Abstract
Background:
Historically, multiple ipsilateral breast cancer (MIBC) has been a contraindication to breast-conserving therapy (BCT). We report the feasibility of BCT in MIBC from the ACOSOG Z11102 trial [Alliance], a single arm non-inferiority trial of BCT for women with 2 or 3 sites of malignancy in the ipsilateral breast.
Methods:
Women who enrolled preoperatively in ACOSOG Z11102 were evaluated for conversion to mastectomy and need for reoperation to obtain negative margins. Characteristics of women who successfully underwent BCT and those who converted to mastectomy were compared. Factors were examined for association with the need for margin re-excision.
Results:
Of 198 patients enrolled preoperatively, 190 (96%) had 2 foci of disease. Median size of the largest tumor focus was 1.5cm (range 0.1-7.0cm), 49 patients (24.8%) had positive nodes. There were 14 women who underwent mastectomy due to positive margins, resulting in a conversion to mastectomy rate of 7.1% (95% CI: 3.9-10.6%). Of 184 patients who successfully completed BCT, 134 completed this in a single operation. Multivariable logistic regression analysis did not identify any factors significantly associated with conversion to mastectomy or need for margin re-excision.
Conclusions:
Breast conservation is feasible in MIBC with 67.6% of patients achieving a margin-negative excision in a single operation and 7.1% of patients requiring conversion to mastectomy due to positive margins. No characteristic was identified that significantly altered the risk of conversion to mastectomy or need for re-excision.
Keywords: multiple ipsilateral, multifocal, multicentric, breast cancer, breast conservation, lumpectomy
Background:
The majority of women with newly diagnosed breast cancer present with unifocal disease. In some cases, however, more than 1 focus of disease is present in the same breast – multiple ipsilateral breast cancers (MIBC). Historically, mastectomy has been recommended for patients with MIBC. This is based on retrospective studies published in the 1980s and early 1990s that reported a higher rate of local-regional recurrence (LRR) in women with multicentric or multifocal breast cancer who underwent breast-conserving therapy (BCT).1-3 These studies were from an era prior to modern technology and multimodality breast cancer care including routine screening mammography, tomosynthesis, breast magnetic resonance imaging (MRI), availability of targeted therapies based on subtype, more precise radiation techniques and comprehensive analysis of surgical margins. Based on the c LRR ratesranging from 23-40% these retrospective studies, many surgeons continue to recommend mastectomy for women with MIBC.
More recently, mammographic quality has improved and utilization of new imaging techniques including breast MRI has increased. More sensitive imaging modalities have increased the detection of previously radiographically occult disease. Local control rates in patients undergoing breast conserving therapy are improving, particularly in those patients with hormone receptor positive disease. At the same time, the finding of MIBC is increasing and is now reported at rates ranging from 13-75%.4-17 With this higher detection rate, patients and clinicians are increasingly faced with the question of how best to surgically manage MIBC.
Retrospective series reporting outcomes for women treated more recently, in the 2000s and 2010s, have found rates of LRR comparable to those reported for unifocal disease n appropriately selected patients with MIBC.18-22 The American College of Surgeons Oncology Group (ACOSOG) Z11102 prospectively evaluated the feasibility and safety of breast conservation in women with MIBC. The primary endpoint of Z11102 is LRR at 5 years. Secondary endpoints include rate of conversion to mastectomy. Multifocal breast cancer has been traditionally defined as two or more foci of disease in a single quadrant of the breast. Multicentric disease has been defined as two or more foci in more than one quadrant of the breast. This distinction is relatively arbitrary as multicentric lesions may have minimal separation (particularly when closer to the nipple) while multifocal tumors may be separated by significant distance within the same quadrant of a large breast. Therefore, Z11102 used the term multiple ipsilateral breast cancer and included patients with tumors separated by 2 cm or greater of normal breast tissue.16
Herein we report the feasibility of breast conservation for MIBC and assess factors associated with re-excision and conversion to mastectomy in the Z11102 trial.
Methods:
ACOSOG Z11102 is a prospective, single arm non-inferiority trial designed to assess the feasibility of breast conservation in women with 2 or 3 sites of malignancy in a single breast. ACOSOG is now part of the Alliance for Clinical Trials in Oncology. All sites received approval from their institutional review boards and written informed consent was obtained from patients prior to study enrollment. Inclusion criteria required biopsy of all suspicious lesions prior to surgery and a minimum of one site of invasive carcinoma. The remaining lesions could be ductal carcinoma in situ (DCIS) or invasive carcinoma. Additional eligibility criteria included female gender, life expectancy >5 years, age over 40 years, and cN0 or N1 disease. Initially, magnetic resonance imaging (MRI) within 60 days prior to surgery was required in addition to mammogram. The MRI requirement was removed in an amendment activated May, 2015. Exclusion criteria included: pregnancy, neoadjuvant endocrine or chemotherapy, a single radiographic site of disease larger than 5cm, bilateral breast cancer (synchronous or metachronous), co-morbidity precluding whole breast radiation, history of prior ipsilateral breast irradiation, plan for partial breast irradiation, or a known BRCA mutation. Patients were initially all registered for the study preoperatively. An amendment effective May, 2015 allowed postoperative registration if this occurred prior to initiation of radiation therapy. These patients were not included in this analysis. For women registered prior to surgery, enrollment was based on radiographic distance between lesions. The protocol required a minimum of 3cm of normal appearing breast tissue between lesions prior to an amendment (effective in January, 2014) that decreased the minimum distance between lesions to 2cm.
Excision through a single or multiple incisions was allowed. At the outset of the trial, negative margins were defined as a minimum of 2mm excepting anterior and posterior margins if skin and/or fascia were taken. Re-excision was recommended for patients with margins <2mm. After the publication of the Society of Surgical Oncology/American Society of Radiation Oncology consensus guidelines on margins, the was protocol was amended, effective May 2015, defining negative margin for invasive breast cancer as “no ink on tumor”. For patients with persistently positive margins after attempted BCT, mastectomy was recommended. Oncoplastic reconstruction was allowed. Adjuvant systemic therapy was prescribed at the discretion of the treating oncologist. Hormone receptor positivity was defined per individual institutional policies.
Radiation target delineation was performed according to the RTOG contouring consensus definitions. Radiation was delivered with standard fractionation of 1.8 to 2.0 Gy daily for a total whole breast dose of 45-50 Gy. A radiation boost of 10-16 Gy in 2.0 Gy daily fractions to each tumor bed was mandatory. The protocol required that no more than 40% of the target breast tissue receive over 60 Gy. Hypofractionation was not allowed. Women who could not undergo radiation boost were excluded from the trial. All radiation plans were submitted for central review.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. The Alliance Data Safety Monitoring Board reviewed safety data for this trial at least twice a year.
Statistical analysis
The data for this analysis were last updated on 2/28/2018. Comparison of categorical variables between women who completed BCT and those who converted to mastectomy was performed with a chi-square test or a Fisher’s exact test, when appropriate. Continuous variables were compared using a two-sample t-test or Wilcoxon rank sum test, as appropriate. The association of variables with a dichotomous outcome (conversion to mastectomy or need for re-excision) was evaluated with a logistic regression model. Multivariable logistic regression was to be performed using all variables found to be significant in the univariate analysis. The a priori level of significance was 0.05, and all p-values are two-sided. The analyses were performed using SAS (9.4.).
Results
Two hundred twenty-three eligible patients were enrolled between July 23, 2012 and August 19, 2016. Of this group, 198 enrolled preoperatively and are the subjects of this report. Patient characteristics can be found in table 1. The median age was 62 years (range 40-87). The majority of patients (190, 96.0%) had 2 foci of disease identified on preoperative biopsy while 8 patients (4.0%) had 3. In the 190 women with 2 sites of disease, in 112 patients (59.0%), all sites of disease were invasive ductal carcinoma (IDC). There were 42 patients (22.1%) with one site of ductal carcinoma in situ (DCIS) and one site of IDC, 16 patients (8.4%) had invasive lobular carcinoma (ILC) at all sites, 18 patients (9.5%) had one site of IDC site and one of ILC and 2 patients (1.0%) had one site of DCIS site and one ILC. In the 8 women with 3 sites of disease, 4 had IDC at all sites, 2 had IDC and DCIS, 1 had a combination of IDC and ILC and 1 had ILC and DCIS. The majority of patients had at least one tumor that was estrogen receptor (ER) positive (95.5%) and 91.4% had progesterone receptor (PR) positive tumors. Twenty women (10.1%) had at least one site of HER2+ disease. Forty-nine patients (24.8%) had node positive disease. Median size of the largest lesions on preoperative imaging was 1.7cm (range 0.8-5.0cm). Median pathologic size of the largest single focus of tumor (invasive or DCIS) was 1.5cm (range 0.12-7.0cm). On final pathology, 8 patients (4.0%) had one single contiguous lesion. Six of these 8 patients had successful BCT and 2 (25%) converted to mastectomy. In contrast, 12 of 190 (6.3%) women who did not have a single contiguous lesion converted to mastectomy (p=0.10).
Table 1–
Analysis of factors associated with successful completion of breast conserving surgery vs conversion to mastectomy for MIBC
| All patients enrolled preoperatively (n=198) |
Completed BCS (n=184) |
Converted to mastectomy (n=14) |
p-value | ||
|---|---|---|---|---|---|
| Patient Age | 0.094 | ||||
| Median (range) | 62 (40 – 87) | 63 (40 – 87) | 57.5 (42 – 76) | ||
| Patient had preoperative MRI | 0.60 | ||||
| Yes | 186 (93.9%) | 173 (94.0%) | 13 (92.9%) | ||
| No | 12 (6.1%) | 11 (6.0%) | 1 (7.1%) | ||
| Number of lesions (preoperative imaging) | 0.20 | ||||
| 1 | 3 (1.5%) | 3 (1.6%) | 0 | ||
| 2 | 186 (93.9%) | 174 (93.6%) | 12 (85.7%) | ||
| 3 | 9 (4.6%) | 7 (3.8%) | 2 (14.3%) | ||
| Number of lesions (preoperative biopsy) | 0.45 | ||||
| 2 | 190 (96.0%) | 177 (96.2%) | 13 (92.9%) | ||
| 3 | 8 (4.0%) | 7 (3.8%) | 1 (7.1%) | ||
| Number of lesions (pathology) | 0.47 | ||||
| 0 | 1 (0.5%) | 1 (0.5%) | 0 | ||
| 1 | 10 (5.0%) | 10 (5.4%) | 0 | ||
| 2 | 172 (86.9%) | 160 (87.0%) | 12 (85.7%) | ||
| 3 | 14 (7.1%) | 12 (6.5%) | 2 (14.3%) | ||
| 4 | 1 (0.5%) | 1 (0.5%) | 0 | ||
| Size of largest lesion (preoperative) | 0.97 | ||||
| Median (range) | 1.7 (0.8 – 5.0) | 1.7 (0.8 – 5.0) | 1.6 (0.8 – 4.5) | ||
| Size of largest lesion (pathology) | 0.17 | ||||
| Median (range) | 1.5 (0.1 – 7.0) | 1.4 (0.1 – 6.5) | 2.1 (0.1 – 7.0) | ||
| Minimum distance between lesions (preoperative) | 0.82 | ||||
| Median (range) | 3.7 (2.0 – 14.0) | 3.8 (2.0 – 14.0) | 3.4 (2.2 – 8.0) | ||
| ER Status | 0.82 | ||||
| All positive | 179 (90.4%) | 167 (90.8%) | 12 (85.7%) | ||
| All negative | 9 (4.5%) | 8 (4.3%) | 1 (7.1%) | ||
| Mixed | 10 (5.1%) | 9 (4.9%) | 1 (7.1%) | ||
| PR Status | 0.57 | ||||
| All positive | 162 (81.8%) | 152 (82.6%) | 10 (71.4%) | ||
| All negative | 17 (8.6%) | 15 (8.2%) | 2 (14.3%) | ||
| Mixed | 19 (9.6%) | 17 (9.2%) | 2 (14.3%) | ||
| Any HER2 positive disease | 0.37 | ||||
| Yes | 20 (10.4%) | 20 (11.2%) | 0 | ||
| No | 172 (89.6%) | 158 (88.8%) | 14 (100%) | ||
| Not done | 6 | 6 | 0 | ||
| Histology | 0.64 | ||||
| Ductal | 116 (58.6%) | 108 (58.7%) | 8 (57.1%) | ||
| Lobular | 16 (8.1%) | 14 (7.6%) | 2 (14.3%) | ||
| Ductal/DCIS | 44 (22.2%) | 42 (22.8%) | 2 (14.3%) | ||
| Lobular/DCIS | 3 (1.5%) | 3 (1.6%) | 0 | ||
| Ductal/Lobular | 19 (9.6%) | 17 (9.2%) | 2 (14.3%) | ||
| Tumor Grade | 0.17 | ||||
| G1 (Low) | 53 (26.8%) | 46 (25.0%) | 7 (50.0%) | ||
| G2 (Intermediate) | 97 (49.0%) | 91 (49.5%) | 6 (42.9%) | ||
| G3 (High) | 46 (23.2%) | 45 (24.5%) | 1 (7.1%) | ||
| GX (Grade cannot be assessed) | 2 (1.0%) | 2 (1.1%) | 0 | ||
| Type of first surgery | 0.65 | ||||
| Single lumpectomy | 58 (29.3%) | 55 (29.9%) | 3 (21.4%) | ||
| Two lumpectomies | 137 (69.2%) | 126 (68.5%) | 11 (78.6%) | ||
| Three lumpectomies | 3 (1.5%) | 3 (1.6%) | 0 | ||
| Axillary surgery done | 0.008 | ||||
| No axillary surgery | 3 (1.5%) | 3 (1.6%) | 0 | ||
| SLND only | 161 (81.3%) | 154 (83.7%) | 7 (50.0%) | ||
| ALND only | 10 (5.0%) | 18 (9.8%) | 1 (7.1%) | ||
| Both SLND and ALND | 24 (12.1%) | 9 (4.9%) | 6 (42.9%) | ||
| Pathologic N Stage | 0.17 | ||||
| N0 | 148 (74.7%) | 141 (76.6%) | 7 (50.0%) | ||
| N1 | 41 (20.7%) | 35 (19.0%) | 6 (42.9%) | ||
| N2 | 5 (2.5%) | 4 (2.2%) | 1 (7.1%) | ||
| N3 | 3 (1.5%) | 3 (1.6%) | 0 | ||
| NX | 1 (0.5%) | 1 (0.5%) | 0 | ||
ALND – axillary lymph node dissection; DCIS – ductal carcinoma in situ; ER – estrogen receptor; G – grade; HER2 – human epidermal growth factor receptor; N – node; PR – progesterone receptor; SLND – sentinel lymph node dissection
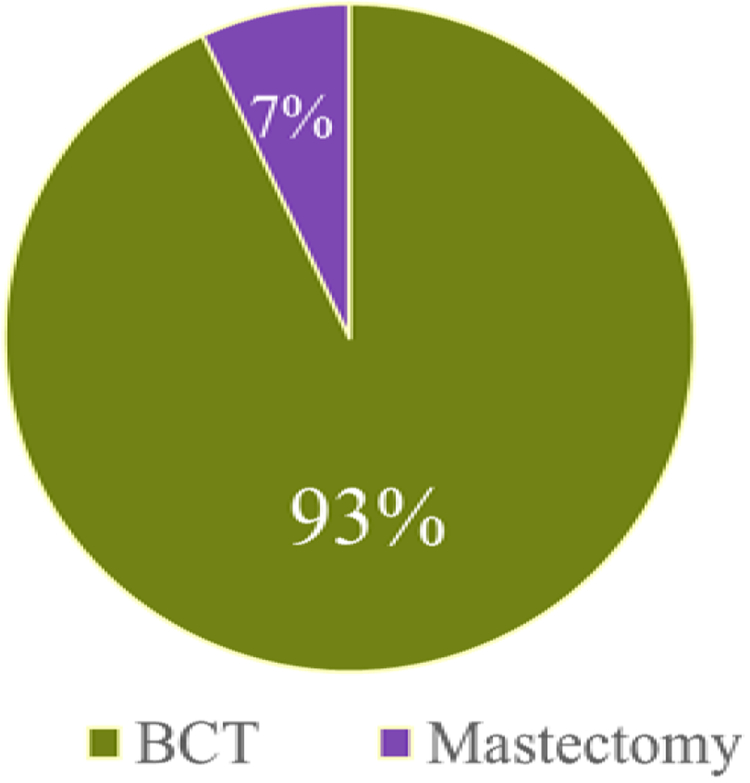
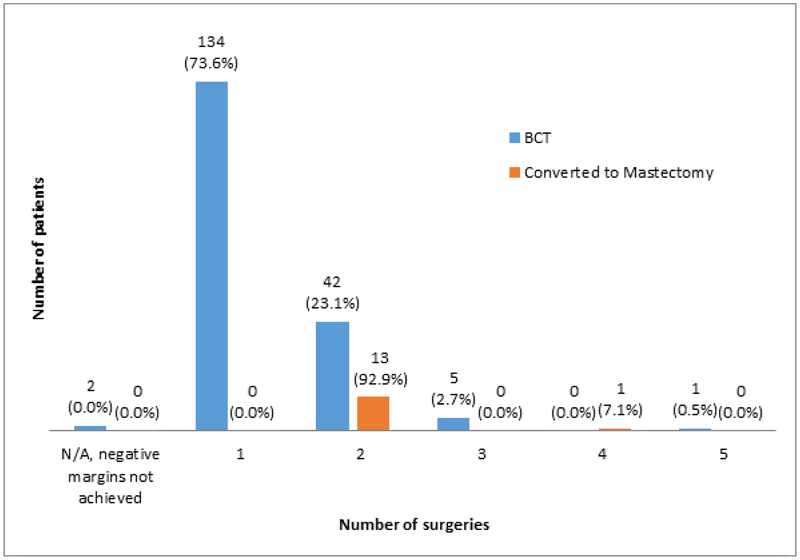
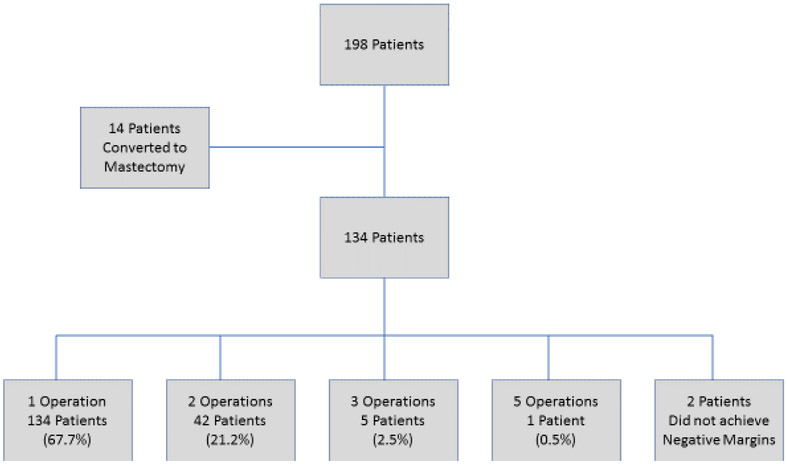
Of the 198 patients, 184 (92.9%, 95% CI: 89.4 to 96.5) successfully completed BCT while 14 patients (7.1%, 95% CI: 3.5 to 10.6) ultimately underwent mastectomy due to persistently positive margins (FIGURE 1). Of the patients who successfully completed BCT, the majority (134, 73.6%; 95% CI: 67.2 to 80.0) successfully completed breast conservation in a single operation, 42 patients (23.1%) had a second operation to achieve negative margins, 5 (2.7%) required 3 operations and a single patient (0.5%) underwent 5 procedures to obtain negative margins. Two patients in the BCT group did not achieve negative margins; of these, 1 declined additional surgery and 1 had a focally positive deep margin after excision of fascia and no additional surgery was recommended. In the conversion to mastectomy group, 13 (93%) women underwent 2 operations and one underwent 4 operations (FIGURE 2). After the margin amendment, the total number of patients completing BCT in a single operation trended up from 64.1% to 74.6% (p=0.12). For the 184 patients who completed BCT, 154 (84.2%) had routine closure by breast surgeon, 23 (12.6%) had local tissue rearrangement by a breast surgeon, 3 (1.6%) had breast reshaping by a plastic surgeon, and 3 (1.6%) had simultaneous breast reduction by a plastic surgeon. Data were missing for 1 patient. In the mastectomy group, one woman opted for contralateral prophylactic mastectomy. There were 16 patients in the BCT group who had contralateral surgery (4 had excisional biopsy, 4 had mastopexy, 6 had breast reduction, 2 had a contralateral procedure that was not specified).
Figure 1.
Proportion of women with multiple ipsilateral breast cancers that converted to mastectomy
Figure 2.
Total Number of Operations Performed to Obtain Negative Margins stratified by BCT vs mastectomy patients
Factors potentially associated with conversion to mastectomy were assessed including patient age, use of preoperative MRI, number of foci of disease identified preoperatively, median pathologic tumor size, number of lumpectomies, nodal status, tumor grade, hormone receptor and HER2 status, and histology including DCIS or ILC at one or more sites of disease. None of these factors were associated with conversion to mastectomy on univariate analysis. The median largest radiographic tumor size was 1.7cm in the BCT group and 1.6cm in the group that converted to mastectomy (p=0.97). In the BCT group, median pathologic size was 1.4 cm compared to 2.1 cm in the group that converted to mastectomy (p=0.17). In the BCT group, 75% of the patients had low or intermediate grade tumors while in the conversion to mastectomy group 93% of the patients had low or intermediate grade tumors (p=0.17). Node positive disease was found in 50% (7/14) of the women converted to mastectomy compared to 23% (42/184) in the BCT group (p=0.17).
Of the 116 patients with IDC at all sites, the conversion rate to mastectomy was 8/116 (6.9%), whereas in cases with ILC at both sites the conversion rate was 12.5% (2/16), and in cases with one site of IDC and one site of ILC it was 10.5%. In aggregate, patients with ILC at any site had a 10.5% (4/38; 95% CI: 0.9-22.0) rate of conversion to mastectomy. In the 47 patients with DCIS at one site and invasive disease at the other site(s), the conversion rate to mastectomy was 4.3% (2/47; 95% CI: 0-10.0). Neither the presence of ILC 4/38 (10.5%) versus 10/160(6.3%); p=0.47 nor DCIS 2/47 (4.3%) vs 8/116 (6.9%); p=0.73 were significantly associated with conversion to mastectomy.
HER2 status was also not found to be significantly associated with conversion to mastectomy in women with MIBC (p=0.37). In the successful BCT cohort, 11.2% (20/178) had at least one HER2-positive lesion. None of the 14 patients with mastectomy had HER2-positive disease. (TABLE 1).
The same factors were assessed for association with re-operation for margin re-excision. None of the variables analyzed were found to be associated with number of operative procedures (TABLE 2).
Table 2.
Comparison of characteristics of patients requiring one vs multiple operations to achieve negative margins (2 patients with BCT despite positive margins excluded)
| Single surgery (n=134) | 2 or more surgeries (n=62) | p-value | ||
|---|---|---|---|---|
| Patient Age | 0.49 | |||
| Median (range) | 63 (40 – 83) | 60.5 (42 – 87) | ||
| Registration date | 0.12 | |||
| Before margins amendment | 75 (56.0%) | 42 (67.7%) | ||
| After margins amendment | 59 (44.0%) | 20 (32.3%) | ||
| Patient had preoperative MRI | 0.74 | |||
| Yes | 127 (94.8%) | 58 (93.6%) | ||
| No | 7 (5.2%) | 4 (6.4%) | ||
| Number of lesions (preoperative imaging) | 0.76 | |||
| 1 | 3 (2.2%) | 0 | ||
| 2 | 125 (92.3%) | 59 (95.2%) | ||
| 3 | 6 (4.5)% | 3 (4.8%) | ||
| Number of lesions (preoperative biopsy) | 0.99 | |||
| 2 | 128 (95.5%) | 60 (96.8%) | ||
| 3 | 6 (4.5%) | 2 (3.2%) | ||
| Number of lesions (pathology) | 0.49 | |||
| 0 | 1 (0.8%) | 0 | ||
| 1 | 7 (5.2%) | 2 (3.2%) | ||
| 2 | 118 (88.1%) | 53 (85.5%) | ||
| 3 | 7 (5.2%) | 7 (11.3%) | ||
| 4 | 1 (0.8%) | 0 | ||
| Size of largest lesion (preoperative) | 0.43 | |||
| Median (range) | 1.7 (0.8 – 5.0) | 1.8 (0.8 – 5.0) | ||
| Size of largest lesion (pathology) | 0.094 | |||
| Median (range) | 1.4 (0.1 – 6.5) | 1.6 (0.1 – 7.0) | ||
| Min distance between lesions (preoperative) | 0.88 | |||
| Median (range) | 3.9 (2.0 – 10.0) | 3.5 (2.0 – 14.0) | ||
| ER Status | 0.92 | |||
| All positive | 121 (90.3%) | 57 (91.9%) | ||
| All negative | 6 (4.5%) | 3 (4.8%) | ||
| Mixed | 7 (5.2%) | 2 (3.2%) | ||
| PR Status | 0.19 | |||
| All positive | 114 (85.1%) | 47 (75.8%) | ||
| All negative | 11 (8.2%) | 6 (9.7%) | ||
| Mixed | 9 (6.7%) | 9 (14.5%) | ||
| Any HER2 positive disease | 0.48 | |||
| Yes | 15 (11.5%) | 5 (8.2%) | ||
| No | 115 (88.5%) | 56 (91.8%) | ||
| Histology | 0.098 | |||
| Ductal | 84 (62.7%) | 32 (51.6%) | ||
| Lobular | 9 (6.7%) | 6 (9.7%) | ||
| Ductal/DCIS | 23 (17.2%) | 20 (32.3%) | ||
| Lobular/DCIS | 3 (2.2%) | 0 | ||
| Ductal/Lobular | 15 (11.2%) | 4 (6.4%) | ||
| Tumor Grade | 0.71 | |||
| G1 (Low) | 38 (28.4%) | 15 (24.2%) | ||
| G2 (Intermediate) | 63 (47.0%) | 33 (53.2%) | ||
| G3 (High) | 32 (23.9%) | 13 (21.0%) | ||
| GX (Grade cannot be assessed) | 1 (0.8%) | 1 (1.6%) | ||
| Type of first surgery | 0.31 | |||
| Single lumpectomy | 41 (30.6%) | 16 (25.8%) | ||
| Two lumpectomies | 92 (68.7%) | 44 (71.0%) | ||
| Three lumpectomies | 1 (0.8%) | 2 (3.2%) | ||
| Axillary surgery performed | 0.15 | |||
| No axillary surgery | 2 (1.5%) | 1 (1.6%) | ||
| SLND only | 111 (82.8%) | 49 (79.0%) | ||
| ALND only | 9 (6.7%) | 1 (1.6%) | ||
| Both SLND and ALND | 12 (9.0%) | 11 (17.7%) | ||
| Pathologic N Stage | 0.26 | |||
| N0 | 102 (76.7%) | 45 (72.6%) | ||
| N1 | 24 (18.0%) | 16 (25.8%) | ||
| N2/N3 | 7 (5.3%) | 1 (1.6%) | ||
ALND – axillary lymph node dissection; DCIS – ductal carcinoma in situ; ER – estrogen receptor; G – grade; HER2 – human epidermal growth factor receptor; N – node; PR – progesterone receptor; SLND – sentinel lymph node dissection
Discussion
Breast conserving surgery is technically feasible in women with MIBC, with a low rate of conversion to mastectomy (7.1%) and with most women successfully achieving breast conservation with negative margins in a single operation (67.6%). Long-term LRR data are necessary to assess oncologic outcomes; these will be reported from the ACOSOG Z11102 trial when the data are sufficiently mature.
Several retrospective studies have reported acceptable LRR rates following BCT in the MIBC population. One of the largest was a review of 476 patients treated with BCT for MIBC between 1997 and 2002 with a 5.1% LRR rate at five years.20 This cohort had more advanced disease with 55% of all patients being node positive compared to 25% in the Z11102 trial. Ataseven et al. reviewed the surgical management of women with MIBC treated with neoadjuvant chemotherapy. 22 Patients with operable or locally advanced breast cancer who were enrolled on several neoadjuvant cooperative group trials were evaluated for local recurrence-free survival (LRFS), disease-free survival (DFS) and overall survival (OS). Of 6134 patients accrued, 22.9% were found to have MIBC. Patients who achieved margin negative resection or a pathologic complete response had no statistically significant difference in LRFS when comparing unifocal to MIBC. Of note, the Z11102 trial excluded patients treated with neoadjuvant therapy.
The data from Z11102 establish the feasibility of performing BCT for MIBC with a rate of conversion to mastectomy being acceptably low (7.1%). This is consistent with studies which report the rate of conversion to mastectomy in women with unifocal breast cancer ranging between 4% (after SSO/ASTRO consensus guidelines regarding surgical margins) and 11%.23,24 Risk factors for conversion to mastectomy cited in other studies include nodal positivity, invasive lobular carcinoma and DCIS. Additionally, studies have documented HER2 status and tumor size as risk factors for margin re-excision.25,26,27 In our study, none of these previously identified risk factors influenced the need for conversion to mastectomy. This is likely due to the small size of the conversion to mastectomy cohort; which is a significant limitation of the study as the study was not powered for rates of conversion to mastectomy.
The re-excision rate in Z11102 of 32.4% is slightly higher than expected compared with patients with unifocal disease23,28,29,30. This rate decreased after the guideline amendment supporting no ink on tumor. Additionally, only 3.6% of patients required >2 operations in this study. Although multiple surgical procedures have been shown to be oncologically safe,18 they are costly, are stressful to patients, potentially compromise cosmetic outcome and may delay systemic or local therapy. No variables were identified that predicted the need for a subsequent procedure for MIBC.
Conclusions
The Z11102 trial demonstrates acceptably low (7.1%) risk of conversion to mastectomy in women with MIBC. The majority of these women (67.6%) require one operation to successfully complete BCT with negative margins. These data may inform conversations between patients and surgeons regarding management of MIBC. Based on these data, no specific patient, imaging or tumor factor accurately predicts patients at highest risk for failure of BCT in the MIBC population. Additional data are awaited from this trial regarding LRR following BCT for women with MIBC.
Figure 3.
Number of operations required to complete surgery
Synopsis:
Z11102 is a prospective single arm trial assessing the oncologic safety and surgical feasibility of breast conservation therapy (BCT) in women with multiple ipsilateral breast cancer. Here we present initial feasibility data assessed by conversion to mastectomy and re-excision rates.
Acknowledgements:
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180790, U10CA180854, and U10CA180858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
We have no conflicts of interest to disclose.
ClinicalTrials.gov Identifier: NCT01556243
References:
- 1.Wilson LD, Beinfield M, McKhann CF, Haffty BG. Conservative surgery and radiation in the treatment of synchronous ipsilateral breast cancers. Cancer 1993;72:137–42. [DOI] [PubMed] [Google Scholar]
- 2.Leopold KA, Recht A, Schnitt SJ, et al. Results of conservative surgery and radiation therapy for multiple synchronous cancers of one breast. International journal of radiation oncology, biology, physics 1989;16:11–6. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz JM, Jacquemier J, Amalric R, et al. Breast-conserving therapy for macroscopically multiple cancers. Annals of surgery 1990;212:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008;26:3248–58. [DOI] [PubMed] [Google Scholar]
- 5.Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology 2002;224:569–76. [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Gilbreath PL. Multicentric and multifocal cancer: whole-breast US in preoperative evaluation. Radiology 2000;214:59–66. [DOI] [PubMed] [Google Scholar]
- 7.Morrow M, Harris JR. More mastectomies: is this what patients really want? J Clin Oncol 2009;27:4038–40. [DOI] [PubMed] [Google Scholar]
- 8.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. Journal of the American College of Surgeons 2009;209:180–7; quiz 294–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999;213:881–8. [DOI] [PubMed] [Google Scholar]
- 10.Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging.Cancer 2003;98:468–73. [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Orel SG, Czerniecki BJ, Solin LJ, Schnall MD. MRI before reexcision surgery in patients with breast cancer. Ajr 2004;182:473–80. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 2007;25:5203–9. [DOI] [PubMed] [Google Scholar]
- 13.Bendifallah S, Werkoff G, Borie-Moutafoff C, et al. Multiple synchronous (multifocal and multicentric) breast cancer: clinical implications. Surgical oncology; 19:e115–23. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson LS, Given-Wilson R, Hall T, Potts H, Sharma AK, Smith E. Increasing the diagnosis of multifocal primary breast cancer by the use of bilateral whole-breast ultrasound. Clinical radiology 2005;60:573–8. [DOI] [PubMed] [Google Scholar]
- 15.Berg WA, Madsen KS, Schilling K, et al. Breast Cancer: Comparative Effectiveness of Positron Emission Mammography and MR Imaging in Presurgical Planning for the Ipsilateral Breast. Radiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009;27:4082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Annals of surgical oncology 2009;16:2682–90. [DOI] [PubMed] [Google Scholar]
- 18.Hartsell WF, Recine DC, Griem KL, Cobleigh MA, Witt TR, Murthy AK. Should multicentric disease be an absolute contraindication to the use of breast-conserving therapy? International journal of radiation oncology, biology, physics 1994;30:49–53. [DOI] [PubMed] [Google Scholar]
- 19.Cho LC, Senzer N, Peters GN. Conservative surgery and radiation therapy for macroscopically multiple ipsilateral invasive breast cancers. American journal of surgery 2002;183:650–4. [DOI] [PubMed] [Google Scholar]
- 20.Gentilini O, Botteri E, Rotmensz N, et al. Conservative surgery in patients with multifocal/multicentric breast cancer. Breast cancer research and treatment 2009;113:577–83. [DOI] [PubMed] [Google Scholar]
- 21.Bauman L, Barth RJ, Rosenkranz KM. Breast conservation in women with multifocal-multicentric breast cancer: is it feasible? Annals of surgical oncology;17:325. [DOI] [PubMed] [Google Scholar]
- 22.Ataseven B, Lederer B, Blohmer JU et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival in 6134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Onc. 2015. April;22(4): 1118–27 [DOI] [PubMed] [Google Scholar]
- 23.Morrow M, Abrahamse P, Hofer T. Trends in reoperation after initial lumpectomy for breast cancer. Jama Onc 2017:3(10): 1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walijee JF, Hu ES, Newman LA et al. Predictors of re-excision in women undergoing breast-conserving therapy for cancer. Ann Surg Onc 2008;May 15(5);1297–1303 [DOI] [PubMed] [Google Scholar]
- 25.O’Sullivan MJ, Li T, Freedman G et al. The effect of multiple re-excisions on the risk of breast cancer recurrence after breast conserving therapy. Ann Surg Oncol 2007; 3: 3133–3140. [DOI] [PubMed] [Google Scholar]
- 26.Haixia J, Weijaun, Yang T et al. HER2 positive breast cancer is aassociated with an increased risk of positive cavity margins after initial lumpectomy. World Journal of Oncology 2014:12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurnlawan E, Windle I, Wong M et al. Predictors of surgcal margin status in breast-conserving surgery within a breast screening program. Ann Surg Onc 15(9):2542–9 [DOI] [PubMed] [Google Scholar]
- 28.Coopey S, Smith B, Hanson S et al. The safety of multiple re-excisions after lumpectomy for breast cancer. Ann Surg Oncol. 2011. December; 18(13):3797–801 [DOI] [PubMed] [Google Scholar]
- 29.Sabel MS, Rogers K, Griffith K et al. Residual disease after re-excision lumpectomy for close margins. J Surg Oncol. 2009. February 1; 99(2): 99–103 [DOI] [PubMed] [Google Scholar]
- 30.Hadzikadic G, McGuire KP, Ozmen T et al. Margin width is not predictive of residual disease on re-excision in breast conserving therapy. J Surg Oncol 2014. April; 109(5):426–30 [DOI] [PubMed] [Google Scholar]