Abstract
Background:
Painful events are the leading cause of hospitalizations for patients with sickle cell disease. Individualized pain plans targeting patient-specific maximum opioid dosing may shorten hospitalization length and are recommended by national guidelines. Prior to implementing individualized sickle cell pain plans, we tested the hypothesis that a shorter time to achieve a maximum opioid dose would improve hospitalization outcomes.
Procedure:
Two-year IRB-approved, retrospective study of pediatric patients admitted for vaso-occlusive crisis (VOC). We recorded the ED admission time, order entry time for the maximum opioid dose during the hospitalization, and time of discharge orders. We categorized patients as infrequent if they required < 3 admissions for VOC over two years and patients as frequent if they required ≥ 3 admissions for VOC over two years. To account for multiple admissions, generalized linear modeling was performed.
Results:
We identified 236 admissions for acute pain observed in 108 patients. Achieving an earlier maximum opioid dose was significantly associated with shorter length of hospitalization for frequent and infrequent pain patients (both p≤0.0001). As total hospitalization length can be impacted by the time a maximum opioid order was placed, we also analyzed hospitalization length after the maximum opioid order was placed. Frequent pain patients that achieved earlier analgesia had a significantly shorter hospitalization from the time the maximum opioid order was placed (p=0.03) while no association was found for infrequent pain patients (p=0.84).
Conclusions:
Early achievement of maximum analgesia improved hospitalization outcomes and warrant further investigation in prospective studies of individualized pain plans.
Keywords: Sickle Cell Anemia, Outcomes, Pain
Introduction
Acute painful events are the leading cause of both emergency department (ED) visits and admissions for patients with sickle cell disease (SCD).1,2 Patients with SCD report dissatisfaction with the quality of care received in the ED during vaso-occlusive crises (VOC); one such reason cited for dissatisfaction are delays in receiving opioid therapy for analgesia.3–6 High-dose opioid protocols may be safe and more appropriate alternatives for treatment for opioid tolerant patients but ED physicians may have a lower comfort level with aggressive opioid dosing.7–9 In an attempt to impact pain outcomes for SCD patients in the ED, the 2014 NHLBI Expert Panel Report on the Management of Sickle Cell Disease recommended prompt assessment and administration of analgesia within 30 minutes of triage with frequent assessments and re-administration of opioid therapy every 30 minutes until analgesia is achieved.10 Unfortunately, due to the complexity of cases in the ED and possible limited resources to meet the needs of all patients experiencing emergencies, achieving appropriate and timely analgesia as suggested by the NHLBI is rarely accomplished in a clinical ED.11–13 Potential strategies to improve patient reported outcomes and lower admission rates in the ED include utilizing patient-specific pain plans and higher initial opioid dosing. 7,14
Once patients are admitted to the hospital for acute pain, the NIH SCD guidelines recommend using an individualized prescribing protocol or SCD-specific pain protocol.10 This recommendation for utilizing an individualized prescribing protocol or SCD-specific pain protocol is based on expert opinion as there is little evidence to suggest a benefit on inpatient pain outcomes. Despite the limitation in evidence, patients have expressed a desire to receive individualized opioid dosing plans during acute pain events.15 These individual prescribing protocols would include specific analgesic medication recommendations including drugs, dosage, route and frequency of medication delivery. The major hypothesized benefit of an individualized pain plan is that patients receive a patient-specific opioid dosing strategy based on historical opioid doses during acute pain events. This information could allow for higher upfront doses of opioid rather than a lower, suboptimal initial opioid dose and slow titration to analgesia. Prior review and approval of individualized pain plans by the primary hematologist could improve adoption of higher initial opioid doses by the admitting inpatient team.
The goal of individualized pain plans is to achieve earlier analgesia as measured by time from ED triage until the patient receives his/her maximum opioid dose during their admission (as defined by maximum hourly dose in oral morphine equivalents). To begin to address this limitation in the field, we tested the hypothesis that earlier achievement of a maximum opioid dose would result in a shorter length of hospitalization. We also sought to test the hypothesis that those receiving SCD modifying therapies would have shorter lengths of hospitalization attributable to their therapy.
Methods
Study Population:
We conducted an IRB-approved, retrospective cohort study of all pediatric patients with SCD admitted from the ED for VOC from Jan 2015 through Dec 2016. Patients with a diagnosis of SCD [hemoglobin SS, SC, SB+, SB0, verified by electronic medical record (EMR) review] were included in the study if their primary diagnosis for admission was VOC or sickle pain crises identified by ICD-10 code [D57.0, D57.21, D57.41, or D57.81]. Next, we performed chart reviews for each patient to confirm that the admission was associated with VOC and excluded patients in which another complication (i.e. fever, acute chest syndrome, or priapism) was the primary compliant. We considered that some patients with frequent acute pain events might have different outcomes than patients with rare acute pain events. Therefore, we a priori categorized patients as infrequent if they required < 3 admissions for VOC over two years and patients as frequent if they required ≥ 3 admissions for VOC over two years.1,16 To categorize patients by current SCD modifying therapy, we documented their current SCD modifying therapy from their most recent outpatient clinic note. Patients were categorized as either receiving no SCD modifying therapy, hydroxyurea, or transfusion therapy. Ten of the twelve patients on transfusion therapy were receiving blood transfusion for frequent pain events, in addition to prescribing hydroxyurea, to maintain a goal HbS% between 30–50%. These patients on transfusion therapy and prescribed hydroxyurea were categorized as transfusion therapy for statistical power in this analysis.
Clinical Investigation:
Individual clinicians directed opioid management and discharge during hospitalizations for acute pain events using their clinical acumen. Protocols for titration of opioids to maximum dose or discharge criteria after achieving analgesia are not standardized at our institution. The primary outcome for this study was length of hospitalization. To measure this objective, we recorded the time of ED admission, time of the order entry for the maximum opioid dose (mg) during the hospitalization, and time of entry for the discharge orders. Specifically, hospital admission length was defined as the difference in time between the emergency department triage and the time discharge orders were placed in the EMR. We defined the maximum opioid dose as the highest hourly total dose a patient received in oral morphine equivalents including patients receiving patient-controlled analgesia (PCA). For patients treated via PCA, the maximum opioid dose for the admission was calculated as the maximum total cumulative dose within an hour, including both continuous infusion opioid and patient-controlled boluses.
Statistical Analysis.
The unit of observation in the analyses was pain admission. Each pain admission was considered an independent event. We summarized the demographic characteristics of the patients and admissions by presenting count and percentages for categorical variables and observed means and standard deviations for continuous variables. To account for individual patients with multiple admissions, generalized linear mixed modeling based on the gamma distribution with log link function and random intercept was fitted to examine the association between outcomes (time to achieve a maximum opioid dose, length of hospitalization and length of hospitalization after achieving a maximum opioid dose) and explanatory variables (genotype, sickle cell therapy, and frequent/infrequent pain patients). Estimated mean outcomes and p-values were obtained by fitting the models separately for each outcome and explanatory variable unadjusted for other explanatory variables. All analyses were performed using JMP Pro 12 and SAS v4.0 (Cary, NC). All p-values <0.05 were deemed significant.
Results
We identified 236 admissions for acute pain observed in 108 patients over the 24-month period from January 2015 and December 2016. The total number of pain events per patient during the study period ranged from 1 to 14 events (mean: 2.18 ± 2.3) hospitalizations. Of the 108 patients included in this study, we identified 21 patients as frequent pain patients (≥ 3 admissions/2 years). One hundred and twenty-seven (54%) of the total admissions occurred in these 21 patients. One hundred and twenty (51%) acute pain events occurred in females. The mean age for these acute pain admissions was 13.5 years (range: 0.5–19 years). Genotypes included eighty patients with HbSS or HbSB0 thalassemia (74%), twenty-four with HbSC (22%), and four patients with HbSB+ thalassemia (4%). Of the 236 admissions for VOC, 122 events (52%) occurred in patients prescribed hydroxyurea (HU) alone at the time of admission, 28 (12%) were receiving chronic transfusion alone, 22 (9%) were prescribed HU and receiving chronic transfusion, and 64 (27%) admissions occurred in patients that were not receiving any disease modifying therapy. (Table 1).
Table 1:
Study Demographics
| Genotype | Patients (%) N=108 |
Admissions (%) N=236 |
|---|---|---|
| Hb SS/Sβ0 thalassemia | 80 (74) | 182 (77) |
| Hb SC | 24 (22) | 45 (19) |
| HbSβ+ thalassemia | 4 (4) | 9 (4) |
| SCD Modifying Therapy | ||
| Hydroxyurea | 53 (49) | 122 (52) |
| HU/Transfusion | 10 (9) | 50 (21) |
| No therapy | 45 (42) | 64 (27) |
| VOC Frequency | ||
| < 3 admissions | 87 (81) | 109 (46) |
| ≥ 3 admissions | 21 (19) | 127 (54) |
Those with frequent pain admissions had an observed mean (± SD) length of hospitalization of 104.8 (± 78.8) hours compared to those with infrequent admissions who had an observed mean length of hospitalization of 90.6 (± 63.8) hours (p=0.24). Those with frequent pain admissions also had an observed mean time to maximum opioid dose of 27.6 (± 44.4) hours that was not significant when compared to those with infrequent admissions 18.2 (± 34) hours (p=0.06). Lastly, those with frequent pain admissions were discharged a mean of 66.9 (± 55.3) hours after receiving their maximum opioid dose which is similar to those with infrequent admissions, who were discharged 61.1 (± 43.8) hours later (p= 0.67) (Table 2).
Table 2:
Comparison of Outcomes by Frequency of Visits
| < 3 admissions (Infrequent) Observed Mean |
≥ 3 admissions (Frequent) Observed Mean |
p-value* | |
|---|---|---|---|
| Total Length | 90.6 ± 63.8 | 104.8 ± 78.8 | 0.2369 |
| Time to Max Order | 18.2 ± 34 | 27.6 ± 44.4 | 0.0567 |
| Time to Discharge after Max Opioid | 61.1 ± 43.8 | 66.9 ± 55.3 | 0.6690 |
p-value was obtained using generalized linear mixed model (gamma distribution; log link; random intercept)
Impact of Achieving Early Maximum Opioid Dose on Outcomes.
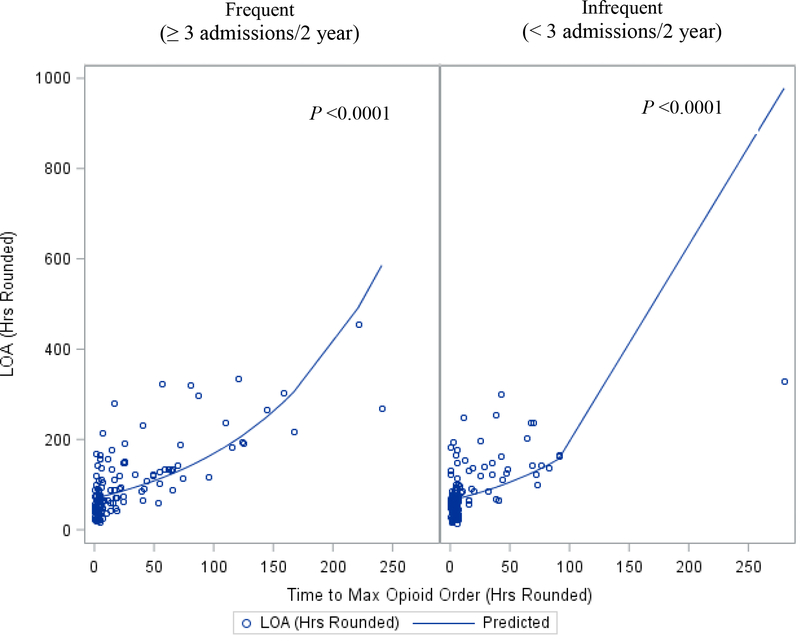
Among all study participants, the observed mean total hospitalization for acute pain among was 98 ± 72 hrs. Using generalized linear modelling to account for patients with multiple admissions, achieving an earlier maximum opioid dose was significantly associated with shorter estimated total length of hospitalization for both frequent (p≤0.0001) and infrequent pain patients (p≤0.0001) (Figure 1).
FIGURE 1:
Scatterplot and predicted curve for Length of Admissions (LOA) and Time to Maximum Opioid Order. (Predicted curve is based on the marginalized linear predictor.)
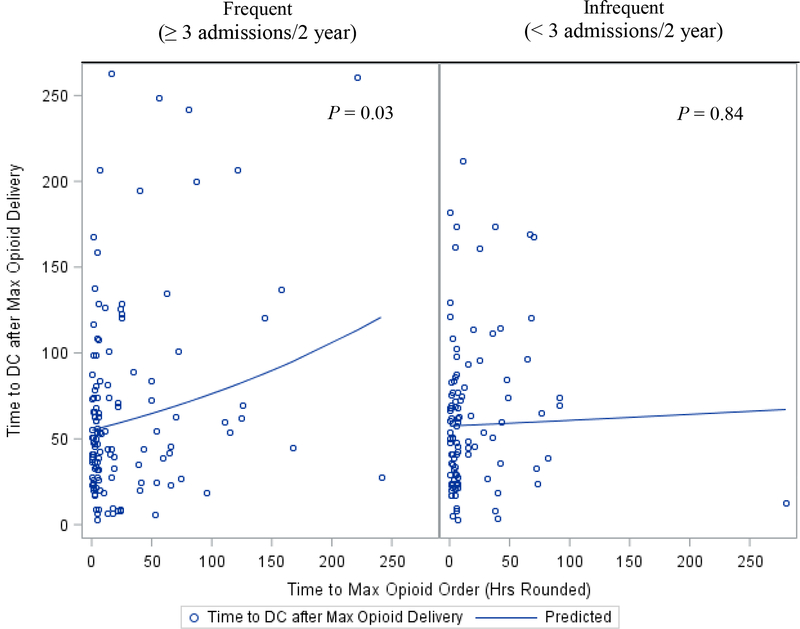
We also evaluated the length of hospitalization following the placement of the maximum opioid order. Among all patients the observed mean (± SD) time to discharge following the administration of the maximum opioid dose was 64 (± 50) hours. Frequent pain patients that achieved earlier analgesia had a significantly shorter estimated hospitalization from the time the maximum opioid order was placed (p=0.03). However, for infrequent pain patients, we identified no significant association between time to discharge after maximum opioid order and the time the maximum opioid order was placed (p=0.84) (Figure 2).
FIGURE 2:
Scatterplot and predicted curve for Time to Discharge after Maximum Opioid Delivery and Time to Maximum Opioid Order. (Predicted curve is based on the marginalized linear predictor.)
Differences in Hospitalization Length by Pain Frequency and SCD Therapy:
Using generalized linear modelling to account for multiple admissions, we identified no significant difference in the total hospitalization length for patients frequently admitted to the hospital for pain as compared to patients with infrequent hospitalizations for pain (estimated mean 95.2 vs 81.6 hrs, p=0.24). However, a significant impact for type of SCD modifying therapy on total length of hospitalization (p=0.027) was seen. Specifically, those receiving transfusion had a significantly estimated shorter hospitalization (65.6 hrs) compared to patients prescribed HU (89.6 hrs, p=0.0076). Length of hospitalization was not different for patients on HU vs no SCD modifying therapy (89.6 vs 85.5 hrs, p=0.68).
Differences in Time to Achieve a Maximum Opioid Order by Pain Frequency and SCD Therapy:
Among all patients, the observed mean time to achieve maximum opioid dose was 23.3±40 hrs (range: 5–280 hours). Using generalized linear modelling, the time to achieve a maximum opioid dose was not different between patients frequently admitted to the hospital as compared to patients with infrequent hospitalizations (21.5 vs 12.7 hours, p=0.057). We identified a significant association between the estimated time to achieve a maximum opioid dose and SCD therapy (p=0.047). Patients on transfusion therapy had a significantly shorter estimated time to achieve a maximum opioid dose than patients prescribed HU (10 vs 18 hours, p=0.023). There were no significant differences in time to achieve a maximum opioid order for patients with no SCD modifying therapy as compared to transfusion (12 vs 10 hours, p= 0.057) or HU (12 vs 18 hours, p= 0.13).
Discussion
Overall, our data shows that earlier titration of opioid to a maximum dose for analgesia during an inpatient admission for acute pain correlates with improved outcomes for hospital admission length. This result suggests that either rapid assessment or titration of their pain regimen or higher initial opioid doses could decrease hospitalization length. Additionally, patients with frequent pain represent the majority of our pain admissions and our findings suggest the strongest impact on early analgesia was in these patients with frequent hospitalizations. Therefore, this frequent pain cohort is the ideal candidate for the development and pilot testing of individualized pain plans as they have a more severe impact from their pain and have several admission records to review to determine an optimal opioid dosing strategy.
While there are limited inpatient studies of patient-specific opioid dosing in hospitalized patients, there is more data from the ED. In a recent pediatric VOC trial in the ED setting, an association between earlier initial opioid dosing in the ED and length of hospitalization was not identified.14 However, other studies have suggested that individualized pain management plans in the ED are associated with decreased hospital admission rates, shorter hospitalization, and higher levels of patient satisfaction.17 Similarly, an adult ED study suggested that a patient-specific opioid dosing strategy allowed a greater reduction in pain scores and lower admission rates for acute pain than those treated with standard weight-based dosing strategies. 7 As the ED studies have inherent differences in outcome measures, these trials may not be applicable to an inpatient setting, but do demonstrate that additional prospective research should be conducted in both settings.
Patients treated with transfusion have the shortest hospitalization for acute pain as compared to HU alone or no therapy. This finding is comparable to previous studies that show a protective role of transfusion in the development of acute pain events. 18 While HU decreases the incidence of VOC admissions, our study did not appreciate a benefit from HU therapy to shorten the length of hospital admission.19,20
While our data suggests a benefit to achieving early analgesia, some limitations inherent in a retrospective review are worth noting. As a single-institution study, we were unable to account for admissions outside of our hospital system. Second, this retrospective study could not capture rigorous methods for determining home opioid use or recent hydroxyurea adherence. Third, individual provider practices for opioid titration and discharge practices were not standardized. Fourth, we did not evaluate the impact of early, aggressive opioid management in the ED for patients presenting with VOC that were discharged without admission. Finally, length of hospitalization is a standard measure for hospital outcomes; however, there are some limitations in using length of stay as other factors independent of pain management could influence this metric including psychosocial issues, opioid metabolism, and differences in patient reported outcomes of pain.
In conclusion, our data suggests that earlier achievement of analgesia as measured by time to maximum opioid dose is associated with shorter hospitalizations. Importantly, among patients with frequent pain admissions, achievement of earlier maximum opioid dose decreased total hospitalization length and time to discharge after a maximum opioid dose was administered. Prospective studies are needed on individualized pain plans for VOC admissions to confirm our findings. To address this limitation, our institution is currently evaluating individualized pain plans that use an initial dosing regimen based on the mean maximal opiod dose from the three prior admissions for frequent pain patients. Additionally, to adequately assess individualized pain plans with higher initial opiod dosing, prospective data is needed on the safety of such regimens as well as the benefit in patient reported outcomes. Ultimately, it is our goal to provide appropriate pain management and relieve the suffering children with SCD experience during VOC.
Acknowledgements:
The authors are grateful to the contribution of Anna Arthur, PharmD for her initial work on this project. The authors acknowledge the members of the Sickle Cell team for their work in the care of these patients including the Nurse Practitioners Kristen Osborn, Heather Carlton, Susan Dobbins, Lindsey Evans, and Brandi Pernell. The authors acknowledge the Pediatric Research Office for their assistance.
Funding Source: NIH K23 5K23HL127100; NIH K23HL114636
Abbreviation Key:
- SCD
Sickle cell disease
- VOC
Vaso-occlusive pain crisis
- ED
Emergency department
- PCA
Patient-controlled analgesia
- HU
Hydroxyurea
Footnotes
This work was presented previously at the American Society of Hematology Annual Meeting December 2017.
Conflicts of Interest Statement: The authors have no conflicts of interest to report relevant to this study. Dr. Lebensburger is funded by NIH K23 5K23HL127100 and Dr. Amanda Brandow is funded by NIH K23HL114636
References
- 1.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. [DOI] [PubMed] [Google Scholar]
- 2.Bou-Maroun LM, Meta F, Hanba CJ, Campbell AD, Yanik GA. An analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomes. Pediatr Blood Cancer. 2018;65(1). [DOI] [PubMed] [Google Scholar]
- 3.Lebensburger JD, Bemrich-Stolz CJ, Howard TH. Barriers in transition from pediatrics to adult medicine in sickle cell anemia. J Blood Med. 2012;3:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bemrich-Stolz CJ, Halanych JH, Howard TH, Hilliard LM, Lebensburger JD. Exploring Adult Care Experiences and Barriers to Transition in Adult Patients with Sickle Cell Disease. Int J Hematol Ther. 2015;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med. 2007;14(5):419–425. [DOI] [PubMed] [Google Scholar]
- 6.Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molokie RE, Montminy C, Dionisio C, et al. Opioid doses and acute care utilization outcomes for adults with sickle cell disease: ED versus acute care unit. Am J Emerg Med. 2018;36(1):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanabe P, Martinovich Z, Buckley B, Schmelzer A, Paice JA. Safety of an ED High-Dose Opioid Protocol for Sickle Cell Disease Pain. J Emerg Nurs. 2015;41(3):227–235. [DOI] [PubMed] [Google Scholar]
- 10.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 11.Mathias MD, McCavit TL. Timing of opioid administration as a quality indicator for pain crises in sickle cell disease. Pediatrics. 2015;135(3):475–482. [DOI] [PubMed] [Google Scholar]
- 12.Kavanagh PL, Sprinz PG, Wolfgang TL, et al. Improving the Management of Vaso-Occlusive Episodes in the Pediatric Emergency Department. Pediatrics. 2015;136(4):e1016–1025. [DOI] [PubMed] [Google Scholar]
- 13.Zempsky WT, Loiselle KA, McKay K, Lee BH, Hagstrom JN, Schechter NL. Do children with sickle cell disease receive disparate care for pain in the emergency department? J Emerg Med. 2010;39(5):691–695. [DOI] [PubMed] [Google Scholar]
- 14.Brandow AM, Nimmer M, Simmons T, et al. Impact of emergency department care on outcomes of acute pain events in children with sickle cell disease. Am J Hematol. 2016;91(12):1175–1180. [DOI] [PubMed] [Google Scholar]
- 15.Lin RJ, Evans AT, Wakeman K, Unterbrink M. A Mixed-Methods Study of Pain-related Quality of Life in Sickle Cell Vaso-Occlusive Crises. Hemoglobin. 2015;39(5):305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr. 2012;160(2):286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnamurti L, Smith-Packard B, Gupta A, Campbell M, Gunawardena S, Saladino R. Impact of individualized pain plan on the emergency management of children with sickle cell disease. Pediatr Blood Cancer. 2014;61(10):1747–1753. [DOI] [PubMed] [Google Scholar]
- 18.Miller ST, Wright E, Abboud M, et al. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139(6):785–789. [DOI] [PubMed] [Google Scholar]
- 19.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–1322. [DOI] [PubMed] [Google Scholar]
- 20.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet (London, England). 2011;377(9778):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]