Abstract
Introduction
Hemorrhagic shock is a major cause of death after trauma. An additional blunt chest trauma independently contributes to mortality upon the development of an acute lung injury (ALI) by aggravating pathophysiological consequences of hemorrhagic shock. The maintenance of hydrogen sulfide availability is known to play an important role during hemorrhage and ALI. We therefore tested the impact of a genetic 3-mercaptopyruvate sulfurtransferase mutation (Δ3-MST) in a resuscitated murine model of traumatic-hemorrhagic shock.
Methods
Anesthetized wild type (WT) and Δ3-MST mice underwent hemorrhagic shock with/without blunt chest trauma. Hemorrhagic shock was implemented for 1h followed by retransfusion of shed blood and intensive care therapy for 4h including lung-protective mechanical ventilation, fluid resuscitation, and noradrenaline titrated to maintain a mean arterial pressure ≥50mmHg. Systemic hemodynamics, metabolism, and acid-base status were assessed together with lung mechanics, and gas exchange. Post-mortem tissue samples were analyzed for immunohistological protein expression and mitochondrial oxygen consumption.
Results
3-MST-deficient mice showed similar results in parameters of hemodynamics, gas exchange, metabolism, acid base status, and survival compared to the respective WT controls. Renal albumin extravasation was increased in Δ3-MST mice during hemorrhagic shock, together with a decrease of LEAK respiration in heart tissue. In contrast, mitochondrial oxygen consumption in the uncoupled state was increased in kidney and liver tissue of Δ3-MST mice subjected to the combined trauma.
Conclusion
In summary, in a resuscitated murine model of traumatic-hemorrhagic shock, 3-MST-deficiency had no physiologically relevant impact on hemodynamics and metabolism, which ultimately lead to unchanged mortality regardless of an additional blunt chest trauma.
Keywords: 3-mercaptopyruvate sulfurtransferase, blunt chest trauma, hemorrhagic shock, mitochondrial oxygen consumption, hydrogen sulfide
Introduction
Hemorrhagic shock (HS) is responsible for about 30–40% of trauma related deaths (1). Mortality following traumatic-hemorrhagic shock is mostly due to blood loss-related tissue ischemia, hypoxia, and inflammation, eventually leading to multi-organ failure (2–4). The restoration of blood flow represents an ischemia/reperfusion (I/R) type of injury and may further aggravate tissue damage, which is associated with increased radical stress, inflammation, and mitochondrial dysfunction(2, 5, 6). An additional blunt chest trauma (TxT) with the development of acute lung injury (ALI) adds to the severity of pathophysiological consequences(7). Lung contusion following blunt chest trauma aggravates pulmonary and systemic inflammatory responses due to alveolar hypoxia and hypoxemia. Besides, the direct physical trauma to the lung promotes oxidative stress and apoptotic events(8).
There is ample evidence in animal models that maintenance of hydrogen sulfide (H2S) availability (9), assumes major protective importance in ALI(10–12), and hemorrhagic shock(13). This mainly applies to the expression and activity of one of the best-characterized H2S producing enzymes, cystathionine-γ-lyase (CSE). In contrast, the role of other H2S-producing enzymes, particularly 3-mercaptopyruvate sulfurtransferase (3-MST), which is primarily responsible for mitochondrial H2S production, are less explored (14, 15). Ahmad et al. recently reported, that mice with a mutation of 3-MST, showed no effect in a model of endotoxin-induced multiple organ injury (16) . In another study, 3-MST was suggested to assume particular importance during hemorrhage, as rat strains with longer survival after controlled hemorrhage presented with higher cardiac mitochondrial 3-MST expression (17) . It should be noted however, that the animal models used in former studies were spontaneously breathing rodents, which did not receive mechanical ventilation or standard intensive care unit therapy.
Therefore, the aim of the current study was to examine whether the genetic mutation of 3-MST (Δ3-MST) influenced lung and kidney injury in a resuscitated murine model of traumatic-hemorrhagic shock, comprising circulatory support and lung-protective mechanically ventilation (12).
Material and Methods
The study was authorized by the federal authorities for animal research of the Regierungspräsidium Tübingen (approved animal experimentation number: 1190, 24.09.2014), Baden-Württemberg, Germany, and the Animal Care Committee of the University of Ulm, Baden-Württemberg, Germany, and performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals and the European Union "Directive 2010/63 EU on the protection of animals used for scientific purposes". The experiments were implemented using C57BL/6J (WT) mice that were received from Charles River laboratories Germany (Wilmington, MA, USA) and homozygous (Δ3-MST) mutant mice (C57BL/6N.15T13671A1) bred in-house [gift from Prof. Csaba Szabó, University of Texas Medical Branch; animal pairs from Dr. Noriyuki Nagahara, Nippon Medical School, did not breed and where therefore excluded from our experiments (18) ]. The Δ3-MST mouse strain is, as shown by Ahmad (16) , characterized by a pronounced 3-MST deficiency in the lung and in the spleen, whereas its expression is hardly affected in liver and kidney. Groups comprised 10 animals (WT HS nonTxT), 11 animals (WT HS TxT), 8 animals (Δ3-MST HS nonTxT), and 7 animals (Δ3-MST HS TxT). Animals were kept under standardized conditions, and were equally distributed in age (20–25 weeks). Body weight was 31 ± 2g in the WT groups, 27 ± 5g in the Δ3-MST group without trauma, and 33 ± 5g in the Δ3-MST group with trauma. Due to breeding difficulties, we did not get the sufficient number of descendants to form Δ3-MST groups with exclusively male animals. Thus, the WT groups contained only male animals, whereas the Δ3-MST group without trauma contained three female animals and the Δ3-MST group with trauma contained one female animal. The experiments of the current study were carried out simultaneously as a recently published manuscript on the impact of cigarette smoke exposure-induced COPD on murine hemorrhagic shock and resuscitation with/without blunt chest trauma (19). Therefore, the data of the wild type animals of the present study are the same as those of the latter study in order to comply with the German regulations to animal protection requesting to minimize the number of animals according to the 3R-prinicple (20). 11 mice (WT HS nonTxT: 4; WT HS TxT: 4; Δ3-MST HS nonTxT: 0; Δ3-MST HS TxT: 3) were not included in the final data analysis, as they died due to hematothorax and pericardial tamponade subsequent to blunt chest trauma, uncontrollable bleeding during surgery or technical problems. Thus, the subsequent data refer to the 36 mice included in the final data analyses.
Implementation of general anesthesia,,surgical instrumentation, hemorrhagic shock, and blast wave induced blunt chest trauma
Anesthesia, surgical instrumentation as well as implementation of hemorrhagic shock and blunt chest trauma have been described in detail previously (19). Briefly, after induction of anesthesia with sevoflurane and buprenorphine mice underwent blast wave-induced blunt chest trauma or sham procedure. Immediately afterwards, mice received intraperitoneal ketamine, midazolam and fentanyl and were placed on a procedure bench incorporating a closed-loop-system for body temperature control (19, 21, 22). Animals were mechanically ventilated via a tracheostomy with an identical, pressure-controlled, lung-protective respiratory strategy including hourly recruitment manoeuvres as described previously (8, 12, 19). Surgical instrumentation comprised arterial central venous and urinary bladder catheterization (8, 12, 19). General anesthesia was maintained by continuous intravenous administration of ketamine, midazolam, and fentanyl to reach deep sedation and to guarantee complete tolerance to noxious stimuli. After the surgical instrumentation, hemorrhagic shock was induced for 1h by blood withdrawal adjusted to either the maximum volume (30µL·gbody weight−1) or when mean arterial blood pressure (MAP) reached 35mmHg (19). After 1h of hemorrhagic shock, mice were resuscitated by re-transfusion of shed blood, hydroxyethyl starch, and if necessary continuous i.v. norepinephrine to reach a MAP≥50mmHg. At the end of the experiment the animals were exsanguinated, tissue samples were taken immediately thereafter, and prepared for further analyses. Heart apex, diaphragm, a third of the right kidney (cross section), and a part of the liver (lobus medialis sinister) were harvested for mitochondrial respiration measurements, left lung and the left kidney were taken for immunohistochemistry analyses.
Parameters of lung mechanics, hemodynamics, gas exchange and metabolism
Systemic hemodynamics, blood gas tensions, body temperature, and static thoracopulmonary compliance were recorded hourly, acid-base status, glycemia, and lactatemia were assessed at the end of the 4h period after re-transfusion (19).
Urine output together with the measurement of plasma and urine creatinine concentrations allowed for calculation of creatinine clearance as described previously (19, 23, 24). Whole blood sulfide concentrations were determined using gas chromatography/mass spectrometry as described previously (19, 25).
Immunohistochemistry
Immunohistochemistry (IHC) for extravascular albumin (anti-albumin; Santa Cruz Biotechnology, Dallas, USA) accumulation, and nitrotyrosine formation (anti-nitrotyrosine, Merck Millipore, Darmstadt, Germany) in lung and kidney was done as described previously(8). Primary antibodies were detected by secondary antibodies conjugated to AP (Alkaline Phosphate-conjugated antibody, Jackson, ImmunoResearch, West Grove, USA) and visualized with a red chromogen (Darko REAL Detection System Chromogen Red), and Mayers hematoxylin (Sigma, Taufkirchen, Germany). Visualization was performed using the Zeiss Axio Imager A1 microscope (Zeiss, Jena, Germany). Four distinct 800.000 µm2 regions were quantified for intensity of signal by using the Axio Vision 4.8 software. Results are presented as densitometric sum (8, 21).
Mitochondrial respiration
The activity of the mitochondrial oxygen consumption was measured using high-resolution respirometry (Oxygraph-2k respirometer, Oroboros instruments Corp, Innsbruck, Austria) (19, 26, 27). After the mouse was exsanguated, a piece of the respective organ was carefully removed. The tissue samples was placed in ice-cold custodiol®. Briefly, post-mortem homogenized samples of heart (1.5mg), diaphragm (2mg), kidney (2mg) and liver (2mg) were measured with mechanically disrupted cell membranes and intact mitochondria. Therefore, the tissue samples were weighed and placed into a homogenization glass filled with 2ml of respiration medium. The homogenization glass was placed in a water container (4°C), followed by tissue homogenization using a potter homogenizer. The same respiratory medium composed of EGTA 0.5mM, MgCl2·6H2O 3mM, Lactobionic acid 60mM, Taurine 20mM, KH2PO4 10mM, HEPES 20mM, Sucrose 110mM, and bovine serum albumin 1g/l was used for the homogenization as well as for the respiration measurement in the oxygraph chambers.
We were able to assess various states of mitochondrial function upon the addition of a defined sequence of substrates and inhibitors. Thus, oxygen flux was measured for: complex I activity after the addition of pyruvate 10mM, malate 5mM, glutamate 10mM, and ADP 5mM; maximum oxidative capacity (OxPHOS) after the addition of octanoyl-carnitine 1mM and succinate 10mM; the integrity of the outer mitochondrial membrane by cytochrome c 10µM; LEAK state after the inhibition of the ATP-synthase by oligomycin 2.5µM; the maximal oxygen consumption in the uncoupled state, after titrating 4-(trifluoromethoxy)phenylhydrazone (FCCP, final concentration 1.5µM); and complex IV activity after adding ascorbate 2mM and N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride (TMPD) 0.5mM to avoid uncontrolled autoxidation.
Statistical analysis
Unless stated otherwise, all data are presented as median (quartiles). The sample sizes were based on our previous experiments (8, 12, 21) : a statistical power analysis was performed with the PaO2/FiO2 ratio, lung tissue NF-κB activation, and thoracopulmonary compliance as main criteria and yielded, based on two-sided testing, α=0.05, power 80% and non-parametric analysis of variance, a minimum of n=8–10 for eight experimental groups. Intergroup differences were analyzed using the Kruskal-Wallis on ranks test and subsequently the Dunn’s test for multiple comparison (two-tailed). Survival curves were compared using the Log-rank (Mantel-Cox) test. The significance level was set to p<0.05. Quantitative graphical presentations and statistical analyses were performed by using GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA).
Results
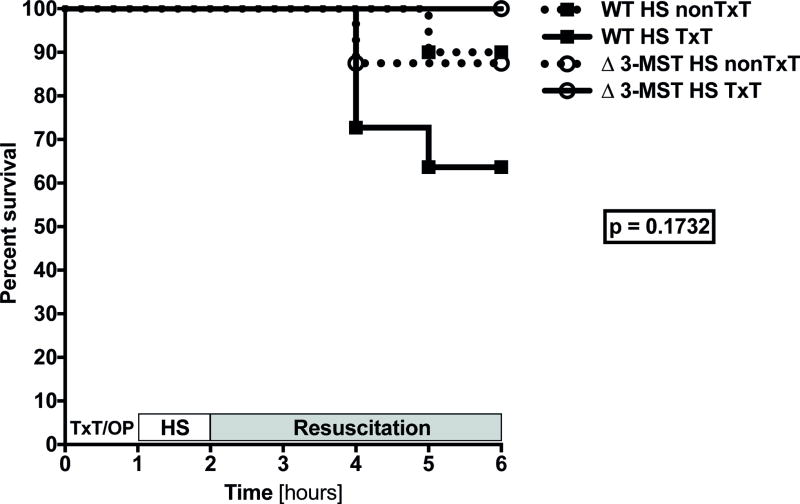
The Kaplan-Meier curve (Figure 1) shows that early survival of mice undergoing hemorrhagic shock did not significantly differ between groups no matter the presence of hemorrhagic shock in combination with a blunt chest trauma and/or 3-MST mutation until the end of the 4h resuscitation period.
Figure 1. Clinical impact of 3-MST deficiency on survival in murine hemorrhagic shock and in combination with blunt chest trauma.
Survival curves were compared using the Log-rank (Mantel-Cox) test. Abbreviations: HS: hemorrhagic shock, 3-MST: 3-mercaptopyruvate sulfurtransferase, nonTxT: no TxT, OP: operation, TxT: blunt chest trauma, WT: wild type.
Parameters of hemodynamics, metabolism and organ function are shown in Table 1. Δ3-MST mice had the lowest thoracopulmonary compliance, whereas the other parameters of systemic hemodynamics, gas exchange, metabolism, and acid base status showed no significant intergroup difference except for lactate levels, which were also lowest in these animals. Δ3-MST mice subjected to blunt chest trauma and subsequent hemorrhagic shock had the highest urinary output. Nevertheless, creatinine plasma levels were higher in both Δ3-MST groups, whereas creatinine clearance showed no significant intergroup difference. Sulfide concentrations in whole blood did not show any significant differences either.
Table 1. Clinical impact of 3-MST deficiency in murine hemorrhagic shock and in combination with blunt chest trauma.
Illustration of systemic hemodynamics, lung mechanics, pulmonary gas exchange, metabolism, acid-base status, creatinine and sulfide concentrations. Presented parameters were raised at the end of the 4 hours of pressure-controlled mechanical ventilation in WT and 3-MST deficient mice during hemorrhagic shock and in combination with a blunt chest trauma. All data are presented as median (quartiles). Abbreviations: FiO2 fraction of inspired oxygen, 3-MST: 3-mercaptopyruvate sulfurtransferase, nonTxT: no TxT, paCO2 partial pressure of carbon dioxide, paO2 partial pressure of oxygen, TxT: blunt chest trauma, WT: wild type.
| Parameters | WT nonTxT | WT TxT | Δ3-MST nonTxT | Δ3-MST TxT | p-value K-W- ANOVA |
|---|---|---|---|---|---|
| Hemorrhagic shock | |||||
| Heart rate [min−1] | 410 (348; 543) | 420 (370; 480) | 434 (317; 539) | 370 (314; 521) | 0.9666 |
| Mean arterial pressure [mmHg] | 60 (54; 63) | 54 (40; 61) | 53 (50; 60) | 60 (52; 73) | 0.2958 |
| Noradrenaline requirement [µg·kg−1·min−1] | 0.090 (0.006; 0.194) | 0.000 (0.000; 0.475) | 0.133 (0.019; 0.279) | 0.000 (0.000; 0.057) | 0.2452 |
| Hemoglobin [mg·dl−1] | 7.5 (6.4; 8.1) | 6.6 (6.0; 7.4) | 6.8 (5.8; 7.5) | 7.7 (6.3; 7.9) | 0.2718 |
| Static thoracopulmonary compliance [ml·cmH2O−1] | 0.12 (0.11; 0.13) | 0.11 (0.08; 0.12) | 0.09 (0.08; 0.11) | 0.09 (0.08; 0.11) | 0.0537 |
| PaCO2 [mmHg] | 41 (38; 45) | 36 (33; 47) | 38 (36; 48) | 37 (35; 45) | 0.7364 |
| PaO2 [mmHg] | 78 (69; 89) | 89 (83; 96) | 87 (74; 99) | 83 (65; 98) | 0.4685 |
| PaO2/FiO2 [mmHg] | 370 (327; 426) | 418 (386; 457) | 415 (350; 472) | 378 (310; 425) | 0.4235 |
| Glucose [mg·dl−1] | 92 (67; 95) | 78 (69; 88) | 100 (96; 115) § | 90 (78; 114) | 0.0472 |
| Arterial pH | 7.30 (7.25; 7.35) | 7.29 (7.16; 7.38) | 7.36 (7.25; 7.37) | 7.36 (7.28; 7.43) | 0.4786 |
| Arterial base excess [mmol·L−1] | −5.9 (−7.5; −3.9) | −5.8 (−14.5; −4.7) | −4.8 (−7.1; −3.2) | −3.4 (−7.0; −2.6) | 0.1574 |
| Lactate [mmol·L−1] | 0.9 (0.8; 1.6) | 1.2 (1.0; 4.6) | 0.7 (0.6; 0.8) § | 0.7 (0.6; 0.9) § | 0.0004 |
| Urinary output [ml] | 0.67 (0.42; 2.17) | 0.97 (0.51; 1.61) | 1.74 (1.34; 2.59) | 2.82 (1.98; 3.23) #§ | 0.0086 |
| Creatinine concentration in plasma [µg·ml−1] | 1.84 (1.49; 2.80) | 2.27 (1.82; 2.92) | 3.32 (1.62; 3.68) | 3.33 (3.22; 3.70) # | 0.0470 |
| Creatinine Clearance [ml·min−1] | 0.31 (0.17; 0.52) | 0.29 (0.20; 0.42) | 0.18 (0.12; 0.37) | 0.20 (0.10; 0.22) | 0.1193 |
| Sulfide concentration in whole blood [µM] | 1.69 (1.50; 2.02) | 1.58 (1.33; 1.69) | 1.79 (1.48; 2.36) | 2.08 (1.73; 2.51) | 0.0803 |
significant to WT nonTxT (p <0.05);
significant to WT TxT (p <0.05);
significant to Δ3-MST nonTxT (p<0.05)
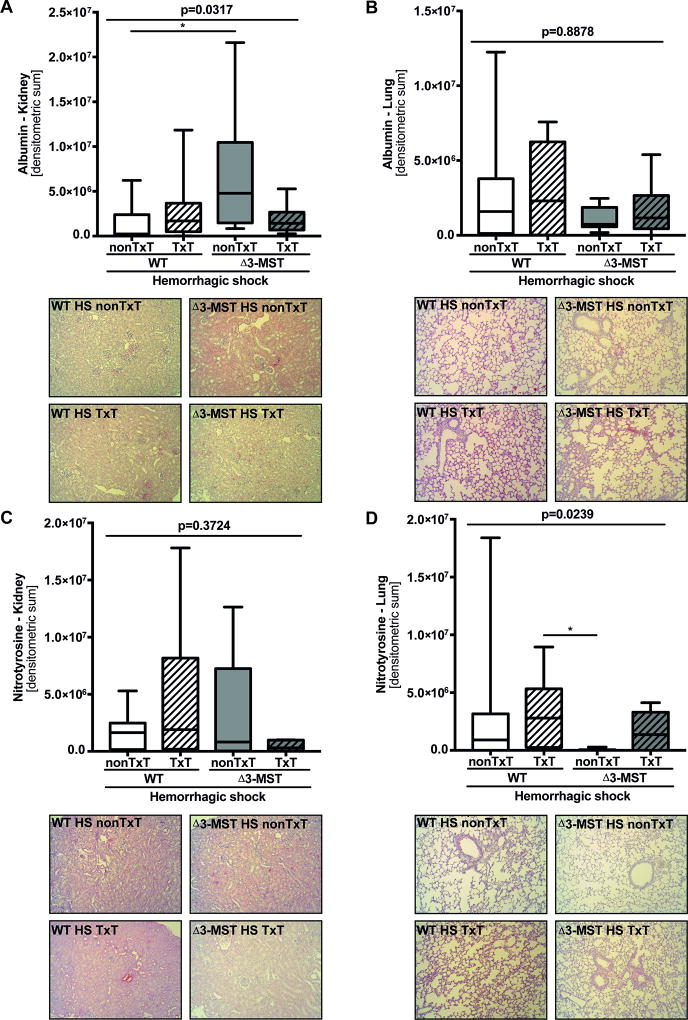
Immunohistochemistry (Figure 2) revealed that albumin extravasation was increased in the kidney (Figure 2 A) of Δ3-MST mice with hemorrhagic shock but not in the lung (Figure 2 B), whereas nitrotyrosine formation did not differ in either tissue compared to the respective control (Figure 2 C-D). Δ3-MST with blunt chest trauma and subsequent hemorrhagic shock neither affected the tissue albumin extravasation nor the expression of nitrotyrosine in lung or kidney.
Figure 2. The impact of 3-MST deficiency on protein expression patterns in murine hemorrhagic shock and in combination with blunt chest trauma.
Immunohistochemical protein expression levels of extravascular albumin accumulation in kidney (A) and lung (B) tissue and nitrotyrosine expression levels in kidney (C) and lung (D) tissue are presented as quantitative results of densitometry analysis. All tissue samples were obtained after 4 hours of pressure-controlled mechanical ventilation in WT and 3-MST deficient mice during hemorrhagic shock and in combination with a blunt chest trauma. All data are presented as median (quartiles, min/max). Abbreviations: HS: hemorrhagic shock, 3-MST: 3-mercaptopyruvate sulfurtransferase, nonTxT: no TxT, TxT: blunt chest trauma, WT: wild type.
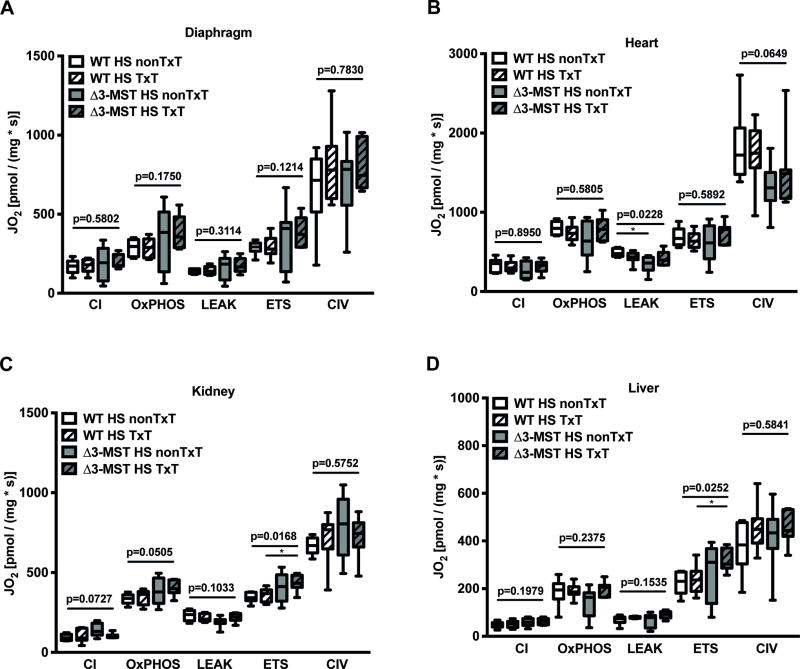
3-MST mutation during hemorrhagic shock coincided with a decrease in mitochondrial LEAK respiration in heart tissue (Figure 3 B), while it had no influence on complexes I, II, CIV, and the ETS state, nor on any of the measured states in the diaphgram, kidney, and liver biopsies (Figure 3 A, C-D). In contrast, oxygen consumption in the uncoupled state was significantly increased in kidney and liver tissue of Δ3-MST TxT mice subjected to the combined trauma (Figure 3 C-D), while no effect was present on oxygen flux in diaphragm, and heart (Figure 3 A-B).
Figure 3. Mitochondrial effects of 3-MST deficiency in murine hemorrhagic shock and in combination with blunt chest trauma.
Illustration of mitochondrial oxygen consumption (JO2) in homogenized post-mortem specimen from the diaphgram (A), heart (B), kidney (C), and liver (D). JO2 was measured in terms of complex I activity, OxPHOS- (maximum respiratory capacity in the state of oxidative phosphorylation (coupled state)), LEAK- (respiration after ATP-synthase inhibition, thereby compensating for proton leakage or slipping), ETS-capacity (maximal respiratory capacity of the electron transfer system in the uncoupled state), and CIV activity. All data are presented as median (quartiles, min/max). Abbreviations: CI: complex I, CIV: complex IV, HS: hemorrhagic shock, 3-MST: 3-mercaptopyruvate sulfurtransferase, nonTxT: no TxT, TxT: blunt chest trauma, WT: wild type.
Discussion
In this study, we tested the hypothesis whether the absence of 3-MST aggravates lung and kidney injury caused by blunt chest trauma and subsequent hemorrhage in mice. The main findings were that in the present study 3-MST-deficiency did not affect hemodynamics, mitochondrial respiration and metabolism in a physiologically relevant matter early after trauma, which ultimately lead to unchanged early mortality when compared to WT animals.
Generally, after hemorrhagic shock, there were no intergroup differences between WT animals and Δ3-MST mice without additional blunt chest trauma with respect to hemodynamics, gas exchange, acid base status, or metabolism. These findings were mirrored by the unaffected Kaplan-Meier survival curves (Figure 1). Hemorrhage and subsequent resuscitation represent an I/R injury and particularly affect lung and kidney function (28). Besides, an additional blunt chest trauma may further aggravate lung damage due to severe lung contusion (8, 29, 30). Interestingly, however, apart from the reduced compliance in Δ3-MST mice, we did not see any differences in pulmonary gas exchange, although all groups were subjected to hemorrhagic shock (Table 1). Neither pulmonary tissue extravascular albumin accumulation nor nitrotyrosine formation showed any intergroup difference (Figure 2 B-D), no matter the presence of an additional blunt chest trauma, hence indicating that 3-MST deficiency modulated neither nitrosative stress nor pulmonary barrier dysfunction (31) . These results confirm most recent data investigating the effect of endotoxin on organ function and injury in the same Δ3-MST mouse strain used in our experiment: despite particularly pronounced 3-MST deficiency in the lung, endotoxin had no effect on lung tissue myeloperoxidase content and malondialdehyde formation (16).
In respect to kidney function, Δ3-MST mice without trauma showed significantly higher renal albumin extravasation, at first glance suggesting more pronounced endothelial barrier dysfunction in this tissue (31) (Figure 2 A). Moreover, plasma creatinine levels were higher in both Δ3-MST groups, the difference being significant in the Δ3-MST TxT mice (Table 1). It should be noted, however, that the 3-MST deficiency in the kidney is hardly detectable in the mouse strain we used (16) , hence, making a direct effect of 3-MST deficiency unlikely in the kidney. This latter reasoning is supported by the results on creatinine clearance and renal tissue nitrotyrosine formation: none of these parameters showed any intergroup difference, no matter the presence of an additional blunt chest trauma. Nevertheless, due to the data discussed above it remains open, why the Δ3-MST animals with the combined trauma showed a significantly increase in urinary output.
3-MST deficiency was associated with significantly lower lactate concentrations at the end of the experiment (Table 1). Again, in our experiment it is unlikely that this finding reflects major differences with respect to cell metabolism: based on the estimation of the anion gap, lactate concentrations did not explain the overall metabolic acidosis present in WT animals, no matter the group assignment, hence, most likely excluding inadequate tissue perfusion. Finally, the degree of metabolic acidosis did not show any significant intergroup difference either.
With regard to mitochondrial oxygen consumption, our data do not indicate a compromised respiratory activity in any of the investigated tissues of the Δ3-MST deficient groups compared to the corresponding controls. This overall result was unexpected, since the importance of Δ3-MST for supporting basal mitochondrial respiration has been convincingly shown previously (34). Only in heart tissue of Δ3-MST deficient mice, which were solely subjected to hemorrhagic shock (Figure 3 B) we found a reduced mitochondrial oxygen consumption in the LEAK state. This effect, however, did not affect the ratio between maximum respiratory activity and LEAK nor the ATP production-related oxygen consumption, suggesting only a minor effect in terms of bioenergetics. In contrast, our data even suggest an increase in mitochondrial oxygen consumption especially in kidney and liver tissue (Figure 3 C-D). In fact, in both tissues ETS capacity, and thus maximal mitochondrial oxygen flux, was enhanced in the uncoupled state of Δ3-MST mice with the combined trauma compared to the WT animals. The poorly affected expression of Δ3-MST in kidney and liver of the Δ3-MST-deficient mouse strain we used obviously could explain why this genetic modification did not particularly impair mitochondrial function especially in these organs. Nevertheless, the striking fact that mitochondrial respiration in the uncoupled state was not only maintained, but even higher in these cases suggests the presence of additional mechanisms partially compensating for the reduced function of Δ3-MST. For example, we also noticed highest sulfide concentrations in the Δ3-MST deficient mice (Table 1), which may originate from increased sulfur release. In respect to the bell-shape concentration response curve from sulfide it is tempting to speculate that increased sulfide levels generated independently from the activity of Δ3-MST may have exerted stimulatory effects on the mitochondrial respiratory chain, resulting in increased oxygen consumption especially in kidney and liver (33).
Conclusion
In the present study, we tested the hypothesis, whether genetic 3-MST deficiency affects organ function after murine hemorrhagic shock and resuscitation with and without blunt chest trauma. Confirming previous experiments in murine endotoxemia, our study did not show a major effect of 3-MST deficiency, thus demonstrating that - unlike CSE - 3-MST does not seem to play a major role during resuscitation from traumatic-hemorrhagic shock in mice.
Acknowledgments
This project was supported by the DFG (CRC 1149). CH was supported by the GEROK and Gender Program (CRC1149) and Hertha-Nathorff Program, University of Ulm. MW was supported by the GEROK Program (CRC1149), TM was supported by the PhD Program (International Graduate School).
Footnotes
All authors declare no conflicts of interest.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Angele MK, Schneider CP, Chaudry IH. Bench-to-bedside review: latest results in hemorrhagic shock. Crit Care. 2008;12:218. doi: 10.1186/cc6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckbert SR, Vedder NB, Hoffman W, Winn RK, Hudson LD, Jurkovich GJ, Copass MK, Harlan JM, Rice CL, Maier RV. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denk S, Weckbach S, Eisele P, Braun CK, Wiegner R, Ohmann JJ, Wrba L, Hönes FM, Kellermann P, Radermacher P, et al. Role of hemorrhagic shock in experimental polytrauma. Shock. 2018;49:154–163. doi: 10.1097/SHK.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 6.Douzinas EE, Livaditi O, Tasoulis MK, Prigouris P, Bakos D, Goutas N, Vlachodimitropoulos D, Andrianakis I, Betrosian A, Tsoukalas GD. Nitrosative and oxidative stresses contribute to post-ischemic liver injury following severe hemorrhagic shock: the role of hypoxemic resuscitation. PLoS One. 2012;7:e32968. doi: 10.1371/journal.pone.0032968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Crit Care Med. 2008;36:2309–2315. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 8.Wagner K, Gröger M, McCook O, Scheuerle A, Asfar P, Stahl B, Huber-Lang M, Ignatius A, Jung B, Duechs M, et al. Blunt chest trauma in mice after cigarette smoke-exposure: Effects of mechanical ventilation with 100% O2. PLoS One. 2015;10:e0132810. doi: 10.1371/journal.pone.0132810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, Wang P, Yang G, Cao Q, Wang R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am J Pathol. 2013;182:1188–1195. doi: 10.1016/j.ajpath.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008;82:1196–1202. doi: 10.1016/j.lfs.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Aslami H, Pulskens WP, Kuipers MT, Bos AP, van Kuilenburg AB, Wanders RJ, Roelofsen J, Roelofs JJ, Kerindongo RP, Beurskens CJ, et al. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS One. 2013;8:e63497. doi: 10.1371/journal.pone.0063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann C, Hafner S, Scheuerle A, Möller P, Huber-Lang M, Jung B, Nussbaum B, McCook O, Gröger M, Wagner F, et al. The role of cystathionine-γ-lyase in blunt chest trauma in cigarette smoke exposed mice. Shock. 2017;47:491–499. doi: 10.1097/SHK.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 13.Issa K, Kimmoun A, Collin S, Ganster F, Fremont-Orlowski S, Asfar P, Mertes PM, Levy B. Compared effects of inhibition and exogenous administration of hydrogen sulfide in ischemia-reperfusion injury. Crit Care. 2013;17:R129. doi: 10.1186/cc12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura H. Hydrogen sulfide and polysulfides as biological mediators. Molecules. 2014;19:16146–16157. doi: 10.3390/molecules191016146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal. 2015;22:362–376. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad A, Gero D, Olah G, Szabo C. Effect of endotoxemia in mice genetically deficient in cystathionine-γ-lyase, cystathionine-β-synthase or 3-mercaptopyruvate sulfurtransferase. Int J Mol Med. 2016;38:1683–1692. doi: 10.3892/ijmm.2016.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemcke HG, DeKroon RM, Mocanu M, Robinette JB, Alzate O. Cardiac mitochondrial proteomic expression in inbred rat strains divergent in survival time after hemorrhage. Physiol Genomics. 2013;45:243–255. doi: 10.1152/physiolgenomics.00118.2012. [DOI] [PubMed] [Google Scholar]
- 18.Nagahara N, Nagano M, Ito T, Shimamura K, Akimoto T, Suzuki H. Antioxidant enzyme, 3-MST KO mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Sci Rep. 2013;3:1986. doi: 10.1038/srep01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartmann C, Gröger M, Noirhomme JP, Scheuerle A, Möller P, Wachter U, Huber-Lang M, Nussbaum B, Jung B, Merz T, McCook O, et al. In-depth characterization of the effects of cigarette smoke exposure on the acute trauma response and hemorrhage in mice. Shock. doi: 10.1097/SHK.0000000000001115. epub ahead of print Feb 8. [DOI] [PubMed] [Google Scholar]
- 20.Lilley E, Armstrong R, Clark N, Gray P, Hawkins P, Mason K, Lopez-Salesansky N, Stark AK, Jackson SK, Thiemermann C, et al. Refinement of animal models of sepsis and septic shock. Shock. 2015;43:304–316. doi: 10.1097/SHK.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 21.Wagner F, Scheuerle A, Weber S, Stahl B, McCook O, Knöferl MW, Huber-Lang M, Seitz DH, Thomas J, Asfar P, et al. Cardiopulmonary, histologic, and inflammatory effects of intravenous Na2S after blunt chest trauma-induced lung contusion in mice. J Trauma. 2011;71:1659–1667. doi: 10.1097/TA.0b013e318228842e. [DOI] [PubMed] [Google Scholar]
- 22.Wagner F, Wagner K, Weber S, Stahl B, Knöferl MW, Huber-Lang M, Seitz DH, Asfar P, Calzia E, Senftleben U, et al. Inflammatory effects of hypothermia and inhaled H2S during resuscitated, hyperdynamic murine septic shock. Shock. 2011;35:396–402. doi: 10.1097/SHK.0b013e3181ffff0e. [DOI] [PubMed] [Google Scholar]
- 23.Wagner K, Wachter U, Vogt JA, Scheuerle A, McCook O, Weber S, Gröger M, Stahl B, Georgieff M, Möller P, et al. Adrenomedullin binding improves catecholamine responsiveness and kidney function in resuscitated murine septic shock. Intensive Care Med Exp. 1:2. doi: 10.1186/2197-425X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenzel T, Weidgang C, Wagner K, Wagner F, Gröger M, Weber S, Stahl B, Wachter U, Vogt J, Calzia E, et al. Association of kidney tissue barrier disrupture and renal dysfunction in resuscitated murine septic shock. Shock. 2016;46:398–404. doi: 10.1097/SHK.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 25.McCook O, Radermacher P, Volani C, Asfar P, Ignatius A, Kemmler J, Möller P, Szabó C, Whiteman M, Wood ME, et al. H2S during circulatory shock: some unresolved questions. Nitric Oxide. 2014;41:48–61. doi: 10.1016/j.niox.2014.03.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumgart K, Wagner F, Gröger M, Weber S, Barth E, Vogt JA, Wachter U, Huber-Lang M, Knöferl MW, Albuszies G, et al. Cardiac and metabolic effects of hypothermia and inhaled hydrogen sulfide in anesthetized and ventilated mice. Crit Care Med. 2010;38:588–595. doi: 10.1097/ccm.0b013e3181b9ed2e. [DOI] [PubMed] [Google Scholar]
- 27.Gröger M, Matallo J, McCook O, Wagner F, Wachter U, Bastian O, Gierer S, Reich V, Stahl B, Huber-Lang M, et al. Temperature and cell-type dependency of sulfide effects on mitochondrial respiration. Shock. 2012;38:367–374. doi: 10.1097/SHK.0b013e3182651fe6. [DOI] [PubMed] [Google Scholar]
- 28.Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194:402–414. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 29.Fröhlich S, Boylan J, McLoughlin P. Hypoxia-induced inflammation in the lung: a potential therapeutic target in acute lung injury? Am J Respir Cell Mol Biol. 2013;48:271–279. doi: 10.1165/rcmb.2012-0137TR. [DOI] [PubMed] [Google Scholar]
- 30.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32:122–130. doi: 10.1097/SHK.0b013e31819c385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opal SM, van der Poll T. Endothelial barrier dysfunction in septic shock. J Intern Med. 2015;277:277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 32.Modis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Hellmich MR, Radermacher P, Bouillaud F, Szabo C. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171:2123–2146. doi: 10.1111/bph.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann C, Nussbaum B, Calzia E, Radermacher P, Wepler M. Gaseous mediators and mitochondrial function: The future of pharmacologically induced suspended animation? Front Physiol. 2017;8:691. doi: 10.3389/fphys.2017.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo C, Ransy C, Módis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]