Abstract
Objective:
To examine the frequency of and factors associated with emergency department (ED) ICP monitor placement in severe pediatric TBI.
Methods:
Retrospective, multicenter, cohort study of children<18 years admitted to the ED with severe TBI and intubated for >48 hours from 2007–2011.
Results:
224 children had severe TBI and 75% underwent either ED, operating room (OR) or pediatric intensive care unit (PICU) ICP monitor placement. 4/5 centers placed ICP monitors in the ED, mostly (83%) fiberoptic. Nearly 40% of patients receiving an ICP monitor had it placed in the ED (29% overall). Factors associated with ED ICP monitor placement were: age 13 to<18 years olds compared to infants (aRR 2.02;95% CI 1.37, 2.98), longer ED length of stay (LOS)(aRR 1.15;95% CI 1.08, 1.21), trauma center designation pediatric only I/II compared to adult/pediatric I/II (aRR 1.71;95% CI 1.48, 1.98) and higher mean pediatric TBI patient volume (aRR 1.88;95% CI 1.68, 2.11). Adjusted for center, higher bedside ED staff was associated with longer ED LOS (aRR 2.10;95% CI 1.06, 4.14).
Conclusion:
ICP monitors are frequently placed in the ED at pediatric trauma centers caring for children with severe TBI. Both patient and organizational level factors are associated with ED ICP monitor placement.
Keywords: emergency department, intracranial pressure monitor, pediatrics, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality in children and adolescents [1]. Between 2002–2006, over half a million children 0 to 14 years sustained TBI annually in the United States, resulting in approximately 473,947 emergency department (ED) visits [2]. Children 0–4 years had the highest rate of TBI related ED visits (1,256 per 100, 000 population), followed by adolescents 15–19 years, (757 per 100, 000 population) [2]. Intracranial pressure (ICP) monitoring is considered an important strategy to guide goal directed management of high ICP in severe TBI care [3] because ICP monitoring may result in treatment that improves cerebral perfusion and improve outcomes [4]. Additionally, ICP monitor placement earlier in the course of treatment such as in the ED may be important, especially in children with severe TBI who may experience delay due to transfer procedures, availability of operating room (OR) and/or bed availability in the pediatric intensive care unit (PICU). Early placement of ICP monitors may also help guide management of patients with intracranial hypertension or at higher likelihood of developing intracranial hypertension as well as in decision making regarding transfer locations to OR or PICU. However, there is a paucity of information on when and where ICP monitors are placed after severe TBI. Moreover, the Brain Trauma Foundation (BTF) guidelines do not address timing or location of ICP monitor placement [3,5].
Despite national recommendations, there are large national variations in the use of ICP monitors in the care of children with severe TBI. This variation may be attributed to the lack of conclusive evidence that ICP monitors affect outcomes, as well as a complex interplay between patient and organizational level factors that may affect the decision to place an ICP monitor [6,7]. While patient level factors such as older age [6–8], higher head abbreviated injury severity (AIS) score, higher injury severity score (ISS) [6,7,9] and the presence of intracranial hemorrhage on head CT, and organizational factors such as trauma center designation [6,7] and higher patient volume [6], have separately been associated with higher ICP monitor utilization, no study has simultaneously considered how these factors may be related to the timing or location of ICP monitor placement. To increase our understanding of the influence of these factors on location of ICP monitor placement, we examined the frequency of and patient and organizational level factors associated with ICP monitor placement in the ED versus later in the operating room (OR) or pediatric intensive care unit (PICU) in children with severe TBI. We also qualitatively assessed clinician perspectives on location of ICP monitoring to provide context and understanding of the practice of ED ICP monitor placement. We hypothesized that ED ICP monitor placement may be infrequent given the notion that the ED is a much lesser sterile environment compared to the PICU with increased emphasis on quick stabilization and transfer of patients to the PICU. We also hypothesized that both patient and organizational level factors may be associated with ICP monitor placement in the ED.
Materials and Methods
Study center selection and data sources
Data were collected from 224 (94.9%) children with complete ED records from the parent Pediatric Guideline Adherence and Outcomes (PEGASUS) study of 236 patients [4]. The PEGASUS study was a retrospective, multicenter cohort study that included data abstracted from five regional pediatric trauma centers (PTC) affiliated with academic medical centers located in 5 different U.S. states between 2007–2011 [4]. These centers were recruited for the parent study based on willingness to participate, a priori ability to contribute data from 30 to 50 children with severe TBI annually, electronic medical records, representation in the Pediatric Neurocritical Care Research Group (http://www.pncrg.org/), PTC publications, regional PTC status, and recognized expertise severe TBI care. Study centers included the University of Washington’s Harborview Medical Center, Seattle, WA (lead and data coordinating center); Children’s Hospital of Pittsburgh, Pittsburgh, PA; Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Harbor University of California, Los Angeles Medical Center, Torrance, CA; and Nationwide Children’s Hospital, Columbus, OH [4]. Human subjects’ committee approval was obtained at each site. Written or verbal informed consent was not required for this study per the Institutional Review Board, as this was a retrospective chart review. Patient records and information was anonymized and de-identified before analysis at each site.
Study population
Eligible participants were patients < 18 years with TBI, defined as having one or more International Classification of Diseases, 9th edition, discharge diagnosis code: 800.0–801.9, 803.0–804.9, 850.0–854.1, 959.01, 950.1–950.3, and 995.55 [10]. Criteria for severe TBI required: a minimum head Abbreviated Injury Severity (AIS) score ≥ 3, post-resuscitation Glasgow Coma Scale (GCS) score ≤ 8, alive with tracheal intubation in the intensive care unit (ICU) > 48 hours from ICU admission, trauma history, and abnormal admission head computed tomography (CT) findings. Type of ICP monitoring (external ventricular drain [EVD] or fiberoptic device) was recorded.
Covariates and outcomes (Quantitative analysis)
Patient and organizational level factors that may be associated with the decisions to place an ICP monitor in the ED were determined a priori based on published literature and knowledge of the care of children with severe TBI.
Table 1 shows the patient and organizational characteristics of 224 children admitted to the ED with severe TBI in the study. Patient level factors included: age [6–8], head abbreviated injury severity score and injury severity score [6,7,9], inflicted injury [6,7,11], epidural or subdural or subarachnoid hemorrhage [7], intraventricular hemorrhage [9], prehospital hypotension [9], ED hypotension, decompressive craniectomy [6], direct admission to trauma center, intracerebral hemorrhage, and contusion on head CT scan. Hypotension was defined as systolic blood pressure < 70 + 2*age [years]).
Table 1:
Characteristics of 224 Children from Five Trauma Centers Admitted to the Emergency Department (ED) with Severe Traumatic Brain Injury (TBI).
| Characteristics | N=224 N (%) |
|---|---|
| Demographic | |
| Age (years) | |
| < 1y | 42 (19) |
| 1 to < 5y | 48 (21) |
| 5 to <13y | 47 (21) |
| 13 to <18y | 87 (39) |
| Gender | |
| Male | 157 (70) |
| Clinical | |
| Head abbreviated injury severity score | |
| 3 or 4 | 100 (45) |
| 5 or 6 | 124 (55) |
| Injury severity score | |
| <15 | 15 (7) |
| ≥15 | 209 (93) |
| Inflicted injury | 50 (22) |
| Direct admit | 126 (56) |
| Head CT findings | |
| Epidural (EDH), subdural hematoma (SDH) or subarachnoid hemorrhage (SAH) | 192 (86) |
| Intracerebral hemorrhage | 70 (31) |
| Intraventricular hemorrhage | 46 (21) |
| Contusion | 89 (40) |
| Prehospital (PH) hypotension only (n = 200)* | 41 (21) |
| ED hypotension**only | 29 (13) |
| PH or ED hypotension | 57 (25) |
| Decompressive craniectomy± (n = 112) | 69 (62) |
| Outcome | |
| In-hospital mortality | 27 (12) |
| Glasgow outcome scale score at discharge for survivorsβ (n=197) | |
| Poor | 128 (65) |
| Good | 69 (35) |
| Organizational | |
| ED Length of stay (LOS) median (IQR) hours | 1.3 (0.9–2.7) |
| Trauma center designation | |
| Adult and pediatric I/II (2 centers) | 102 (46) |
| Pediatric only I/II (3 centers) | 122 (54) |
| Mean pediatric TBI patient volume per center≠ | |
| < 200 (2 centers) | 93 (42) |
| ≥ 200 (3 centers) | 131 (58) |
| Total number of ED bedside staff | |
| < 8 (2 centers) | 87 (39) |
| ≥ 8 (3 centers) | 137 (61) |
| Neurosurgery resident present in ED (2 centers) | 91 (41) |
Prehospital (PH) includes EMS (flight + ambulance or ambulance only) and Index hospital combined; PH blood pressure data missing for 24 children
Systemic hypotension definition= systolic blood pressure < 70 + 2 (age [years])
Decompressive craniectomy for high intracranial pressure (ICP), EDH, SDH or cerebral edema within 48 hours of trauma center admission; High intracranial hypertension (ICP) is defined as presence of cerebral herniation (unequal pupils or hypertension and bradycardia, as determined clinically), administration of mannitol or hypertonic saline, or ICP > 20 mm Hg if ICP monitor was placed
Glasgow outcome scale (GOS) score at discharge for survivors (n=197) is dichotomized to poor GOS (vegetative state & major impairment) and good GOS (minor impairment & baseline status)
Mean pediatric TBI patient volume per center = Total TBI (head abbreviated injury severity score > 1) patient (age <18 years) hospital volume for the period 2007–2011 (5 years) divided by 5 for each center
Organizational level factors were: trauma center designation [6,7], mean pediatric TBI patient volume per center [6,7], number of bedside ED staff, neurosurgery resident physically present as part of the trauma team in the ED and ED length of stay (LOS). All factors were considered as categorical variables except for ED LOS, which was examined as a continuous variable.
The main outcome of interest was ED ICP monitor placement, which was examined as a dichotomous variable. We also examined any ICP monitor placement (yes/no) which was defined as placement of ICP monitor in any treatment location (ED, OR or PICU). Other outcomes were: in-hospital mortality (yes/ no) and discharge Glasgow Outcome Scale (GOS) score (dichotomous variable among survivors with poor [vegetative state or major impairment] and good [minor impairment or baseline status] GOS) [12].
Reasons for ED ICP monitor placement (Qualitative analysis)
Common reasons for ED ICP monitor placement were collected from study investigators of the 4 centers which reported ED ICP monitor placement. Study investigators asked local neurosurgeons to identify whether patient (demographics, clinical characteristics/conditions), organizational (structural or process i.e., ED LOS) or other factors commonly affected decisions to place an ICP monitor in the ED rather than in the OR or PICU. Two questions posed to the chief of neurosurgery or faculty neurosurgeons (if > 1 faculty member) were: 1) What drives ICP monitor placement in the ED at your center?, 2) Are there patient CT findings such as intracerebral hemorrhage or anticipated longer ED LOS that drive your clinical decision making? Study investigators JG, RM, MW, MSV then used this information to develop a common scenario process flow, as previously described [13,14], which includes both the decision logic involved in ED ICP monitor placement and the proportion of patients who underwent ED ICP monitor placement. Majority responses were used to attribute ED LOS as either an exposure or outcome in the association between ED LOS and ED ICP monitor placement.
Statistical analysis
Demographic, clinical, outcome and organizational characteristics of the entire cohort (N = 224) were examined in univariate analyses and described using frequencies and proportions. As none of the patients from center 1 received an ED ICP monitor, we included only centers 2–5 for the bivariate and multivariable analyses to examine factors associated with ED ICP monitor placement. We performed bivariate and multivariable analyses using generalized linear mixed models (GLMM) with Poisson distribution and robust error variance with centers specified for random effect to estimate risk ratios (RRs) and 95% confidence intervals (CIs) [15–18]. This model allows each center to have its own intercept that is used to provide weighted population effect sizes and standard errors, thus assuming a constant effect of the factor across centers. Statistically significant factors (p < 0.05) from the bivariate models were considered for the final multivariable model. Multivariable GLMMs were performed to examine fixed and random effects of the factors on outcomes and yield adjusted relative risk (aRR) estimates. Individual random intercepts for each center were predicted in post-estimation and examined to assess between-center differences in the factors with the outcomes. Stata/MP 14 was used for analysis [19].
Results
Patient characteristics
The majority were five years or older (60%), male (70%) and directly admitted (56%; Table 1). Most (93%) children had ISS of 15 and above, and many (55%) had a head AIS score of five or six. Prehospital or ED hypotension was common (25%). Fifty (22%) children had inflicted injury and 192 (86%) had a head CT finding of epidural hematoma (EDH), subdural hematoma (SDH) or subarachnoid hemorrhage (SAH). Intracerebral hemorrhage (31%) and contusion (40%) were common. Sixty nine (62%) of the 112 children with high ICP, EDH, SDH or SAH within 48 hours of admission underwent decompressive craniectomy. In-hospital mortality was 12% (27), and among 197 survivors, 65% had poor GOS. Median ED LOS was 1.3 hours (IQR 0.9–2.7).
Frequency, location and type of ICP monitors placed
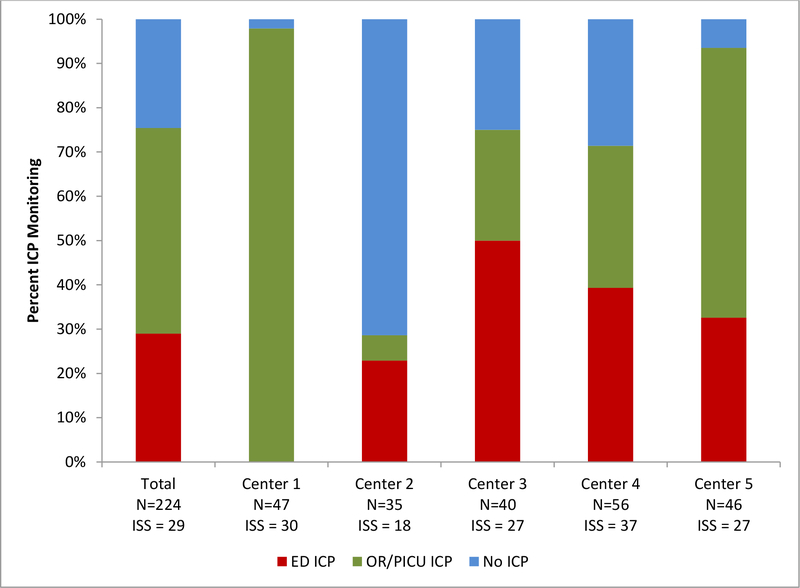
Among 224 children in our study, 169 (75%) underwent any ICP monitor placement and 65 (29%) underwent ED ICP monitor placement (Figure 1). Except for center 2, most centers placed an ICP monitor irrespective of location in majority of the TBI patients, the frequency of any ICP monitor placement ranging from 29% to 98% (Table 2; Figure 1). There was large between center variation in the frequency of ED ICP monitor placement (p < 0.001; Figure 1). Patients in center 1 underwent ICP monitor placement only in the OR or PICU and had a shorter median ED LOS (0.9 hours) compared to other centers, while the majority of patients in centers 2, 3, 4 who had any ICP monitors (80%, 67% & 55% respectively) underwent ICP monitor placement in the ED (Table 2).
Figure 1.
is a stacked bar graph showing the frequency and location (emergency department [ED], operating room [OR] or pediatric intensive care unit [PICU]) of intracranial pressure (ICP) monitor placement by center in 224 children with severe traumatic brain injury.
Table 2: Characteristics and Clinical Care of 224 Children from Five Trauma Centers Admitted to the Emergency Department (ED) with Severe Traumatic Brain Injury (TBI).
Any intracranial pressure (ICP) monitor placement = placement of ICP monitor in the Emergency Department (ED) or Operating Room (OR) or Pediatric Intensive Care Unit (PICU). Center 1 does not place ED ICP monitors whereas Centers 2–5 reported placing ICP monitors in the ED.
| Center 1 N = 47 N (%) | Center 2 N = 35 N (%) | Center 3 N = 40 N (%) | Center 4 N = 56 N (%) | Center 5 N = 46 N (%) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| < 1y | 3 (6) | 19 (54) | 11 (28) | 4 (7) | 5 (11) |
| 1 to < 5 | 11(23) | 7 (20) | 10 (25) | 10 (18) | 10 (22) |
| 5 to <13y | 16 (34) | 5 (14) | 7 (18) | 7 (13) | 12 (26) |
| 13 to <18y | 17 (36) | 4 (11) | 12 (30) | 35 (63) | 19 (41) |
| Head abbreviated injury severity score | |||||
| 3 or 4 | 22 (47) | 32 (91) | 20 (50) | 10 (18) | 16 (35) |
| 5 or 6 | 25 (53) | 3 (9) | 20 (50) | 46 (82) | 30 (65) |
| Injury severity score | |||||
| < 15 | 1 (2) | 10 (29) | 1 (2) | 1 (2) | 2 (4) |
| ≥ 15 | 46 (98) | 25 (71) | 39 (98) | 55 (98) | 44 (96) |
| Inflicted injury (yes/no) | 11 (23) | 18 (51) | 13 (33) | 2 (4) | 6 (13) |
| Direct admit (yes/no) | 23 (49) | 6 (17) | 20 (50) | 32 (57) | 45 (98) |
| Head CT findings | |||||
| Epidural or subdural hematoma or subarachnoid hemorrhage | 41 (87) | 30 (86) | 32 (80) | 47 (84) | 42 (91) |
| Intracerebral hemorrhage | 9 (19) | 4 (11) | 10 (25) | 29 (52) | 18 (39) |
| Intraventricular hemorrhage | 7 (15) | 3 (9) | 7 (18) | 21 (38) | 8 (17) |
| Contusion | 27 (57) | 7 (20) | 6 (15) | 26 (46) | 23 (50) |
| Prehospital (PH) hypotension only [n=39 (Center 1); n=34 (Center 2); n=32 (Center 3); n=55 (Center 4); n=40 (Center 5)] | 8 (21) | 6 (18) | 6 (19) | 19 (35) | 2 (5) |
| ED hypotension only (yes/no) | 2 (4) | 3 (9) | 7 (18) | 11 (20) | 6 (13) |
| PH or ED hypotension | 10 (21) | 8 (23) | 10 (25) | 22 (39) | 7 (15) |
| Decompressive craniectomy [n=31 (Center 1); n=15 (Center 2); n=15 (Center 3); n=30 (Center 4); n=21 (Center 5)] | 14 (45) | 10 (67) | 14 (93) | 11 (37) | 20 (95) |
| ED Length of stay (LOS) median (IQR) hours | 0.9 (0.7–1.1) | 1.1 (0.7–2.1) | 1.3 (1.1–1.8) | 2.5 (1.4–3.7) | 2.5 (1.3–5.8) |
| Any ICP monitor placement | 46 (98) | 10 (29) | 30 (75) | 40 (71) | 43 (93) |
| Fiberoptic* | 6 (13) | 6 (60) | 16 (53) | 38 (95) | 15 (35) |
| External ventricular drain (EVD)* | 40 (87) | 4 (40) | 14 (47) | 2 (5) | 28 (65) |
| ED ICP placement [n=46 (Center 1); n=10 (Center 2); n=30 (Center 3); n=40 (Center 4); n=43 (Center 5)] | 0 (0) | 8 (80) | 20 (67) | 22 (55) | 15 (35) |
| Fiberoptic** | 0 (0) | 6 (75) | 16 (80) | 22 (100) | 10 (67) |
| EVD** | 0 (0) | 2 (25) | 4 (20) | 0 (0) | 5 (33) |
| OR or PICU ICP placement [n=46 (Center 1); n=10 (Center 2); n=30 (Center 3); n=40 (Center 4); n=43 (Center 5)] | 46 (100) | 2 (20) | 10 (33) | 18 (45) | 28 (65) |
| Fiberoptic*** | 6 (13) | 0 (0) | 0 (0) | 16 (89) | 5 (18) |
| EVD*** | 40 (87) | 2 (100) | 10 (100) | 2 (11) | 23 (82) |
Fiberoptic and EVD Any ICP monitor types have n (%) out of n=46 (Center 1), n=10 (Center 2), n=30 (Center 3), n=40 (Center 4), n=43 (Center 5)
Fiberoptic and EVD ED ICP monitor types have n (%) out of n=0 (Center 1), n=8 (Center 2), n=20 (Center 3), n=22 (Center 4), n=15 (Center 5)
Fiberoptic and EVD OR or PICU ICP monitor types have n (%) out of n=46 (Center 1), n=2 (Center 2), n=10 (Center 3), n=18 (Center 4), n=28 (Center 5)
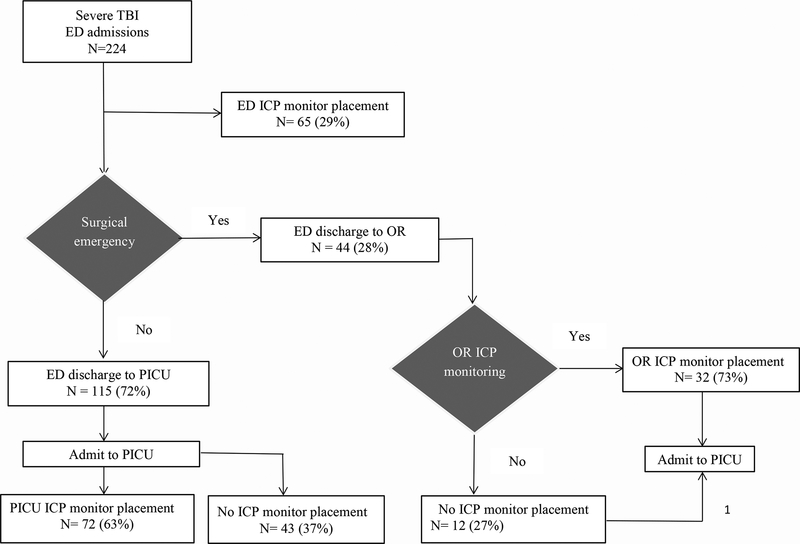
Among trauma centers 2–5 that placed an ICP monitor in the ED, 123 children underwent any ICP monitor placement and more than half (65/123; 53%) received an ICP monitor in the ED. Out of 65 children, 50 (77%) underwent ICP monitor placement within four hours of ED admission. There were 159 children discharged from the ED without an ICP monitor and 44 children directly transferred to the OR. Of these patients, 32 (73%) received OR ICP monitor placement. Among 115 children transferred from the ED, 72 (63%) underwent PICU ICP monitor placement (Figure 2).
Figure 2.
is a common scenario process flow for location (emergency department [ED], operating room [OR] or pediatric intensive care unit [PICU]) of intracranial pressure (ICP) monitor placement in children with severe traumatic brain injury across five centers.
Overall, fiberoptic ICP monitors were most common (83%), except for center 1 which placed EVD (87%) catheters (Table 2). The majority (74%) who underwent ICP monitor placement in OR or PICU in centers 1, 2, 3 and 5 received an EVD, whereas patients in center 4 almost exclusively (95%) underwent fiberoptic (95%) ICP monitor placement, irrespective of location.
Reasons for ED ICP monitor placement
Figure 2 shows the consensus-based, most common scenario process flow and decision logic regarding ED ICP monitor placement and the proportion of patients who underwent ED ICP monitor placement. Organizational factors influencing ED ICP monitor placement were a policy of placement only in the OR or PICU, anticipated PICU transfer delays, anticipated or knowledge of longer ED LOS and PICU transfer after repeat head CT (centers 1, 3, 4 & 5). Patient level factors at centers 2, 3 and 4 were injury severity, head CT findings such as intracerebral hemorrhage and sedation and paralysis. Since patient ED LOS was a factor cited by 4/5 centers, organizational preferences for patient management related to a longer ED LOS led to the consideration of ED LOS as an organizational factor (exposure variable) for the analysis examining factors associated with ED ICP monitor placement (outcome).
Patient level factors associated with ED ICP monitor placement
Among trauma centers 2–5 that placed ICP monitors in the ED, bivariate analyses of patient level factors showed that compared to children < 1year, those who were 13 to < 18 years old were more likely to receive an ED ICP monitor (Crude RR 1.75; 95% CI 1.41, 2.18; Table 3) and also, were more likely to receive an ICP monitor irrespective of location (Crude RR 2.05; 95% CI 1.35, 3.11). Although inflicted injury was not a factor associated with ED ICP monitor placement (Table 3), age was associated with inflicted trauma with infants more likely to have inflicted injury (p<0.001). Multivariable analyses showed a dose response with children > 13 years more likely to receive a monitor in the ED than infants (adjusted relative risk [aRR] 2.02; 95% CI 1.37, 2.98; Table 4). ED hypotension was associated with a higher likelihood of ED ICP monitor placement (Crude RR 1.29; 95% CI 1.02, 1.64; Table 3), however, this factor was not statistically significant in the multivariable analyses.
Table 3: Bivariate Analyses of Factors Associated with Emergency Department (ED) Intracranial Pressure (ICP) Monitor Placement (N=65) Among the Four Trauma Centers (Centers 2–5) That Placed ICP monitor in the ED (Total N = 123).
OR = Operating Room. PICU = Pediatric Intensive Care Unit.
| Factors | OR or PICU ICP monitor placement N = 58 (33%; 58/177) N (%) | ED ICP monitor placement N = 65 (37%; 65/177) N (%) | Crude RR (95% CI)* of ED ICP monitor placement |
|---|---|---|---|
| Age (years) | |||
| < 1y | 9 (16) | 5 (8) | 1 [Reference] |
| 1 to < 5y | 14 (24) | 14 (22) | 1.43 (0.70, 2.95) |
| 5 to <13y | 13 (22) | 12 (18) | 1.41 (0.99, 2.02) |
| 13 to <18y | 22 (38) | 34 (52) | 1.75 (1.41, 2.18) |
| Head abbreviated injury severity score | |||
| 3 or 4 | 19 (33) | 22 (34) | 1 [Reference] |
| 5 or 6 | 39 (67) | 43 (66) | 0.99 (0.78, 1.26) |
| Injury severity score | |||
| <15 | 4 (7) | 3 (5) | 1 [Reference] |
| ≥15 | 54 (93) | 62 (95) | 1.32 (0.47, 3.68) |
| Inflicted injury | 10 (17) | 8 (12) | 0.79 (0.58, 1.08) |
| Direct admit | 43 (74) | 44 (68) | 0.88 (0.52, 1.49) |
| Head CT findings | |||
| Epidural or subdural hematoma or subarachnoid hemorrhage | 52 (90) | 56 (86) | 0.88 (0.57, 1.34) |
| Intracerebral hemorrhage | 18 (31) | 27 (42) | 1.28 (0.89, 1.84) |
| Intraventricular hemorrhage | 11 (19) | 18 (28) | 1.24 (0.96, 1.60) |
| Contusion | 24 (41) | 26 (40) | 1.00 (0.87, 1.15) |
| Prehospital (PH) hypotension only (ED ICP monitor placement n = 56; OR or PICU ICP monitor placement n = 57) | 9 (16) | 10 (18) | 0.98 (0.86, 1.11) |
| ED hypotension only | 5 (9) | 10 (15) | 1.29 (1.02, 1.64) |
| PH or ED hypotension | 12 (21) | 16 (25) | 1.09 (0.94, 1.25) |
|
Decompressive craniectomy (ED ICP monitor placement n = 35; OR or PICU ICP monitor placement n = 29) |
22 (76) | 24 (69) | 0.85 (0.43, 1.70) |
| ED Length of stay (LOS) median (IQR) hours | 1.5 (1.2, 2.5) | 2.5 (1.3, 5.8) | 1.12 (1.05, 1.20) |
| Trauma center designation | |||
| Adult and pediatric I/II | 46 (79) | 37 (57) | 1 [Reference] |
| Pediatric only I/II | 12 (21) | 28 (43) | 1.57 (1.08, 2.29) |
| Mean pediatric TBI patient volume per center≠ | |||
| < 200 | 28 (48) | 15 (23) | 1 [Reference] |
| ≥ 200 | 30 (52) | 50 (77) | 1.79 (1.52, 2.11) |
| Total number of bedside ED staff | |||
| < 8 | 10 (17) | 20 (31) | 1 [Reference] |
| ≥ 8 | 48 (83) | 45 (69) | 0.73 (0.51, 1.04) |
| Neurosurgery resident present in ED | 20 (34) | 30 (46) | 1.25 (0.71, 2.20) |
Crude RR is relative risk with 95% confidence interval for factors associated with ED ICP monitor placement
Inflicted injury, direct admit, head CT findings, PH hypotension only, ED hypotension only, PH or ED hypotension, decompressive craniectomy are dichotomous variables (Yes/No) with Reference group = No
Table 4:
Factors Associated with Emergency Department (ED) Intracranial Pressure (ICP) Monitor Placement (N=65) Among Four Trauma Centers (Centers 2–5) Which Placed ICP monitor in the ED (Total N = 123).
| Factors | aRR (95% CI)* |
|---|---|
| Age (years) | |
| < 1y | 1 [Reference] |
| 1 to 4 | 1.53 (0.77, 3.04) |
| 5 to 12 | 1.60 (0.80, 3.22) |
| 13 to 17 | 2.02 (1.37, 2.98) |
| ED Length of stay (LOS) | 1.15 (1.08, 1.21) |
| Trauma center designation | |
| Adult and pediatric I/II | 1 [Reference] |
| Pediatric only I/II | 1.71 (1.48, 1.98) |
| Mean pediatric TBI patient volume per center≠ | |
| < 200 | 1 [Reference] |
| ≥ 200 | 1.88 (1.68, 2.11) |
*aRR is adjusted relative risk with 95% confidence interval for factors associated with ED ICP monitor placement
Out of 224 children, 67% (149) with EDH, SDH or SAH received ICP monitoring; 93 (62%) children had ICP monitor placement after ED discharge in the OR or PICU. Over 1/3 of children (32/93) who underwent ICP monitoring after ED discharge were emergently transferred to the OR. However, head CT findings including intracerebral and intraventricular hemorrhage were not associated with ED ICP monitor placement (Table 3).
Organizational level factors associated with ED ICP monitor placement
Longer ED LOS was associated with a higher likelihood of ED ICP monitor placement (aRR 1.15; 95% CI 1.08, 1.21; Table 4). Higher bedside ED staff (aRR 2.10; 95% CI 1.06, 4.14) was associated with longer ED LOS. Compared to adult and pediatric I/II trauma centers, pediatric only I/II trauma centers were more likely to place an ICP monitor in the ED (aRR 1.71; 95% CI 1.48, 1.98; Table 4). Higher mean pediatric TBI patient volume was associated with ED ICP monitor placement (aRR 1.88; 95% CI 1.68, 2.11; Table 4).
Exploring ED ICP monitor placement and discharge outcomes
There was no difference in in-hospital mortality (Crude RR 1.34; 95% CI 0.63, 2.84) or discharge GOS score (Crude RR 1.24; 95% CI 0.98, 1.57) in children between those who underwent ICP monitor placement in the ED versus in the OR/PICU among those with severe TBI admitted to trauma centers 2–5.
Discussion
In this study, we examined the frequency of and factors associated with ED ICP monitor placement in children with severe TBI. Main findings are: 1) ED ICP monitor placement is common, 2) Older age was associated with higher likelihood of ED ICP monitor placement, 3) Longer ED LOS is associated with ED ICP monitor placement and higher ED bedside staff was associated with longer ED LOS. 4) Patients admitted to pediatric only I/II trauma centers and centers with higher mean pediatric TBI patient volume were more likely to receive an ED ICP monitor. Given the surprisingly high frequency of ED ICP monitor placement, we complemented the quantitative analysis to include a qualitative component to understand reasons for ED ICP monitor placement. Our use of mixed-methods shows that both patient and organizational level factors may affect the clinical decision regarding location of ICP monitor placement in children with severe TBI.
The role of ICP monitoring after TBI is controversial. Prior studies have not reported on location or timing of ICP monitor placement [6,20–24]. While we did not have documented times of ICP monitor placement, we used location as a surrogate to more generally evaluate early receipt of ICP monitors. Surprisingly, approximately 30% of the cohort and nearly 40% of those who underwent ICP monitoring underwent ICP monitor placement in the ED. Of these patients, 77% received an ICP monitor within four hours of ED admission. In this study, four out of five centers placed an ICP monitor in the ED. The one center that did not place any ICP monitors in the ED had shorter ED LOS and mostly inserted EVD rather than fiberoptic catheters. Therefore, it is possible that organizational preference for one type of ICP monitoring is related to location of monitor placement.
We found that older age was associated with ED ICP monitor placement. In this study, infants were less likely to have an ICP monitor placed that is consistent with earlier studies [6–8]. Several reasons for this observation have been suggested, including lower technical feasibility in infants, physician perceptions of open fontanelles serving as a pressure “pop off” mechanism as well as perceived futility of care in infants with inflicted TBI [11]. While previous studies have only examined placement of ICP monitor irrespective of timing or location, our study found that compared to infants, children greater than 13 years were more likely to receive an ICP monitor in the ED location. Although inflicted TBI was not directly associated with ED ICP monitor placement, we found that majority of infants had inflicted TBI which may account for the fact that they were less likely to receive an ICP monitor early on in the care of TBI. The majority of patients in our study with EDH, SDH or SAH underwent ICP monitor placement later in the OR or PICU, with over a third undergoing emergent neurosurgery [25,26]. In this study, intracerebral hemorrhage was not associated with ED ICP monitor placement, probably because of the small sample size. However, this head CT finding was likely a surrogate for head injury severity [27] and was reported as a factor prompting early ICP monitor placement in the ED by study investigators at two centers. Additionally, not examined in this study, findings identified use of sedatives and paralytics, which limit conduct of an early neurological examination, as a reason for ED ICP monitor placement. These data suggest that patient level factors affect decision-making regarding ED ICP monitor placement in this population.
Our study found that ED LOS was a factor associated with ICP monitor placement in the ED. Limited intensive care unit bed availability [28] and suboptimal ED transfer procedures [29] are shown to be associated with longer ED LOS in adult severe TBI care. We initially assumed that clinical pressures for short ED LOS and the more sterile PICU environment would result in low ED ICP monitor placement rates. Many patients, however, underwent ED ICP monitor placement even though median ED LOS was under two hours. We also observed center variability in ED LOS, and variability in the reasons for prolonged ED LOS, both of which were reported as reasons for ED ICP monitor placement. While we cannot exclude reverse causality (i.e., ICP monitor placement leads to longer ED LOS), we found that ED LOS can be an organizational variable affecting decisions to place an ICP monitor in the ED. We are not able to comment on ED ICP monitor placement and outcomes. While plausible reasoning suggests a longer ED LOS would delay receipt of best practice care for severe TBI that would or should otherwise be provided in the PICU, which includes ICP monitoring [3], our data indicate that many children with severe TBI receive timely ICP monitoring regardless of location and despite anticipated long ED LOS. While our study suggests that higher ED staffing is associated with longer ED LOS, we recognize that these associations may be institution-specific and may be surrogates for other center-specific practices that we were not able to examine.
Organizational factors such as trauma center designation and TBI patient volumes were associated with ED ICP monitor placement in our study. While previous studies examining organizational factors in relation to ICP monitoring have found that trauma centers with higher patient volume and adult I/II designation compared to adult/pediatric I/II only were more likely to place ICP monitors in children with TBI [6,7], these studies did not examine the location or timing of ICP monitor placement. We found that children admitted to trauma centers with higher mean pediatric TBI volumes were more likely to receive a monitor in the ED. Probable reason being that these trauma centers may be better equipped with trained staffs that are more familiar with the care of children with severe TBI in the ED. Among the four centers that placed ICP monitors in the ED in our study, two centers had pediatric only I/II trauma center designation and were 71% more likely to place ED ICP monitors. While one factor alone may not explain this finding, we suspect that there may a combination of factors that may include not only patient level factors such as injury severity, mechanism or head CT findings, but also physician and institutional practices that may result in ED ICP monitor placement.
Limitations
Only five trauma centers were included, which may not be sufficient to assess the full spectrum of organizational factors associated with the location of ICP monitor placement. While we recognize that our assumption of ED ICP monitor placement may not imply that the patient received the monitor at an earlier time, majority of the children who had an ICP monitor placed in the ED received it within four hours of ED admission. We did not have the exact recorded times of monitor placement and therefore were unable to assess the factors associated with the timing of ICP monitor placement. The decision to place an ICP monitor in the ED may also depend on physician preference or practice, as well as on organizational culture around placement of other advanced monitoring techniques relevant to severe TBI care. As we relied on existing data, we were unable to study whether physician level factors [30] were associated with ED ICP monitor placement. Finally, because we excluded patients who died within the first 48 hours of admission to trauma center in the parent PEGASUS study, select factors in our study may not have shown significant associations with ED ICP monitor placement. The present study was not powered or designed to examine the relationship between ED ICP monitoring and patient level outcomes. Larger studies involving more trauma centers may shed more light on patient level outcomes as well as generalizability of findings. Despite these limitations, our study provides new information and a first look on the frequency of and patient and organizational level factors associated with ED ICP monitor placement in severe pediatric TBI.
Conclusion
This is the first report of ED ICP monitor placement in severe TBI. Our study shows that ICP monitor placement in the ED was quite common at select leading pediatric trauma centers. However, there was considerable variation in the frequency of ED ICP monitor placement that may be related to patient level as well as organizational level factors associated with ED ICP monitor placement. Future studies to examine other factors that may influence clinical decision making with regards to ICP monitor placement in children with severe TBI are warranted.
Acknowledgements:
We would like to thank the following staff who contributed to the data collection reported in the manuscript: Rachelle Bell, RN, University of Pittsburgh Medical Center, Pittsburgh, PA; Kristi Schmidt, MD, Ann & Robert H. Lurie Children’s Hospital, Chicago, IL; Alma Ramirez, Los Angeles BioMedical Research Institute, Harbor-UCLA Medical Center, Torrance, CA; and Sheila Giles, RN, Nationwide Children’s Hospital, Columbus, OH. We would also like to thank the neurosurgeons at the study centers from whom the qualitative data was collected.
Footnotes
Declaration of Interest:
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper. This study was funded by the National Institute of Neurological Disorders and Stroke (NINDS) R01 NS072308–06.
References
- 1.Coronado VG, McGuire LC, Faul M, Sugerman DE, Pearson WS. Traumatic brain injury epidemiology and public health issues. Brain injury medicine: Principles and practice 2012;84. [Google Scholar]
- 2.Faul M, Xu L, Wald MM, Coronado V. Traumatic brain injury in the United States. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 3.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B. . Indications for intracranial pressure monitoring. Pediatric Critical Care Medicine 2012;13:S11–S17. [Google Scholar]
- 4.Vavilala MS, Kernic MA, Wang J, Kannan N, Mink RB, Wainwright MS, Groner JI, Bell MJ, Giza CC, Zatzick DF. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Critical care medicine 2014;42(10):2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelson P, Bratton S, Carney N, Chesnut R, Du Coudray H, Goldstein B, Kochanek P, Miller H, Partington M, Selden N. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2003;4(3 Suppl):S19–24. [PubMed] [Google Scholar]
- 6.Bennett TD, Riva-Cambrin J, Keenan HT, Korgenski EK, Bratton SL. Variation in intracranial pressure monitoring and outcomes in pediatric traumatic brain injury. Archives of pediatrics & adolescent medicine 2012;166(7):641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cleve W, Kernic MA, Ellenbogen RG, Wang J, Zatzick DF, Bell MJ, Wainwright MS, Groner JI, Mink RB, Giza CC. National variability in intracranial pressure monitoring and craniotomy for children with moderate to severe traumatic brain injury. Neurosurgery 2013;73(5):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA, Group UPTBIS, Group PICSS. Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive care medicine 2006;32(10):1606–1612. [DOI] [PubMed] [Google Scholar]
- 9.Bailey BM, Liesemer K, Statler KD, Riva-Cambrin J, Bratton SL. Monitoring and prediction of intracranial hypertension in pediatric traumatic brain injury: clinical factors and initial head computed tomography. Journal of Trauma and Acute Care Surgery 2012;72(1):263–270. [DOI] [PubMed] [Google Scholar]
- 10.Buck C International Classification of Diseases 9th Revision Clinical Modification for Physicians. St. Louis, MO, Saunders: Elsevier, American Medical Association; 2010. [Google Scholar]
- 11.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2005;6(5):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. The Lancet 1976;307(7968):1031–1034. [DOI] [PubMed] [Google Scholar]
- 13.Ajdari A, Boyle LN, Kannan N, Rowhani-Rahbar A, Wang J, Mink R, Ries B, Wainwright M, Groner JI, Bell MJ. Examining Emergency Department Treatment Processes in Severe Pediatric Traumatic Brain Injury. Journal for Healthcare Quality 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ries BS, Boyle LN, Vavilala MS, Kannan N, Saxe H, Kernic MA, Rivara FP, Zatzick DF, Bell MJ, Wainwright M. Assessing Clinical Care using Interactive Value Stream Mapping. 2013. SAGE Publications; p 1536–1540. [Google Scholar]
- 15.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC medical research methodology 2003;3(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenland S Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. American Journal of Epidemiology 2004;160(4):301–305. [DOI] [PubMed] [Google Scholar]
- 17.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American journal of epidemiology 2003;157(10):940–943. [DOI] [PubMed] [Google Scholar]
- 18.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 19.StataCorp. 2015Stata Statistical Software: Release 14. StataCorp LP. [Google Scholar]
- 20.Agrawal D, Raghavendran K, Schaubel DE, Mishra MC, Rajajee V. A Propensity Score Analysis of the Impact of Invasive Intracranial Pressure Monitoring on Outcomes After Severe Traumatic Brain Injury. Journal of neurotrauma 2016;33(9):853–858. [DOI] [PubMed] [Google Scholar]
- 21.Alali AS, Fowler RA, Mainprize TG, Scales DC, Kiss A, de Mestral C, Ray JG, Nathens AB. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. Journal of neurotrauma 2013;30(20):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhoury F, Kyriakides TC. Intracranial Pressure Monitoring in Children With Severe Traumatic Brain Injury: National Trauma Data Bank–Based Review of Outcomes. JAMA surgery 2014;149(6):544–548. [DOI] [PubMed] [Google Scholar]
- 23.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J. A trial of intracranial-pressure monitoring in traumatic brain injury. New England Journal of Medicine 2012;367(26):2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawes AJ, Sacks GD, Cryer HG, Gruen JP, Preston C, Gorospe D, Cohen M, McArthur DL, Russell MM, Maggard-Gibbons M. Intracranial pressure monitoring and inpatient mortality in severe traumatic brain injury: A propensity score–matched analysis. journal of trauma and acute care surgery 2015;78(3):492–502. [DOI] [PubMed] [Google Scholar]
- 25.Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J. Surgical management of depressed cranial fractures. Neurosurgery 2006;58(3):S2–56. [DOI] [PubMed] [Google Scholar]
- 26.Compagnone C, Murray GD, Teasdale GM, Maas AI, Esposito D, Princi P, D’avella D, Servadei F. The management of patients with intradural post-traumatic mass lesions: a multicenter survey of current approaches to surgical management in 729 patients coordinated by the European Brain Injury Consortium. Neurosurgery 2005;57(6):1183–1192. [DOI] [PubMed] [Google Scholar]
- 27.Mendelow AD, Gregson BA, Rowan EN, Francis R, McColl E, McNamee P, Chambers IR, Unterberg A, Boyers D, Mitchell PM. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH [Trauma]): the first randomized trial. Journal of neurotrauma 2015;32(17):1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell KJ, Richards CF, Daya M, Bernell SL, Weathers CC, Lowe RA. Effect of increased ICU capacity on emergency department length of stay and ambulance diversion. Annals of emergency medicine 2005;45(5):471–478. [DOI] [PubMed] [Google Scholar]
- 29.Elliott DJ, Williams KD, Wu P, Kher HV, Michalec B, Reinbold N, Coletti CM, Patel BJ, Dressler RM. An interdepartmental care model to expedite admission from the emergency department to the medical ICU. The Joint Commission Journal on Quality and Patient Safety 2015;41(12):542–549. [DOI] [PubMed] [Google Scholar]
- 30.Brolliar SM, Moore M, Thompson HJ, Whiteside LK, Mink RB, Wainwright MS, Groner JI, Bell MJ, Giza CC, Zatzick DF. A qualitative study exploring factors associated with provider adherence to severe pediatric traumatic brain injury guidelines. Journal of neurotrauma 2016;33(16):1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]