Abstract
Rationale:
Ischemic heart disease (IHD) is a leading cause of mortality. The most effective intervention for IHD is reperfusion, which ironically causes ischemia reperfusion (I/R) injury mainly due to oxidative stress-induced cardiomyocyte death. The exact mechanism and site of reactive oxygen species (ROS) generation during I/R injury remain elusive.
Objective:
We aim to test the hypothesis that Complex I-mediated forward and reverse electron flows are the major source of ROS in I/R injury of the heart.
Methods and Results:
We used a genetic model of mitochondrial Complex I deficiency, in which a Complex I assembling subunit, Ndufs4 was knocked out in the heart (Ndufs4H−/−). The Langendorff perfused Ndufs4H−/− hearts exhibited significantly reduced infarct size (45.3 ± 5.5% in wild type vs 20.9 ± 8.1% in Ndufs4H−/−), recovered contractile function, and maintained mitochondrial membrane potential after no flow ischemia and subsequent reperfusion. In cultured adult cardiomyocytes from Ndufs4H−/− mice, I/R mimetic treatments caused minimal cell death. Reintroducing Ndufs4 in Ndufs4H−/− cardiomyocytes abolished the protection. Mitochondrial NADH declined much slower in Ndufs4H−/− cardiomyocytes during reperfusion suggesting decreased forward electron flow. Mitochondrial flashes, a marker for mitochondrial respiration, were inhibited in Ndufs4H−/− cardiomyocytes at baseline and during I/R, which was accompanied by preserved aconitase activity suggesting lack of oxidative damage. Finally, pharmacological blockade of forward and reverse electron flow at Complex I inhibited I/R-induced cell death.
Conclusions:
These results provide the first genetic evidence supporting the central role of mitochondrial Complex I in I/R injury of mouse heart. The study also suggests that both forward and reverse electron flows underlie oxidative cardiomyocyte death during reperfusion.
1. Introduction
Ischemic heart disease (IHD) is the leading cause of death in the US and worldwide. The most clinically effective treatment for IHD is to reestablish blood supply to the ischemic myocardium. However, reperfusion causes additional damage to cardiomyocytes, known as ischemia reperfusion (I/R) injury [1]. Currently, there is no effective therapy for I/R injury. Numerous studies have explored the mechanisms underlying I/R injury in the heart and brain. The leading causes are oxidative stress and Ca2+ overload, both of which can trigger opening of the mitochondrial permeability transition pore (mPTP) leading to cell death [1]. Surprisingly, chronic ablation of the major mitochondrial Ca2+ uptake channel, mitochondrial Ca2+ uniporter (MCU) offered no protection against I/R injury, while short-term MCU knockout or enhancing Ca2+ efflux showed protection [2–4]. These studies raised an intriguing question that mitochondrial Ca2+ may be dispensable for I/R injury. On the other hand, oxidative stress due to excessive reactive oxygen species (ROS) generation has been shown universally in I/R models and causally linked to cell death.[5] However, there are debates over the exact mechanisms and sites of ROS generation during I/R injury [5].
Numerous sites within mitochondrial matrix and in the cytosol can generate ROS under various conditions [5]. Inside mitochondria, Complex I and Complex III are the major ROS generating sites under both resting conditions and after I/R [6–8]. The mechanisms and sites within Complex I that are associated with ROS generation have been extensively studied in vitro [7, 9]. During forward electron flow, a high level of NADH can drive superoxide generation at the flavin mononucleotide moiety located near the NADH binding subunit. In addition, reverse electron flow driven by a reduced ubiquinone (ubiquinol) pool and high proton motive force can generate ROS when electrons flow back from ubiquinol to Complex I. In vitro studies showed that reverse electron flow can drive maximal ROS generation [10]. Recent reports suggested that reverse electron flow through Complex I is a major source of ROS in I/R injury [11, 12]. However, whether this is the major cause of I/R injury is under debate [13]. Genetic evidence supporting the key role of Complex I in oxidative stress and cardiac I/R injury is still missing.
In mammalian cells, Complex I contains 14 core subunits and 30 accessory subunits. A nucleus encoded accessory subunit, NADH:ubiquinone oxidoreductase subunit S4 (Ndufs4) is an 18 kDa protein responsible for the final assembly of Complex I [14]. Mutations in this gene cause Leigh syndrome [15]. Pan-tissue knockout of Ndufs4 resulted in 90% decrease in Complex I activity in the brain and severe neural defects that mimic Leigh syndrome [16]. Heart specific Ndufs4 knockout (Ndufs4H−/−) mice, however, were viable despite 60–70% decrease in Complex I activity in the heart. We have reported that the hearts of Ndufs4H−/− mice exhibited normal energetics and function and less ROS generation at baseline, but increased protein acetylation and sensitivity to pressure overload [17]. In this study, we found that Ndufs4H−/− hearts endured less damage and exhibited better functional recovery after I/R. Mechanistically, Ndufs4H−/− cardiomyocytes were protected from I/R injury due to less active mitochondrial respiration and ROS generation via both forward and reverse electron flows.
2. Methods
2.1. Animals
All the animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington and conform to the NIH guidelines (Guide for the care and use of laboratory animals). The heart specific Ndufs4 knockout (Ndufs4−/−) mice were generated by crossing C57BL/6 mice bearing modified ndufs4 gene containing lox P sites with transgenic mice expressing CRE driven by α-MHC promoter [17]. The Ndufs4H−/− mice were further crossed with mt-cpYFP transgenic mice for measuring mitochondrial flashes in Langendorff perfused heart [18].
2.2. Ischemia reperfusion treatment in Langendorff perfused heart
Langendorff perfusion of mouse hearts followed protocols we reported before.[18–20] Briefly, mouse (>3 months old) was euthanized (pentobarbital, 270 mg/kg, i.p.). The heart was rapidly removed, cannulated via ascending aorta and perfused with Krebs-Henseleit buffer containing (in mM): NaCl 118, NaHCO3 25, KCl 5.3, CaCl2 2, MgSO4 1.2, EDTA 0.5, glucose 10, and pyruvate 0.5 and equilibrated with 95% O2 and 5% CO2 at 37°C (pH 7.4). After 30 min, the perfusion was stopped (no-flow ischemia) for 30 min followed by resumed perfusion for 60 min (reperfusion). The left ventricle (LV) of the heart was cut into 6 cross-section slices along the long axis (~1 mm thick) by using a heart slicer. The slices were stained with 1% triphenyltetrazolium chloride (TTC) and imaged using a digital camera. The weight of each slice was measured and the LV area and infarct area in each slice were determined by using ImageJ (NIH). The infarct weight was calculated as: (infarct area) / (LV area) x (slice weight). The ratio between total infarct weight and total LV weight from the 6 slices was expressed as infarct size (percentage). In a subset of experiments, contractile function of the perfused heart was evaluated by Powerlab system (ADInstruments) following our previous reported protocol [21].
2.3. Confocal imaging of Langendorff perfused heart
Confocal imaging was done by using the Leica TCS SP8 inverted confocal microscope (Leica, Germany) with a 40× 1.3 NA oil-immersion objective. The heart from adult mt-cpYFP transgenic mouse (20–30 g) was perfused in Langendorff mode (see above) and mounted on confocal stage by using a custom made chamber as we previously reported [18, 20]. To minimize motion artifact during imaging, 10 μM (−)-Blebbistatin (Toronto Research Chemicals) was included in the perfusion solution. During the imaging, left ventricle was gently pressed against the coverslip at the bottom of the chamber to further suppress motion artifact. We also included TMRM (150 nM) in the perfusion solution for mitochondrial membrane potential measurement. The mt-cpYFP indicator was excited at 405 and 488 nm and emissions collected at 505–550 nm. TMRM was excited at 552 nm and emission collected at >560 nm. Time-lapse 2D images were collected at a sampling speed of ~1 s per frame for 100 frames for flash. Two dimensional images of TMRM were obtained during 30–60 min of reperfusion.
2.4. Generation of adenovirus construct containing Ndufs4 gene
The Ndufs4 gene was cloned into pDC316 shuttle plasmid in between EcoRI and SacI sites and co-transfected with pBHG helper plasmid into HEK293 cells for adenovirus packaging. The packaged adenoviruses were amplified several times to achieve an estimated pfu of ~109/ml.
2.5. Adult mouse cardiomyocyte isolation, treatments and imaging
Adult mouse cardiomyocytes were isolated by enzyme digestion methods as described previously [22, 23]. The cardiomyocytes were plated on laminin coated coverslips and adenovirus mediated Ndufs4 gene expression or mitochondria-targeted hydrogen peroxide indicator, mt-HyPer [24] overexpression were done at an estimated MOI of 10–100 for 2 days in serum free M199 medium. Ischemia reperfusion mimetic treatment of cultured cardiomyocytes were done by perfusing the cells with oxygen- and glucose-deprived solution containing (in mM): NaCl 137, KCl 4.9, CaCl2 1, MgSO4 1.2, NaH2PO4 1.2, HEPES 20, and NaS2O4 2 (pH 6.2), for 30 min (except for NADH autofluorescence measurements, which is 15 min). Reperfusion was achieved by switch back to normal solution without NaS2O4 and with 5 mM glucose (pH 7.4) [21]. The cells were stained with 0.4% Trypan Blue. Rod shaped cells without any staining were determined live cells and round shaped and blue stained cells dead cells [25]. Apoptosis was evaluated by using a caspase-3/7 detection kit (Invitrogen) and confocal imaging used Leica TCS SP8 inverted confocal microscope. To monitor mitochondrial NADH level, we used an Olympus FV1000 2-photon microscope with a 25x water immersion objective. Serial 2D scanning images were taken with 710 nm excitation and at a sampling rate of 30 s per frame during cellular I/R treatment. The mt-HyPer was excited by alternating excitation at 405 and 488 nm and collecting emissions at >505 nm. The ratio of emission fluorescence at 488 nm and 405 nm excitations was used to indicate mitochondrial ROS generation during I/R treatment. In addition, cardiomyocytes were loaded with MitoSox (Invitrogen, 5 μM for 10 min at 37˚C) and fluorescence monitored by confocal microscope during I/R with 405 and 488 nm excitation and emissions collected at >505 nm.
2.6. Western Blot Analysis
The cultured adult cardiomyocytes with or without adv-Ndufs4 infection were lysed with RIPA buffer containing the protease inhibitor cocktail. Protein samples were denatured and separated via SDS-PAGE, and transferred to PVDF membranes according to standard procedures. The blots were probed with primary antibodies: anti-Ndufs4 (1:1000, Cell Signaling), anti-VDAC1 (1:10000, Cell Signaling) or anti-β actin (1:10000, Sigma) followed by appropriate second antibodies.
2.7. Aconitase activity, lactate and glycogen measurements
These measurements used commercial available kits for aconitase activity assay (Sigma), and lactate (BioVision) or glycogen content in cells (BioVision).
2.8. Blue native gel and in gel complex I activity
Isolated mitochondria from adult mouse heart (100 μg) were solubilized in cold 4x NativePAGE Sample Buffer containing 5% digitonin and 5% coomassie blue G-250 sample additive and centrifuged to remove insoluble particles. The samples were loaded on NativePAGE Novex 3–12% Bis-Tris Gel and run at 100 V for 1 hr, then at 300 V for 2 hr. The gel was stained with coomassie blue overnight and de-stained for >10 hr (buffer changed every ~2 hr) before imaging (Bio-Rad imaging system). The in gel complex I activity was evaluated by adding NADH (5 mM) as substrate and nitrotetrazolium blue (NBT, 0.5 mM) after native gel electrophoresis and washed with water for 3–5 time before imaging.
2.9. Statistics
Data are shown as mean ± SEM. One-way ANOVA was used for experiments with more than 2 groups and followed by Tukey’s post hoc analysis. P < 0.05 was considered statistically significant.
3. Results
3.1. Ndufs4 knockout protected the heart against I/R injury
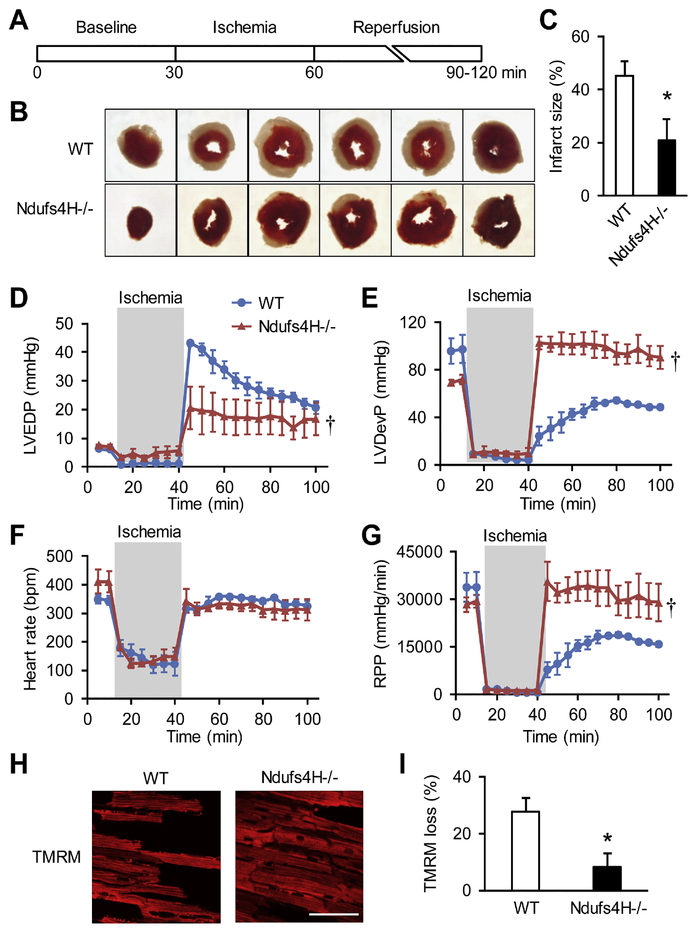
We first confirmed the Ndufs4H−/− mouse model, which showed lack of Ndufs4 protein, decreased mitochondrial respiration chain supercomplexes assembly and in gel Complex I activity at the age of 3–4 months (Online Fig. 1) consistent with previous report [17]. When perfused in Langendorff mode with oxygenated physiological solutions, Ndufs4H−/− hearts exhibited normal contractile function as wild type (WT) controls. After I/R treatment (30 min no-flow followed with 30–60 min reperfusion. Figure 1A), the infarct area was 50% smaller in Ndufs4H−/− hearts compared to WT hearts (45.3 ± 5.5% in WT vs. 20.9 ± 8.1% in Ndufs4H−/−, P < 0.05) (Fig. 1B, C). I/R treatment also compromised contractile function in WT hearts as reflected by partial recovery of left ventricular end diastolic pressure (LVEDP), left ventricular developed pressure (LVDevP) and rate-pressure product (RPP) (Fig. 1D-G). These contractile parameters were fully recovered in Ndufs4H−/− hearts (Fig. 1D-G). Finally, mitochondrial membrane potential was largely maintained in Ndufs4H−/− hearts after I/R, while ~30% of cardiomyocytes in WT hearts lost mitochondrial membrane potential (Fig. 1 H-I). These results support that genetic inhibition of Complex I activity protected against I/R injury in the heart.
Figure 1. Ndufs4H−/− hearts were protected against ischemia reperfusion injury (I/R).
A, Scheme of I/R protocol. B, Representative photographs of heart slices with TTC staining after I/R. C, Quantification of myocardial infarct size. *: P ˂ 0.05 vs wild type (WT). N = 5–6 mice. D-G, Left ventricular end-diastolic pressure (LVEDP), left ventricular developed pressure (LVDevP), heart rate, and rate-pressure product (RPP) were measured in Langendorff perfused hearts during I/R. †: P ˂ 0.05 vs WT. N = 3. H, Representative images of the myocardium of perfused hearts loaded with TMRM and right after I/R. I, Summarized data showing the loss of TMRM signal after I/R. *: P ˂ 0.05 vs WT. N = 4.
3.2. Ndufs4 knockout ameliorated I/R-induced cardiomyocyte death
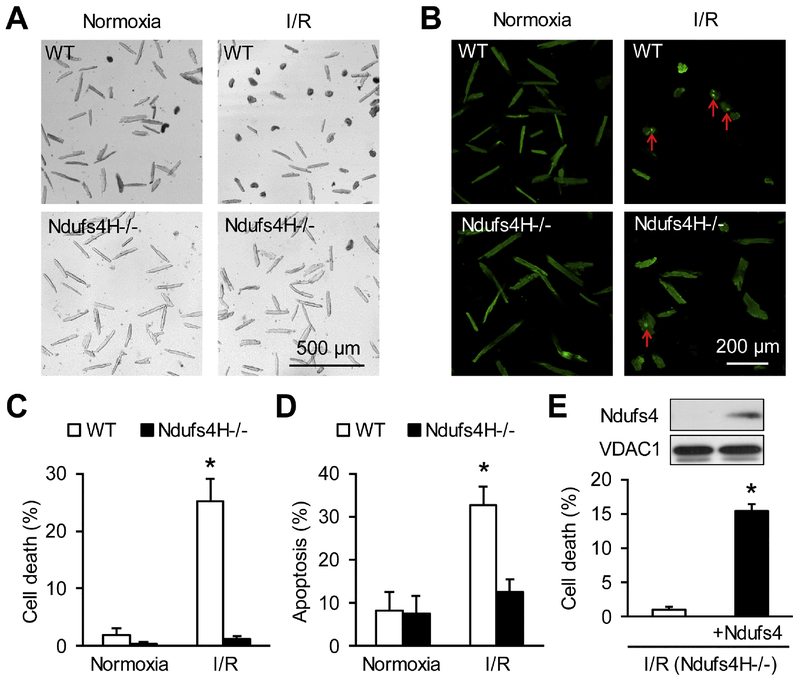
To further explore the potential mechanisms underlying the protection of Ndufs4H−/− against I/R-induced functional decline and infarction, we used an in vitro I/R mimetic model on cultured adult cardiomyocytes [20, 21]. I/R treatment induced significant cell death in adult cardiomyocytes, which was largely abolished in Ndufs4H−/− cardiomyocytes (27.3 ± 2.3% in WT vs. 3.3 ± 1.6% in Ndufs4H−/−, P < 0.05) (Fig. 2A&C). Similarly, I/R-induced much less apoptosis in Ndufs4H−/− cardiomyocytes (Fig. 2B&D). Finally, we re-introduced Ndufs4 gene in cultured Ndufs4H−/− cardiomyocytes and determined the re-expression of Ndufs4 protein in whole cell lysate (insert of Fig. 2E). Re-introducing Ndufs4 gene partially restored I/R-induced cell death (Fig. 2E) suggesting that short-term restoration of Complex I activity enhanced I/R injury.
Figure 2. Adult cardiomyocytes from Ndufs4H−/− mice were protected after I/R.
A-B, Trypan blue staining (A) or caspase-3/7 staining (B) of cardiomyocytes with or without I/R showing cell death and apoptosis. C, Quantification of cardiomyocyte death after I/R. *: P ˂ 0.05 vs Normoxia. N = 8–9. D, Quantification of cardiomyocyte apoptosis after I/R. *: P ˂ 0.05 vs Normoxia. N = 3. E, Re-introducing Ndufs4 in Ndufs4H−/− cardiomyocytes partially restored cell death after I/R. Insert in E showed the detection of re-expressed Ndufs4 by Western blots in whole cell lysate 2 days after adenovirus mediated gene expression. *: P ˂ 0.05 vs Ndufs4H-/−. N = 3 in each group.
3.3. Ndufs4 knockout inhibited mitochondrial respiration and oxidative stress after I/R
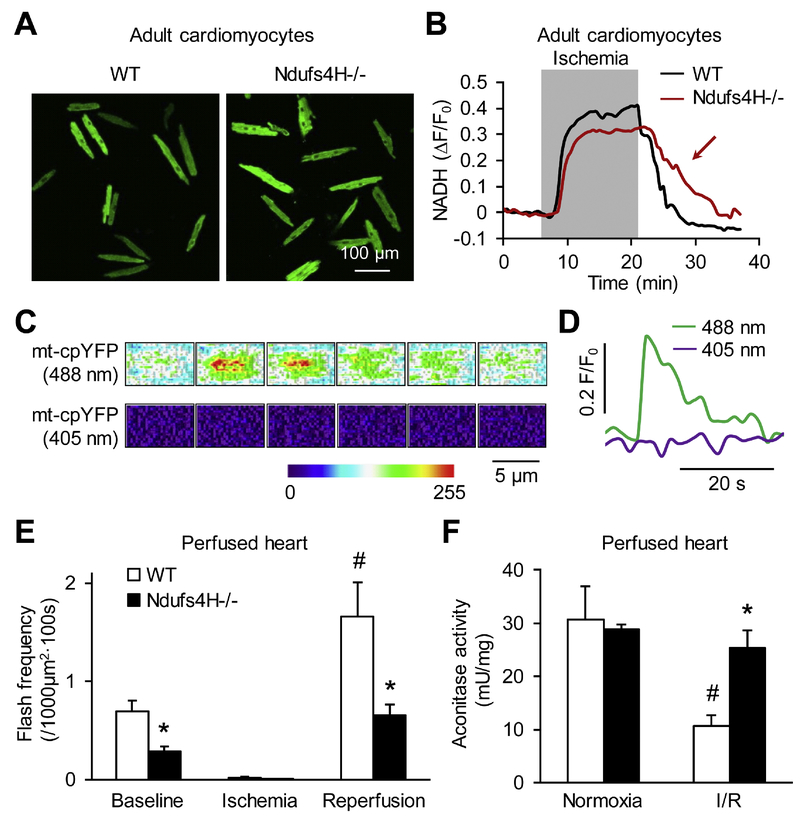
Complex I is the primary site in mitochondrial respiratory chain that accepts electrons from NADH. To determine whether the protective effect of Ndufs4H−/− is associated with inhibited respiration, we first monitored the autofluorescence of NADH, mainly from mitochondria, during I/R treatment. NADH accumulated during ischemia due to blocked respiration. Upon reperfusion, NADH declined quickly in WT cardiomyocytes due to restored respiration. However, NADH declined much slower in Ndufs4H−/− cardiomyocytes suggesting a slower rate of respiration restoration (Fig. 3A-B). In support of these results, mitochondrial flash frequency was lower in Ndufs4H−/− hearts at baseline (0.7 ± 0.1 vs. 0.3 ± 0.1 flashes per 1000 μm2 per 100 s in WT or Ndufs4H−/−, respectively) and during early reperfusion (1.7 ± 0.3 vs. 0.7 ± 0.1 flashes per 1000 μm2 per 100 s in WT or Ndufs4H−/−, respectively) suggesting single mitochondrial respiration was less active in Ndufs4H−/− hearts (Fig. 3C-D) [26, 27]. Since mitochondrial respiration is a major source for reperfusion ROS generation, we used mt-HyPer indicator [24] and MitoSox to monitor mitochondrial ROS levels during I/R treatment. The results showed that ischemia caused a decline in mitochondrial ROS level (Online Fig. 2). Upon reperfusion, there was a rebound increase of mitochondrial ROS in WT but not Ndufs4H−/− cardiomyocytes suggesting that Complex I deficiency prevented bursting mitochondrial ROS generation in early reperfusion (Online Fig. 2). To determine whether preventing reperfusion ROS generation led to less oxidative stress, we monitored aconitase activity. The Ndufs4H−/− hearts had a much higher aconitase activity after I/R supporting that genetic inhibition of Complex I attenuated oxidative stress through preventing reperfusion ROS generation by mitochondria.
Figure 3. Ndufs4H−/− cardiomyocytes exhibited lower mitochondrial respiration and oxidative stress after I/R.
A, Representative images of NADH autofluorescence in cardiomyocytes right after I/R. B, Ndufs4H−/− cardiomyocytes exhibited slower NADH decline in early reperfusion as indicated by the red arrow. Similar results are from 5 independent experiments. C, Heat maps showing time-dependent fluorescence change during a typical mitochondrial flash. Interval between each image is 2 s. D, Traces showing real-time fluorescence change during a flash. E, Mitochondrial flash frequency in perfused heart at baseline and during I/R. *: P ˂ 0.05 vs WT. #: P ˂ 0.05 vs baseline. N = 28–55 scans in 4 hearts for each group. F, Aconitase activity in normal heart tissues and after I/R. *: P ˂ 0.05 vs WT. #: P ˂ 0.05 vs Normoxia. N = 3.
As for other potential mechanisms that may mediate the protection of Ndufs4H−/− against I/R injury, we thought that systemic metabolic remodeling is unlikely a contributor. First, the activity of other respiration complexes is unchanged in Ndufs4H−/− heart [17]. In addition, lactate and glycogen levels were similar between wild type and Ndufs4H−/− cardiomyocytes after I/R (Online Fig. 3) suggesting unchanged glycolysis. Sirt3 activity is decreased in Ndufs4H−/− hearts, which sensitizes the heart to chronic stress [17]. However, previous report showed that decreased Sirt3 activity may worsen rather than protect heart from I/R injury [28].
3.4. Complex I dependent forward and reverse electron flows underlay oxidative cell death
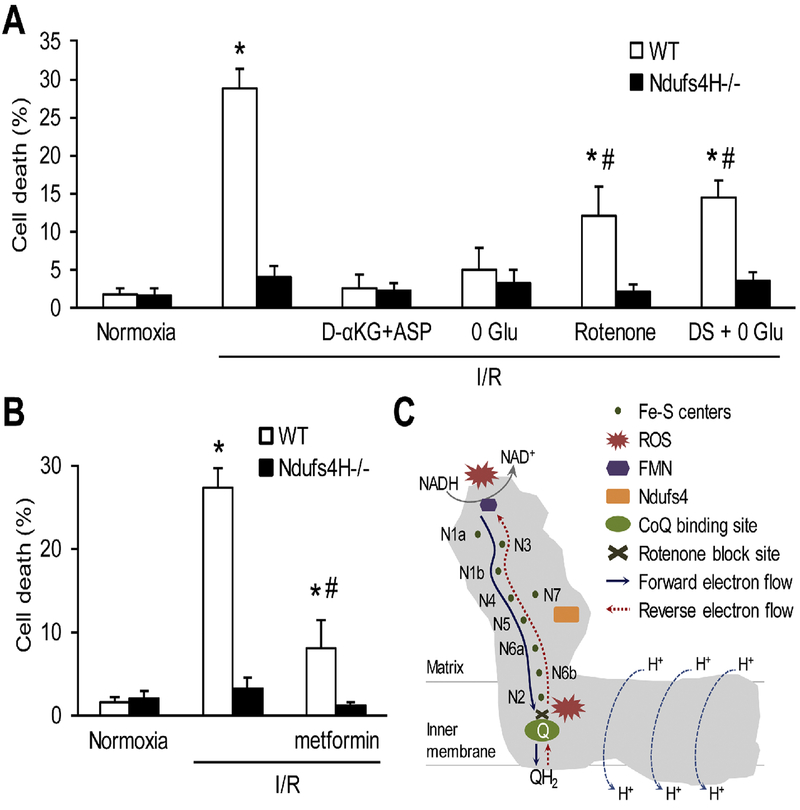
To further determine the mechanism underlying Complex I-dependent oxidative cell death in I/R injury, we used various metabolic perturbations (Fig. 4A). First, using dimethyl α-ketoglutarate (D-αKG, 5 mM) plus aspartate (5 mM) as the fuel in reperfusion, which generate ATP bypassing respiration chain [29], largely prevented cell death in WT cardiomyocytes suggesting the key role of respiration chain in reperfusion injury. In addition, removing glucose, the only metabolic substrate in the reperfusion solution, also blocked cardiomyocyte death. Rotenone (1 μM), which blocks forward and reverse electron flow in Complex I, also attenuated cardiomyocyte death indicating forward electron flow contributes to I/R injury. A recent report showed that reverse electron flow driven by accumulated succinate is a source of reperfusion ROS generation [11]. Using dimethyl-succinate (DS, 5 mM), a membrane-permeable derivative of succinate, as the sole substrate during reperfusion resulted in less cell death compared to 5 mM Glu (~30% in 5 mM Glu and ~15% in 0 Glu+DS group). However, DS still caused significant cell death (~15%) compared to 0 Glu group supporting the importance of reverse electron flow in I/R injury. Finally, metformin a Complex I inhibitor and anti-diabetic drug [30] protected I/R-induced cardiomyocyte death (Fig. 4B). Taken together, Complex I-mediated both forward and reverse electron flows contribute to ROS generation and oxidative stress-induced cardiomyocyte death in I/R.
Figure 4. Complex I-dependent forward and reverse electron flows underlay oxidative cell death.
A, Manipulating respiration chain electron flows by using different substrates or inhibitors during reperfusion to ameliorate I/R-induced cardiomyocyte death. D-αKG+ASP: dimethyl α-ketoglutarate (5 mM) plus aspartate (5 mM). 0 Glu: zero glucose. Rotenone: 1 μM. DS: dimethyl succinate (5 mM). *: P ˂ 0.05 vs normoxia, #: P ˂ 0.05 vs I/R. N = 3–11 in each group. B, Metformin (5 mM) protected I/R injury in cardiomyocytes. *: P ˂ 0.05 vs normoxia. #: P ˂ 0.05 vs I/R. N = 5–11 in each group. C, Diagram showing the sites and electron flows within Complex I for ROS generation. Knockout of Ndufs4 impaired Complex I assembly, resulted in fewer functional Complex I, and prevented supercomplexes formation leading to decreased ROS generation under resting condition and after I/R in the heart. CoQ: ubiquinone. QH2: ubiquinol. FMN: flavin mononucleotide. N1-N7: Fe-S centers.
4. Discussion
In this study, we reported that Complex I deficiency protected the heart from acute I/R injury. I/R mimetic treatment caused oxidative stress, cell death and heart dysfunction in ex vivo perfused hearts and in vitro cultured adult cardiomyocytes, all of which were markedly attenuated by Ndufs4 knockout. These results provide the first molecular evidence in mouse heart supporting a central role of Complex I in cardiac I/R injury. We further showed that Complex I mediated forward and reverse electron flows both contributed to ROS generation in I/R injury (Fig. 4C). Finally, the protective effect of Complex I deficiency on acute I/R injury is in contrast to its detrimental effect on chronic pressure overload-induced heart failure indicating that redox imbalance impacts cardiac pathology in a context dependent manner.
Previous work has proposed that oxidative stress is an important contributor to human diseases including cardiovascular disease, metabolic disease, and neurodegeneration [31]. Ironically, antioxidants tested in in vitro and animal models are largely ineffective or even cause adverse effects in clinical trials [32]. This huge gap in translating the knowledge from basic research to medical practice may be rooted in the facts that intracellular redox regulation is incompletely understood and that ROS play multiple roles in cellular physiology and pathology. Cardiac I/R injury is one of the most studied human diseases directly linked to oxidative stress [1, 5]. Among the numerous cytosolic and mitochondrial sources for reperfusion ROS generation [5], Complex I in mitochondrial respiration chain has been regarded as a major site [7, 9]. Previous studies using pharmacological approaches or indirectly manipulating Complex I, such as metformin [33], amobarbital [34], acidic environment [35], or activating mitochondrial STAT signaling [36],showed that Complex I inhibition lowered reperfusion ROS generation. Here, we showed that direct targeting Complex I assembly ameliorated cardiac I/R injury. Importantly, inhibiting Complex I activity by ~70% led to 50% smaller infarction and fully recovered cardiac contractility right after I/R. The remaining cell death could be attributed to the 30% Complex I activity and/or other ROS generation sites such as Complex III [37]. Future studies are warranted to determine whether 50% attenuation of infarction can lead to improved long-term functional recovery and heart remodeling in vivo in the chronic phase of IHD.
Besides the sites of ROS generation in I/R injury, the mode or mechanism of ROS generation within the major sites, such as Complex I, is also not fully understood. In vitro studies have indicated that two modes of electron flow can promote ROS generation at two sites within Complex I: forward electron flow at the NADH binding site driven by a high NADH supply, or reverse electron flow (electrons passed from Complex III or II to ubiquinone) at ubiquinone binding site driven by succinate, blocked forward electron flow, and a high membrane potential [7, 9]. Our results are in line with a recent report showing reperfusion ROS generation via reverse electron flow [11]. Since succinate-induced cell death is much less than glucose and NADH is also accumulated during ischemia, we conclude that forward electron flow also contributes to I/R injury. Indeed, previous reports have shown that either blocking forward or reverse electron flow protected against I/R injury [12, 38]. Future studies will determine whether the different modes of electron flow act in tandem or in concert (e.g., in different respiratory supercomplexes) for ROS generation during I/R.
The intracellular redox homeostasis is essential for normal function of the cell and stress responses. It is interesting that imbalanced redox can lead to diverse and even opposite outcomes depending on the tissue and nature of stress. The Complex I deficient (Ndufs4H−/−) mice exhibit a reductive state, which is responsible for increased sensitivity to chronic stress-induced heart hypertrophy and failure [17]. On the contrary, the hearts from these mice endured less oxidative damage in response to acute I/R injury due to the same reductive intracellular environment. Finally, pan-tissue knockout of Ndufs4 exerted neurodegeneration, which was ameliorated by low level of oxygen in the environment [39]. Very recently, a report showed that heterozygous Ndufs4 deficiency in whole body rendered the heart more susceptible to I/R injury [40]. Although the results seem contrary to ours, there are significant differences between the two studies. In that report, the partial loss of Ndufs4 caused mild decrease in some ETC components and no change in baseline ROS generation. Unfortunately, Complex I activity was not reported in that paper. Surprisingly, they found mitochondrial ROS generation by Complex I or II substrates was all significantly increased after I/R, which led them to attribute the worsened I/R injury to increased ROS generation by Complex III. In contrast, we have fully characterized the Ndufs4H−/− mouse model [17], which showed complete loss of Ndufs4 and decreased Complex I activity and ROS generation at baseline and after I/R. Thus, our model specifically tested the role of Complex I-mediated ROS generation in I/R injury. Collectively, these studies point out the complicated, tissue- and scenario-dependent role of Complex I deficiency or intracellular redox homeostasis in human disease, which could partially explain the failure of antioxidants in clinical trials.
Mitochondrial Ca2+ and ROS are tightly coupled in cell metabolism [41] and play synergistic roles in cardiac I/R injury [1]. Although not addressed in this study, mitochondrial Ca2+ may be impacted in the Ndufs4H−/− model due to inhibited mitochondrial respiration and ROS generation. First, decreased ROS may increase the threshold of Ca2+ to trigger mPTP opening during reperfusion. Second, respiration inhibition may compromise the reestablishment of electrochemical gradient across inner membrane and through which attenuate mitochondrial Ca2+ uptake during reperfusion. The interplay among mitochondrial energetics, ROS and Ca2+ and the involvement of mitochondrial Ca2+ in the protection of Ndufs4H−/− hearts against I/R injury warrant further investigation.
In summary, by using a genetic mouse model of heart specific Complex I deficiency, we reported that mitochondrial respiration chain, especially Complex I plays an essential role in I/R injury in the heart. We further showed that both reverse and forward electron flows contribute to ROS generation and oxidative cell death in I/R. These new findings support that targeting mitochondrial respiration chain Complex I assembly could be effective in treating I/R injury.
Supplementary Material
Highlights:
Genetic inhibition of mitochondrial Complex I protected I/R injury in mouse heart.
Complex I deficiency inhibited respiration, ROS generation and cardiomyocyte death.
Complex I mediated forward and reverse electron flow contributes to I/R injury.
Acknowledgements
We thank Drs. Philp G. Morgen and Ernst-Bernhard Kayser for technical support and helpful discussions. This study was partially supported by grants from NIH (HL114760 and HL37266 to W.W., HL110349 to R.T., P01AG001751 and R56AG055114 to P.S.R.), American Heart Association (18EIA33900041 to W.W.) and Glenn Foundation for Medical Research Postdoctoral Fellowship to H.Z..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
Reference
- [1].Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemiareperfusion injury. Physiol Rev. 2008;88:581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, et al. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015;12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, et al. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545:93–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem. 2017;292:16804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–80. [DOI] [PubMed] [Google Scholar]
- [10].Scialo F, Fernandez-Ayala DJ, Sanz A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol. 2017;8:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brand MD, Goncalves RL, Orr AL, Vargas L, Gerencser AA, Borch Jensen M, et al. Suppressors of Superoxide-H2O2 Production at Site IQ of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab. 2016;24:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP. The role of succinate and ROS in reperfusion injury - A critical appraisal. J Mol Cell Cardiol. 2017;110:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mimaki M, Wang X, McKenzie M, Thorburn DR, Ryan MT. Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta. 2012;1817:851–62. [DOI] [PubMed] [Google Scholar]
- [15].Petruzzella V, Vergari R, Puzziferri I, Boffoli D, Lamantea E, Zeviani M, et al. A nonsense mutation in the NDUFS4 gene encoding the 18 kDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum Mol Genet. 2001;10:529–35. [DOI] [PubMed] [Google Scholar]
- [16].Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr., Suthammarak W, Gong G, et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gong G, Wang W. Confocal imaging of single mitochondrial superoxide flashes in intact heart or in vivo. J Vis Exp. 2013:e50818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sun CL, Zhang H, Liu M, Wang W, Crowder CM. A screen for protective drugs against delayed hypoxic injury. PLoS One. 2017;12:e0176061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang H, Shang W, Zhang X, Gu J, Wang X, Zheng M, et al. Beta-adrenergic-stimulated L-type channel Ca(2)+ entry mediates hypoxic Ca(2)+ overload in intact heart. J Mol Cell Cardiol. 2013;65:51–8. [DOI] [PubMed] [Google Scholar]
- [21].Yu Q, Lee CF, Wang W, Karamanlidis G, Kuroda J, Matsushima S, et al. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc. 2014;3:e000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang H, Wang P, Bisetto S, Yoon Y, Chen Q, Sheu SS, et al. A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration. Cardiovasc Res. 2017;113:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1667–74. [DOI] [PubMed] [Google Scholar]
- [24].Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–6. [DOI] [PubMed] [Google Scholar]
- [25].Xu S, Wang P, Zhang H, Gong G, Gutierrez Cortes N, Zhu W, et al. CaMKII induces permeability transition through Drp1 phosphorylation during chronic beta-AR stimulation. Nat Commun. 2016;7:13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gong G, Liu X, Zhang H, Sheu SS, Wang W. Mitochondrial flash as a novel biomarker of mitochondrial respiration in the heart. Am J Physiol Heart Circ Physiol. 2015;309:H1166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemiareperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol. 2014;306:H1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sgarbi G, Casalena GA, Baracca A, Lenaz G, DiMauro S, Solaini G. Human NARP mitochondrial mutation metabolism corrected with alpha-ketoglutarate/aspartate: a potential new therapy. Arch Neurol. 2009;66:951–7. [DOI] [PubMed] [Google Scholar]
- [30].El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8. [DOI] [PubMed] [Google Scholar]
- [31].Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Sci Transl Med 2016;8:326rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. [DOI] [PubMed] [Google Scholar]
- [34].Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–15. [DOI] [PubMed] [Google Scholar]
- [35].Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y, et al. Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol. 2014;306:C1142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–6. [DOI] [PubMed] [Google Scholar]
- [38].Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–47. [DOI] [PubMed] [Google Scholar]
- [39].Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kuksal N, Gardiner D, Qi D, Mailloux RJ. Partial loss of complex I due to NDUFS4 deficiency augments myocardial reperfusion damage by increasing mitochondrial superoxide/hydrogen peroxide production. Biochem Biophys Res Commun. 2018. [DOI] [PubMed] [Google Scholar]
- [41].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.