Abstract
Breast cancer is the most common cancer of adolescents and young adult (AYA) women aged 15 to 39 years, accounting for 5.6% of all invasive breast cancer in women. In comparison to older women, AYAs are more likely to have familial cancer predisposition genes, larger breast tumors, unfavorable biological characteristics, distant metastatic disease at diagnosis, and adverse outcome. Endocrine therapy and some chemotherapy recommendations differ between young and older women. AYAs require coordinated multidisciplinary care, treatment regimens that minimize late effects such as premature menopause and osteoporosis, and proactive management of psychological and sexual health during and after cancer treatment.
Keywords: Age, Young adult, Breast, Neoplasm, Pregnancy
Introduction
This article updates our 2009 review of breast cancer in young women [1], discussing the epidemiology, risk factors, protective factors, clinicopathologic characteristics, treatment, outcomes, and distinctive age-related challenges of breast cancer in adolescents and young adults (AYAs) aged 15–39 years, including the risk of premature menopause, bone health, breast cancer during pregnancy, and psychosocial issues. References cited were obtained by PubMed search for the topics above in combination with the terms breast cancer, young, early-onset, and premenopausal. Incidence and survival data presented in the tables and graphs of this article were obtained from the 2017 United States Surveillance, Epidemiology and End Results (SEER) database [2] using SEER*Stat version 8.3.4.2–5 and DevCan - Probability of Developing or Dying of Cancer. Surveillance Research Program, Statistical Methodology and Applications, National Cancer Institute, DevCan 6.7.5 [3].
Epidemiology
Breast cancer is the most common cancer among women worldwide [4]. It is the most common cancer among AYAs in the United States (US), accounting for 15% of all invasive cancer and 30% of cancer in females (SEER). In 2013, approximately 11,000 AYA women in the US were diagnosed with invasive breast cancer and approximately 1,000 died of the disease [5].
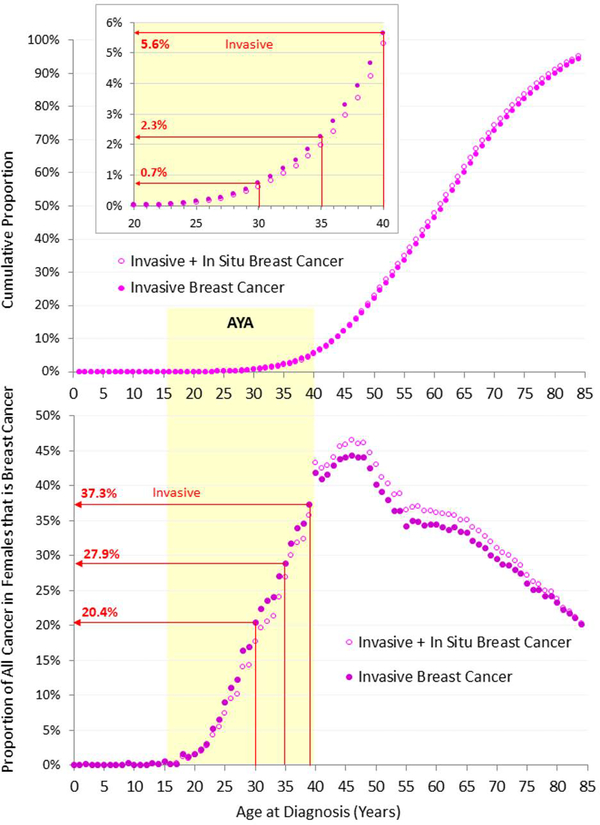
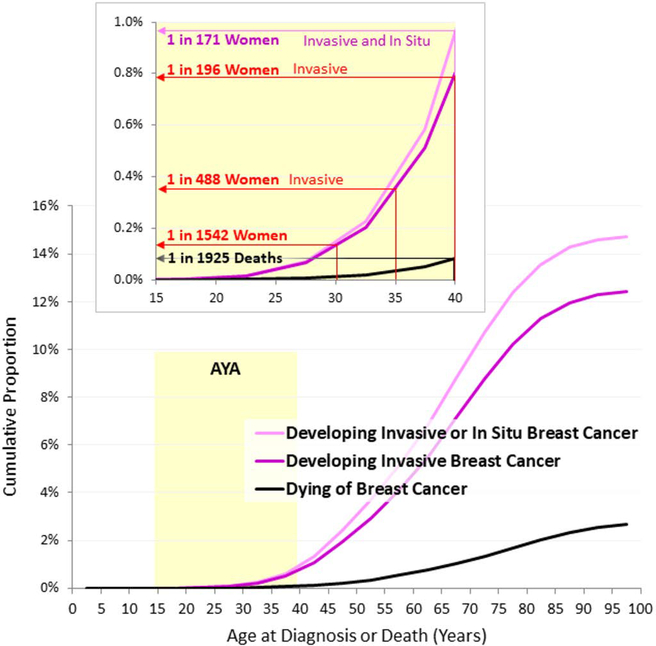
In 2000–2014 in the US, 5.6% of all invasive breast cancer was diagnosed in women aged <40 (Figure 1, upper panel). During 2012–2014, the individual average risk of a US woman developing invasive breast cancer by 40 was 1 in 196 (Figure 2, inset).
Figure 1.
Cumulative Proportion of Number of Women with Breast Cancer by Single Year of Age, 2010–2014, SEER18 (upper panel) and Proportion of All Invasive Cancer in Females that is Breast Cancer, by Single Year of Age, 2000–2014, SEER18 (lower panel), by Invasive* and Invasive + In Situ Breast Cancer. *Malignant, according to SEER classification. Data Source: Surveillance, Epidemiology and End Results (SEER) database [2]
Figure 2.
Cumulative Proportion of Females Who Developed or Died of Breast Cancer during 2012–2014, SEER18, by Single Year of Age. Data Source: DevCan: Probability of developing or dying of cancer software, Version 6.7.5. [3]
The percentage of all cancer that is breast cancer increases rapidly among AYA women during the third and fourth decades of life, from 2% at age 20 to more than 40% by age 40. (Figure 1, lower panel). The abrupt increase at age 40 is attributable to routine screening mammography. Breast cancer incidence is similar among AYA women in both developed and developing countries [6]. Breast cancer is in males rare and has an older age predominance. There are 30–40 new cases annually in AYA males, comprising 0.1% of all cancer and 2.6% of breast cancer in AYAs. Breast cancer in males is not further reviewed in this article.
In the US, the incidence of invasive breast cancer has slightly increased among AYA women since 1992 (SEER). Between 1990 and 2008, the incidence of breast cancer increased by about 1.2% percent per year among European in AYA women, with the greatest increases in women under 35 [7].
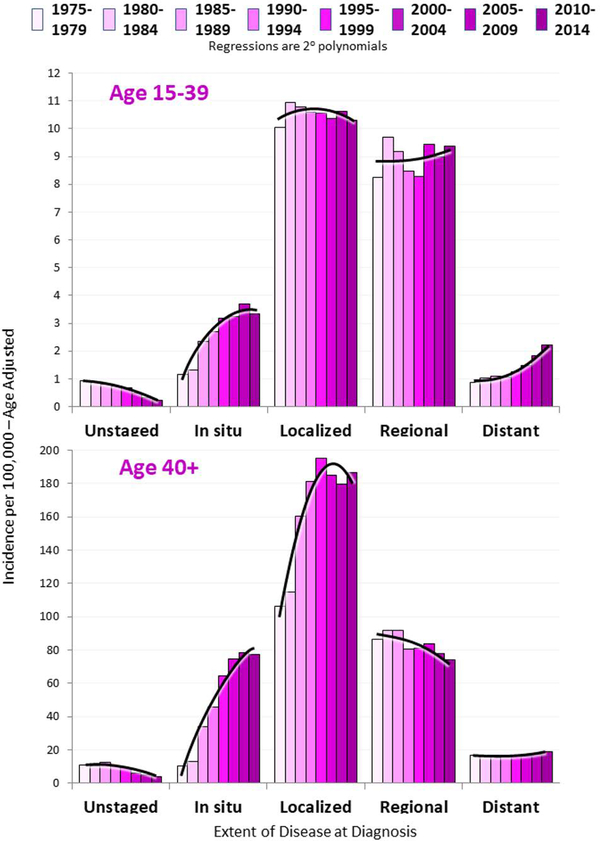
Figure 3 shows the incidence trends since 1975 by age and extent of disease at diagnosis. The incidence of local and regional disease has remained stable among AYAs, but distant disease has increased and the rate of increase is rising (upper panel). Although screening mammography for women aged 40+ aims to diagnose cancer in its early stages, distant disease has never decreased in this older population (lower panel) whereas diagnosis of early stage disease has increased dramatically. The recent slight uptick in distant disease among older women probably reflects the reported small increases in the 40–55 year-old age group [8, 9].
Figure 3.
Incidence of Breast Cancer in Women 15 to 39 Years of Age and 40 Years and Older by Extent of Disease at Diagnosis and 5-Calendar Year Intervals, 1975–2014, SEER9
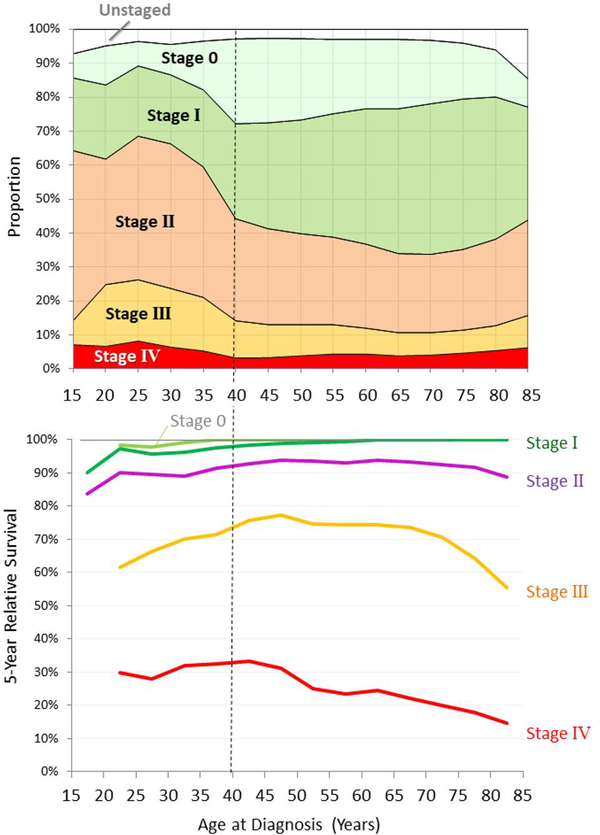
Figure 4 (upper panel) depicts American Joint Committee on Cancer (AJCC) stage of breast cancer as a function of age at diagnosis, in 2000–2014 (SEER). A higher percentage of AYAs than older women present with non-localized regional or distant (stage II, III or IV) disease. Two out of three 25- to 29-year-olds have stage II-IV disease at diagnosis, compared to slightly more than one in three women aged 40+. Compared to older women a higher, and increasing, percentage of AYAs have distant (stage IV) disease at diagnosis [9].
Figure 4.
Incidence (upper panel) and 5-Year Relative Survival (lower panel) of Women with Invasive Breast Cancer, SEER18, 2000–2014, by Breast Adjusted AJCC6 Stage.
Breast cancer incidence varies according to race and ethnicity in US AYAs. In comparison to young white women, black women aged <35 have a higher incidence of invasive breast cancer and three times greater breast cancer mortality (SEER and National Center for Health Statistics) [10, 11] In contrast, breast cancer is less frequent among Native American women of all ages compared to the general US population [12]. Advanced disease at diagnosis is more common among AYA black, Hispanic, and Native American women, and in women of all ages with low socioeconomic status, [13–15].
Risk Factors
Familial
AYA breast cancer is more common among women with a family history of early onset breast cancer (RR 3.22 for women <35) [10]. Early onset breast cancer is especially common among women harboring BRCA1 mutations [16], 80% of whom present with aggressive triple negative tumors. Germline BRCA1, BRCA2 or TP53 mutations are found in about 50% of AYAs who are diagnosed with breast cancer before age 30 and have a strong family history of breast cancer [17]. Germline cancer predisposition mutations are found in approximately 10% of all breast cancer patients [18]. PALB2 mutations increase risk for AYA breast cancer by at least 8-fold [19]. Germline mutations in many additional genes, including PTEN, BARD1, STK11/LKB1, CDH1, CHEK2, ATM, MRE11, NBS1, BRIP1, FANCA, FANCC, FANCM, RAD50, RAD51, RAD51B, RAD51C, RAD51D, and XRCC2 impart a high risk or moderately increased risk for breast cancer [18, 20].
Hormonal
Breast cancer risk in AYAs increases with early menarche, oral contraceptive use [10] (particularly for teenage BRCA1 carriers) [21], anovulatory infertility [22], later age (>30 years) at first birth, and for several months following the delivery of a child [23]. Breastfeeding decreases the risk for AYA breast cancer [24].
Personal and lifestyle
Prior mantle irradiation [25] and alcohol consumption [26] are well-established risk factors for breast cancer at all ages. A >10 pack-years smoking history increases the risk of Estrogen receptor (ER)-positive breast cancer by 1.6-fold among women aged 20–44. [27]. Although low body mass index increases risk [10] for AYA breast cancer, substantial weight gain (BMI increase of ≥10kg/m2) after age 18 also raises the risk of ER-negative breast cancer by 2-fold [28]. The triad of high caloric intake, inactivity and obesity increases risk in premenopausal women [29], whereas regular vigorous exercise decreases risk [30, 31].
A diet high in energy-rich foods, such as red meat or fast food, increases premenopausal breast cancer risk [4, 10, 32, 33]. In contrast, a whole food, plant-based, low-energy density diet reduces the risk for AYA breast cancer [4, 32, 33]. Substituting legumes for one serving of meat per day lowers breast cancer risk by 19% among premenopausal women [34].
High mammographic density is a risk factor for breast cancer at all ages [35]. Breast density in premenopausal women is affected by dietary intake of alcohol, vitamin D, and calcium, [36], by IL-6 levels, [37] and by dehydroepiandrosterone sulfate (DHEAS) levels in early adolescence [38]. Low vitamin D intake is associated with increased risk for premenopausal breast cancer and with the development of larger, higher grade tumors [39].
Clinicopathologic Features
Subtype and grade
The distribution of breast cancer subtype and grade changes with age [40], with more aggressive phenotypes among AYA compared to older women. Among 185 pre-menopausal women with invasive breast cancer <50 years of age, women <35 had a greater risk of developing of ER negative, progesterone receptor (PR) negative, and pathologic grade 3 tumors, as well as vascular or lymphatic invasion. [41] Basal-like and Her2-enriched breast tumors are more common in young compared to older women.[42]
Gene Expression
Comprehensive gene expression profiling has the capacity to predict outcome and detect age-related differences in breast tumor biology. Azim et al. showed that, compared to older women, AYAs more frequently developed aggressive triple negative tumors with stromal-related signatures. Risk of relapse was also higher in AYAs, following adjustment for subtype, grade, tumor size and nodal status. A candidate gene approach, adjusting for subtype, identified over 12 gene sets were associated with young age [43].
Anders et al. compared clinically-annotated global gene expression data from >700 early stage breast cancers within two cohorts (≤ 45 and ≥ 65 years). The younger group demonstrated differential expression of ER, PR, Her2/neu and epithelial growth factor receptor (EGFR), and Gene Set Enrichment Analysis identified 367 significant gene sets enriched among the younger cohort [44]. However, after correction for subtype and clinicopathologic features such as tumor grade, age-related gene expression differences were no longer seen [45].
A subsequent study reported age-dependent expression of individual breast cancer genes, using a candidate gene approach and after correction for tumor grade and subtype. Compared to older women, AYAs had relative overexpression of BUB1, KRT5, and MYCN and under-expression of CXCL2. Within tumor subtypes, gene expression had an age-related impact on outcome. [46].
Thus, age-related biological differences in breast tumors exist but may be largely driven by the higher frequency of aggressive basal-like and Her2-enriched subtypes among AYAs. The underlying reason for over-representation of aggressive subtypes among AYAs is not known and merits research.
Treatment and Management
Early Stage Disease
, AYAs often choose breast-conserving cancer surgery for reasons including body image, lactation, sexual function, and quality of life. However, the risk of local breast cancer recurrence after conservative surgery is 9-fold higher among women diagnosed <age 35 compared to >age 60 [47]. Nevertheless, conservative surgery in young women has not been associated with a negative impact on survival. In AYAs, breast-conserving surgery should always be followed by radiation. Following mastectomy, adjuvant radiation is used more often in AYAs compared to older women [48]. BRCA mutation carriers may have a higher incidence of radiation-induced malignancies [49].
Because of their higher risk for distant recurrence, adjuvant systemic therapies are usually warranted in AYAs with breast cancer [50]. ER-negative tumors, which benefit more from chemotherapy, are more frequent in AYAs than older women. Among patients <50 years old, use of adjuvant chemotherapy reduces the relative risk (RR) of recurrence by 35% and of death by 27% [51]. Choice of chemotherapy regimen is based on stage and clinical subtype.
For ER-positive early stage breast cancers, adjuvant endocrine therapy reduces the risk of recurrence by approximately 50% in young women [52]. The ATLAS and aTTom trials found that ten years of tamoxifen were superior to five for all age groups [53, 54]. Adding ovarian suppression to tamoxifen has the most potential to prevent recurrence among AYAs aged <35 and premenopausal patients who receive chemotherapy, and addition of an aromatase inhibitor even further reduces recurrence risk [55].Both tamoxifen and aromatase inhibitors may cause menopausal symptoms such as hot flashes. [56]
Metastatic disease
In both AYAs and older women, metastatic breast cancer is treated with sequential systemic therapy regimens, with each regimen aiming to palliate symptoms and slow disease progression for as long as possible. Premenopausal women with ER-positive disease should also either receive an ovarian-suppressing medication for the duration of endocrine therapies (including tamoxifen, aromatase inhibitor, fulvestrant, etc.) or else undergo bilateral salpingoophorectomy. [57–59].
Outcomes
Age disparities
During 2012–2014 in the U.S., the individual average risk of a woman dying from breast cancer by the age of 40 was 1 in 1925 and the probability 0.052% (SEER). Age <35 years at diagnosis is an independent predictor of time to recurrence (RR 1.70) and overall mortality (RR, 1.50) [60]. Following a diagnosis of early stage breast cancer, AYAs are 39% more likely to die compared to older women [61].
For all stages, survival rates are lower among AYAs compared to older women diagnosed with breast cancer. Of all age groups, women aged 40–55 have the best 5-year relative survival for stage II-IV disease (Figure 4) [62]. Among AYAs, survival is inversely proportional to age at every stage, with the poorest survival rates among the women diagnosed at the youngest ages. Stage IV breast cancer has the worst prognosis by far (Figure 4, lower panel).
For every histologic subtype of breast cancer, survival is worse for AYAs than for older women. This outcome disparity is most pronounced for invasive ductal carcinoma, but it is present for medullary, inflammatory and lobular breast cancer, as well as Paget’s disease of the breast (SEER) [63]. AYAs have inferior outcomes with all subgroups of hormone receptor expression. For patients with ER negative tumors, incidence of relapse peaks at 2 years post-therapy. For patients with ER-positive tumors steadily declines over an 8 year period. [64].
Since 1985, the improvement of the rate of breast cancer survival among AYA women has exceeded that of older women for all stages at diagnosis (SEER). The greatest relative gains have been in AYAs who present with distant disease, possibly due to improved strategies for palliative and supportive care.
Breast cancer outcomes for AYAs in Asia are not inferior to those of older women despite higher grade tumors and more advanced disease at diagnosis in younger patients [65]. This geographic variation in age-related outcome differences could be related to either environmental or genetic factors.
Ethnic disparities
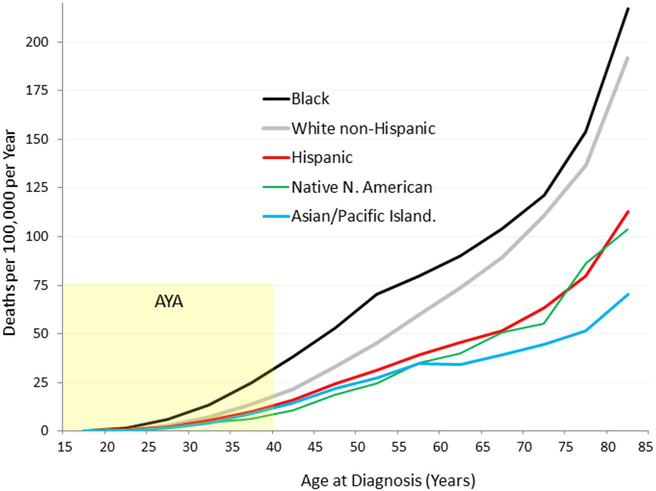
Among AYA women with breast cancer, African Americans have the worst survival rates, followed by white non-Hispanics, followed by Latinas and Native Americans; Asians have the lowest rates of death (Figure 5). Black AYAs have disproportionately high mortality compared to white AYAs (RR 1.94 for localized, 1.58 for regional, and 2.32 for metastatic disease), with larger mean tumor size and higher rates of ER-negativity, medullary tumors and metastatic disease at presentation in blacks [66]. Between 1975 and 2000, decline in mortality was three times greater for white than for black AYA women in the US [32]. In United Kingdom, blacks have worse outcomes than whites despite equal access to health care [67].
Figure 5.
Death Rate of Breast Cancer in Women, U.S., 2000–2011, by Race/Ethnicity and Age. Source: National Center for Health Statistics. Note: Hispanic may overlap with Black, Asian/PI, Native North American
Risk factors for death
Young age at diagnosis is a risk factor for death following local recurrence. Progression to metastatic disease is more likely in AYA compared to older women who present with early stage breast cancer. Among AYAs, the risk of cancer-related death is higher in patients with triple negative [Hazard ratio (HR) 2.7] and hormone receptor negative, HER2 enriched (HR 1.6) breast cancer subtypes compared to those with hormone receptor positive, HER2 non-enriched disease [68].
Somatic mutations in exons 5 to 8 of the tumor suppressor gene TP53, which occur in approximately 30% of all malignant breast tumors, are more common in tumors of AYAs than older women, and confer increased risk of tumor-related death (RR 2.27), particularly for patients with hormone-receptor negative disease [69]. Germline mutations in PALB2 are associated with strong predisposition to early onset breast cancer and inferior breast cancer survival [70].
Co-morbid medical conditions may increase breast cancer mortality in AYAs. Among premenopausal breast cancer patients, obesity is associated with poor prognosis [71], and type 2 diabetes mellitus [72] is associated with inferior DFS, increased recurrence and metastasis compared to age-matched breast cancer patients without these conditions.
Second Malignancies
Breast cancer is the most common secondary malignancy arising in females treated for childhood cancer. Risk is highest following chest radiation, anthracyclines, or alkylating agents. At-risk survivors should be managed according to the Children’s Oncology Group breast cancer surveillance guidelines. [73]
Contralateral breast cancer is the most common second malignancy by far in survivors of primary breast cancer, [74] and risk increases with younger age at diagnosis. Compared to women whose primary diagnosis occurred at age 40+, women initially diagnosed with breast cancer at age 29 or younger have a HR of 2.8 for the development of contralateral breast cancer [75]. Very early age at diagnosis (<30 years) was associated with a very high risk (RR>30) compared to the general population. Relative risk is 10.2 for women diagnosed between 30–34 years of age and 6.9 in women diagnosed between 35–39 years of age. In comparison, RR decreases to 1.1 for women diagnosed at 60–64 years of age [76, 77]. For women diagnosed between 15–44 years of age, the cumulative RR for developing a contralateral breast cancer is 5.4 [76, 77].
Risk for contralateral breast cancer is significantly heightened in women diagnosed with breast cancer <45 years who have a either family history of bilateral breast cancer (RR 3.6) or a first degree relative with breast cancer under age 45 (RR 2.5), compared to those without a family history of breast cancer [78]. For AYAs radiation therapy following lumpectomy, compared to after mastectomy, imparts an additional 50% increased risk of contralateral breast cancer. [79]. For all ages, adjuvant chemotherapy decreases the risk of contralateral breast cancer for 5 years following treatment, but not subsequently [79].
AYAs treated for breast cancer also have disproportionately high rates of myelodysplastic syndrome (RR 30.4) [80] and second malignancies in other sites, including bone, thyroid, ovary, colon, corpus uteri, lung, kidney, melanoma and non-melanoma skin cancer, leukemia, and lymphoma [77].
Special Issues for AYAs with breast cancer
Fertility Preservation
AYAs face potential long-term infertility resulting from breast cancer therapy [81], and ASCO guidelines mandate counseling about this risk at the time of diagnosis [82]. Before the start of any systemic therapy, all AYAs with breast cancer should be offered referral to a reproductive endocrinologist for either embryo or oocyte cryopreservation. Preimplantation genetic diagnosis, selecting unaffected embryos for implantation, is an option for carriers of BRCA mutations or other cancer predisposition syndromes [83]. Ovarian suppression with goserelin during chemotherapy is an option for AYAs with hormone-receptor negative breast cancer, decreasing the incidence long-term ovarian failure and possibly improving pregnancy outcomes [84].
Inherited Breast Cancer
Genetic evaluation is an essential component of multidisciplinary care for AYAs with breast cancer and should be initiated promptly following diagnosis. Patients require both pre-test counseling, to assess family history and evaluate risk, and post-test genetic counseling to accurately interpret test results. Because numerous genes may predispose AYAs to early onset breast cancer, next generation sequencing panels–which evaluate numerous genes simultaneously–are preferable to single gene testing [85]. Women with familial breast cancer syndromes require individualized counseling, evidence-based recommendations regarding tumor surveillance and prophylactic mastectomy/oophorectomy, ongoing support for decision-making, and lifelong tumor surveillance. [86]
Pregnancy during and after breast cancer
Pregnant women who receive standard chemotherapies for breast cancer during the second and third trimester have outcomes comparable to non-pregnant breast cancer patients [87, 88].
The desire for future pregnancy can affect selection of treatment protocol. One multicenter study showed that 19% of young breast cancer patients refused endocrine therapy, or chose one chemotherapy regimen over another, based on the wish to bear children in the future [81]. Pregnancy following a diagnosis of breast cancer does not impact mortality. A large meta-analysis showed a lower relative risk of death (RR=0.59) among women who bore a child after a breast cancer diagnosis [89]. The POSITIVE study is prospectively evaluating outcomes of pregnancy following breast cancer.
Survivorship Issues
When considering adjuvant endocrine and chemotherapy, clinicians should consider the long-term impact of bone loss, including osteoporosis and potentially disabling fractures. Bisphosphonate therapy has the potential to reduce bone loss [90] and improve breast cancer outcome [91], decreasing distant metastases and bone recurrences among post-menopausal women [92]. It is unclear if AYAs would derive similar benefit. Formal guidelines for bisphosphonate use in AYAs are eagerly awaited.
Most AYAs who survive breast cancer will live for decades following treatment and are at significant risk for second breast cancers and other malignancies [93]. Providers should proactively encourage AYA breast cancer survivors to adopt risk-reduction strategies including regular vigorous exercise, limitation of alcohol, and a diet low in energy-density and rich in plants [4, 30, 31].
Psychosocial Issues
AYAs are at significant risk for emotional and psychosocial sequelae during and after breast cancer treatment. They require age--specific psychosocial support, ideally in the context of coordinated multidisciplinary care teams [94, 95]. A retrospective study of >500 breast cancer survivors aged 25–50 years showed that long-term difficulties with emotional and social functioning increased with decreasing age at diagnosis. Younger breast cancer survivors experienced lower vitality and higher rates of depression [90]. Another study showed that, in comparison to both age-matched healthy controls and women who were older at diagnosis,, AYAs who had completed adjuvant chemotherapy for breast cancer 3–8 years earlier were at increased risk for difficulties with anxiety, sleep, marital satisfaction, and body image [96].
Concerns about fertility, sexuality, body image, disruptions in peer and romantic relationships, financial and occupational difficulties, and death from cancer are more pronounced in AYAs than older survivors and may contribute to distress [81, 97, 98]. Interventions to enhance psychosocial outcomes are currently being developed, including mindfulness meditation and online supports for patients and their families [99, 100].
Limitations
Young women have only recently been studied as a specific subgroup of breast cancer patients. There are few large scale, prospective studies focusing on this population. We present emerging data on statistically significant risk factors and evidence-based treatment recommendations for breast cancer in AYAs
Conclusion
Breast cancer is the most common malignancy among AYA women, who tend to present with more aggressive breast cancer subtypes and have worse outcomes compared to older women with breast cancer. Optimal care for this population requires a multidisciplinary team to maximize outcome, reserve quality of life and foster healthy survivorship. Multidisciplinary care algorithms for AYAs with breast cancer should include upfront investigation of familial cancer susceptibility, tailoring of the medical regimen to maximize fertility in patients who desire future children, management of reproductive issues--including the potential for post-treatment infertility, premature ovarian failure, or pregnancy, counseling for psychosocial and sexual issues, and long-term survivorship care.
Acknowledgement.
This publication was made possible by support for KJR from CTSA Grant Number KL2 TR002379 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- AJCC
American Joint Committee on Cancer
- AYA
Adolescent and Young Adult
- AYAs
Adolescents and Young Adults
- DFS
Disease-free Survival
- DHEAS
Dehydroepiandrosterone Sulfate
- EGFR
Epithelial Growth Factor Receptor
- ER
Estrogen Receptor
- HR
Hazard Ratio
- PR
Progesterone Receptor
- RR
Relative Risk
- SEER
United States Surveillance, Epidemiology and End Results database
- US
United States
Footnotes
Conflict of Interest Statement. The authors report no conflicts of interest in relation to this study. RHJ serves on the speaker bureau and as an occasional consultant for Shire Pharmaceuticals and Jazz Pharmaceuticals.
References
- 1.Anders CK, Johnson R, Litton J, et al. , Breast cancer before age 40 years. Semin Oncol, 2009. 36(3): p. 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program (www.seer.cancer.gov). SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (2000–2012) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2013 Counties. released April 2015, based on the November 2014 submission.
- 3.DevCan - Probability of Developing or Dying of Cancer. Surveillance Research Program SMaA, National Cancer Institute, DevCan 6.7.5. http://surveillance.cancer.gov/devcan/ Accessed October 29, 2017.
- 4.Shapira N, The potential contribution of dietary factors to breast cancer prevention. Eur J Cancer Prev, 2017. 26(5): p. 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis C, Ma J, Bryan L, et al. , Breast cancer statistics, 2013. CA Cancer J Clin, 2014. 64(1): p. 52–62. [DOI] [PubMed] [Google Scholar]
- 6.Narod SA, Breast cancer in young women. Nat Rev Clin Oncol, 2012. 9(8): p. 460–70. [DOI] [PubMed] [Google Scholar]
- 7.Leclere B, Molinie F, Tretarre B, et al. , Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol, 2013. 37(5): p. 544–9. [DOI] [PubMed] [Google Scholar]
- 8.Bleyer A and Welch HG, Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med, 2012. 367(21): p. 1998–2005. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RH, Chien FL, and Bleyer A, Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA, 2013. 309(8): p. 800–5. [DOI] [PubMed] [Google Scholar]
- 10.Althuis MD, Brogan DD, Coates RJ, et al. , Breast cancers among very young premenopausal women (United States). Cancer Causes Control, 2003. 14(2): p. 151–60. [DOI] [PubMed] [Google Scholar]
- 11.Shavers VL, Harlan LC, and Stevens JL, Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer, 2003. 97(1): p. 134–47. [DOI] [PubMed] [Google Scholar]
- 12.Weir HK, Jim MA, Marrett LD, et al. , Cancer in American Indian and Alaska Native young adults (ages 20–44 years): US, 1999–2004. Cancer, 2008. 113(5 Suppl): p. 1153–67. [DOI] [PubMed] [Google Scholar]
- 13.Baquet CR, Mishra SI, Commiskey P, et al. , Breast cancer epidemiology in blacks and whites: disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc, 2008. 100(5): p. 480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg LX, Reichman ME, Miller BA, et al. , Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control, 2009. 20(4): p. 417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingo PA, King J, Swan J, et al. , Breast cancer incidence among American Indian and Alaska Native women: US, 1999–2004. Cancer, 2008. 113(5 Suppl): p. 1191–202. [DOI] [PubMed] [Google Scholar]
- 16.Antoniou A, Pharoah PD, Narod S, et al. , Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet, 2003. 72(5): p. 1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalloo F, Varley J, Moran A, et al. , BRCA1, BRCA2 and TP53 mutations in very early-onset breast cancer with associated risks to relatives. Eur J Cancer, 2006. 42(8): p. 1143–50. [DOI] [PubMed] [Google Scholar]
- 18.Couch FJ, Shimelis H, Hu C, et al. , Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol, 2017. 3(9): p. 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoniou AC, Casadei S, Heikkinen T, et al. , Breast-cancer risk in families with mutations in PALB2. N Engl J Med, 2014. 371(6): p. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleibl Z and Kristensen VN, Women at high risk of breast cancer: Molecular characteristics, clinical presentation and management. Breast, 2016. 28: p. 136–44. [DOI] [PubMed] [Google Scholar]
- 21.Kotsopoulos J, Lubinski J, Moller P, et al. , Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers. Breast Cancer Res Treat, 2014. 143(3): p. 579–86. [DOI] [PubMed] [Google Scholar]
- 22.Suba Z, Circulatory estrogen level protects against breast cancer in obese women. Recent Pat Anticancer Drug Discov, 2013. 8(2): p. 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez AO, Chew H, Cress R, et al. , Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol, 2008. 112(1): p. 71–8. [DOI] [PubMed] [Google Scholar]
- 24.Warner ET, Colditz GA, Palmer JR, et al. , Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat, 2013. 142(1): p. 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway JL, Connors JM, Tyldesley S, et al. , Secondary Breast Cancer Risk by Radiation Volume in Women With Hodgkin Lymphoma. Int J Radiat Oncol Biol Phys, 2017. 97(1): p. 35–41. [DOI] [PubMed] [Google Scholar]
- 26.Allen NE, Beral V, Casabonne D, et al. , Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst, 2009. 101(5): p. 296–305. [DOI] [PubMed] [Google Scholar]
- 27.Kawai M, Malone KE, Tang MT, et al. , Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer, 2014. 120(7): p. 1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai M, Malone KE, Tang MT, et al. , Height, body mass index (BMI), BMI change, and the risk of estrogen receptor-positive, HER2-positive, and triple-negative breast cancer among women ages 20 to 44 years. Cancer, 2014. 120(10): p. 1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvera SA, Jain M, Howe GR, et al. , Energy balance and breast cancer risk: a prospective cohort study. Breast Cancer Res Treat, 2006. 97(1): p. 97–106. [DOI] [PubMed] [Google Scholar]
- 30.Boeke CE, Eliassen AH, Oh H, et al. , Adolescent physical activity in relation to breast cancer risk. Breast Cancer Res Treat, 2014. 145(3): p. 715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slattery ML, Edwards S, Murtaugh MA, et al. , Physical activity and breast cancer risk among women in the southwestern United States. Ann Epidemiol, 2007. 17(5): p. 342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000., Bleyer A, et al. , Editors. 2006, National Cancer Institute (Publication Number 06–5767): Bethesda, MD. [Google Scholar]
- 33.Cho E, Chen WY, Hunter DJ, et al. , Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med, 2006. 166(20): p. 2253–9. [DOI] [PubMed] [Google Scholar]
- 34.Farvid MS, Cho E, Chen WY, et al. , Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ, 2014. 348: p. g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack VA and dos Santos Silva I, Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev, 2006. 15(6): p. 1159–69. [DOI] [PubMed] [Google Scholar]
- 36.Lindgren J, Dorgan J, Savage-Williams J, et al. , Diet across the Lifespan and the Association with Breast Density in Adulthood. Int J Breast Cancer, 2013. 2013: p. 808317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozhand A, Lee E, Wu AH, et al. , Variation in inflammatory cytokine/growth-factor genes and mammographic density in premenopausal women aged 50–55. PLoS One, 2013. 8(6): p. e65313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung S, Egleston LB, Chandler DW, et al. , Adolescent endogenous sex hormones and breast density in early adulthood. Cancer Epidemiol Biomarkers Prev, 2015. 24(4): p. 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Manson JE, Lee IM, et al. , Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med, 2007. 167(10): p. 1050–9. [DOI] [PubMed] [Google Scholar]
- 40.El Saghir NS, Seoud M, Khalil MK, et al. , Effects of young age at presentation on survival in breast cancer. BMC cancer, 2006. 6(1): p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colleoni M, Rotmensz N, Robertson C, et al. , Very young women (< 35 years) with operable breast cancer: features of disease at presentation. Annals of Oncology, 2002. 13(2): p. 273–279. [DOI] [PubMed] [Google Scholar]
- 42.Parker JS, Mullins M, Cheang MCU, et al. , Supervised risk predictor of breast cancer based on intrinsic subtypes. Journal of Clinical Oncology, 2009. 27(8): p. 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azim HA Jr, Michiels S, Bedard PL, et al. , Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clinical Cancer Research, 2012. 18(5): p. 1341–1351. [DOI] [PubMed] [Google Scholar]
- 44.Anders CK, Hsu DS, Broadwater G, et al. , Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. Journal of Clinical Oncology, 2008. 26(20): p. 3324–3330. [DOI] [PubMed] [Google Scholar]
- 45.Anders CK, Fan C, Parker JS, et al. , Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol, 2011. 29(1): p. e18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson RH, Hu P, Fan C, et al. , Gene expression in “young adult type” breast cancer: a retrospective analysis. Oncotarget, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voogd A, Nielsen M, Peterse J, et al. , Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials. J Clin Oncol, 2001. 19(6): p. 1688–97. [DOI] [PubMed] [Google Scholar]
- 48.Dragun AE, Huang B, Gupta S, et al. , One decade later: trends and disparities in the application of post-mastectomy radiotherapy since the release of the American Society of Clinical Oncology clinical practice guidelines. Int J Radiat Oncol Biol Phys, 2012. 83(5): p. e591–6. [DOI] [PubMed] [Google Scholar]
- 49.Drooger JC, Hooning MJ, Seynaeve CM, et al. , Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: a critical review of the literature. Cancer Treat Rev, 2015. 41(2): p. 187–96. [DOI] [PubMed] [Google Scholar]
- 50.Goldhirsch A, Glick J, Gelber R, et al. , Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J Clin Oncol 2001. 19(18): p. 3817–27. [DOI] [PubMed] [Google Scholar]
- 51.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet, 1998. 352(9132): p. 930–42. [PubMed] [Google Scholar]
- 52.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet, 1998. 351(9114): p. 1451–67. [PubMed] [Google Scholar]
- 53.Davies C, Pan H, Godwin J, et al. , Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet, 2013. 381(9869): p. 805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray RG, Rea G, Handley K, et al. , aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. 2013, Chicago, IL.: 2013 ASCO Annual Meeting. [Google Scholar]
- 55.Francis PA, Regan MM, Fleming GF, et al. , Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med, 2015. 372(5): p. 436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torino F, Barnabei A, De Vecchis L, et al. , Chemotherapy-induced ovarian toxicity in patients affected by endocrine-responsive early breast cancer. Crit Rev Oncol Hematol, 2014. 89(1): p. 27–42. [DOI] [PubMed] [Google Scholar]
- 57.Bartsch R, Bago-Horvath Z, Berghoff A, et al. , Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer, 2012. 48(13): p. 1932–8. [DOI] [PubMed] [Google Scholar]
- 58.Cheung KL, Agrawal A, Folkerd E, et al. , Suppression of ovarian function in combination with an aromatase inhibitor as treatment for advanced breast cancer in pre-menopausal women. Eur J Cancer, 2010. 46(16): p. 2936–42. [DOI] [PubMed] [Google Scholar]
- 59.Klijn J, Blamey R, Boccardo F, et al. , Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. Journal of Clinical Oncology, 2001. 19(2): p. 343–353. [DOI] [PubMed] [Google Scholar]
- 60.Nixon AJ, Neuberg D, Hayes DF, et al. , Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. Journal of Clinical Oncology, 1994. 12(5): p. 888–894. [DOI] [PubMed] [Google Scholar]
- 61.Margenthaler J, Younger Women Diagnosed with Early-Stage Breast Cancer More Likely to Die than Older Women.. 2008 American College of Surgeons Clinical Congress, 2008. [Google Scholar]
- 62.Bleyer A, O’Leary M, Barr R, et al. , Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 years of age, including SEER incidence and survival: 1975–2000. National Cancer Institute, NIH Pub. No. 06–5767; Bethesda, MD., 2006. [Google Scholar]
- 63.Bleyer A, Barr R, Hayes-Lattin B, et al. , The distinctive biology of cancer in adolescents and young adults. Nature Reviews Cancer, 2008. 8(4): p. 288–298. [DOI] [PubMed] [Google Scholar]
- 64.Copson E, Eccles B, Maishman T, et al. , Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. J Natl Cancer Inst, 2013. 105(13): p. 978–88. [DOI] [PubMed] [Google Scholar]
- 65.Foo CS, Su D, Chong CK, et al. , Breast cancer in young Asian women: study on survival. ANZ J Surg, 2005. 75(7): p. 566–72. [DOI] [PubMed] [Google Scholar]
- 66.Newman L, Bunner S, Carolin K, et al. , Ethnicity related differences in the survival of young breast carcinoma patients. Cancer, 2002. 95(1): p. 21–7. [DOI] [PubMed] [Google Scholar]
- 67.Copson E, Maishman T, Gerty S, et al. , Ethnicity and outcome of young breast cancer patients in the United Kingdom: the POSH study. Br J Cancer, 2014. 110(1): p. 230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keegan TH, Press DJ, Tao L, et al. , Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res, 2013. 15(5): p. R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olivier M, Langerod A, Carrieri P, et al. , The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res, 2006. 12(4): p. 1157–67. [DOI] [PubMed] [Google Scholar]
- 70.Cybulski C, Kluzniak W, Huzarski T, et al. , Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol, 2015. 16(6): p. 638–44. [DOI] [PubMed] [Google Scholar]
- 71.Cleary MP, Impact of obesity on development and progression of mammary tumors in preclinical models of breast cancer. J Mammary Gland Biol Neoplasia, 2013. 18(3–4): p. 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma FJ, Liu ZB, Qu L, et al. , Impact of type 2 diabetes mellitus on the prognosis of early stage triple-negative breast cancer in People’s Republic of China. Onco Targets Ther, 2014. 7: p. 2147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derman YE, Clinical Practice Recommendations Based on an Updated Review of Breast Cancer Risk Among Women Treated for Childhood Cancer. J Pediatr Oncol Nurs, 2018. 35(1): p. 65–78. [DOI] [PubMed] [Google Scholar]
- 74.Zhang W, Becciolini A, Biggeri A, et al. , Second malignancies in breast cancer patients following radiotherapy: a study in Florence, Italy. Breast Cancer Res, 2011. 13(2): p. R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li CI, Malone KE, Porter PL, et al. , Epidemiologic and molecular risk factors for contralateral breast cancer among young women. Br J Cancer, 2003. 89(3): p. 513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prior P and Waterhouse JA, Incidence of bilateral tumours in a population-based series of breast-cancer patients. I. Two approaches to an epidemiological analysis. Br J Cancer, 1978. 37(4): p. 620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Thompson W, Semenciw R, et al. , Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev, 1999. 8(10): p. 855–61. [PubMed] [Google Scholar]
- 78.Reiner AS, John EM, Brooks JD, et al. , Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women’s Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol, 2013. 31(4): p. 433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hooning MJ, Aleman BM, Hauptmann M, et al. , Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol, 2008. 26(34): p. 5561–8. [DOI] [PubMed] [Google Scholar]
- 80.Kaplan HG, Malmgren JA, Li CI, et al. , Age related risk of myelodysplastic syndrome and acute myeloid leukemia among breast cancer survivors. Breast Cancer Res Treat, 2013. 142(3): p. 629–36. [DOI] [PubMed] [Google Scholar]
- 81.Ruddy KJ, Gelber SI, Tamimi RM, et al. , Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol, 2014. 32(11): p. 1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loren AW, Mangu PB, Beck LN, et al. , Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol, 2013. 31(19): p. 2500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woodson AH, Muse KI, Lin H, et al. , Breast cancer, BRCA mutations, and attitudes regarding pregnancy and preimplantation genetic diagnosis. Oncologist, 2014. 19(8): p. 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore HC, Unger JM, Phillips KA, et al. , Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med, 2015. 372(10): p. 923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maxwell KN, Wubbenhorst B, D’Andrea K, et al. , Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med, 2015. 17(8): p. 630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pruthi S, Gostout BS, and Lindor NM, Identification and Management of Women With BRCA Mutations or Hereditary Predisposition for Breast and Ovarian Cancer. Mayo Clin Proc, 2010. 85(12): p. 1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphy CG, Mallam D, Stein S, et al. , Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer, 2012. 118(13): p. 3254–9. [DOI] [PubMed] [Google Scholar]
- 88.Amant F, von Minckwitz G, Han SN, et al. , Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol, 2013. 31(20): p. 2532–9. [DOI] [PubMed] [Google Scholar]
- 89.Azim HA, Jr., Santoro L, Pavlidis N, et al. , Safety of pregnancy following breast cancer diagnosis: a meta-analysis of 14 studies. Eur J Cancer, 2011. 47(1): p. 74–83. [DOI] [PubMed] [Google Scholar]
- 90.Ganz P, Greendale G, Petersen L, et al. , Breast cancer in younger women: reproductive and late health effects of treatment. Journal of Clinical Oncology, 2003. 21(22): p. 4184–93. [DOI] [PubMed] [Google Scholar]
- 91.Gnant M, Mlineritsch B, Stoeger H, et al. , Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol, 2011. 12(7): p. 631–41. [DOI] [PubMed] [Google Scholar]
- 92.Coleman R, Gnant M, Paterson A, et al. , Abstract S4–07: Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women with early breast cancer: A meta-analysis of individual patient data from randomised trials Cancer Res, 2013. 73. [Google Scholar]
- 93.Lee JS, DuBois SG, Coccia PF, et al. , Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer, 2016. 122(1): p. 116–23. [DOI] [PubMed] [Google Scholar]
- 94.Shannon C and Smith IE, Breast cancer in adolescents and young women. Eur J Cancer, 2003. 39(18): p. 2632–42. [DOI] [PubMed] [Google Scholar]
- 95.Partridge A, Gelber S, Peppercorn J, et al. , Web-based survey of fertility issues in young women with breast cancer. Journal of Clinical Oncology, 2004. 22(20): p. 4174–83. [DOI] [PubMed] [Google Scholar]
- 96.Champion VL, Wagner LI, Monahan PO, et al. , Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer, 2014. 120(15): p. 2237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miaja M, Platas A, and Martinez-Cannon BA, Psychological Impact of Alterations in Sexuality, Fertility, and Body Image in Young Breast Cancer Patients and Their Partners. Rev Invest Clin, 2017. 69(4): p. 204–209. [DOI] [PubMed] [Google Scholar]
- 98.Park EM and Rosenstein DL, Depression in adolescents and young adults with cancer. Dialogues Clin Neurosci, 2015. 17(2): p. 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bower JE, Crosswell AD, Stanton AL, et al. , Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer, 2015. 121(8): p. 1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fergus KD, McLeod D, Carter W, et al. , Development and pilot testing of an online intervention to support young couples’ coping and adjustment to breast cancer. Eur J Cancer Care (Engl), 2014. 23(4): p. 481–92. [DOI] [PubMed] [Google Scholar]