Abstract
Achieving undetectable MRD (U-MRD) status after chemoimmunotherapy predicts longer progression-free and overall survival. The predictive factors and timing of relapse in patients with U-MRD and value of interim MRD analysis are ill-defined. This was a prospective study of 289 patients with CLL treated first-line with FCR. MRD analysis was performed after course 3 (C3) and at end-of-therapy (EOT) in bone marrow using 4-color flow cytometry (sensitivity 10−4). Eighteen percent of patients had U-MRD after C3 and 48% at EOT. U-MRD status at EOT was associated with longer PFS (median NR vs 38mo, p<0.001). MRD level (≤1% vs. >1%) after C3 predicted greater likelihood of U-MRD status at EOT (64% vs. 9%, p<0.001). PFS was significantly longer for patients with MRD ≤1% vs. >1% after C3 (median 73mo vs 41mo, p<0.001), but similar for <0.01% vs. 0.01–1%. Interim MRD status may therefore be used for risk stratification and to individualize therapy. Eighty-five patients with U-MRD status at EOT had yearly blood MRD monitoring; MRD re-emerged in 38/85, a median of 48mo after EOT and preceded clinical progression by a median of 24 months, which may allow development of early intervention strategies.
Keywords: Chronic Lymphocytic Leukemia, CLL, FCR, MRD, IGHV mutation status
Introduction
Achieving undetectable minimal residual disease (U-MRD) status with chemoimmunotherapy (CIT) is an important and independent predictor for longer progression-free survival (PFS)(1–5) and overall survival (OS).(1, 6) We and others(6–8) reported a plateau on the PFS curve after FCR therapy for patients with favorable baseline characteristics, particularly for patients with mutated IGHV(6) who lack del(11q) or del(17p).(7) U-MRD status post-treatment predicts prolonged PFS and is likely a pre-requisite for “cure.” Notably, pre-treatment characteristics retain prognostic significance even in patients who achieve U-MRD status with FCR: PFS was almost 80% at 13 years in patients with mutated IGHV who achieved U-MRD status at end-of-treatment (EOT), but most patients with unmutated IGHV relapse even having achieved U-MRD status at EOT.(6)
While the prognostic value of post-treatment MRD analysis is well-established, the value of interim MRD analysis and post-treatment serial analysis are less clear. In our most recent study of FCR, MRD analysis (10−4 sensitivity) was performed in bone marrow (BM) after course 3 (C3) and at EOT. We previously reported early results from this study.(3) There were several notable findings. First, patients who achieved U-MRD status after 3 courses of FCR had similarly favorable PFS whether further courses of FCR were given or not; this raised the possibility that patients who achieve U-MRD status early could potentially receive a reduced number of chemotherapy courses without compromising disease control. Second, patients who achieved U-MRD status after 6 course of FCR had very favorable outcomes, even if MRD-positive after 3 courses.
With further follow up in this study, many patients had yearly MRD analysis, using standard 4-color FLC in blood to detect subclinical disease relapse. This was not protocol-mandated. In the current analysis, 3 years after original publication, in addition to confirming the previous observations with more mature follow-up, our aims were as follows: first, to determine whether MRD assessment in BM after 3 courses can predict clinical outcomes, including U-MRD status at the EOT and subsequent PFS (and therefore whether it may have utility in personalizing therapy); to describe the time-course of sub-clinical and clinical relapse in patients who achieved U-MRD status at the EOT; and to identify predictive factors for relapse in patients achieving U-MRD status at the EOT.
Patients and methods
Patients
The study (NCT00759798) was approved by and conducted according to The University of Texas M.D. Anderson Cancer Center Institutional Review Board guidelines and in accordance with the Declaration of Helsinki. Two hundred eighty-nine patients were enrolled and treated with up to 6 courses of standard FCR(3, 9, 10) from 2008–15. The primary aim of the study was to prospectively evaluate associations between pre-treatment prognostic factors and CR; secondary aims were to evaluate associations between pre-treatment prognostic factors and time to treatment failure and U-MRD status in BM.
Baseline pre-treatment evaluation included clinical assessment for lymphadenopathy and constitutional symptoms, β2-microglobulin level and BM aspirate and biopsy. Fluorescence in situ hybridization (FISH) for common CLL-associated chromosomal abnormalities,(11) determination of immunoglobulin heavy chain variable region (IGHV) somatic hypermutation status (IGHV-MS) and immunohistochemistry for zeta-associated protein-70 (ZAP70)(12) were performed on BM. Stimulated karyotype was generated on BM in the majority of patients. CT scans for pre-treatment work-up and response assessment were not mandated as they were not the standard for assessment at the time of study design; nodal assessments were performed routinely by physical examination. All patients had adequate renal and hepatic function. Response assessments were performed according to the 1996 National Cancer Institute Working Group criteria.(13)
MRD analysis
BM examination was performed after C3 and at the EOT in all patients. MRD analysis was performed in BM using the international standardized methodology of the European Research Initiative on CLL (ERIC). The assay is quantitative, with a sensitivity down to 0.01%.(14) If MRD was undetectable, but sensitivity of at least 0.01% was not achieved, results were excluded from the analysis (i.e. data was treated as missing, given it was not possible in such cases to determine whether patients had truly achieved U-MRD). Serial analyses for MRD were done after the EOT on blood at yearly intervals during follow-up in many patients who achieved U-MRD status in BM at the EOT; however, this was not mandated by the protocol.
Statistical analysis
PFS was defined as the time from the start of treatment until disease progression or death from any cause. OS was defined as the time from the start of treatment until death from any cause. Survival curves were calculated using the Kaplan-Meier method and cross-group comparisons were made using the log rank test. Univariable and multivariable analyses were performed using Cox regression; predictors with univariable p-value <0.1 were included in the multivariable model. 3- and 6-month landmark analyses (6 months representing the EOT in the majority of patients) were performed for PFS and OS to determine the association between MRD status at the EOT and time-to-event endpoints. Univariable and multivariable analyses for binary outcomes were performed using logistic regression analysis; predictors with univariable p-value <0.1 were included in the multivariable model. All p-values are 2-sided, with a significance level of ≤0.05. Classification and regression tree models,(15, 16) fit using the rpart package in R, were used to identify prognostically important covariates and potential interactions among them, for the following: PFS according to pre-treatment characteristics, likelihood of achieving U-MRD status at the EOT according to pre-treatment characteristics, and PFS from the 6-month landmark according to MRD status and pre-treatment characteristics.
Results
Pretreatment characteristics are shown in Table 1.
Table 1.
Pre-treatment characteristics.
| Characteristic (N=289) | Number (%) unless stated |
|---|---|
| Age, median (range) | 59 (32–84) |
| Gender | |
| Male | 184 (64) |
| Female | 105 (36) |
| Rai Stage | |
| 0–II | 174 (60) |
| III–IV | 115 (40) |
| White cell count (×109/L) | 81.5 (1.4–452.2) |
| Hemoglobin (g/dL) | 12.2 (6.9–16.5) |
| Platelet count (×109/L | 139 (10–398) |
| FISH hierarchy, n=282 | |
| Del(13q) | 95 (34) |
| Negative | 62 (22) |
| Trisomy 12 | 50 (18) |
| Del(11q) | 58 (21) |
| Del(17p) | 17 (6) |
| IGHV-MS, n=285 | |
| Mutated | 105 (37) |
| Unmutated | 149 (52) |
| No polymerase chain reaction (PCR) product obtained | 31 (11) |
| ZAP70 expression by IHC, n=258 | |
| Negative | 92 (36) |
| Positive | 166 (64) |
| β2-microglobulin, n=284 | |
| <4.0mg/L | 166 (59) |
| ≥4.0mg/L | 118 (41) |
| Complex metaphase karyotype, n=230 | |
| No complex karyotype | 210 (91) |
| Complex karyotype | 20 (9) |
Abbreviations: FISH, fluorescence in situ hybridization; IGHV-MS, IHC, immunohistochemistry; immunoglobulin heavy chain variable somatic hypermutation status; ZAP70 – Zeta-associated protein 70
Response, PFS and OS
Median follow-up for the total cohort was 57 months (range 3–93). Overall response rate was 96% (64% CR). One hundred eighteen patients came off study during follow-up, for the following reasons: disease progression (n=94), primary refractory disease (n=10), Richter transformation (n=7) and death in CR/PR (n=7). Twenty-five patients were censored for PFS for the following reasons: received systemic therapy for another cancer (MDS/AML, n=4, other, n=7), treatment on another clinical trial (lenalidomide, n=4, ofatumumab, n=3 or alemtuzumab n=3) for eradication of MRD (n=10), allogeneic stem cell transplant in remission (n=3), received treatment for autoimmune hemolytic anemia (n=1). Patients censored for PFS were followed for OS.
Univariable associations between pre-treatment characteristics, PFS and survival are shown in Supplementary Table 1. In multivariable analysis, the following were significantly associated with shorter PFS: unmutated IGHV [HR 3.31 (1.62–6.78), p=0.001], del(17p) [HR 10.04 (2.81–38.86), p<0.001] and β2-microglobulin ≥4.0mg/L [HR 1.77 (1.07–2.91), p=0.03]. Patients with trisomy 12 had longer PFS [HR 0.37 (0.16–0.89), p=0.03]. In multivariable analysis, the only pre-treatment characteristic significantly associated with inferior overall survival (OS) was unmutated IGHV [HR 3.38 (1.07–10.72), p=0.04]. There was a trend toward inferior OS in patients with del(17p) [HR 2.78 (0.90–8.61), p=0.08]
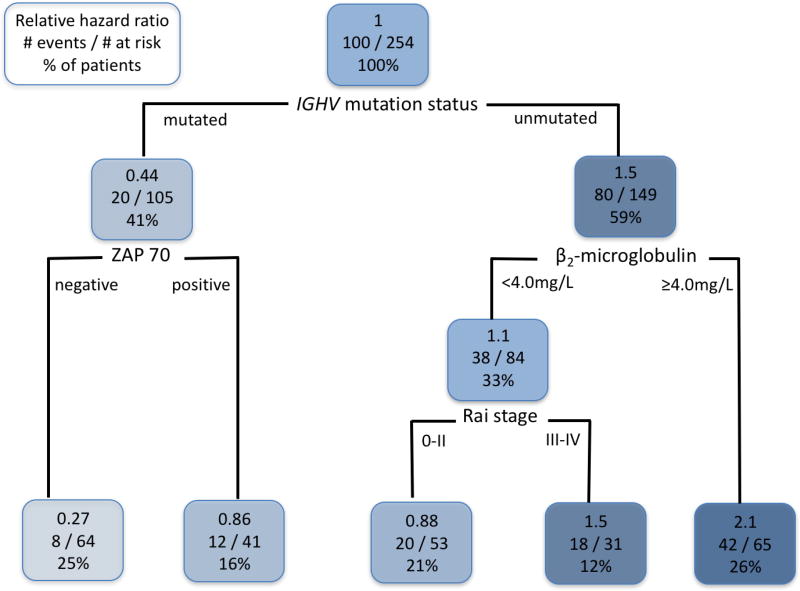
We performed classification and regression tree (CART) analysis to analyze potential interactions between pre-treatment prognostic variables and PFS (Figure 1). Analyses identified IGHV-MS as the most important determinant of PFS and also demonstrated that the outcome for patients with mutated IGHV could be further stratified according to ZAP70 status; patients who were negative for ZAP70 had particularly favorable PFS (Figure 2A). We confirmed these data in univariable and multivariable Cox Regression models, which were performed separately for patients with mutated and unmutated IGHV (Supplementary Tables 2–5). In patients with mutated IGHV, on multivariable Cox Regression analysis, patients who were ZAP70 positive by IHC (39% of patients with mutated IGHV) had shorter PFS [HR 2.88 (1.03–8.06), p=0.04]. In patients with unmutated IGHV, B2M ≥4.0mg/L [1.88 (1.09–3.26), p=0.02), advanced Rai stage [1.92 (1.92–3.27), p=0.02] and del(17p) [19.64 (3.73–103.44), p<0.001] were all associated with inferior PFS.
Figure 1.
Classification and Regression Tree model of association between pre-treatment characteristics and progression-free survival (PFS). The following variables were included in the model: Age ≥65 vs. <65, Rai stage III–IV vs. 0–II, B2M ≥4.0mg/L vs. <4.0mg/L, ZAP70-positive vs. negative, FISH hierarchy [del(17p) vs. other], unmutated IGHV vs. mutated IGHV. For the purposes of this analysis, patients with missing IGHV-mutation status values were excluded. Thus, a total of 254 patients were analyzed. Each node is organized as follows: the top line shows the hazard ratio for PFS, relative to all patients within the cohort; the middle line shows the number of patients in each group who had an event/number at risk; the bottom line shows the percentage of the total patients who fall within that node. This classification demonstrates that patients with mutated IGHV can be subdivided according to whether they are ZAP70-negative or positive; patients with mutated-IGHV who are also ZAP70-negative have the most favorable outcome. For patients with unmutated IGHV, those with a B2M ≥4.0 had an inferior outcome to those with B2M <4.0; there was no difference in outcome according to ZAP70. Abbreviations: IGHV, immunoglobulin heavy chain variable gene; ZAP70, zeta-associated protein-70, by immunohistochemistry in BM.
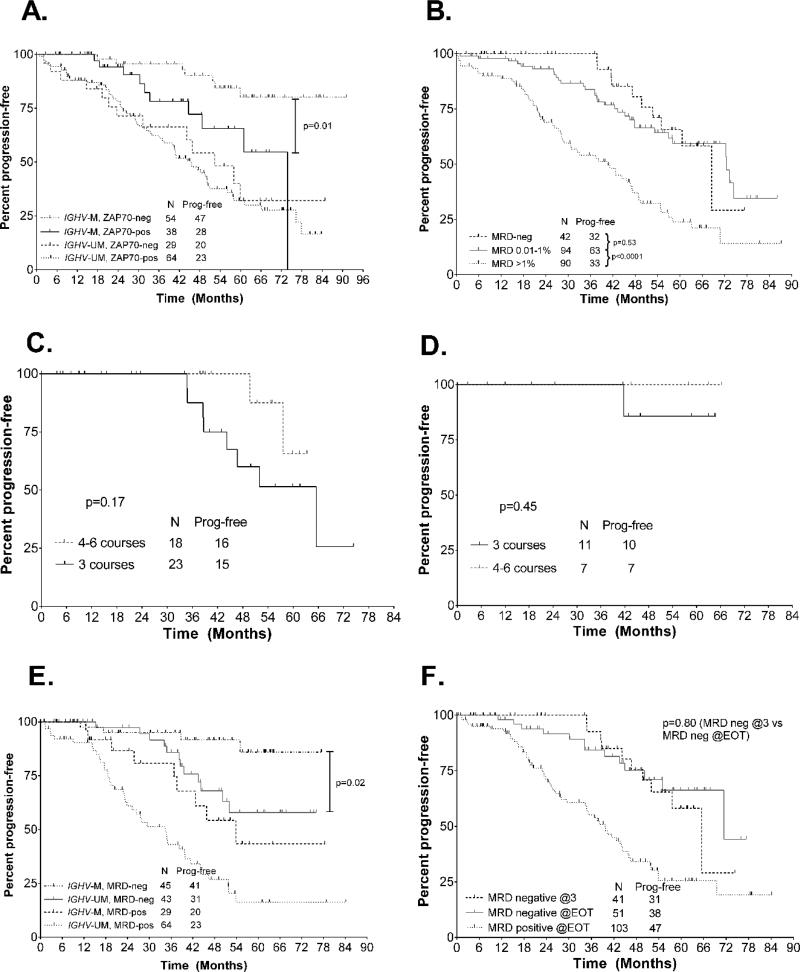
Figure 2.
A. Progression-free survival (PFS) according to IGHV-MS and ZAP70 expression. B. PFS from the 3-month landmark according to minimal residual disease (MRD) level in bone marrow (BM) after course 3 in the whole patient cohort. C. PFS analysis from the 3-month landmark in all patients who achieved undetectable MRD (U-MRD) status in BM after course 3, according to number of total FCR courses received. D. PFS analysis from the 3-month landmark in mutated-IGHV patients who achieved U-MRD status in BM after course 3, according to number of FCR courses received. E. PFS from the 6-month landmark according to IGHV-MS and MRD positive vs undetectable. F. PFS from the 6-month landmark according to timing of achieving U-MRD status. U-MRD @EOT denotes that the patient was MRD-positive after course 3 and had U-MRD at the end of therapy (EOT).
MRD-level in BM after 3 courses and likelihood of converting to U-MRD by EOT
The level of MRD after course 3 indicates the degree of chemosensitivity and may correlate with PFS and OS.(1) After course 3, 236 patients had BM MRD results available. Forty-two of 236 patients (18%) with evaluable results achieved U-MRD status; 94(40.5%) had 0.01–1% MRD and 96 (41.5%) had >1% MRD. An additional 21 patients were excluded from the analysis: 6 cases with U-MRD were excluded as they did not have sensitivity of at least 0.01%; reported sensitivity for these patients ranged from 0.02–0.15%. Univariable associations between pre-treatment characteristics and U-MRD status after 3 courses are shown in Supplementary Table 6. On multivariable analysis, trisomy 12 [OR – odds ratio - 4.14 (1.16–14.78), p=0.03] and negative FISH [OR 4.29 (1.29–14.26), p=0.02] were significantly associated with an increased likelihood of achieving U-MRD status after 3 courses.
One hundred fifty-four patients who were MRD-positive after 3 courses had evaluable MRD results at the EOT; 52 (34%) converted to U-MRD status at the EOT. The likelihood of converting to U-MRD status was strongly associated with the level of MRD in the BM after 3 courses. Among patients with a detectable MRD level of ≤1%, 44/69 (64%) converted to U-MRD status. In contrast, only 7/85 (8%) converted to U-MRD status if the MRD level was >1%, [OR 17.2 (7.1–41.7), p<0.001]. On multivariable analysis, only an MRD level of ≤1% was significantly associated with the likelihood of converting to U-MRD status at EOT [OR 17.6 (HR 6.8–45.6), p<0.001]. Even among patients with mutated IGHV, only 4/21 (19%) who had MRD level >1% converted to U-MRD status at EOT.
Association between MRD level in BM after 3 courses and subsequent PFS
To determine the association between interim MRD level after 3 courses and subsequent PFS, we performed a 3-month landmark PFS analysis. Patients with MRD >1% had a markedly inferior PFS compared to those with MRD 0.01–1% or <0.01% (p<0.001); however, there was no difference in PFS for those with MRD 0.01–1% versus <0.01% (Figure 2B). When only patients with mutated IGHV were analyzed, results were similar (Supplementary Figure 1). When we included pre-treatment characteristics in a multivariable model with MRD level, the only characteristics significantly associated with inferior PFS from the 3-month landmark were MRD >1% [HR 3.4 (1.9–6.1), p<0.001], unmutated IGHV [HR 2.4 (1.2–5.0), p=0.02] and del(17p) [HR 4.8 (1.3–17.8), p=0.02].
Association between number of courses of FCR and PFS in patients with U-MRD in BM after 3 courses
For the subgroup of patients who achieved U-MRD status after 3 courses, we previously reported that PFS was excellent, even if only 3 courses of FCR were given,(3) but with limited follow-up duration; we therefore repeated this analysis, now with a median follow-up of 56 months (range 3–93). Of the 42 patients who had U-MRD after course 3, 23/42 (55%) received 3 courses of FCR and only 9/42 (21%) received the full 6 courses. There was a trend toward inferior PFS in patients who received only 3 courses (p=0.17), Figure 2C. However, when we analyzed only the 18 patients with mutated IGHV, the results were highly favorable. Only one patient relapsed, despite the fact that 11/18 patients received only 3 courses of therapy and only 2/18 received the full 6 courses (Figure 2D). The single relapse occurred in a patient who received 3 courses of FCR and developed progressive disease after 42 months.
Association between achieving U-MRD status in BM by EOT and pre-treatment characteristics
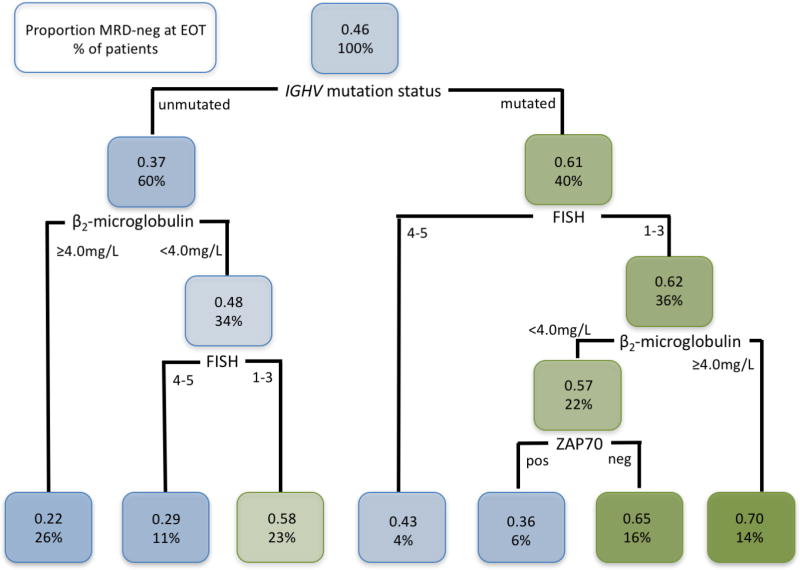
At the EOT, 105/219 (48%) patients with evaluable MRD results from BM achieved U-MRD status. An additional 17 cases with U-MRD were excluded because the assay sensitivity did not reach 0.01%. Univariable associations between pre-treatment characteristics and achieving U-MRD status at the EOT are shown in Supplementary Table 7. On multivariable analysis, the following were significantly associated with achieving U-MRD status at the EOT: mutated IGHV [OR 2.5 (1.2–5.5), p=0.02], negative FISH [OR 2.6 (1.04–6.4), p=0.04] and trisomy 12 [OR 2.7 (1.1–6.8), p=0.03]. Among patients with mutated IGHV, there were no other pre-treatment characteristics significantly associated with the likelihood of achieving U-MRD status at the EOT, but there was a trend toward increased likelihood in patients with trisomy 12 [HR 4.5 (0.87–23.35), p=0.07]. We explored potential interactions between pre-treatment characteristics and the likelihood of achieving U-MRD status at the EOT using a classification and regression tree model. This demonstrated that mutated IGHV was the best pre-treatment predictor of achieving U-MRD status at the EOT, but there were several modifying factors (Figure 3).
Figure 3.
Classification and Regression Tree model of association between pre-treatment characteristics and likelihood of achieving U-MRD status at the end of treatment (EOT). Subjects with missing IGHV-MS or missing MRD result at the EOT were excluded, resulting in a set of 192 patients for analysis. Each node is organized as follows: the top line shows the proportion of patients within that node who achieved U-MRD status at the EOT; the bottom line shows the percentage of total patients in each node. The highest likelihood of achieving U-MRD status at the EOT was for patients with mutated IGHV, lacking del(11q) or del(17p) who had a β2-microglobulin <4.0mg/L. Abbreviations: FISH, fluorescence in situ hybridization [1–3 represents del(13q), no abnormalities and trisomy 12; 4–5 represents del(11q) and del(17p)]; IGHV, immunoglobulin heavy chain variable gene; ZAP70, zeta-associated protein-70, by immunohistochemistry in BM.
Association between achieving U-MRD status in BM at the EOT and PFS
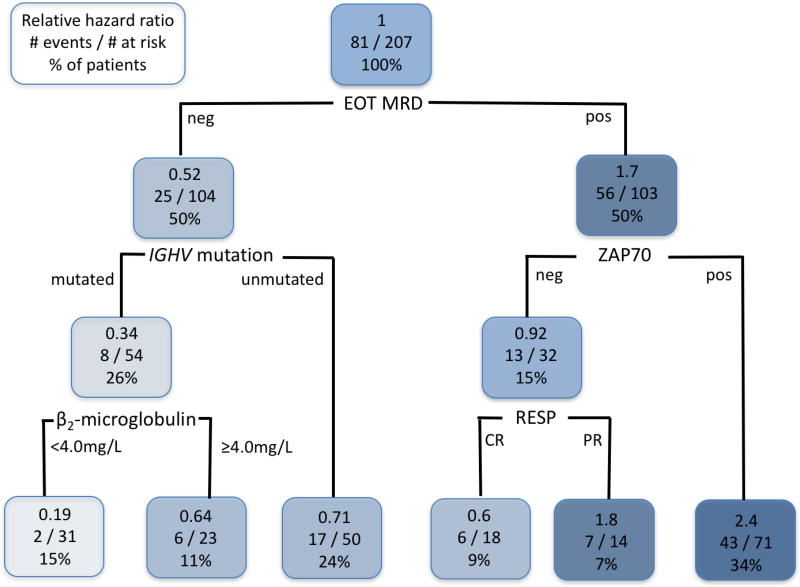
On 6-month landmark analysis, failure to achieve U-MRD status at the EOT was strongly associated with inferior PFS [HR 3.50 (2.17–5.62), p<0.001]. Multivariable analysis was performed, including NCI-WG response category (CR vs. PR; 10 patients who were primary refractory to FCR were excluded) and the following pre-treatment characteristics, which were significantly associated with PFS from the 6-month landmark univariable analyses: age ≥65, β2-microglobulin ≥4.0mg/l, ZAP70-positivity, unmutated-IGHV, FISH subgroup. Failure to achieve U-MRD status at EOT was strongly and independently associated with inferior PFS [HR 3.39 (1.69–6.85), p=0.001]. Failure to achieve CR was also associated with inferior PFS [HR 2.77 (1.42–5.37), p=0.003]. Among pre-treatment characteristics, B2M ≥4.0mg/L [HR 2.33 (1.30–4.17), p=0.005] and ZAP70-positivity [HR 2.86 (1.39–5.86), p=0.004] were also independently associated with inferior PFS.
We performed classification and regression tree analysis to identify interactions between PFS and the following: achieving U-MRD status, response category (CR vs. PR), and pre-treatment characteristics. This confirmed that achieving U-MRD status was the most important predictor of PFS and in patients achieving U-MRD status, PFS was superior in patients who had mutated IGHV and had a B2M <4.0mg/L (Figure 4). When only patients who achieved U-MRD status at the EOT were analyzed in a Cox Regression model, the only significant association with longer subsequent PFS was mutated IGHV [HR 0.29 (0.09–0.90), p=0.03], Figure 2E. There was no difference in outcome for patients achieving U-MRD status according to CR vs. PR [HR 0.77 (0.18–3.24), p=0.72].
Figure 4.
Classification and Regression Tree model of association between pre-treatment characteristics, U-MRD status at end of treatment, IWCLL response category (PR vs. CR), and 6-month landmark progression free survival (PFS). Subjects with PFS <6 months or missing MRD status were excluded. Thus, a total of 207 subjects were analyzed. Each node is organized as follows: the top line shows the hazard ratio for PFS, relative to all patients within the cohort; the middle line shows the number of patients in each group who had an event/number at risk; the bottom line shows the percentage of the total patients who fall within that node. The most favorable PFS was for patients who achieved U-MRD status post-treatment, had mutated IGHV, and pre-treatment B2M <4.0mg/L. Abbreviations: EOT MRD; end-of-treatment minimal residual disease in BM; IGHV, immunoglobulin heavy chain variable gene; RESP, response (CR, complete response; PR, partial response); ZAP70, zeta-associated protein-70, by immunohistochemistry in BM.
Timing of achieving U-MRD status in BM and PFS
We compared outcomes for patients who achieved U-MRD status after 3 courses to those who were MRD-positive after course 3, but had U-MRD at the EOT. There was no difference in PFS between the two groups (p=0.80), Figure 2F. However, as noted above, of the 42 patients who achieved U-MRD status after 3 courses, 23 received only 3 courses and only 9 received the full 6 courses of FCR.
Association between achieving U-MRD status in BM and overall survival
Univariable associations between U-MRD status, baseline characteristics and overall survival are shown in Supplementary Table 8. On multivariable analysis, the following were significantly associated with inferior OS, from the 6-month landmark: failure to achieve U-MRD status [HR 3.48 (1.01–12.05), p=0.048] and del(17p) [HR 3.52 (1.02–12.11), p=0.046].
Time to reemergence of MRD in patients who achieved U-MRD status in BM at EOT
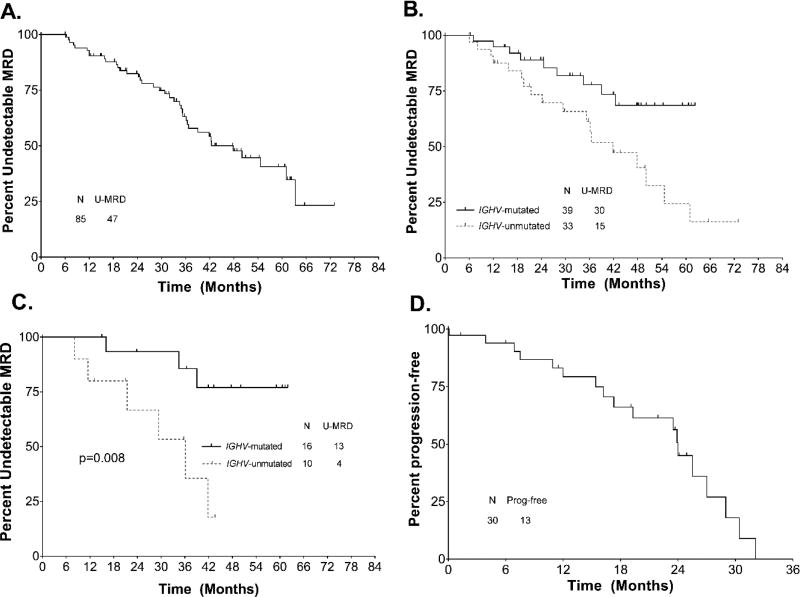
Re-emergence of MRD in blood was detected in 38/85 patients achieving U-MRD status, at a median of 48 months (range 6.1–65.1 months) after EOT, Figure 5A. Patients with unmutated IGHV showed more rapid reemergence of MRD (median 42 months vs. not reached, p=0.01), Figure 5B. Among patients with mutated IGHV who achieved U-MRD status after 3 courses, 3/16 had subsequent reemergence of MRD; all 3 had received 3 courses of FCR. Patients with mutated IGHV who achieved U-MRD status after course 3 had significantly longer time to MRD reemergence than patients with unmutated IGHV [HR 5.59 (1.37–22.85), p=0.02], Figure 5B. There were no other pre-treatment characteristics which were significantly associated with time to MRD reemergence in patients who achieved U-MRD status by C3 or at EOT. Eighteen patients who achieved U-MRD status at the EOT and had MRD monitoring subsequently developed clinical disease progression; of these patients, MRD reemergence in blood preceded clinical disease progression in 17/18 (94%). Reemergence of MRD preceded clinical relapse by a median of 24 months (range 0.1–32.1 months), Figure 5D; there was no difference in time to clinical relapse after reemergence of MRD according to mutation status or other pre-treatment characteristics (data not shown). MRD doubling time was calculable in 14 patients; median doubling time was 2.6 months (range 1.1–7.2). Absolute MRD level at first detection ranged from 0.001 to 0.56 × 109/L and numbers of events were small. As a result, there was no correlation between MRD doubling time and time to clinical relapse.
Figure 5.
A. Time to MRD (minimal residual disease)-reemergence for total cohort. B. Time to MRD reemergence in patients who achieved U-MRD status at end-of-treatment by mutation status. C. Time to MRD reemergence in patients who achieved U-MRD status after course 3, by mutation status. D. Time to clinical progression after reemergence of MRD. Abbreviations: IGHV, immunoglobulin heavy chain variable gene; U-MRD, undetectable minimal residual disease.
Discussion
Achieving long-term remission after first-line therapy for CLL, without requiring maintenance therapy, is desirable. This is achievable with CIT in the majority of the subgroup of patients with mutated-IGHV.(6–8) Accurate pre-treatment determination of prognosis is now particularly important, given the availability of novel, targeted therapies such as the BTK inhibitor ibrutinib and the Bcl-2 inhibitor venetoclax, which are effective in patients with high-risk biological features such as unmutated IGHV and del(17p).(17–21) Our data confirm that patients with mutated IGHV have the most favorable outcomes after FCR CIT. Notably, our data also suggest that certain biomarkers may have prognostic relevance only within specific subgroups of patients; patients with mutated IGHV who are ZAP70-positive by IHC in BM have inferior outcomes to those who are ZAP70 negative but there was no difference in PFS according to ZAP70 expression in patients with unmutated-IGHV. This study pre-dated routine availability of molecular testing for common CLL-associated gene mutations. Assessment of the impact of these prognostic features in larger cohorts of patients with mutated IGHV may add further nuance to pre-treatment assessment of prognosis for patients with mutated IGHV.
MRD analysis provides an in vivo assessment of disease sensitivity. Our data confirm previous observations (6, 22–24) that achieving U-MRD status at the EOT is associated with favorable long-term PFS and OS. Among patients with U-MRD at the EOT, IGHV-MS was the only pre-treatment characteristic that remained prognostic for PFS; patients with mutated IGHV who had U-MRD at the EOT had particularly favorable PFS. In addition to confirming the prognostic importance of post-treatment MRD level, we demonstrated that interim MRD level in BM stratified patients into 3 groups and propose that these 3 groups of patients could potentially benefit from different treatment strategies: first, patients with MRD <0.01% had a favorable PFS, despite most receiving <6 courses of FCR; patients achieving U-MRD status with mutated IGHV had a particularly favorable PFS; this group of patients could potentially stop treatment after 3 courses and be observed; second, patients with MRD 0.01–1% also had a favorable PFS and a high likelihood of achieving U-MRD status at the EOT; this group of patients could complete a further 3 courses of FCR and be reassessed with BM MRD analysis at the EOT; third, patients with MRD >1% had unfavorable PFS and a very low likelihood of achieving U-MRD status at the EOT; this group of patients likely has disease relatively insensitive to CIT. As such, further courses of FCR may be of limited benefit and potentially harmful; instead, these patients may benefit from transitioning to salvage therapy with novel, targeted agents. Finally, we showed that in patients who achieve U-MRD status at the EOT, a yearly assessment of MRD in the blood can detect sub-clinical relapse. Median time to progression after detection of MRD was 24 months, but inter-patient variability was substantial. We did not have sufficient MRD time points and patient numbers to accurately determine a relationship between rate of change in MRD level and time to progression, although such a relationship likely exists. Additionally the relatively short time-to-progression from first detection of MRD in some patients suggests that more frequent MRD analysis (eg. every 6 rather than every 12 months) may be required for most accurate prediction of time to progression.
The optimal treatment-approach in the first-line setting in patients fit for CIT is not clear, including for those with favorable-risk biomarkers (IGHV-mutated, no high-risk FISH).(7, 25) On the one hand, favorable risk patients are potentially curable with CIT;(6–8) on the other, they also have generally favorable survival outcomes, even when CIT is not given as initial therapy,(25, 26). Optimal management in this situation should be clarified by randomized clinical trials. Two important randomized studies are ongoing, comparing CIT to ibrutinib plus rituximab (the ECOG E1912 – NCT02048813 - and the UK FLAIR studies), and a third, the German CLL Study Group CLL 13 study, compares CIT to venetoclax plus either rituximab, obinutuzumab or ibrutinib and obinutuzumab (NCT02950051). The results will therefore go some way toward establishing the best initial treatment strategies for fit patients with CLL; these studies may not, however, be powered to determine optimal management for all prognostically-relevant sub-groups of patients, particularly those with favorable-risk biomarkers. Notably, the treatment paradigms tested in E1912/FLAIR and CLL13 are different: in the E1912 and FLAIR studies, ibrutinib is given indefinitely and for up to 6 years, respectively; in CLL13, the venetoclax-based combinations are given for a finite period of 1 year. The reason for long-term or indefinite ibrutinib therapy is that during treatment with BTK inhibitor monotherapy, achieving U-MRD status is very rare, which makes MRD analysis of limited utility and likely necessitates indefinite maintenance therapy to prevent relapse.(17–20, 27) In contrast, in the first-line setting, venetoclax plus obinutuzumab(28), venetoclax plus ibrutinib(29) and venetoclax plus ibrutinib and obinutuzumab(30) achieved high rates of U-MRD. As U-MRD is now attainable with novel therapeutic combination strategies, we believe it will have increasing relevance as a therapeutic endpoint for the broader population of patients with CLL, particularly where the intent is to give time-limited treatment. Indeed, the primary endpoint for the venetoclax + obinutuzumab vs CIT comparison in CLL13 is U-MRD. Implementation of novel methods to detect MRD with greater sensitivity, such as high-throughput sequencing(31, 32) may further improve the predictive accuracy of MRD assessment and refine its use as a tool for directing therapeutic decisions.
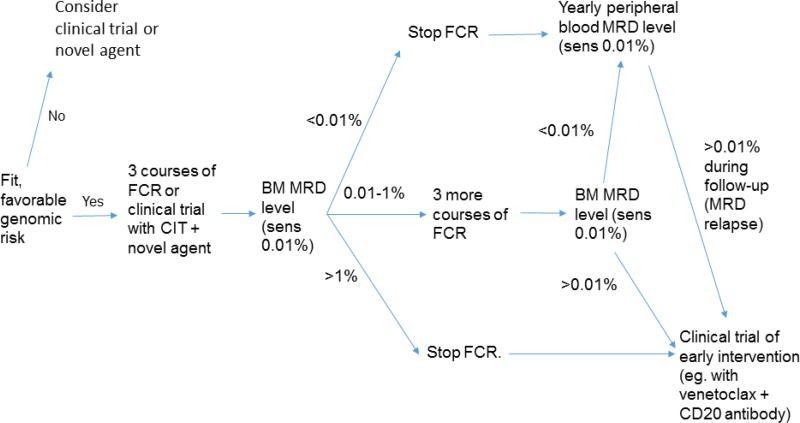
In summary, we propose that, beyond simply providing prognostic information, serial MRD analysis could be combined with knowledge of pre-treatment characteristics to direct adaptive and individualized therapeutic strategies. The aim of such strategies would be to achieve U-MRD status, while overall minimizing therapy and associated toxicity. An example of such a strategy, based on the data we discussed in this paper, is shown schematically in Figure 6. We stress that the concepts outlined here would require systematic testing in carefully designed, randomized studies, before being utilized in routine practice. In particular, it would be important to determine whether cessation of treatment in favorable prognosis patients who achieve U-MRD status after course 3 of FCR still leads to long-term PFS (and “cure”) and whether initiation of MRD-directed salvage therapy in patients MRD-positive after CIT or who have “MRD-relapse” improves PFS and OS.
Figure 6.
Possible adaptive treatment strategy for first-line treatment of CLL, taking into account pre-treatment patient and disease characteristics and MRD results. Favorable genomic risk is defined as mutated IGHV, without del(17p), del(11q) or TP53 mutation. Abbreviations: BM, bone marrow; CIT, chemoimmunotherapy; FCR, fludarabine, cyclophosphamide and rituximab; MRD, minimal residual disease.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions of the late Ms. Susan Lerner and Mark Hess in the maintenance of the CLL database at M.D. Anderson
This was supported, in part, by MD Anderson Cancer Center Support Grant P30 CA016672.
PAT has served as a consultant for Pharmacyclics and AbbVie and has received research funding from AbbVie.
MJK has served as a paid consultant for Roche/Genentech and Pharmacyclics.
SMO has served as a consultant for Janssen Oncology and AbbVie and has received research support from Pharmacyclics.
JAB has served as a consultant for Pharmacyclics and Janssen and has received research funding from Pharmacyclics.
NJ has served as a consultant for Pharmacyclics and has received research funding from Pharmacyclics and AbbVie.
CD has received speaking fees from AbbVie.
WGW has served as a consultant for Pharmacyclics, AbbVie and Roche/Genentech and has received research funding from AbbVie, Pharmacyclics and Genentech.
Footnotes
Supplementary information is available at Leukemia’s website.
Authorship contributions.
PAT collected and analyzed data, provided clinical care to patients and wrote the paper.
CBP performed statistical analysis and co-wrote the paper.
PS collected and analyzed data and co-wrote the paper.
JJ collected and analyzed flow cytometry data and co-wrote the paper.
MJK, SMO, AF, JAB, ZE, NJ, TMK, GB, CD, ND and EJ provided clinical care to patients and co-wrote the paper.
WGW designed and implemented the study, analyzed data, provided clinical care to patients and wrote the paper.
All authors reviewed and authorized the final version
Conflicts of interest.
The remaining authors declare no relevant conflicts of interest.
References
- 1.Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30(9):980–8. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 2.Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–16. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 3.Strati P, Keating MJ, O'Brien SM, Burger J, Ferrajoli A, Jain N, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123(24):3727–32. doi: 10.1182/blood-2013-11-538116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrisqueta P, Villamor N, Terol MJ, Gonzalez-Barca E, Gonzalez M, Ferra C, et al. Rituximab maintenance after first-line therapy with rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) for chronic lymphocytic leukemia. Blood. 2013;122(24):3951–9. doi: 10.1182/blood-2013-05-502773. [DOI] [PubMed] [Google Scholar]
- 5.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PA, Tam CS, O'Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127(3):303–9. doi: 10.1182/blood-2015-09-667675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, et al. Molecular prediction of durable remission after first line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015 doi: 10.1182/blood-2015-05-647925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–15. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 9.Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–80. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 12.Admirand JH, Knoblock RJ, Coombes KR, Tam C, Schlette EJ, Wierda WG, et al. Immunohistochemical detection of ZAP70 in chronic lymphocytic leukemia predicts immunoglobulin heavy chain gene mutation status and time to progression. Mod Pathol. 2010;23(11):1518–23. doi: 10.1038/modpathol.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–7. [PubMed] [Google Scholar]
- 14.Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–64. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 15.Breiman LFJ, Olsen RA, Stone CJ. Classification and Regression Trees: Wadsworth. 1984 [Google Scholar]
- 16.Therneau TAB, Ripley B. R package. Recursive Partitioning and Regression Trees. 4.1-10 ed2015. [Google Scholar]
- 17.Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. New England Journal of Medicine. 2014;371(3):213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–37. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):311–22. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottcher S, Hallek M, Ritgen M, Kneba M. The role of minimal residual disease measurements in the therapy for CLL: is it ready for prime time? Hematol Oncol Clin North Am. 2013;27(2):267–88. doi: 10.1016/j.hoc.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PA, Wierda WG. Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood. 2016;127(3):279–86. doi: 10.1182/blood-2015-08-634816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montserrat E. Treatment of chronic lymphocytic leukemia: achieving minimal residual disease-negative status as a goal. J Clin Oncol. 2005;23(13):2884–5. doi: 10.1200/JCO.2005.11.932. [DOI] [PubMed] [Google Scholar]
- 25.Delgado J, Doubek M, Baumann T, Kotaskova J, Molica S, Mozas P, et al. Chronic lymphocytic leukemia: A prognostic model comprising only two biomarkers (IGHV mutational status and FISH cytogenetics) separates patients with different outcome and simplifies the CLL-IPI. Am J Hematol. 2017;92(4):375–80. doi: 10.1002/ajh.24660. [DOI] [PubMed] [Google Scholar]
- 26.Baumann TDM, Kotaskova J, Molica S, Mozas P, Rivas-Delgado A. Leuk Lymphoma. 2017;58(S1):147–9. [Google Scholar]
- 27.Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):323–32. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flinn IW, Gribben JG, Dyer MJS, Wierda WG, Maris MB, Furman RR, et al. Safety, Efficacy and MRD Negativity of a Combination of Venetoclax and Obinutuzumab in Patients with Previously Untreated Chronic Lymphocytic Leukemia - Results from a Phase 1b Study (GP28331) Blood. 2017;130(Suppl 1):430. [Google Scholar]
- 29.Jain N, Thompson PA, Ferrajoli A, Burger JA, Borthakur G, Takahashi K, et al. Combined Venetoclax and Ibrutinib for Patients with Previously Untreated High-Risk CLL, and Relapsed/Refractory CLL: A Phase II Trial. Blood. 2017;130(Suppl 1):429. [Google Scholar]
- 30.Rogers KA, Huang Y, Stark A, Awan FT, Maddocks KJ, Woyach JA, et al. Initial Results of the Phase 2 Treatment Naive Cohort in a Phase 1b/2 Study of Obinutuzumab, Ibrutinib, and Venetoclax in Chronic Lymphocytic Leukemia. Blood. 2017;130(Suppl 1):431. [Google Scholar]
- 31.Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173–80. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan AC, Zhang B, Narasimhan B, Carlton V, Zheng J, Moorhead M, et al. Minimal residual disease quantification using consensus primers and high-throughput IGH sequencing predicts post-transplant relapse in chronic lymphocytic leukemia. Leukemia. 2013;27(8):1659–65. doi: 10.1038/leu.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.