Abstract
Background
Atrial fibrillation (AF) is a growing financial burden on the healthcare system. Cardiac computed tomographic angiography (CCTA) is needed for pulmonary vein mapping before AF ablation (AFA). CCTA has shown to be an alternative to transesophageal echocardiogram (TEE) to rule-out left atrial appendage thrombus (LAAT) pre-AFA. We aim to examine the safety, cost-effectiveness, and time-efficiency of utilizing CCTA alone to rule out LAAT before AFA.
Methods
We prospectively screened patients with paroxysmal AF undergoing cryoablation. CCTA with delayed enhancement was performed within 72 hours of AFA. Once LAAT was ruled out, patients were enrolled and planned TEE was cancelled. A retrospective control cohort that had both CCTA and TEE prior to AFA was identified. Direct cost data, electrophysiology laboratory utilization time and 30 day stroke outcomes were collected from the EMR, follow up phone calls or clinic visits and comparative analyses were performed.
Results
Seventy patients met the inclusion criteria in the prospective CCTA-only cohort, and seventy- one for the retrospective CCTA+TEE cohort. Baseline characteristics were similar between the two groups. There was a nonsignificant reduction in overall cost ($15,870 ±1,710 vs $16,557 ±2,508, p=0.06) in CCTA-only cohort, whereas the electrophysiology laboratory utilization time was significantly reduced (241.6 ±41.7 vs. 181.3 ±36.4 minutes, p<0.001). There were no strokes reported on 30-day follow-up in the CCTA-only group.
Conclusions
In low-to-intermediate stroke risk patients with paroxysmal AF undergoing cryoablation, eliminating TEE and employing CCTA-only strategy to rule-out LAAT improves electrophysiology laboratory efficiency without influencing peri-procedural cost or increasing post-procedural stroke risk.
Keywords: Atrial fibrillation, Left Atrial Thrombus, CCTA, Transesophageal Echocardiography
Introduction
Atrial Fibrillation (AF) is one of the most common heart rhythm abnormalities, currently affecting 2–3% of the population in Europe and the United States and continues to increase.(1; 2) The presence of AF increases a patient’s risk of death 1.5 to 1.9-fold(3), with most cases due to a stroke caused by embolization of a Left Atrial Appendage Thrombus (LAAT).
Catheter ablation within the left atrium is an increasingly used therapeutic option for patients with symptomatic AF.(4) Imaging of the heart prior to the ablative procedure by cardiac computed tomography angiography (CCTA), or cardiac MRI is valuable in pre-procedural planning, as they allow identification of the number and topology of the pulmonary veins and the relationship of the left atrium to nearby intrathoracic structures such as the esophagus. Also, since patients with AF have an increased risk of stroke from a LAAT,(5) pre-operative imaging of the left atrium is essential to exclude the presence of a thrombus. In current clinical practice, this is done through TEE which is considered to be the “gold standard” imaging tool and is performed usually within 24 to 48 hours of the procedure.(4; 6) Yet, TEE requires sedation and esophageal intubation and does not allow complete visualization and assessment of all of the pulmonary veins nor their precise relationship to other thoracic structures. Furthermore, TEE is a semi-invasive procedure associated with patient discomfort and not without complications such as esophageal perforation, while very infrequent, can potentially be life-threatening.(7) Therefore, prior to a catheter-based AF ablation procedure, patients often undergo evaluation with other imaging modalities such as CCTA, which increases the cost and time surrounding the procedure.
In a cost-analysis study examining Medicare fee-for-service data to evaluate Medicare expenditures before, during, and after catheter ablation for AF from July 2007 to December 2009, it was found that among 11,525 patients who underwent catheter ablation for AF, the mean overall expenditure on the day of the procedure was $14,455.(8) The mean imaging expenditure in the peri-procedural period was $884, accounting for approximately 6% of mean Medicare expenditures for catheter ablation of AF. The challenge becomes determining how to slow the rate of increase in periprocedural costs while preserving high-quality care. In efforts to control expenditures, a cost-conscious clinician should utilize an intervention which provides high value, depending on whether its benefits justify its costs. Therefore, the first step is to decrease or eliminate care that offers no benefit and may even be harmful.(9)
In light of that, CCTA has been increasingly used for LAAT detection. Several studies investigated the use of CCTA in the assessment of LAAT in pre-procedural evaluation for AF ablation.(10–14) A challenge with CCTA is that LAA filling defects may represent thrombus or incomplete contrast mixing with blood. Therefore, pre-bolus of contrast material with delay before the CCTA contrast bolus can help distinguish between thrombus and incomplete contrast mixing.
While prior studies showed non-inferiority of CCTA to TEE, there are no studies to-date examining the cost-effective and time-efficient advantages of safely eliminating the use of pre-procedural TEEs. Therefore, we have devised a study to compare the cost and electrophysiology lab utilization time in patients who undergo both a TEE and CCTA, versus patients who undergo CCTA alone prior to the catheter ablation of AF procedure.
Methods
Cohort Selection
The study was approved by the Institutional Review Board for Human Subjects Research at the University of Buffalo. A retrospective (control) cohort was identified using a query of the electronic medical record. For that group, patients with ablation of AF who underwent both CCTA imaging and TEE prior to ablation during September, 2014 to December, 2015 at Buffalo General Hospital (BGH) and Gates Cardiovascular Institute (GVI) were identified. A comparable experimental cohort was recruited prospectively by consenting patients at the time of their outpatient CCTA referral at BGH/GVI during the period of March, 2016 to February, 2017. Inclusion criteria for the prospective cohort were patients that have paroxysmal AF scheduled for an AFA procedure, and were scheduled to undergo CCTA with LAAT rule out protocol within 3 days prior to their AFA. Exclusion criteria for that cohort were patients who: 1) Refused to consent; 2) Long-persistent AF; 3) Did not undergo AFA for reasons other than TEE or CCTA related; or 4) Patients with history of prior strokes or hypercoagulable state; 5) Patients who were found to have a LAAT or left atrial filling defects on CCTA had to undergo TEE and thus were also excluded from the experimental cohort. For patients who were found to be in AF at the time of the procedure; the clinical protocol and decision whether to perform a TEE was left to the discretion of the attending electrophysiologist who would be performing the ablation procedure. Figure 1 illustrates the study protocol for patient inclusion and exclusion.
Figure 1.
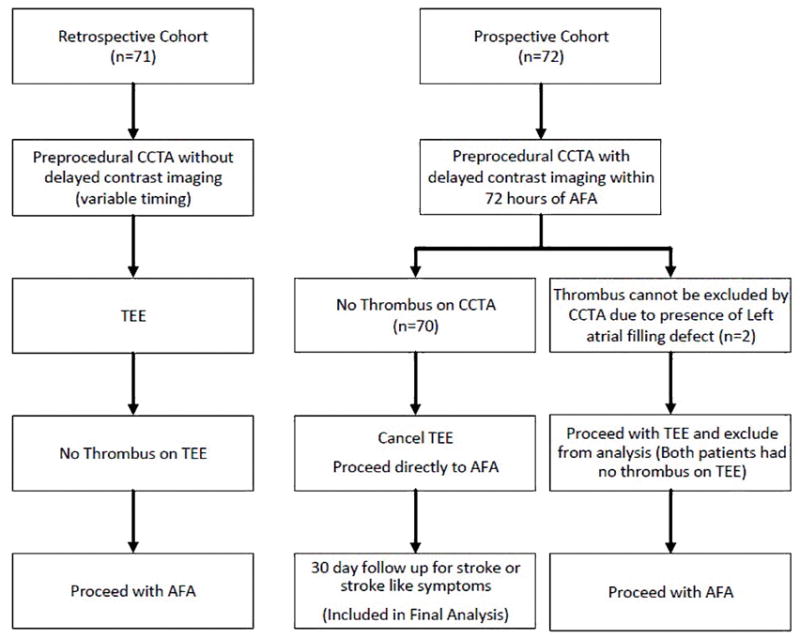
Flow chart illustrating the study protocol for patient inclusion and exclusion.
CCTA Imaging Protocol and Interpretation
Prior to the study period, pre-procedure imaging with CCTA was the standard clinical practice for all eligible patients undergoing catheter ablation of AF at our facility in order to define pulmonary vein anatomy.
The imaging was performed with 320-multidetector scanner (Aquilion ONE, Toshiba Medical Systems, Japan) in the craniocaudal direction and during breath hold. Scanning parameter were as follows: gantry rotation time 350 ms; tube potential, 100kV for the retrospective cohort and 120 kV for the prospective cohort; integrated automatic tube current dose reduction utilizing SUREExposure 3D; display field of view 220 mm2 and slice thickness of 0.5 mm. A retrospective acquisition protocol was used in the retrospective cohort, while a prospective acquisition protocol was used for the prospective cohort. The patient received an injection of 70 milliliters of iodinated contrast Omnipaque 350 (GE Healthcare, USA) at 6 ml/sec followed by 50 ml normal saline at 6 ml/sec. For the initial scan, bolus tracking technique was performed visually with region of interest in the descending aorta. However, to improve the sensitivity of the study to rule out LAAT, delayed imaging was integrated into the standard protocol in the prospective cohort. Delayed images were acquired 20 seconds after the initial scan. Both scans were obtained with prospective electrographic gating during breath hold. Image datasets were transferred to a remote workstation equipped with semi-automated post-processing software (Aquarius, TeraRecon, San Mateo, CA, USA) for further analysis and interpretation. Images were reconstructed at 0.5 mm with 0.4 mm increments.
At our facility, CCTAs are interpreted by cardiac imagers (cardiologists or a cardio-radiologist) experienced in cardiac CT. A positive study was defined as a filling defect both on initial and delayed scans with typical appearance of thrombus. Studies were deemed negative if there was no filling defect or filling defect present on the initial scan and absent from the delayed image (circulatory stasis). A CCTA was read as equivocal if there was a hypointensity in the LAA on delayed imaging that could not be definitively differentiated from a trabeculation or appeared to be possibly caused by an artifact. Another cardiac imaging attending confirmed all equivocal or positive CCTAs and agreement was achieved in all cases.
TEE and AFA Procedural Protocol
In the retrospective cohort TEE was performed after patient had presented to the electrophysiology lab for the AF ablation. Patients were excluded if the TEE was performed the day before the ablation or outside the EP lab on the day of the procedure. A pre-planned TEE was cancelled in the prospective group if CCTA delayed images confirmed no LAAT. Decision to continue or to hold pre-procedural anticoagulation and antiarrhythmic therapy was left to the proceduralist’s discretion. Activated clotting time of >300 seconds was targeted in all cases. All patients were on anticoagulation post procedure for at least the first month of follow up. Pulmonary vein isolation was achieved by cryoballoon ablation strategy in all study cohorts. The procedure duration in the two cohorts was defined as the time patient entered the electrophysiology lab to the time they exited, which includes the TEE and AFA procedures.
Data Collection and Outcome Measures
Data abstraction of the retrospective and prospective cohorts was performed independently by four abstractors (WM, ZS, AS and MA), including patient demographics (age, sex, race, medications), CCTA findings (including rhythm, presence of LAAT, LA size, pulmonary vein anatomy, anomalies, radiation dose, and extra-cardiac findings), TEE and procedural data (including rhythm and presence of LAAT), and clinical history (including stroke risk factors as determined by CHA2DS2VASc score). Procedural cost was obtained through the Federal Identification Number (FIN) assigned to that hospitalization, which includes the entire cost to the hospital for the entire hospital stay. CCTA cost was also obtained through FIN number assigned to that outpatient visit. Collection of procedure-related stroke or transient ischemic (TIA) events were performed for the prospective cohort. Procedure-related strokes and TIAs were defined as events occurring within one month of the procedure that either required hospitalization or patient reported symptoms. This clinical endpoint was assessed one month after the procedure by review of local medical record for hospitalization, calling the patients or at routine clinical follow-up.
Data collected on any incidental lung nodules were reported to the patients by phone calls to inform and advise them to discuss these incidental findings with their primary care physicians.
Statistical Analysis
Demographic and clinical characteristics were summarized using descriptive statistics (means, SD’s, and percentages) for both (CCTA+TEE and CCTA-only) groups and for the overall sample. Statistically significant differences between the groups on demographic and clinical measures were then determined using chi-squared tests for categorical data and Student’s t-tests for continuous data. Finally, a set of analysis of variance (ANOVAs) was performed to determine if there were significant differences on the outcome measures (i.e. total cost, time in lab) between the CCTA+TEE and CCTA-only groups. Any demographic or clinical characteristics that were found to be statistically different between the groups were used as covariates in the ANOVA models. Prior to running the analyses, the distributions of all continuous measures were checked for any violations of normality (e.g., skewness, kurtosis). All alpha levels were set to .05 and all analyses were performed using SPSS v24.
Results
There were a total of 141 patients included in this study. The CCTA+TEE group had 71 patients, and 70 patients were recruited for the CCTA-only group. The baseline demographic and clinical characteristics are shown in Table 1. The majority of the sample were white males, with a little over 60% of the sample being 60 years of age or older. Most of the sample was either overweight (33.3%) or obese (47.5%) and a little over half of the sample had a history of hypertension. The majority of the patients were considered to be high risk with a CHADS2VASC score of 2+. However, none of the patients had prior strokes. Eight patients in the CCTA-only group had CHADS2VASC of 4, with no patients having score above that. Over 80% of the sample was taking cardiac medication(s), with nearly one-third taking more than one medication. The only statistically significant differences between the CCTA+TEE and CCTA-only groups was history of hypertension and aspirin-only use, with the CCTA+TEE group having a significantly higher proportion of patients with history of hypertension and aspirin-only use than the CCTA-only group. The radiation dose (mSV) in the CCTA with delayed imaging was significantly lower when compared to CCTAs done without delayed imaging (8.5 vs 19.9, p<0.001).
Table 1.
Baseline demographic and clinical characteristics
| Overall (N = 141) | CCTA + TEE (N = 71) | CCTA-only (N = 70) | p-value | |
|---|---|---|---|---|
| Age (mean, SD) | 61.7 (10.6) | 61.4 (8.9) | 62.2 (12.1) | .53 |
| Gender (% Male) | 96 (68.1%) | 51 (71.8%) | 45 (64.3%) | .34 |
| Race (% White) | 138 (97.9%) | 68 (95.8%) | 70 (100%) | .08 |
| Body mass index | 30.6 (6.6) | 30.7 (5.6) | 30.4 (7.6) | .82 |
| Diabetes type 2 | 14 (9.9%) | 7 (9.9%) | 7 (10.0%) | .98 |
| Hypertension | 73 (51.8%) | 44 (62.0%) | 29 (41.4%) | .02 |
| Congestive heart failure | 5 (3.5%) | 3 (4.2%) | 2 (2.9%) | .66 |
| CHADS2 VASC | .28 | |||
| 0 | 27 (19.1%) | 13 (18.3%) | 14 (20.0%) | |
| 1 | 45 (31.9%) | 27 (38.0%) | 18 (25.7%) | |
| 2–3 | 53 (37.6%) | 23 (32.4%) | 30 (42.9%) | |
| 4+ | 16 (11.3%) | 8 (11.3%) | 8 (11.4%) | |
| Beta blockers | 79 (56.0%) | 36 (50.7%) | 43 (61.4%) | .20 |
| Calcium channel blockers | 40 (28.4%) | 23 (32.4%) | 17 (24.3%) | .29 |
| ACEi/ARB | 49 (34.8%) | 27 (38.0%) | 22 (31.4%) | .41 |
| Aspirin only | 41 (29.0%) | 15 (21.1%) | 26 (37.1%) | .04 |
| Anticoagulation (Coumadin or NOAC) | 91 (64.5%) | 49 (69%.0) | 42 (60.0%) | .26 |
| No Aspirin or Anticoagulation | 9 (6.4%) | 7 (9.9%) | 2 (2.9%) | .11 |
ACEi – Angiotensin converting enzyme inhibitors; ARB – Angiotensin receptor blocker; NOAC –New oral anticoagulant.
After 30-days from the procedure, with no patients lost to follow up, there were no strokes or TIAs reported in the CCTA-only group, giving the use of CCTA as a LAAT rule-out tool a 100% negative predictive value (NPV). While no patients had a positive CCTA finding of a LAAT in the CCTA-only group, two patients had an equivocal finding of a hypointensity in the LAA on delayed imaging. These patients underwent a confirmatory TEE, and both subsequently had results that were negative for LAAT and went on to have the ablation procedures performed but were excluded from the final time- and cost-efficiency analysis. Since no patient had a positive CCTA for LAAT, its sensitivity and positive predictive value (PPV) in detecting one could not be determined.
The mean comparisons of the outcome measures between the CCTA+TEE and CCTA-only groups is shown in Table 2. There were no significant violations of normality in the distributions of the outcome measures, so parametric tests (i.e., ANOVA) were considered appropriate. On average, the total cost in the CCTA-only group was $687 less than the CCTA+TEE groups. However, a non-significant trend towards reduction in the average total cost was seen in the CCTA-only group compared to the CCTA+TEE group (p =0.06). When the cost was subdivided into imaging (CCTA) and procedural (AFA) costs, the CCTA+TEE group had a significantly lower cost of CCTA than the CCTA-only group. There was no significant difference in the cost of the AFA procedure between the two groups. The CCTA-only group required 60 minutes less time for lab tests than the CCTA+TEE group (p < 0.001).
Table 2.
Comparisons of procedure costs and lab time between CCTA+TEE and CCTA-only patients
| CCTA + TEE (n = 71), SD | CCTA-only (n = 70), SD | p-value | |
|---|---|---|---|
| Total cost (US$) | 16,557, (±2,508) | 15,870, (±1,710) | .060 |
| CCTA cost | 437.3, (±214.1) | 210.1, (±60.2) | < .001 |
| AFA cost | 16,120, (±2,505) | 15,660, (±1,716) | .207 |
| Time in lab (min) | 241.6, (±41.7) | 181.3, (±36.4) | < .001 |
CCTA – Cardiac computed tomorgraphy angiography; AFA – Atrial fibrillation ablation.
We also performed additional analysis in regards to the often-found anomalous pulmonary vein anatomy (Table S1 in the Supplement). There were only 4 patients in the retrospective group with anomalous veins, and 12 in the prospective group. Given the low number of patients in the retrospective group, the analysis was only performed in the prospective group. In the prospective group, there was a difference in regards to the length of the AFA procedure, where a significant prolongation in the EP lab utilization time in patients with an anomalous vein was seen compared to with normal veins (253 min vs 179 min, p<0.001). Nonetheless, the total cost of the AFA and TEE procedures also was not significantly different between patients with anomalous veins and patient with normal veins ($17,553 vs $15,677, p=0.93). Given this finding, we performed a sensitivity analysis for our primary outcome of lab utilization time comparing retrospective (CCTA+TEE) versus prospective (CCTA only) groups excluding patients with anomalous veins, and found that the reduction in lab utilization time in the prospective group remains statistically significant (239 vs 179 min, p<0.001).
Discussion
The results of the safety analysis in our study shows that the use of CCTA only with delayed imaging protocol is enough to rule out a LAAT in patients that are at low to intermediate risk of stroke related to AF. This is in agreement with prior studies such as the one conducted by Wang et al(10) which showed that among 670 CCTA-negative patients for LAAT or stasis, 669 (99%) were negative by TEE with one false-negative CCTA in a patient with grade 2 stasis by TEE but no thrombus, yielding a negative predictive value of 99.9%. The authors of that study concluded that it may be possible to eliminate TEE in up to 80% of AF ablation patients based on negative CCTA findings. A meta-analysis of published prospective studies comparing CCTA with TEE in detecting LAAT prior to March 2014 concluded that for patients with TEE intolerance or contraindications, CCTA may be an alternative method, especially when the delayed imaging, ECG gating and heart rate control were performed.(15) Our study gives further incentive to this method, as it showed improvement in the efficiency of the electrophysiology lab by one hour. However, we were not able to show a significant cost difference when TEE was eliminated from the preprocedural imaging protocol. The CCTA results are consistent with prior literature which shows that the sole use CCTA with delayed imaging is a safe and effective method in ruling out a LAAT, potentially obviating the need for TEE prior to AFA procedure.(10; 15) Prior literature showed that, when using confirmatory intracardiac echocardiography as the reference standard, the use of CCTA-alone with delayed imaging protocol had a 100% positive predictive value (PPV), 100% NPV, 100% sensitivity, and a 100% specificity (when equivocal results were considered negative).(16) However, given that no patient had a positive LAAT on CCTA in our study, we could not determine the sensitivity or the PPV of using CCTA only for detecting a LAAT. Nonetheless, this demonstrates that the incidence of LAAT or the development of periprocedural CVA/TIA is relatively low enough to give a low pre-test probability warranting the safe use of CCTA only. However, given that no patient in the CCTA-only group has scored the CHADS2VASC above 4, we cannot extrapolate these findings to very high risk patients as such. Furthermore, none of patients included in the CCTA-only cohort had a prior history of CVA/TIA or LAAT, and thus these patients may be too high risk to safely obviate the need for a TEE. We do also recognize that approximately two-third of the patients were on anticoagulation, making the pre-test probability low for CVA. Nonetheless, it is also important to note that almost all patients in our study held their anticoagulant 1–3 days prior to the procedure (depending on the proceduralist’s preference). Thus, one might argue that this puts them at a higher risk of a subsequent development of a LAAT which would otherwise be detected on TEE on the day of the procedure could be missed in the prospective (CCTA only) cohort. The absence of CVAs in that cohort proves that doing a CCTA alone without a TEE on the day of the procedure is also safe in patients where the anticoagulant was held for up to 3 days prior to the procedure. Since the publication for uninterrupted dabigatran and feasibility and safety of uninterrupted apixaban in comparison to uninterrupted warfarin, clinical practice may have also begun to change.(17) Therefore, as more AFA are performed on uninterrupted anticoagulation in the future, performing CCTA 1–3 days prior to the ablation in patient cohort represented in our study, forgoing TEE would be feasible. Nonetheless, there is no clear delineation in the literature the optimal timing of preprocedural CCTA when used for ruling out LAAT. Based on available data, 3 days appears to be the average time with variable practice on preprocedural continuation of anticoagulation in prior studies(13; 16). Therefore, a future adequately powered, multi-center, placebo controlled, randomized, double-blinded trial including high risk patients, timing of preprocedural CCTA and continued use of anticoagulant periprocedurally is needed to assess the safety of using CCTA-alone in ruling out LAAT prior to AFA procedure.
In regards to the cost analysis, there was no significant difference in the total cost of the procedure including imaging. This may be due to the large disparity in the cost of the procedure from one patient to another as illustrated by the standard deviation. While there was a nonsignificant trend in reduction of total cost with lower cost in the CCTA-only group, this reduction is attributed to the lower cost of the CCTA in that group. The reason why CCTA was less expensive in the CCTA-only group compared to the CCTA+TEE group is likely due to the timing of cohort sampling. The CCTA+TEE group is a retrospective cohort of patients who presenting during prior time period compared to the prospective CCTA-only cohort. During that time the cost of CCTA was higher on average than the time when the prospective cohort was recruited.
While reduced cost was not found to be an advantage in excluding the use of TEE for ruling out a LAAT, doing so significantly reduced the total lab utilization time by 1 hour. This was about 25% of the average total time it took to perform an AFA. TEEs performed as part of the procedure not only added extra time to the procedure, it also required coordination with echocardiography staff and associated additional risk of complications.
Furthermore, aborting an ablation procedure due to TEE findings after the patient has been taken to the electrophysiology lab and has had induction of general anesthesia causes further risk to the patient and is more inefficient than cancelling the procedure before to the procedure day. Therefore, based on this study, we can determine that low-intermediate risk patients with paroxysmal AF may safely proceed with AFA ablation after using CCTA to rule out LAAT within 3-days from the procedure, eliminating the need for a TEE, thus reducing electrophysiology laboratory utilization time. However, patients with an equivocal or positive CCTA result, and high-risk patients such as ones with history of prior CVA/TIA, LAAT, CHADS2 VASC >4 or persistent AF should still undergo a confirmatory TEE, given their underrepresentation within our study. A flowchart for a proposed clinical protocol for pre-procedural imaging in patients undergoing AFA procedure has been shown in Figure 2.
Figure 2.
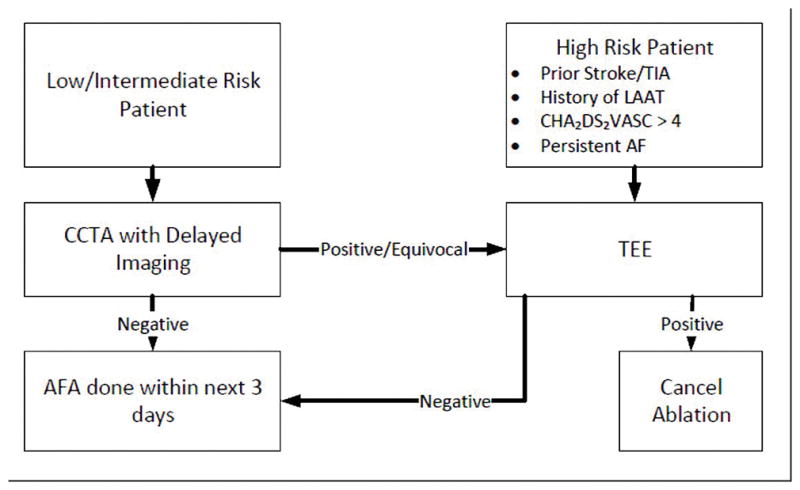
A flowchart for a proposed clinical protocol for pre-procedural imaging in patients undergoing atrial fibrillation procedure to reduce electrophysiology laboratory utilization time.
Given that our study protocol did not include systematically timing our cardiac imagers’ readings of the CCTAs, we cannot give objective data of the additional time to read the LAA with delayed imaging. However, a verbal survey with the cardiac imagers of our facility found that since a second set of images is acquired, the time to perform and read the study increases not more than a few minutes. For CCTA acquisition, it is increased by approximately 20 seconds. In the delayed CCTA images the left heart structures are poorly or not opacified, and hence the focus is truly on evaluating the LAA. This small duration of time spent on evaluating the delayed images is of high yield as it considerably increased the confidence among the readers and the specificity in identifying the presence or absence of a LAA clot. Therefore, one may argue that when the images were difficult to call LAA filling defect due to contrast not making in time, or due to clot in CCTA without delayed imaging, the delayed images helped make that decision quicker and improved the specificity. The additional delayed imaging did not increase the radiation exposure in our study. While we acknowledge that there is a small incremental increase of radiation dose for delayed imaging, but when carefully protocoled, the whole procedure can be accomplished within an acceptable range of radiation exposure. In fact, the CCTA-only group with delayed imaging had a lower radiation exposure in our study. This difference can be attributed to the change in imaging protocol of our center during the time in which the CCTA’s of the retrospective group (2014–2015) and the CCTA’s of the prospective group (2016–2017) were performed. A lower radiation doses and quality were adopted with the newer scans, and the method of acquisition was also changed from retrospective to a prospective acquisition protocol. This proves that an increased radiation dose with delayed imaging protocol can be avoided while the maintaining sensitivity of the procedure.
Although we have attempted to streamline the atrial fibrillation ablation pre-imaging process in our study, patients still had to present a few days prior to the procedure for their cardiac CT. This removed the flexibility in scheduling options for the patient. Intracardiac echocardiography catheters have been utilized to visualize the LAA and for low risk patients in which 3D mapping system provide adequate anatomical pulmonary vein landmark, avoiding CCTA altogether may be a consideration for future studies.
In the prospective group, there was a significant prolongation in the EP lab utilization time in patients with an anomalous vein when compared to patients with normal veins (253 min vs 179 min, p<0.001). This may have been due to differences to the approach of the procedure where supra-numerous or small caliber veins can involve more technically challenging methods for vein isolation. Very rarely, the electrophysiologist had to use radiofrequency energy in addition to the cryoablation to attain PV isolation but those patient’s had not met the inclusion criteria and therefore, none of the study cohort had hybrid radiofrequency and cryoablation.
While this study is the first to examine and demonstrate the cost-effectiveness and time-efficiency of safely eliminating the use of pre-procedural TEEs, there were some limitations. Given that the two cohorts were sampled from two different periods, there may be temporally-related confounding variables. For instance, the difference in timing of sampling between the two cohorts may be a factor as the analysis does not take into consideration possible inflation or deflation in the cost of the AFA procedure. The cost of an AFA procedure performed early in the CCTA+TEE group (i.e. September, 2014) may have been less expensive compared to an AFA procedure performed during the time in which the CCTA-only group was recruited (i.e. February, 2017), thus underestimating the cost reduction of eliminating the TEE. Our study also was not powered enough to capture any patients with a positive LAAT on CCTA, therefore we could not determine the sensitivity or the PPV of using CCTA only for detecting a LAAT. The power of the CCTA+TEE study group also does not allow us to closely examine TEE complications. Additionally, we did not have any stroke outcomes in the prospective group that is likely due to low incidence of thromboembolism in this patient cohort.
Conclusion
The use of CCTA only for ruling out LAAT in patients undergoing AFA procedure can safely obviate the need for a TEE. While no significant cost reduction was detected by eliminating TEEs, there was a 60 minute reduction in electrophysiology lab utilization time. Whether these findings apply to high-risk patients remains to be determined. Therefore a future adequately powered, multi-center trial including high risk patients is needed to assess the safety of using CCTA alone in ruling out LAAT prior to AFA procedure.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. Dr. Sharma also received support from the NIH/NHLBI 1K08HL131987.
Footnotes
Conflict of interest: None
Compliance with Ethical Standards:
Funding Source: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. Dr. Sharma also received support from the NIH/NHLBI 1K08HL131987.
Conflict of Interest: None to declare. Informed consent: Written informed consent was granted by all patients partaking in the prospective study cohort. Ethical approval: The study was approved by the University at Buffalo Institutional Review Board (IRB) and informed consent was obtained from all individual participants included in the study
References
- 1.Thomas M, Munger L-QW, Shenc Win K. Atrial fibrillation. J Biomed Res. 2014 doi: 10.7555/JBR.28.20130191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massimo Zoni-Berisso FL, Carazza Tiziana, Domenicucci Stefano. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014 doi: 10.2147/CLEP.S47385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaspal S, Taggar FM, Lip Gregory YH. Mortality in patients with atrial fibrillation: improving or not? European Society of Cardiology. 2008 doi: 10.1093/europace/eun054. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 5.Agmon Y, Khandheria BK, Gentile F, Seward JB. Clinical and echocardiographic characteristics of patients with left atrial thrombus and sinus rhythm: experience in 20 643 consecutive transesophageal echocardiographic examinations. Circulation. 2002;105:27–31. doi: 10.1161/hc0102.101776. [DOI] [PubMed] [Google Scholar]
- 6.Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD, Johnson RG, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med. 1995;123:817–822. doi: 10.7326/0003-4819-123-11-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Chan KL, Cohen GI, Sochowski RA, Baird MG. Complications of transesophageal echocardiography in ambulatory adult patients: analysis of 1500 consecutive examinations. J Am Soc Echocardiogr. 1991;4:577–582. doi: 10.1016/s0894-7317(14)80216-2. [DOI] [PubMed] [Google Scholar]
- 8.Pokorney SD, Hammill BG, Qualls LG, Steinberg BA, Curtis LH, Piccini JP. Cost analysis of periprocedural imaging in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2014;114:266–271. doi: 10.1016/j.amjcard.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens DK, Qaseem A, Chou R, Shekelle P Clinical Guidelines Committee of the American College of P. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180. doi: 10.7326/0003-4819-154-3-201102010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Kadiyala M, Koss E, Yarramaneni A, Rapelje K, Kampfer S, Reichek N, et al. CTA Detection of Left Atrial Stasis and Thrombus in Patients with Atrial Fibrillation. Pacing Clin Electrophysiol. 2016;39:1388–1393. doi: 10.1111/pace.12959. [DOI] [PubMed] [Google Scholar]
- 11.Teunissen C, Habets J, Velthuis BK, Cramer MJ, Loh P. Double-contrast, single-phase computed tomography angiography for ruling out left atrial appendage thrombus prior to atrial fibrillation ablation. Int J Cardiovasc Imaging. 2017;33:121–128. doi: 10.1007/s10554-016-0973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikegami Y, Tanimoto K, Inagawa K, Shiraishi Y, Fuse J, Sakamoto M, Momiyama Y, et al. Identification of Left Atrial Appendage Thrombi in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation Using Intra-Cardiac Echocardiography and Cardiac Computed Tomography. Circ J. 2017 doi: 10.1253/circj.CJ-17-0077. [DOI] [PubMed] [Google Scholar]
- 13.Martinez MW, Kirsch J, Williamson EE, Syed IS, Feng D, Ommen S, Packer DL, et al. Utility of nongated multidetector computed tomography for detection of left atrial thrombus in patients undergoing catheter ablation of atrial fibrillation. JACC Cardiovasc Imaging. 2009;2:69–76. doi: 10.1016/j.jcmg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Homsi R, Nath B, Luetkens JA, Schwab JO, Schild HH, Naehle CP. Can Contrast-Enhanced Multi-Detector Computed Tomography Replace Transesophageal Echocardiography for the Detection of Thrombogenic Milieu and Thrombi in the Left Atrial Appendage: A Prospective Study with 124 Patients. Rofo. 2016;188:45–52. doi: 10.1055/s-0041-106067. [DOI] [PubMed] [Google Scholar]
- 15.Zou H, Zhang Y, Tong J, Liu Z. Multidetector computed tomography for detecting left atrial/left atrial appendage thrombus: a meta-analysis. Intern Med J. 2015;45:1044–1053. doi: 10.1111/imj.12862. [DOI] [PubMed] [Google Scholar]
- 16.Bilchick KC, Mealor A, Gonzalez J, Norton P, Zhuo D, Mason P, Ferguson JD, et al. Effectiveness of integrating delayed computed tomography angiography imaging for left atrial appendage thrombus exclusion into the care of patients undergoing ablation of atrial fibrillation. Heart Rhythm. 2016;13:12–19. doi: 10.1016/j.hrthm.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calkins H, Nordaby M. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N Engl J Med. 2017;377:495–496. doi: 10.1056/NEJMc1707247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


