Abstract
We have previously found that the transcription factor PPARγ2 contributes to the mechanism of action of the ketogenic diet (KD), an established treatment for pediatric refractory epilepsy. Among the wide-array of genes regulated by PPARγ, previous studies have suggested that antioxidants such as catalase may have prominent roles in KD neuroprotective and antiseizure effects. Here, we tested the hypothesis that the KD increases catalase through activation of PPARγ2, and that this action is part of the mechanism of antiseizure efficacy of the KD. We determined catalase mRNA and protein expression in hippocampal tissue from epileptic Kcna1−/− mice, Pparγ2+/+ mice and Pparγ2−/− mice. We found that a KD increases hippocampal catalase expression in Kcna1−/− and Pparγ2+/+ mice, but not Pparγ2−/− mice. Next, we determined whether catalase contributes to KD seizure protection. We found that the KD reduces pentylenetetrazole (PTZ)-induced seizures; however, pretreatment with a catalase inhibitor occluded KD effects on PTZ seizures. These results suggest that the KD regulates catalase expression through PPARγ2 activation, and that catalase may contribute to the KD antiseizure efficacy.
Keywords: Epilepsy, in vivo, PPAR, PPARgamma, peroxisome proliferator activated receptor, metabolism
1. Introduction:
High fat–low carbohydrate/adequate protein ketogenic diets (KDs) are a broadspectrum anti-seizure therapy. Traditionally, clinical use has been limited to pediatric epilepsy refractory to anti-seizures medications, though it is efficacious against many seizure types, severities, etiologies and patient ages (Bergin, 2017; Johnson and Cervenka, 2017; Neal et al., 2017). Within this difficult-to-treat refractory patient population, KDs impressively reduce ~50% of seizures in approximately half of the patients and ~90% of seizures in up to one-third of patients (Neal et al., 2017). Though a range of KDs have been clinically used for decades, molecular mechanisms underlying therapeutic effects are still being discovered.
In previous studies, we demonstrated that the KD reduces seizures by 75% and increases lifespan of Kcna1-null (Kcna1−/−) mice, a model of severe, chronic, temporal lobe epilepsy and sudden unexpected death in epilepsy (Kim et al., 2015; Simeone et al., 2016; Simeone et al., 2017c). Further studies indicated that a splice variant of the nutritionally-regulated transcription factor peroxisome proliferator activated receptor gamma2 (PPARγ2) is involved in the anti-seizure mechanism of the KD (Simeone et al, 2017b,c). Among the wide-array of genes regulated by PPARγ, antioxidants such as catalase may have prominent roles in KD neuroprotective and antiseizure effects. In fact, catalase expression or activity increases significantly in in vivo experiments where rats were treated with a KD and in in vitro experiments using proposed effectors of various KDs, ketone bodies and decanoic acid (Kim et al., 2010; Hughes et al., 2014; Wang et al., 2017). In the present study, we demonstrate for the first time that in vivo treatment with a KD increases hippocampal catalase mRNA and protein, and that this upregulation requires PPARγ2. Furthermore, we found that upregulation of catalase contributes to the antiseizure effects of the KD.
2. Methods:
2.1. Animals and dietary treatment:
All mice were housed in the Animal Resource Facilities at Creighton University School of Medicine in a temperature (25°C)- and humidity (50–60%)-controlled, pathogen-free environment. Mice were given food and water ad libitum and kept on a 12-hour light/dark cycle. Heterozygous Kcna1+/− mice on a C3HeB/FeJ congenic background were purchased from Jackson Laboratories (Bar Harbor, Maine) and bred to obtain Kcna1−/− mice. Heterozygous Pparγ2+/− mice on a mixed 129sv-C57Bl/6 background were provided by Gema Medina-Gomez (Universidad Rey Carlos, Madrid, Spain) and bred to obtain Pparγ2+/+ and Pparγ2−/−littermates. Genotyping was performed by Transnetyx Inc. (Cordova, TN, U.S.A.). On P21, mice were randomly weaned onto either a standard diet (SD; 0.1:1, fat to carbohydrates plus proteins based on %weight; energy density of 3.1 kcal/g; Teklad 2018S, Envigo, Madison, WI, U.S.A.) or a KD (6.3:1, fat to carbohydrates plus proteins; energy density of 7.2 kcal/g; Bio-Serv F3666, Frenchtown, NJ, U.S.A.) for 10–14 days. Diets were fed ad libitum. Four-week old male C57Bl/6 mice were purchased from Envigo (Indianapolis, IN, U.S.A.). On P35, C57Bl/6 mice were randomly separated into SD or KD groups for 10–14 days. All procedures involving animals were in accordance with National Institutes of Health guidelines, the EU Directive 2010/63/EU and were approved by the Institutional Animal Care and Use Committees at Creighton University School of Medicine.
2.2. Western Blot:
Protein was isolated from one hippocampus from Kcna1−/−, Pparγ2+/+ and Pparγ2−/− mice and quantified with a Bradford Assay. Tissue was prepared as we have described (Simeone et al., 2017c; Simeone et al., 2018). Membranes were incubated overnight with primary antibodies for mouse anti-β-actin (1:8000; 926–42212 LiCor Biosciences) and rabbit anti-catalase (1:500; PA5–29183 Thermo Fisher Scientific, Waltham, MA, U.S.A.) at 4°C. Following PBS-T washes, membranes were incubated in secondary antibodies for one hour: goat anti-rabbit (1:5000; 926–32221, Li-Cor Biosciences) or goat anti-mouse (1:20,000; 926–32210, Li-Cor Biosciences). Samples were run in duplicates, images were captured on an Odyssey FC (Licor Biosciences). Catalase protein signal was normalized to β-actin values and the SD-fed animal that was run on the same gel.
2.3. Reverse transcriptase qPCR:
RNA was isolated from the other hippocampus of Kcna1−/−, Pparγ2+/+ and Pparγ2−/− mice. Quantitative PCR was performed for catalase and housekeeping genes ribosomal protein L22 and L30 (Rpl22 and Rpl30) using a SYBR green PCR master mix (Agilent Technologies, Santa Clara, CA) and a AriaMx Real Time PCR instrument (Agilent Technologies) as we have described (Simeone et al., 2018). Primers were Catalase forward, 5’-GGCAAAGGTGTTTGAGCATATT-3’; Catalase reverse, 5’- GAGTCTGTGGGTTTCTCTTCTG-3’; Rpl22 forward, 5’-GGCCAAACAGAAGAACCAGG-3’; Rpl22 reverse, 5’-CACCTGTCTGCTTCTGAGGA-3’; Rpl30 forward, 5’- AAGGCAAAGCGAAGTTGGTT-3’; Rpl30 reverse, 5’- ACCTGGGTCAATGATAGCCA-3’; (IDT’s Primer Quest software2). Cycling conditions: 95°C-10min, 40cycles of 95°C-30sec, 72°C-30sec. Each real-time PCR was performed using three biological samples in technical triplicates and melt curve analyses were completed to ensure the specificity of amplification. Calculations of the Catalase gene expression relative to averaged Rpl22 and Rpl30 expression were based on the differences in threshold cycles using the 2–ΔΔCt method.
2.4. Pentylenetetrazole (PTZ)-induced seizures:
After 10–14 days fed either SD or KD C57Bl/6 mice were intraperitoneally (i.p.) injected with either saline vehicle (0.1 ml) or the catalase inhibitor sodium azide (SA; 10 mg/kg; Sanchis-Segura et al., 1999) 15 min prior to i.p. injection of either saline vehicle (0.1 ml) or pentylenetetrazole (PTZ; 80 mg/kg). Seizure incidence, duration and severity were measured over 10 min post-PTZ. Seizure severity was scored using a modified Racine scale: 0 - normal; 1- ear and facial twitching; 2 -convulsive waves; 3 - myoclonic jerks; 4 - clonic-tonic convulsions on side; 5 - generalized clonic-tonic convulsions, loss of postural control (Ammon-Treiber et al., 2007; Simeone et al., 2014, 2017c; Roundtree et al., 2016). A cumulative seizure burden for each mouse was calculated using the equation: SB Σ(σδ)i where σ is the severity and δ is the duration for each seizure (i) (Barker-Haliski et al., 2016; Simeone et al., 2014, 2017c; Roundtree et al., 2016).
2.5. Catalase Activity Assay:
Thirty minutes after saline vehicle or SA i.p. injection mice were anesthetized with isoflurane, decapitated and hippocampal tissue was isolated and immediately homogenized in 500 ul of buffer containing 50 mmol/L HEPES, 250 mmol/L sucrose, 1 mmol/L EDTA, 0.1% ethanol, and 10% protease inhibitor cocktail (modified from Yakunin et al., 2014) and centrifuged at 18,000 g for one hour at 4°C. Protein was quantified using a Qubit (Thermo Fisher Scientific). Catalase enzymatic activity was measured using an Amplex Red Catalase Assay kit according to the manufacturer’s protocol (Thermo Fisher Scientific).
2.6. Statistical Analysis:
All data are reported as mean ± standard error. Statistical significance was determined with either an unpaired t-test or a two-way ANOVA using Prism 7 software (Graphpad Software, Inc.).
3. Results:
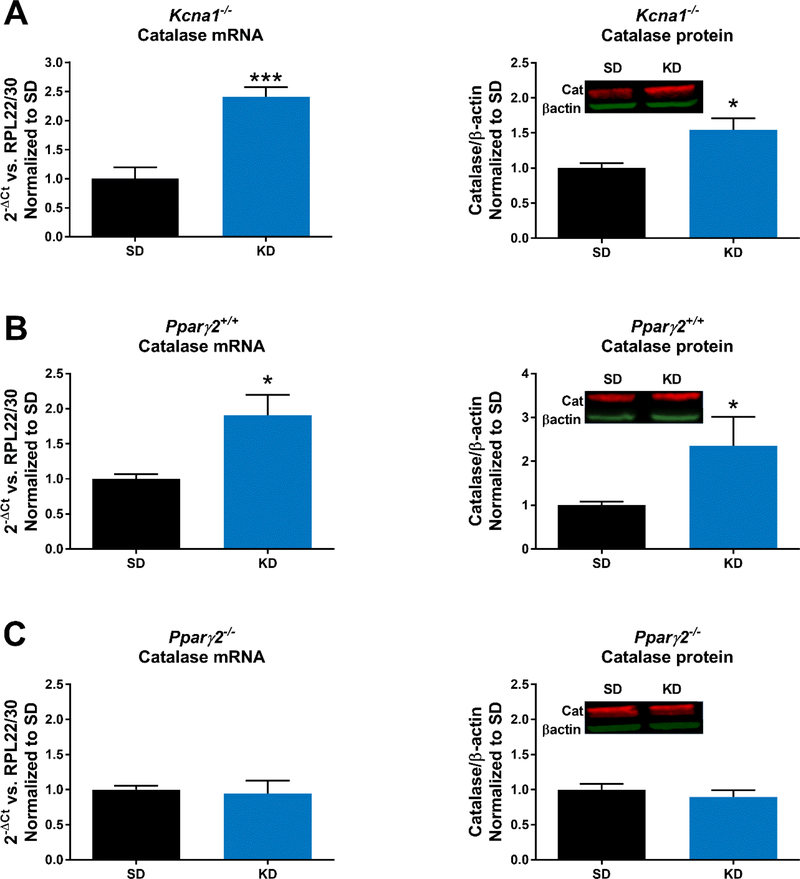
To determine whether the KD regulates catalase we measured mRNA and protein expression in hippocampal tissue from epileptic Kcna1−/− mice. We found a that KD-treatment significantly increased catalase mRNA (p<0.001) and protein (p<0.05) (Fig. 1A) compared to SD. To determine whether PPARγ2 had a role in KD-mediated catalase regulation we repeated this experiment in Pparγ2+/+ and Pparγ2−/− mice. Hippocampal catalase expression in Pparγ2+/+ mice exhibited similar increases as Kcna1−/− mice in response to KD treatment (Fig. 1B); however, the KD failed to increase catalase mRNA and protein in Pparγ2−/− mice (Fig. 1C).
Figure 1.
The ketogenic diet (KD) fails to increase catalase in mice without PPARγ2. Twoweek treatment with a KD increases hippocampal catalase mRNA and protein in (A) epileptic Kcna1−/− mice (n = 3/group) and (B) wildtype Pparγ+/+ mice (n = 3–4/group); however, catalase mRNA and protein did not change with KD treatment of (C) Pparγ−/− mice (n = 3/group). Significance determined by an unpaired t-test, *p<0.05, ***p<0.001.
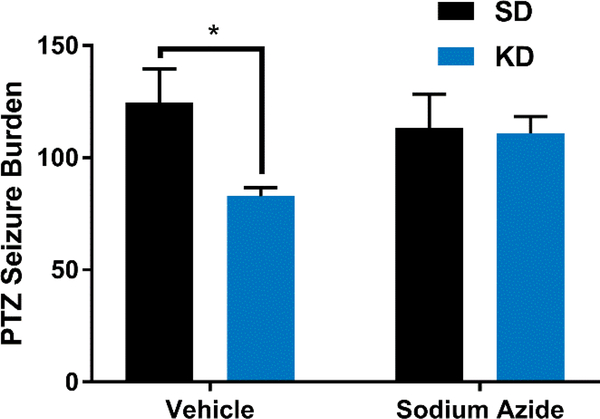
Previously, we demonstrated pharmacologic and genetic loss of Pparγ2 abrogated KD effects on chronic, spontaneous recurrent seizures and chemicallyinduced acute seizures, respectively (Simeone et al., 2017c). Here, we determined whether inhibiting catalase would affect KD antiseizure efficacy. We found that KDtreatment reduced the PTZ-induced seizure burden by ~34% (Figure 2). Sodium azide (SA) has been shown to decrease brain catalase activity by ~53% thirty minutes postinjection i.p. (Sanchis-Segura et al., 1999). Similarly, we found that SA inhibited hippocampal catalase activity by 36.3 ± 1.7% thirty minutes post-injection i.p (112 ± 3 vs. 177 ± 18 mUnits/ml/mg protein, n = 5/group, p < 0.05, unpaired t-test). Pretreating mice with SA prevented the KD reduction of PTZ seizures. Injection of SA without PTZ did not provoke seizures in either SD- or KD-fed mice (n = 5/group).
Figure 2.
Catalase inhibition prevents KD-mediated seizure protection. Mice were administered either vehicle or the catalase inhibitor sodium azide (SA,10 mg/kg; i.p.) fifteen minutes before a second i.p. injection of vehicle or pentylenetetrazole (PTZ, 80 mg/kg). KD-treatment significantly reduced the seizure burden of vehicle-treated mice (n = 5/group), but not SA-treated mice (n = 6/group). Significance determined by a twoANOVA followed by Sidak’s multiple comparisons test, *p<0.05.
4. Discussion
The KD increases long chain fatty acids, which are endogenous ligands of PPARγ, in humans and animals suggesting that fatty acids may have a role in KD action(Dell et al., 2001; Fraser et al., 2003; Taha et al., 2005; Simeone, 2017a). We previously found that the KD increased brain nuclear PPARγ2 and that pharmacological or genetic loss prevented KD antiseizure efficacy against chemically induced acute seizures in non-epileptic mice and spontaneous recurrent seizures in epileptic Kcna1−/− mice (Simeone et al., 2017c). Further supporting an interaction between the KD and PPARγ, co-administration of an ineffective low ratio KD and low dose of a PPARγ agonist provided significant seizure protection (Simeone et al., 2017b). Here, we have begun to explore potential downstream effectors.
PPARγ regulates a multitude of genes involved in mitochondria, antiinflammatory and antioxidant activities, and is a master regulator of adipogenesis. Recently, in vitro studies found that decanoic acid, a component of the medium chain triglyceride KD, increases the antioxidant catalase via PPARγ (Hughes et al., 2014; Kanabus et al., 2016). In the current in vivo studies, we found that the KD increases catalase mRNA and protein, and that this does not occur in mice lacking PPARγ2 splice variant. This matches well with the known differences in properties of PPARγ1 and PPARγ2, specifically PPARγ2 has more effective ligand-independent transactivation, increased ligand binding affinity and upregulates catalase expression to a greater degree than PPARγ1 (Simeone, 2017a).
The enzymatic activity of catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen, thus preventing the formation of the reactive oxygen species (ROS), specifically hydroxyl radicals, and subsequent oxidative damage. ROS have a complicated relationship with seizures and epilepsy, i.e. they can be generated by seizures, they can worsen seizures and they can cause seizures (Souza et al., 2009; Simeone et al., 2014; Waldbaum and Patel, 2010). Compared to other tissues the low levels of catalase limit the brain’s ability to combat ROS (e.g., brain levels of catalase is 10% of liver) (Shin et al., 2011). Moreover, experimental acute PTZ seizures have been shown to decrease catalase and increase ROS (Souza et al., 2009). Therefore, a reasonable expectation is that strategies which reduce ROS will be neuroprotective and have antiseizure effects. This has been born out experimentally (Simeone et al., 2014; Waldbaum and Patel, 2010). Consistent with this hypothesis, the KD has been found to reduce ROS production (Sullivan et al., 2004), reduce spontaneous seizures (Kim et al., 2015; Simeone et al., 2016, 2017c) and here we found that the KD increases catalase and reduces PTZ-induced seizure burden. Furthermore, catalase inhibition occluded the antiseizure effects of the KD.
In conclusion, these data provide evidence that the KD regulates catalase expression, that this regulation involves PPARγ2 activation, and that catalase may contribute to the KD antiseizure efficacy. These data support the notion that ROS plays a significant role in the generation/expression of seizures and that antioxidant pathways are important therapeutic antiseizure targets for consideration.
Highlights.
The ketogenic diet increases hippocampal catalase mRNA and protein.
The ketogenic diet does not increase catalase mRNA or protein in the hippocampus of mice lacking PPARγ2.
Inhibiting catalase occluded the KD antiseizure effects.
Acknowledgements:
Please note, the second to last author has published under the names K Dorenbos, KA Fenoglio, KA Fenoglio-Simeone and KA Simeone.
Funding: This work was supported by Citizens United for Research in Epilepsy Foundation (TAS) NIH NS085389 (TAS) and NIH NS072179 (KAS). The project described was also supported by the National Center for Research Resources grant G20RR024001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Declaration of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ammon-Treiber S, Grecksch G, Angelidis C, Vezyraki P, Hӧllt V, Becker A, 2007. Pentylenetetrazol-kindling in mice overexpressing heat shock 70. Naunyn-Schmiedeberg’s Arch. Pharmacol 375, 115–121. [DOI] [PubMed] [Google Scholar]
- 2.Barker-Haliski ML, Heck TD, Dahle EJ, Vanegas F, Pruess TH, Wilcox KS, White HS, 2016. Acute treatment with minocycline, but not valproic acid, Zimproves long-term behavioral outcomes in the Theiler’s virus model of temporal lobe epilepsy. Epilepsia. 57,1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergin AM, 2017. Ketogenic Diet in Established Epilepsy Indications, in: Masino SA (Ed), Ketogenic diet and metabolic therapies: expanded roles in health and disease. Oxford University press, New York, pp. 40–49. [Google Scholar]
- 4.Dell CA, Likhodii SS, Musa K, Ryan MA, Burnham WM, Cunnane SC, 2001. Lipid and fatty acid profiles in rats consuming different high-fat ketogenic diets. Lipids 36:373–378. [DOI] [PubMed] [Google Scholar]
- 5.Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC, 2003. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology 60:1026–1029. [DOI] [PubMed] [Google Scholar]
- 6.Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O’Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ, 2014. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem 129, 426–433. [DOI] [PubMed] [Google Scholar]
- 7.Johnson EL, Cervenka MC, 2017. Dietary Therapy in Adults: History, Demand and Results, in: Masino SA (Ed), Ketogenic diet and metabolic therapies: expanded roles in health and disease. Oxford University press, New York, pp. 16–25. [Google Scholar]
- 8.Kanabus M, Fassone E, Hughes SD, Bilooei SF, Rutherford T, Donnell MO, Heales SJR, Rahman S, 2016. The pleiotropic effects of decanoic acid treatment on mitochondrial function in fibroblasts from patients with complex I deficient Leigh syndrome. J. Inherit. Metab. Dis 39, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM, 2015. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol 78, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DY, Vallejo J, Rho JM, 2010. Ketones prevent synaptic dysfunctioninduced by mitochondrial respiratory complex inhibitors. J. Neurochem 114, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal E, 2017. “Alternative” Ketogenic Diets, in: Masino SA (Ed), Ketogenic diet and metabolic therapies: expanded roles in health and disease. Oxford University press, New York, pp. 5–15. [Google Scholar]
- 12.Roundtree HM, Simeone TA, Johnson C, Matthews SA, Samson KK,Simeone KA, 2016. Orexin Receptor Antagonism Improves Sleep and Reduces Seizures in Kcna1-null Mice. Sleep. 39, 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchis-Segura C, Miquel M, Correa M, Aragon CM, 1999. The catalaseinhibitor sodium azide reduces ethanol-induced locomotor activity. Alcohol. 19, 37–42. [DOI] [PubMed] [Google Scholar]
- 14.Simeone KA, Matthews SA, Samson KK, Simeone TA, 2014. Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures. Exp. Neurol 251, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeone KA, Matthews SA, Rho JM, Simeone TA, 2016. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 57, e178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simeone KA, Hallgren J, Bockman CS, Aggarwal A, Kansal V, Netzel L, Iyer SH, Matthews SA, Deodhar M, Oldenburg PJ, Abel PW, Simeone TA, 2018. Respiratory dysfunction progresses with age in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia. 59, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeone TA, 2017a. Ketogenic Diet and PPARgamma, in: Masino SA (Ed),Ketogenic diet and metabolic therapies: expanded roles in health and disease. Oxford University press, New York, pp. 167–185. [Google Scholar]
- 18.Simeone TA, Matthews SA, Simeone KA, 2017b. Synergistic protection against acute flurothyl-induced seizures by adjuvant treatment of the ketogenic diet with the type 2 diabetes drug pioglitazone. Epilepsia. 58, 1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simeone TA, Matthews SA, Samson KK, Simeone KA, 2017c. Regulationof brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol 287, 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza MA, Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Lima FD, Dalla Corte LC, Silva LF, Retamoso LT, Dalla Corte CL, Puntel O, de Avila DS, Soares FA, Fighera MR, de Mello CF, Royes LF, 2009. Swimming training prevents pentylenetetrazol-induced inhibition of Na+, K+ATPase activity, seizures, and oxidative stress. Epilepsia. 50, 811–823. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM, 2004. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol 55, 576–580. [DOI] [PubMed] [Google Scholar]
- 22.Taha AY, Ryan MA, Cunnane SC, 2005. Despite transient ketosis, theclassic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism 54:1127–1132. [DOI] [PubMed] [Google Scholar]
- 23.Waldbaum S, Patel M, 2010. Mitochondrial oxidative stress in temporal lobeepilepsy. Epilepsy Res. 88, 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Wu X, Liu Q, Kong G, Zhou J, Jiang J, Wu X, Huang Z, Su W, Zhu Q, 2017. Ketogenic Metabolism Inhibits Histone Deacetylase (HDAC) and Reduces Oxidative Stress After Spinal Cord Injury in Rats. Neuroscience. 366, 3643. [DOI] [PubMed] [Google Scholar]
- 25.Yakunin E, Kisos H, Kulik W, Grigoletto J, Wanders RJ, Sharon R, 2014. The regulation of catalase activity by PPARγ is affected by α-synuclein. Ann. Clin. Transl. Neurol 1, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]