resolution or individual level data are needed (Dalla Costa et al., 2018).
Also in hazard assessment of mixtures, there are major issues with data availability. One option for performing MRA is to apply a whole mixture approach, e.g. in effect-based water monitoring under discussion in the context of the WFD. It has the advantage that all chemicals in the mixture are addressed and interactions such as synergistic effects can be considered. However, the choice of appropriate test and sample preparation methods is crucial. The availability of validated or standardised test methods that can be applied in routine monitoring needs to be improved (Wernersson et al., 2014). Testing mixtures that resemble real co-exposure patterns, e.g. from HBM data as in EDC-MixRisk and in HBM4EU, can support the identification of new biomarkers that can support future epidemiological studies and help establishing links between exposure and health effects.
When component-based approaches are used, those are often limited by the availability of information for the mixture components. Toxicity information, especially for specific endpoints of interest in a mixture assessment, is often lacking. SOLUTIONS case studies demonstrate that missing toxicity data for single chemicals are the most important bottleneck for reaching conclusive evidence on mixture risks. For example, for more than 50% of more than 300 chemicals that have been monitored at 68 different sites in the Danube, toxicity data were missing. Also in a review of recent case studies from the peer reviewed literature, the same issue of data gaps on both, the toxicity and exposure for individual chemicals, hampered MRA and in particular possible refined assessments in most cases (Bopp et al., 2016).
Information needed for effect-based grouping is also often limited. The approach as e.g. proposed by EFSA's PPR Panel for developing CAGs for pesticides can be refined from common target organs, common phenomenological effects, MoA, and mechanism of action (EFSA PPR, 2014). In most cases, information is only available related to the target organ and phenomenological effect. As shown above, the use of NAM for deriving more mechanistic information that can be integrated for example in AOP networks is one step forward supporting MRA.
A clear definition of similarly and dissimilarly acting chemicals is needed, to decide which chemicals should be considered in an assessment group. Emphasis should, when applicable, be placed more on common adverse outcomes (EFSA PPR Panel, 2013).
5.2. Legislative requirements
Requirements for taking combined effects and risks into account have been established for specific types of mixtures and specific protection goals under some pieces of EU chemicals legislation, such as the plant protection product regulation (Regulation (EC) No 1107/2009). However, legal requirements for MRAs are often missing, in particular for unintentional mixtures (Kienzler et al., 2014, Kienzler et al., 2016). Experience from both the US and the EU shows that significant progress in MRA is not achievable without the driving force of corresponding legal provisions.
If chemicals were more consistently addressed as mixtures, considering their combined effects as a group, also regrettable substitutions (i.e. replacing chemicals with structurally different chemicals but still having similar toxicological properties), could be addressed and eventually prevented.
5.3. Harmonisation
Where rules and guidance documents for performing MRAs have already been established under different pieces of European legislation and for different protection goals, both the approaches and the terminology are not always consistent. The need for harmonisation and integration across regulatory sectors is well recognised (Bopp et al., 2015; Evans et al., 2016). Harmonisation could encompass terminology, grouping, data formats, methodology and regulatory approaches including respective guidance.
Several scientific methodologies and regulatory approaches to assessing mixtures have been developed, and a variety of approaches might be warranted as there will be different needs in different contexts, such as for pro- or retrospective MRA, for risk assessment of intentional and unintentional mixtures. However, harmonisation of higher level principles could facilitate more regular considerations of combined exposures.
The EFSA MixTox project worked on the harmonisation of methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2018). The EuroMix project works as one of their objectives on harmonising approaches to MRA globally, using a series of four workshops. The specific objectives of the workshops are to (1) discuss current and impending regulation, across different chemical sectors (e.g. pesticides, contaminants) and regions (e.g. USA, Europe) and (2) how and when new science might impact on future regulation (EuroMix, 2017). Two more harmonisation workshops will be held in 2018/19 aiming to explore in more detail how the results of EuroMix can help further the international harmonisation of MRA.
5.4. Stepwise translation of science into regulation
MRA remains a complex and difficult task. Science in the area of MRA has progressed; several frameworks and methodologies have been developed. The current projects tackling MRA as described in this paper are contributing to further advancing our capacity to address chemical mixtures. Therefore, one must not wait to have a perfect model to address mixtures in regulation, as some tools, data and approaches already exist. MRAs can be developed based on more data rich chemicals, and transferred to data poor chemicals including methods for filling data gaps (e.g. exposure modelling and in silico tools). The more sophisticated MRA models should be adopted over time.
5.5. Conclusions
Over the last years, we have achieved a better understanding of risks from combined exposure to multiple chemicals and methodology to assess those risks (Kortenkamp and Faust, 2018). However, many challenges remain as outlined above and can be addressed by various stakeholders, including researchers, industry, regulators. Data gaps need to be addressed by generating new data and by making existing data more easily accessible and reusable. Translation of new methodologies into regulation needs to be facilitated by close collaboration between scientists and regulators. MRA is needed for both, mixtures within regulatory sectors and overarching regulatory sectors. In case an overarching approach is needed, a question remains on how to divide the acceptable risk per regulatory sector or even per chemical. All stakeholders need to extend the dialogue across different chemical sectors in order to come to more overarching and harmonised approaches.
Declarations of interest
The authors declare no conflict of interest in relation to the preparation of and contribution to the present article. Funding sources are declared in the acknowledgment section.
Acknowledgements
WB, JvG, MF, AK have received funding from the European Union's Seventh Framework Programme for research, technological development and demonstration under grant agreement No 603437 (SOLUTIONS). AB, PED, JR have received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement GA No 634880 (EDC-MixRisk); JvK under GA No 633172 (EuroMix); MKG, RB, EL under GA No 733032 (HBM4EU); BvdW under GA No 681002 (EUToxRisk). JR acknowledges further funding received from the Swedish Research Council; EL and JvK acknowledge further funding received from the Strategic Programme RIVM.
Handling Editor: Robert Letcher
Footnotes
The views expressed are those of the authors and do not necessarily represent the official position of their organisations.
http://edcmixrisk.ki.se Intermediate results are available at https://cordis.europa.eu/project/rcn/193310_en.html.
http://www.solutions-project.eu Intermediate results are available at https://cordis.europa.eu/project/rcn/110817_en.html.
Contributor Information
Stephanie K. Bopp, Email: stephanie.bopp@ec.europa.eu.
Robert Barouki, Email: robert.barouki@parisdescartes.fr.
Werner Brack, Email: werner.brack@ufz.de.
Silvia Dalla Costa, Email: silvia.dalla-costa@ec.europa.eu.
Jean-Lou C.M. Dorne, Email: jean-lou.dorne@efsa.europa.eu.
Paula E. Drakvik, Email: elina.drakvik@swetox.se.
Michael Faust, Email: faust@fb-envico.com.
Tuomo K. Karjalainen, Email: tuomo.karjalainen@ec.europa.eu.
Stylianos Kephalopoulos, Email: stylianos.kephalopoulos@ec.europa.eu.
Jacob van Klaveren, Email: jacob.van.klaveren@rivm.nl.
Marike Kolossa-Gehring, Email: marike.kolossa@uba.de.
Andreas Kortenkamp, Email: andreas.kortenkamp@brunel.ac.uk.
Erik Lebret, Email: erik.lebret@rivm.nl.
Teresa Lettieri, Email: teresa.lettieri@ec.europa.eu.
Sofie Nørager, Email: sofie.norager@ec.europa.eu.
Joëlle Rüegg, Email: joelle.ruegg@swetox.se.
Jose V. Tarazona, Email: jose.tarazona@efsa.europa.eu.
Xenia Trier, Email: xenia.trier@eea.europa.eu.
Bob van de Water, Email: b.water@lacdr.leidenuniv.nl.
Jos van Gils, Email: jos.vangils@deltares.nl.
Åke Bergman, Email: ake.bergman@swetox.se.
References
- Altenburger R., Ait-Aissa S., Antczak P., Backhaus T., Barcelo D., Seiler…Brack W. Future water quality monitoring — adapting tools to deal with mixtures of pollutants in water resource management. Sci. Total Environ. 2015;512:540–551. doi: 10.1016/j.scitotenv.2014.12.057. [DOI] [PubMed] [Google Scholar]
- Altenburger R., Scholze M., Busch W., Escher B.I., Jakobs G., Krauss M.…Kortenkamp A. Mixture effects in samples of multiple contaminants – an inter-laboratory study with manifold bioassays. Environ. Int. 2018;114:95–106. doi: 10.1016/j.envint.2018.02.013. [DOI] [PubMed] [Google Scholar]
- ATSDR . Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services; Atlanta, GA: 2004. Guidance Manual for the Assessment of Joint Toxic Action of Chemical Mixtures (Final) [Google Scholar]
- Baas J., Augustine S., Marques G.M., Dorne J.L. Dynamic energy budget models in ecological risk assessment: from principles to applications. Sci. Total Environ. 2018;628–629:249–260. doi: 10.1016/j.scitotenv.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Backhaus T., Karlsson M. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res. 2014;49(0):157–165. doi: 10.1016/j.watres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Barouki R., Melén E., Herceg Z., Beckers J., Chen J., Karagas M.…Nohara K. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ. Int. 2018;114:77–86. doi: 10.1016/j.envint.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béchaux C., Zetlaoui M., Tressou J., Leblanc J.C., Héraud F., Crépet A. Identification of pesticides mixtures and connection between combined exposure and diet. Food Chem. Toxicol. 2013;59:191–198. doi: 10.1016/j.fct.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Beckers L.-M., Busch W., Krauss M., Schulze T., Brack W. Characterization and risk assessment of seasonal and weather dynamics in organic pollutant mixtures from discharge of a separate sewer system. Water Res. 2018;135:122–133. doi: 10.1016/j.watres.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Birgersson L., Borbely G., Caporale N., Germain P.L., Leemans M., Rendel F.…Testa Giuseppe. 2017. From Cohorts to Molecules: Adverse Impacts of Endocrine Disrupting Mixtures. [DOI] [PubMed] [Google Scholar]
- Boon P.E., van Donkersgoed G., Christodoulou D., Crépet A., D'Addezio L., Desvignes V.…van Klaveren J.D. Cumulative dietary exposure to a selected group of pesticides of the triazole group in different European countries according to the EFSA guidance on probabilistic modelling. Food Chem. Toxicol. 2015;79:13–31. doi: 10.1016/j.fct.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Bopp S., Berggren E., Kienzler A., van der Linden S., Worth A. EUR 27471 EN. 2015. Scientific methodologies for the combined effects of chemicals – a survey and literature review. [Google Scholar]
- Bopp S.K., Kienzler A., van der Linden S., Lamon L., Paini A., Parissis N., Richarz A.-N., Triebe J., Worth A. EUR 27968 EN. 2016. Review of case studies on the human and environmental risk assessment of chemical mixtures. [Google Scholar]
- Bornehag C.G., Gennings C. Deliverable Report D2.1 Report on Critical EDC Mixtures I, WP2 Epidemiology and Biostatistics, EDC-MixRisk, 28 of December 2016. 2016. https://cordis.europa.eu/project/rcn/193310_en.html
- Brack W., Altenburger R., Schüürmann G., Krauss M., López Herráez D., van Gils J.…de Aragão Umbuzeiro G. The SOLUTIONS project: challenges and responses for present and future emerging pollutants in land and water resources management. Sci. Total Environ. 2015;503–504:22–31. doi: 10.1016/j.scitotenv.2014.05.143. [DOI] [PubMed] [Google Scholar]
- Brack W., Ait-Aissa S., Burgess R.M., Busch W., Creussot N., Di Paolo C., Escher B.I.…Kraus M. Effect-directed analysis supporting monitoring of aquatic environments — an in-depth overview. Sci. Total Environ. 2016;544:1073–1118. doi: 10.1016/j.scitotenv.2015.11.102. [DOI] [PubMed] [Google Scholar]
- Brack W., Dulio V., Ågerstrand M., Allan I., Altenburger R., Brinkmann M.…Vrana B. Towards the review of the European Union Water Framework Directive: recommendations for more efficient assessment and management of chemical contamination in European surface water resources. Sci. Total Environ. 2017;576:720–737. doi: 10.1016/j.scitotenv.2016.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch W., Schmidt S., Kühne R., Schulze T., Krauss M., Altenburger R. Micropollutants in European rivers: a mode of action survey to support the development of effect-based tools for water monitoring. Environ. Toxicol. Chem. 2016 doi: 10.1002/etc.3460. [DOI] [PubMed] [Google Scholar]
- Carvalho R.N., Arukwe A., Ait-Aissa S., Bado-Nilles A., Balzamo S., Baun A.…Lettieri T. Mixtures of chemical pollutants at European legislation safety concentrations: how safe are they? Toxicol. Sci. 2014;141(1):218–233. doi: 10.1093/toxsci/kfu118. 2014 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépet A., Tressou J., Graillot V., Béchaux C., Pierlot S., Héraud F., Leblanc J.C. Identification of the main pesticide residue mixtures to which the French population is exposed. Environ. Res. 2013;126:125–133. doi: 10.1016/j.envres.2013.03.008. 2013 Oct. [DOI] [PubMed] [Google Scholar]
- Dalla Costa S., Kephalopoulos S., Bopp S., Kienzler A., Richarz A., Van der Linden S., Korytar P., Backhaus T., Lebret E., Van Klaveren J., Van der Voet H. Vol. 2018. European Commission, Joint Research Centre; Ispra: 2018. JRC Workshop on IPCHEM Supporting the Assessment of Chemical Mixtures - Final Report. (PUBSY No.112434) [Google Scholar]
- Daneshian M., Kamp H., Hengstler J., Leist M., van de Water B. Highlight report: launch of a large integrated European in vitro toxicology project: EU-ToxRisk. Arch. Toxicol. 2016;90(5):1021–1024. doi: 10.1007/s00204-016-1698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zwart D., Posthuma L. Complex mixture toxicity for single and multiple species: proposed methodologies. Environ. Toxicol. Chem. 2005;24(10):2665–2676. doi: 10.1897/04-639r.1. [DOI] [PubMed] [Google Scholar]
- Delmaar C., Bokkers B., Ter Burg W., Schuur G. Validation of an aggregate exposure model for substances in consumer products: a case study of diethyl phthalate in personal care products. J. Expo. Sci. Environ. Epidemiol. 2015;25:317e323. doi: 10.1038/jes.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp J., Gutbier S., Klima S., Hoelting L., Pinto-Gil K., Hsieh J.H.…Leist M. A high-throughput approach to identify specific neurotoxicants/developmental toxicants in human neuronal cell function assays. ALTEX. 2018 doi: 10.14573/altex.1712182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalque L., Charlier C., Pirard C. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol. Lett. 2014;231(2):161–168. doi: 10.1016/j.toxlet.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Dorne J.L., Richardson J., Kass G., Georgiadis N., Monguidi M., Pasinato L.…Robinson T. OpenFoodTox: EFSA's open source toxicological database on chemical hazards in food and feed. EFSA J. 2017;15:3. doi: 10.2903/j.efsa.2017.e15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC European Commission Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities (2007–2013) 2006. http://ec.europa.eu/research/participants/data/ref/fp7/90448/fp7ec_en.pdf
- EC European Commission . Chemical Mixtures, COM. Vol. 2012. 2012. Communication from the Commission to the Council - the combination effects of chemicals; p. 10. [Google Scholar]
- EC European Commission Horizon 2020 - the Framework Programme for Research and Innovation (2014–2020) 2013. http://ec.europa.eu/research/participants/data/ref/h2020/legal_basis/sp/h2020-sp_en.pdf
- EFSA . June 2018. Scientific Committee (2018) “Draft Guidance on Harmonised Methodologies for Human Health, Animal Health and Ecological Risk Assessment of Combined Exposure to Multiple Chemicals” for Public Consultation, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) International framework dealing with human risk assessment of combined exposure to multiple chemicals. EFSA J. 2013;11(7):3313. [Google Scholar]
- EFSA (European Food Safety Authority) Modern methodologies and tools for human hazard assessment of chemicals. EFSA J. 2014;12(4):3638. [Google Scholar]
- EFSA (European Food Safety Authority) EFSA Supporting Publication 2015:EN-784. 2015. Harmonisation of human and ecological risk assessment of combined exposure to multiple chemicals. (39 pp.) [Google Scholar]
- EFSA Panel on Plant Protection Products and their Residues (PPR) Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA J. 2012;10(10):2839. 2012. (95 pp.) [Google Scholar]
- EFSA Panel on Plant Protection Products and their Residues (PPR) Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (2014 update) EFSA J. 2014;11(7):3293. 2013. (131 pp.) [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) Scientific Opinion on relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides residues in food. EFSA J. 2013;11(12):3472. 2013. (40 pp.) [Google Scholar]
- Escher B.I., Aїt-Aїssa S., Behnisch P.A., Brack W., Brion F., Brouwer A.…Neale P.A. Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci. Total Environ. 2018;628-629:748–765. doi: 10.1016/j.scitotenv.2018.01.340. [DOI] [PubMed] [Google Scholar]
- EuroMix . Thon Hotel EU; Rue de la Loi 75, 1040 Bruxelles, Belgium: 2017. Report of EuroMix Second Workshop on International Harmonisation on the Risk Assessment of Combined Exposures to Chemicals, 17 May 2017.http://www.euromixproject.eu/wp-content/uploads/2017/11/Harmonisation-WS-2-Report-Final-for-web.pdf [Google Scholar]
- Evans R.M., Martin O.V., Faust M., Kortenkamp A. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci. Total Environ. 2016;543(Pt A):757–764. doi: 10.1016/j.scitotenv.2015.10.162. [DOI] [PubMed] [Google Scholar]
- Gosens I., Delmaar C.J.E., Burg W., De Heer C., Schuur A.G. Aggregate exposure approaches for parabens in personal care products: a case assessment for children between 0 and 3 years old. 2013;24(2):208–214. doi: 10.1038/jes.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grech A., Brochot C., Dorne J.L., Quignot N., Bois F.Y., Beaudouin R. Toxicokinetic models and related tools in environmental risk assessment of chemicals. Sci. Total Environ. 2017;578:1–15. doi: 10.1016/j.scitotenv.2016.10.146. [DOI] [PubMed] [Google Scholar]
- Groten J.P., Feron V.J., Sühnel J. Toxicology of simple and complex mixtures. Trends Pharmacol. Sci. 2001;22:316–322. doi: 10.1016/s0165-6147(00)01720-x. [DOI] [PubMed] [Google Scholar]
- Hollender J., Schymanski E.L., Singer H.P., Ferguson P.L. Nontarget screening with high resolution mass spectrometry in the environment: ready to go? Environ. Sci. Technol. 2017;51:11505–11512. doi: 10.1021/acs.est.7b02184. [DOI] [PubMed] [Google Scholar]
- Junghans M., Backhaus T., Faust M., Scholze M., Grimme L.H. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006;76(2):93–110. doi: 10.1016/j.aquatox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Karjalainen T., Hoeveler A., Draghia-Akli R. European Union research in support of environment and health: building scientific evidence base for policy. Environ. Int. 2017;103:51–60. doi: 10.1016/j.envint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Kennedy M.C., Glass C.R., Bokkers B., Hart A.D.M., Hamey P.Y., Kruisselbrink J.W.…van Klaveren J.D. A European model and case studies for aggregate exposure assessment of pesticides. Food Chem. Toxicol. 2015;79:32–44. doi: 10.1016/j.fct.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Kienzler A., Berggren E., Bessems J., Bopp S., van der Linden S., Worth A. EUR 26675 EN. 2014. Assessment of mixtures - review of regulatory requirements and guidance. [Google Scholar]
- Kienzler A., Bopp S.K., van der Linden S., Berggren E., Worth A. Regulatory assessment of chemical mixtures: requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016 doi: 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- König M., Escher B.I., Neale P.A., Krauss M., Hilscherová K., Novák J.…Brack W. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ. Pollut. 2017;220(Part B):1220–1230. doi: 10.1016/j.envpol.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr. Opin. Pharmacol. 2014;19:105–111. doi: 10.1016/j.coph.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Faust M. Regulate to reduce chemical mixture risk. Science. 2018;361(6399):24–226. doi: 10.1126/science.aat9219. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A., Backhaus T., Faust M. 2009. State of the Art Report on Mixture Toxicity. Final Report. 070307/2007/485103/ETU/D.1. (391 pp.) [Google Scholar]
- Kruisselbrink J., van der Voet H., van Donkersgoed G., van Klaveren J. EFSA Supporting Publication 2018. 2018. Proposal for a data model for probabilistic cumulative dietary exposure assessments of pesticides in line with the MCRA software.https://www.efsa.europa.eu/en/supporting/pub/1375e Available on. [Google Scholar]
- Liess M., Foit K., Knillmann S., Schaefer R.B., Liess H.D. Predicting the synergy of multiple stress effects. Sci. Rep. 2016;6:32965. doi: 10.1038/srep32965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindim C., van Gils J., Cousins I.T. A large-scale model for simulating the fate and transport of organic contaminants in river basins. Chemosphere. 2016;144:803–810. doi: 10.1016/j.chemosphere.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Malaj E., von der Ohe P.C., Grote M., Kühne R., Mondy C.P., Usseglio-Polatera P.…Schäfer R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. U. S. A. 2014;111(26):9549–9554. doi: 10.1073/pnas.1321082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massei R., Busch W., Wolschke H., Schinkel L., Bitsch M., Schulze T., Krauss M., Brack W. Screening of pesticide and biocide patterns as risk drivers in sediments of major European river mouths: ubiquitous or river basin-specific contamination? Environ. Sci. Technol. 2018;52:2251–2260. doi: 10.1021/acs.est.7b04355. [DOI] [PubMed] [Google Scholar]
- Mav D., Shah R.R., Howard B.E., Auerbach S.S., Bushel P.R., Collins J.B.…Paules R.S. A hybrid gene selection approach to create the S1500+ targeted gene sets for use in high-throughput transcriptomics. PLoS One. 2018;13(2):e0191105. doi: 10.1371/journal.pone.0191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek M.E.B., Boobis A.R., Crofton K.M., Heinemeyer G., Van Raaij M., Vickers C. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul. Toxicol. Pharmacol. 2011;60:S1–S14. doi: 10.1016/j.yrtph.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Moretto A., Bachman A., Boobis A., Solomon K.R., Pastoor T.P., Wilks M.F., Embry M.R. A framework for cumulative risk assessment in the 21st century. Crit. Rev. Toxicol. 2016:1–13. doi: 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- Muschket M., Di Paolo C., Tindall A.J., Touak G., Phan A., Krauss M.…Brack W. Identification of unknown antiandrogenic compounds in surface waters by effect-directed analysis (EDA) using a parallel fractionation approach. Environ. Sci. Technol. 2018;52:288–297. doi: 10.1021/acs.est.7b04994. [DOI] [PubMed] [Google Scholar]
- Muz M., Krauss M., Kutsarova S., Schulze T., Brack W. Mutagenicity in surface waters: synergistic effects of carboline alkaloids and aromatic amines. Environ. Sci. Technol. 2017;51:1830–1839. doi: 10.1021/acs.est.6b05468. [DOI] [PubMed] [Google Scholar]
- Neale P.A., Ait-Aissa S., Brack W., Creusot N., Denison M.S., Deutschmann B.…Escher B.I. Linking in vitro effects and detected organic micropollutants in surface water using mixture-toxicity modeling. Environ. Sci. Technol. 2015;49:14614–14624. doi: 10.1021/acs.est.5b04083. [DOI] [PubMed] [Google Scholar]
- Neale P.A., Altenburger R., Ait-Aissa S., Brion F., Busch W., Umbuzeiro G.D.…Escher B.I. Development of a bioanalytical test battery for water quality monitoring: fingerprinting identified micropollutants and their contribution to effects in surface water. Water Res. 2017;123:734–750. doi: 10.1016/j.watres.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Neale P.A., Munz N.A., Ait-Aissa S., Altenburger R., Brion F., Busch W.…Hollender J. Integrating chemical analysis and bioanalysis to evaluate the contribution of wastewater effluent on the micropollutant burden in small streams. Sci. Total Environ. 2017;576:785–795. doi: 10.1016/j.scitotenv.2016.10.141. [DOI] [PubMed] [Google Scholar]
- Neale P.A., Brack W., Ait-Aissa S., Busch W., Hollender J., Krauss M.…Escher B.I. Solid-phase extraction as sample preparation of water samples for cell-based and other in vitro bioassays. Environ. Sci.: Processes Impacts. 2018;20(3):493–504. doi: 10.1039/c7em00555e. [DOI] [PubMed] [Google Scholar]
- Nowell L.H., Norman J.E., Moran P.W., Martin J.D., Stone W.W. Pesticide Toxicity Index — a tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. Sci. Total Environ. 2014;476–477:144–157. doi: 10.1016/j.scitotenv.2013.12.088. [DOI] [PubMed] [Google Scholar]
- Price P., Han X. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int. J. Environ. Res. Public Health. 2011;2011(8):2212–2225. doi: 10.3390/ijerph8062212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P., Dhein E., Hamer M., Han X., Heneweer M., Junghans M.…Rodriguez C. A decision tree for assessing effects from exposures to multiple substances. Environ. Sci. Eur. 2012;24:26. 2012. [Google Scholar]
- Quignot N., Béchaux C., Amzal B. EFSA Supporting Publication. Vol. 85. 2015. Data collection on toxicokinetic and toxicodynamic interactions of chemical mixtures for human risk assessment. [Google Scholar]
- Quignot N., Grech A., Amzal B. EFSA Supporting Publication. 2015. Data collection on combined toxicity of multiple chemicals for animal health and ecological risk assessment; p. 112. [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna: 2014. R: A Language and Environment for Statistical Computing.http://www.R-Project.org/ [Google Scholar]
- Robinson O., Vrijheid M. The pregnancy exposome. Curr. Environ. Health Rep. 2015;2(2):204–213. doi: 10.1007/s40572-015-0043-2. [DOI] [PubMed] [Google Scholar]
- Sarigiannis D.A. Assessing the impact of hazardous waste on children's health: the exposome paradigm. Environ. Res. 2017;158:531–541. doi: 10.1016/j.envres.2017.06.031. [DOI] [PubMed] [Google Scholar]
- SCHER, SCCS, SCENIHR . 2012. Opinion on the Toxicity and Assessment of Chemical Mixtures. [Google Scholar]
- Schulze T., Krauss M., Novak J., Hilscherova K., Ait-Aissa S., Creusot N.…Brack W. Large volume sampling and effect-based screening. In: Liska I., Wagner F., Sengl M., Deutsch K., Slobodnik J., editors. Joint Danube Survey 3. A Comprehensive Analysis of Danube Water Qiuality. ICPDR - International Commission for the Protection of the Danube River; Vienna, Austria: 2015. pp. 284–295. [Google Scholar]
- Schulze T., Ahel M., Ahlheim J., Ait-Aissa S., Brion F., Di Paolo C.…Brack W. Assessment of a novel device for onsite integrative large-volume solid phase extraction of water samples to enable a comprehensive chemical and effect-based analysis. Sci. Total Environ. 2017;581:350–358. doi: 10.1016/j.scitotenv.2016.12.140. [DOI] [PubMed] [Google Scholar]
- Tarazona J.V., Rodríguez C., Alonso E., Sáez M., González F., San Andrés M.D., Jiménez B., San Andrés M.I. Toxicokinetics of perfluorooctane sulfonate in birds under environmentally realistic exposure conditions and development of a kinetic predictive model. Toxicol. Lett. 2015;232(2):363–368. doi: 10.1016/j.toxlet.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Topping C.J., Craig P.S., de Jong F., Klein M., Laskowski R., Manachini B.…van der Linden T. Towards a landscape scale management of pesticides: ERA using changes in modelled occupancy and abundance to assess long-term population impacts of pesticides. Sci. Total Environ. 2015;537:159–169. doi: 10.1016/j.scitotenv.2015.07.152. [DOI] [PubMed] [Google Scholar]
- Toropov A.A., Toropova A.P., Marzo M., Dorne J.L., Georgiadis N., Benfenati E. QSAR models for predicting acute toxicity of pesticides in rainbow trout using the CORAL software and EFSA's OpenFoodTox database. Environ. Toxicol. Pharmacol. 2017;53:158–163. doi: 10.1016/j.etap.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Toropova A.P., Toropov A.A., Marzo M., Escher S.E., Dorne J.L., Georgiadis N., Benfenati E. The application of new HARD-descriptor available from the CORAL software to building up NOAEL models. Food Chem. Toxicol. 2018;112:544–550. doi: 10.1016/j.fct.2017.03.060. [DOI] [PubMed] [Google Scholar]
- Turner M.C., Vineis P., Seleiro E., Dijmarescu M., Balshaw D., Bertollini R., EXPOSOMICS Consortium EXPOSOMICS: final policy workshop and stakeholder consultation. BMC Public Health. 2018;18(1):260. doi: 10.1186/s12889-018-5160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L.N., Ågerstrand M., Beronius A., Beausoleil C., Bergman Å., Bero L.A.…Rudén C. A proposed framework for the systematic review and integrated assessment (SYRINA) of endocrine disrupting chemicals. Environ. Health. 2016;14(15(1)):74. doi: 10.1186/s12940-016-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson A.N., Carere M., Maggi C. Technical report on Aquatic Effect-based Monitoring Tools. Technical Report - 2014 – 077. 2014. https://circabc.europa.eu/sd/a/0d78bbf7-76f0-43c1-8af2-6230436d759d/Effect-based%20tools%20CMEP%20report%20main%2028%20April%202014.pdf
- van der Voet H., de Boer W., Kruisselbrink J., van Donkersgoed G., van Klaveren J.(.R.I.V.M). MCRA Made Scalable for Large Cumulative Assessment Groups. 2016. https://www.efsa.europa.eu/en/supporting/pub/910e EFSA supporting publication available on.
- van der Voet H., de Boer W.J., Kruisselbrink J.W., van Lenthe M.S., Crépet A., Kennedy M.C., Sprong C., van Klaveren J. Model Integration Into a Web-based Model and Data Toolbox. 2017. Demonstration prototype of the EuroMix model toolbox.https://zenodo.org/record/1066087#.WuixZtJlI5s Available on. [Google Scholar]
- van Wezel A.P., ter Laak T.L., Fischer A., Bauerlein P.S., Munthe J., Posthuma L. Mitigation options for chemicals of emerging concern in surface waters; operationalising solutions-focused risk assessment. Environ. Sci.: Water Res. Technol. 2017;3:403–414. [Google Scholar]
- WHO/IPCS . World Health Organization; Geneva: 2004. IPCS Risk Assessment Terminology, Part 1: IPCS/OECD Key Generic Terms Used in Chemical Hazard/Risk Assessment. [Google Scholar]
- Wink S., Hiemstra S.W., Huppelshoten S., Klip J.E., van de Water B. Dynamic imaging of adaptive stress response pathway activation for prediction of drug induced liver injury. Arch. Toxicol. 2018 doi: 10.1007/s00204-018-2178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]