Abstract
Purpose
Heat shock factor 1 (HSF1) is a key regulator of the heat shock response and plays an important role in various cancers. However, the role of HSF1 in gastric cancer is still unknown. The present study evaluated the function of HSF1 and related mechanisms in gastric cancer.
Materials and Methods
The expression levels of HSF1 in normal and gastric cancer tissues were compared using cDNA microarray data from the NCBI Gene Expression Omnibus (GEO) dataset. The proliferation of gastric cancer cells was analyzed using the WST assay. Transwell migration and invasion assays were used to evaluate the migration and invasion abilities of gastric cancer cells. Protein levels of HSF1 were analyzed using immunohistochemical staining of tissue microarrays from patients with gastric cancer.
Results
HSF1 expression was significantly higher in gastric cancer tissue than in normal tissue. Knockdown of HSF1 reduced the proliferation, migration, and invasion of gastric cancer cells, while HSF1 overexpression promoted proliferation, migration, and invasion of gastric cancer cells. Furthermore, HSF1 promoted the proliferation of gastric cancer cells in vivo. In Kaplan-Meier analysis, high levels of HSF1 were associated with poor prognosis for patients with gastric cancer (p=0.028).
Conclusion
HSF1 may be closely associated with the proliferation and motility of gastric cancer cells and poor prognosis of patients with gastric cancer. Accordingly, HSF1 could serve as a prognostic marker for gastric cancer.
Keywords: Heat shock factor 1 (HSF1), gastric cancer, prognostic marker
INTRODUCTION
Worldwide, gastric cancer incidence ranks second among men and third among women, and the cancer is the second leading cause of cancer-related deaths.1,2,3 Despite greater understanding of genetic and epigenetic various cancer events, the absence of biomarkers for identification of gastric cancer has remained a problem.4 Also, among various anti-cancer therapies for gastric cancer, chemotherapy, which is the most common therapy, shows limited efficacy.5 Therefore, identifying prognosis marker and targeted therapy for gastric cancer may improve the survival of advanced gastric cancer patients.6 Although many studies have suggested the involvement of various molecular signaling pathways in gastric tumorigenesis and related prognostic markers or therapeutic targets, the actual molecular mechanisms thereof have not been fully elucidated.7,8 Therefore, reliable and feasible prognostic markers or therapeutic targets for gastric cancer remain an unmet need.
Heat shock factor 1 (HSF1) is a key transcription factor that initiates the expression of heat shock proteins (HSPs) by binding to consensus motifs, such as heat shock elements and other genes, in response to cellular stress, thereby allowing the cell to adapt and prolong survival. HSPs, such as HSP27, HSP70, and HSP90, are important chaperone proteins that promote proper folding, transportation, and degradation of proteins within cells.9,10,11,12 HSF1 activation is repressed by interaction with overexpressed HSP in non-tumor cells.9,10,11,12,13 However, this feedback inhibition may be ineffective in tumor cells. Several studies have linked increased HSF1 activity to malignant cell growth.9 This pro-malignant activity is induced upon the binding of HSF1 to the promoters and the subsequent initiation of the expression of certain genes independent of heat shock.11,14 Several studies have demonstrated that HSF1 is overexpressed in solid tumor cancers, including esophageal squamous cell, breast, hepatocellular, osteosarcoma, non-small cell lung, and pancreatic cancer.15,16,17,18 Furthermore, evidence implies that the elevated expression of HSF1 is correlated with poor survival in patients with cancer.18 We prepared HSF1-expressing gastric cancer cells and studied their proliferation and motility and evaluated HSF1 expression. The same experiments were extended in tissues from patients with gastric cancer to determine the clinical significance of HSF1 expression in gastric cancer.
MATERIALS AND METHODS
Cell culture
The human gastric cancer cell lines AGS and MKN28 were obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) at 37℃ in a humidified incubator with a 5% CO2 atmosphere.
Cell proliferation assay
AGS and MKN28 cells were plated in 96-well plates (3×103 cells/well). After incubation for 24 hours, the cells were transfected with siRNA (scRNA or HSF1 siRNA) and vector plasmid (pcDNA_EV or pcDNA_HSF1). WST-1 solution (Daeil Lab Services Co., Ltd, Seoul, Korea) was added to each well 48 hours after transfection. The plates were incubated for another 1–2 hours and gently shaken. Absorbance was then measured at a wavelength of 450 nm.
Transwell migration and invasion assays
AGS and MKN28 cells were transfected with siRNA (scRNA or HSF1 siRNA) and vector plasmid (pcDNA_EV or pcDNA_HSF1). After 24-hour transfection, 1×104 cells from each well were isolated and added to the upper transwell chamber (Corning Costar, Tewksbury, MA, USA) that carried a filter coated with 0.5 mg/mL of collagen type I (BD Biosciences, Seoul, Korea) for the migration assay or a filter coated with Matrigel (1:15) (BD Biosciences) for the invasion assay. RPMI-1640 containing 10% FBS and 1% antibiotics was added to the lower chamber, and the plates were incubated for 20 hours. Cells that migrated and invaded were quantified after hematoxylin and eosin staining, as previously described.18 For quantification, cells were counted in five randomly selected areas of each well using wide-field microscopy. Data are expressed as mean±SEM from three independent experiments.
Transfection of siRNA and HSF1 construction
Transfection of human HSF1 siRNAs or scRNA was performed using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's protocol. The coding strands of HSF1 siRNAs purchased from Genolution Inc. (Seoul, Korea) were as follows: 1) 5′-GAACGACAGUGGCUCAGCAUU-3′ and 2) 5′-CCACUUGGAUGCUAUGGACUU-3′. Human HSF1 was cloned into the pcDNA-3.0-Flag plasmid between EcoR1 and Xho1 restriction enzyme sites to obtain the pcDNA_HSF1 construct. The full-length HSF1 cDNA was amplified with polymerase chain reaction (PCR) using the primers 5′-GAATTCATGGATCTGCCCGTGGGCCC-3′ (sense) and 5′-CTCGAGCTAGGAGACAGTGGGGTCCT-3′ (antisense). The clone for HSF1 cDNA was provided by the Korea Human Gene Bank (Medical Genomics Research Center, KRIBB, Daejeon, Korea).
RNA isolation and reverse transcription-polymerase chain reaction
Total RNA was extracted from human gastric cancer cells and tissues obtained from patients using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. Reverse transcription (RT) was carried out using a RT system (Toyobo, Osaka, Japan), and PCR was performed using Ex-Taq DNA Polymerase (Takara Bio Inc, Shiga, Japan). The primer sequences were as follows: HSF1, 5′-GACATAAAGATCCGCCAGGA-3′ (sense) and 5′-CTGCACCAGTGAGATCAGGA-3′ (antisense); β-actin:5′-AGCCTCGCCTTTGCCGA-3′ (sense) and 5′-CTGGTGCCTGGGGCG-3′ (antisense).
Western blot analysis
Western blot analysis was carried out as described previously.19 Briefly, AGS and MKN28 cells were lysed by radioimmunoprecipitation assay buffer (Biosesang Inc, Seongnam, Korea) containing a protease inhibitor cocktail and phosphatase inhibitor cocktail (GeneDEPOT, Barker, TX, USA), followed by sonication on ice. A total of 20 µg of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane (Merck, Kenilworth, NJ, USA). After blocking with 5% skim milk for 1 hour, the membrane was incubated overnight with anti-HSF1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibody in 5% bovine serum albumin at 4℃. Anti-β-actin (Santa Cruz Biotechnology) was used as a control. The membrane was then incubated with a secondary antibody (goat-anti-rabbit IgG) for 90 min, followed by detection of protein bands with an enhanced chemiluminescence kit (Bio-Rad, Hercules, CA, USA) using Supernova-Q1800.
Human gastric cancer tissue microarray analyses
For immunohistochemical (IHC) analysis, core tissue biopsy specimens (2-mm diameter) were obtained from individual paraffin-embedded gastric carcinomas (donor blocks) and arranged in new recipient paraffin blocks (tissue array blocks) using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea). IHC analysis of HSF1 was performed as described previously.20 Immunohistochemistry of HSF1 revealed nuclear positivity and no cytoplasmic reaction in either normal mucosa cell or gastric cancer cells. The intensity of HSF1 staining was initially scored from 0 to 3 as follows: 0, no nuclear staining; 1, weak; 2, moderate; and 3, strong nuclear positivity. No gastric cancer samples were negative for HSF1 immunohistochemistry. Then, gastric cancer samples were grouped into those with staining intensities of 1–2 (low expression) and 3 (high expression) for statistical correlation and survival rate calculation.
Gene expression profiles using cDNA microarray data and analysis of public dataset
GSE13861, GSE13195, and GSE30727, as available datasets, were downloaded from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/). GSE13861 was developed from 65 primary gastric adenocarcinomas, six gastrointestinal stromal tumors, and 19 surrounding normal fresh frozen tissues. GSE13195 and GSE30727 were developed, respectively, from 25 pairs and 30 matching pairs of gastric cancer and normal surrounding tissue. These three datasets were normalized using GEO2R, and a scatter plot for the expression pattern analysis was obtained.
Kaplan-Meier analysis of overall survival
Kaplan-Meier analysis of overall survival for patients with gastric cancer was performed using the online resource Kaplan-Meier Plotter, as a public database (http://kmplot.com/analysis).21 To investigate the prognostic value of HSF1, the patient cohorts were divided into two groups according to their median (or upper/lower quartile) expression of HSF1, and the database was analyzed by a PostgreSQL server (https://www.postgresql.org/about/servers/). The two patient cohorts were compared by a Kaplan-Meier survival plot, and hazard ratios with 95% confidence intervals and log rank p values were calculated.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). The data were analyzed by Student's t-test and one-way ANOVA, unless otherwise specified. Differences in patient survival were determined using the Kaplan-Meier method. Results are presented as a mean±SEM. Values of p<0.05 were considered statistically significant.
RESULTS
Analysis of HSF1 expression and survival rate using cDNA microarray data in gastric cancer patients
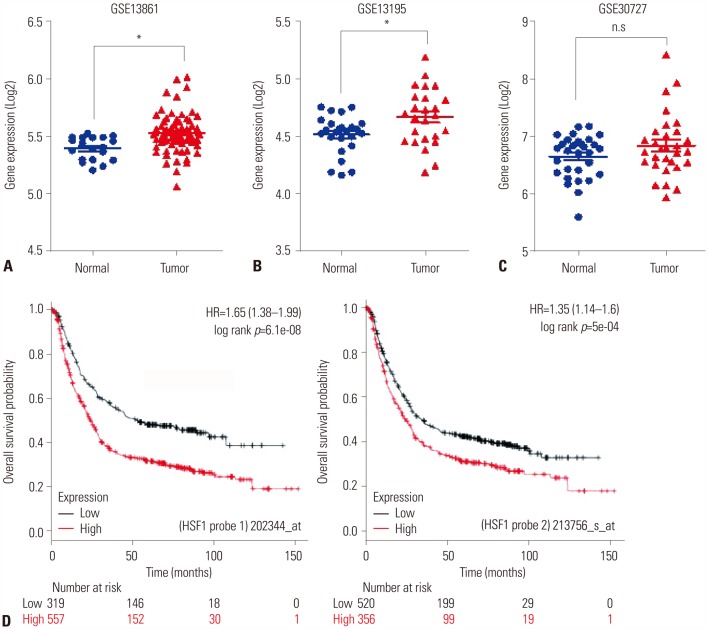
First, we aimed to identify relationships between high expression of HSF1 and poor survival in gastric cancer using the GEO database (Fig. 1A–C) and Kaplan-Meier analysis for gastric cancer patients (Fig. 1D). We collected three (GSE13861, GSE13195, and GSE30727) public datasets of patients with gastric cancer and compared the expression levels of HSF1 in tumor and normal gastric tissues (Fig. 1A–C). In all three datasets, the expression of HSF1 was higher in tumor tissue than in normal tissue. Expression was found to be statistically significant in two of the datasets (Fig. 1A and B; p<0.001). Fig. 1C depicts a trend in higher expression of HSF1 in tumor tissues than in normal tissues (p=0.061). Furthermore, statistical analysis of tumor tissue from the public database of patients with gastric cancer revealed a correlation between high expression of HSF1 and a significantly lower probability of survival (Fig. 1D).
Fig. 1. Evaluation of the correlation between HSF1 expression and survival rate of patients with gastric cancer using public databases. (A–C) Expression levels of HSF1 mRNA in tumor and normal tissues from the GEO database. Public datasets are presented as a scatter diagram: (A) GSE13861, (B) GSE13195, and (C) GSE30727. p values were calculated using Student's t-test (A and B: *p<0.001; C, p=0.061). (D) Kaplan-Meier survival plots demonstrate the poor prognostic effect of HSF1 upregulation, which was correlated with worse overall survival, in patients with gastric cancer (probe 1: 202344_at, n=876; probe 2: 213756_s_at, n=876). HSF1, heat shock factor 1; HR, hazard ratio.
HSF1 protein expression upregulated in patients with gastric cancer and correlated with poor survival
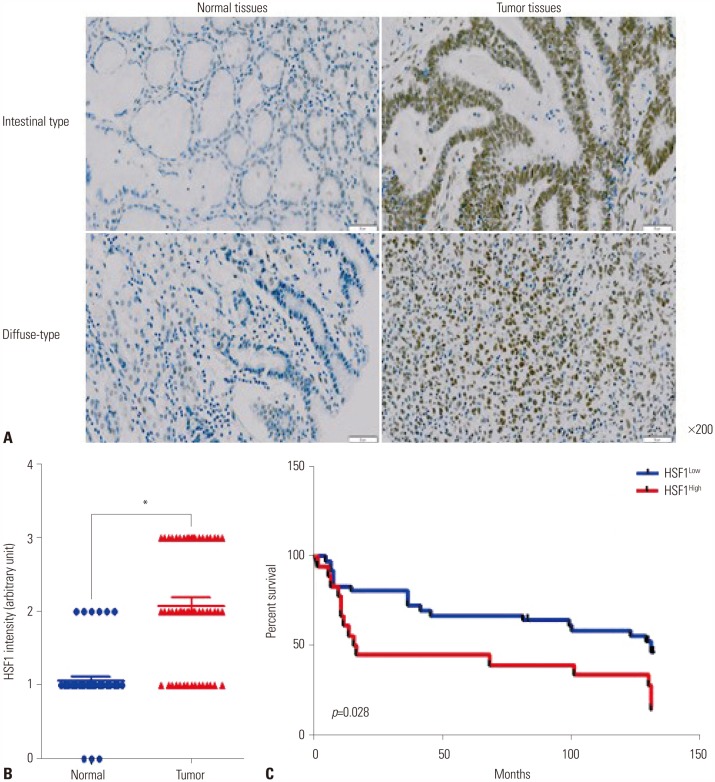
To determine HSF1 protein expression levels in patients with gastric cancer, a tissue microarray (TMA) of 54 patients was prepared and IHC analysis was performed (Table 1 and Fig. 2A). High expression of HSF1 was detected in tumor tissues from the patients with intestinal and diffuse types of gastric cancer, and most HSF1 expression was localized in the nucleus. HSF1 expression was notably upregulated in tumor tissues from patients with gastric cancer (Fig. 2A). Quantitative analysis of HSF1 staining confirmed that patients with gastric cancer exhibited higher expression of HSF1 in tumor tissues than in normal tissues (Fig. 2B). As shown in Table 1, the expression levels of HSF1 were analyzed with respect to clinical factors. Based on the expression level of HSF1, gastric cancer tissues were further classified as high (score 3) and low (score 1 and 2) groups. TMA results confirmed the absence of any significant correlation between HSF1 expression and clinicopathological findings (Table 1). However, the expression of HSF1 was significantly higher in tumor tissue than in the paired normal tissue in patients with gastric cancer (Fig. 2B). The probability of survival was significantly lower for patients with gastric cancer exhibiting high HSF1 expression levels in tumor tissues (Fig. 2C). These data strongly indicate that HSF1 expression is upregulated during the progression of gastric cancer.
Table 1. Correlation between HSF1 Expression and Clinicopathological Parameters in Gastric Cancer.
| Characteristic | HSF1 in tumor tissues | ||
|---|---|---|---|
| Low (n=35) | High (n=19) | p value* | |
| Sex | 0.533 | ||
| Male | 26 | 12 | |
| Female | 9 | 7 | |
| Age (yr) | 1 | ||
| <60 | 16 | 8 | |
| ≥60 | 19 | 11 | |
| Differentiation | 0.935 | ||
| Well | 15 | 7 | |
| Moderately | 9 | 5 | |
| Poorly | 11 | 7 | |
| LN metastasis | 0.134 | ||
| Positive | 19 | 15 | |
| Negative | 16 | 4 | |
| Lauren's classification | 0.766 | ||
| Intestinal | 24 | 12 | |
| Diffuse | 11 | 7 | |
| T classification | 0.327 | ||
| T1 | 12 | 4 | |
| T2 | 5 | 1 | |
| T3 | 11 | 11 | |
| T4 | 7 | 3 | |
| TNM stage | 0.095 | ||
| I | 14 | 3 | |
| II | 8 | 5 | |
| III | 13 | 9 | |
| V | 0 | 2 | |
*Fisher's exact test was used to analyse the significance of the associations between Tumor groups and each characterisitcs.
HSF1, heat shock factor 1; LN, lymph node.
HSF1 high-expression (scored 3) and low-expression (scored 1 and 2) groups.
Fig. 2. High expression of HSF1 associated with poor survival in patients with gastric cancer. (A) In situ expression of HSF1 in normal and tumor tissues from patients with intestinal and diffuse types of gastric cancer was detected by immunohistochemistry and hematoxylin and eosin staining (magnification: ×200). (B) Comparative analysis of HSF1 expression in normal and tumor tissues from patients with gastric cancer based on staining intensity. p values were calculated using Student's t-test and significant differences are indicated by * (*p<0.001). (C) Kaplan-Meier analysis of survival curves for patients with gastric cancer according to the expression of HSF1 in tumor tissues (n=54). HSF1 high-expression group (score 3) had a much lower survival rate at 125 months than the HSF1 low-expression group (score 1 or 2). HSF1, heat shock factor 1.
Knockdown of HSF1 reduces the proliferation, migration, and invasion of gastric cancer cells
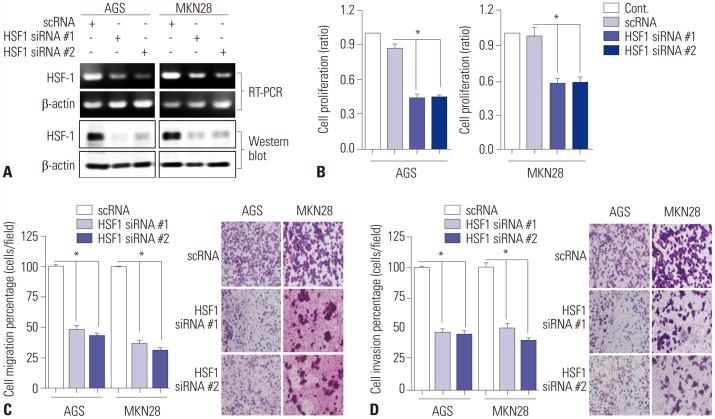
We have previously confirmed the overexpression of HSF1 protein in gastric cancer cell lines.20 We examined the effects of loss- and gain-of-function of HSF1 in AGS and MKN28 cells by evaluating the mRNA and protein expressions of HSF1 (Fig. 3A). The mRNA and protein expressions of HSF1 were knocked down with two specific siRNAs, and the efficiency of interference was confirmed by RT-PCR and Western blot analysis (Fig. 3A). After the knockdown of HSF1, cell proliferation was analyzed by WST assay. As a result, we found that the knockdown of HSF1 expression significantly reduced the proliferation of gastric cancer cells (Fig. 3B). Transwell migration and invasion assays showed that HSF1 knockdown inhibited the migration and invasion of gastric cancer cells (Fig. 3C and D). These results indicate that HSF1 induces the progression of gastric cancer.
Fig. 3. Downregulation of HSF1 expression reduces proliferation, migration, and invasion of gastric cancer cells. AGS and MKN28 cells were transfected with a scrambled siRNA (scRNA) or two small-interfering RNAs (siRNAs) specific for HSF1 (siRNA #1 and #2). (A) HSF1 protein expression was detected by Western blot analysis. β-actin was used as a loading control. (B) WST assay was performed to detect cell viability. (C and D) Transwell assays to evaluate (C) migration and (D) invasion of cells (×200). The histogram is represented as mean±SEM (n=3). p values were calculated using ANOVA and statistically significant differences are indicated as * (*p<0.001). HSF1, heat shock factor 1.
Overexpression of HSF1 induces proliferation, migration, and invasion of gastric cancer cells
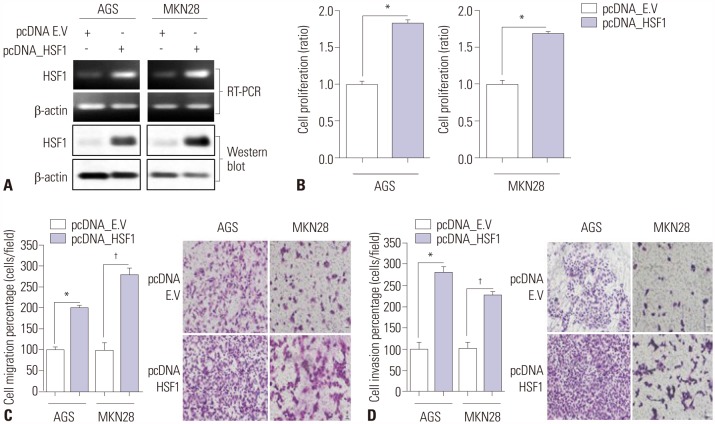
The effects of the gain-of-function of HSF1 in AGS and MKN28 gastric cancer cells were examined. The cells were transiently transfected with a HSF1 overexpression vector or empty vector for 48 hours. Overexpression of HSF1 was confirmed by RT-PCR and Western blot analysis (Fig. 4A). Overexpression of HSF1 significantly increased gastric cancer cell proliferation (Fig. 4B), migration (Fig. 4C), and invasion (Fig. 4D). These findings suggest that HSF1 participates in the progression of gastric cancer.
Fig. 4. Overexpression of HSF1 promotes proliferation, migration, and invasion of gastric cancer cells. Overexpression of HSF1 in AGS and MKN28 cells was achieved by transfection with an empty vector (pcDNA_EV) or HSF1 overexpression vector (pcDNA_HSF1). (A) Expressions of HSF1 mRNA and protein were detected by Western blot analysis. β-actin was used as a loading control. (B) WST assay was performed to detect cell viability. (C and D) Transwell migration assays to evaluate (C) migration and (D) invasion activity of cells (×200). Data are represented as mean±SEM (n=3). p values were calculated using Student's t-test (*p<0.001, †p=0.001). HSF1, heat shock factor 1.
DISCUSSION
Our study demonstrated that the silencing of HSF1 expression results in a reduction in the proliferation, migration, and invasion of gastric cancer cells, while HSF1 overexpression elicits the opposite effects on gastric cancer cells. Furthermore, the high expression of HSF1 was found to be correlated with poor prognosis in patients with gastric cancer.
HSF1 regulates the expression of HSPs by binding to consensus motifs, such the heat shock elements. HSPs are highly conserved stress proteins that are expressed and induced in response to heat shock or other stress factors or unfavorable conditions, such as inflammation, ischemia, infection, or carcinogenesis.12,22,23 In gastric cancer, HSP70 has been reported as a high-risk factor, and HSP90 inhibitor has been used for therapeutic purposes.24,25 We previously reported that HSF1 regulates neogenin-1 expression to promote motility of gastric cancer cells by binding with galectin-3.20 These studies show that HSP and HSF1 contribute to the tumorigenesis or progression of gastric cancer. However, the role of HSF1 in gastric cancer has remained unclear.
The protein HSF1 was recently shown to play a significant role in the development and progression of a variety of cancers.9 In particular, HSF1 was described as playing an essential role in the development of lymphoma in p53-deficient mouse and carcinomas in a Ras tumor model.26,27 In hepatocellular carcinoma (HCC), HSF1 has been found to directly activate miR-135b expression and to promote HCC cell motility.28 Furthermore, IHC analysis has revealed HSF1 as a prognostic marker for breast cancer.15,29 High level expression of HSF1 was also reported in cervical, colon, lung, pancreatic, osteosarcoma, and prostate cancers.16,18,30,31 These studies suggest that HSF1 may serve as a key regulator of tumorigenesis in a variety of cancers, including breast cancer.
Using three sets of GEO datasets, our study confirmed increased levels of HSF1 mRNA expression in tumor tissues from patients with gastric cancer. Furthermore, the high level of HSF1 expression was correlated with poor overall survival. We confirmed the protein expression level of HSF1 in 54 tumor samples from patients with gastric cancer and found that the expression level of HSF1 protein was consistent with the expression levels of HSF1 mRNA.
Although we observed no significant correlation between HSF1 expression and clinicopathological findings, HSF1 expression was increased in tumor tissues and was correlated with poor overall survival. To determine whether HSF1 expression is important for tumor growth and cell motility, we performed loss-of-function and gain-of-function analyses in gastric cancer cells. Downregulation of HSF1 significantly delayed the growth and motility of gastric cancer cells, while the overexpression of HSF1 increased cell growth and motility. Further studies evaluating the role of HSF1 in gastric cancer using large scale clinical data are warranted.
In conclusion, the present study demonstrated increases in the expression levels of HSF1 in gastric cancer tissues, compared to paired normal tissue. We deemed that HSF1 promotes the proliferation, migration, and invasion of gastric cancer cells and confirmed that HSF1 expression is correlated with poor prognosis in patients with gastric cancer. Thus, our study documents the essential role of HSF1 in gastric cancer progression, suggesting its potential role as a prognostic maker for gastric cancer.
ACKNOWLEDGEMENTS
This work was supported by The International Research & Development Program of the NRF funded by the Ministry of Education, Science and Technology (MEST) of Korea (NRF-2016K1A3A1A47921595) and research fund from Chosun University, 2017.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–4490. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: current insights and future perspectives. World J Gastroenterol. 2018;24:3313–3329. doi: 10.3748/wjg.v24.i30.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji X, Yan Y, Bu ZD, Li ZY, Wu AW, Zhang LH, et al. The optimal extent of gastrectomy for middle-third gastric cancer: distal subtotal gastrectomy is superior to total gastrectomy in short-term effect without sacrificing long-term survival. BMC Cancer. 2017;17:345. doi: 10.1186/s12885-017-3343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan DD, Zhu ZX, Zhang X, Liu J. Targeted therapy for gastric cancer: current status and future directions (Review) Oncol Rep. 2016;35:1245–1254. doi: 10.3892/or.2015.4528. [DOI] [PubMed] [Google Scholar]
- 7.Kanat O, O'Neil B, Shahda S. Targeted therapy for advanced gastric cancer: a review of current status and future prospects. World J Gastrointest Oncol. 2015;7:401–410. doi: 10.4251/wjgo.v7.i12.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y, Wu Z, Guo W, Li J. Gene mutations in gastric cancer: a review of recent next-generation sequencing studies. Tumour Biol. 2015;36:7385–7394. doi: 10.1007/s13277-015-4002-1. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK. HSF1, a versatile factor in tumorogenesis. Curr Mol Med. 2012;12:1102–1107. doi: 10.2174/156652412803306675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002;205(Pt 5):677–685. doi: 10.1242/jeb.205.5.677. [DOI] [PubMed] [Google Scholar]
- 11.Neueder A, Gipson TA, Batterton S, Lazell HJ, Farshim PP, Paganetti P, et al. HSF1-dependent and -independent regulation of the mammalian in vivo heat shock response and its impairment in Huntington's disease mouse models. Sci Rep. 2017;7:12556. doi: 10.1038/s41598-017-12897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem. 2002;86:376–393. doi: 10.1002/jcb.10232. [DOI] [PubMed] [Google Scholar]
- 13.Gómez AV, Córdova G, Munita R, Parada GE, Barrios ÁP, Cancino GI, et al. Characterizing HSF1 binding and post-translational modifications of hsp70 promoter in cultured cortical neurons: implications in the heat-shock response. PLoS One. 2015;10:e0129329. doi: 10.1371/journal.pone.0129329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma P, Pfister JA, Mallick S, D'Mello SR. HSF1 protects neurons through a novel trimerization- and HSP-independent mechanism. J Neurosci. 2014;34:1599–1612. doi: 10.1523/JNEUROSCI.3039-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang MJ, Yun HH, Lee JH. KRIBB11 accelerates Mcl-1 degradation through an HSF1-independent, Mule-dependent pathway in A549 non-small cell lung cancer cells. Biochem Biophys Res Commun. 2017;492:304–309. doi: 10.1016/j.bbrc.2017.08.118. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Qian W, Li J, Jiang Z, Cheng L, Yan B, et al. Loss of AMPK activation promotes the invasion and metastasis of pancreatic cancer through an HSF1-dependent pathway. Mol Oncol. 2017;11:1475–1492. doi: 10.1002/1878-0261.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan T, Shao J, Hu B, Liu G, Luo P, Zhou Y. Prognostic role of HSF1 overexpression in solid tumors: a pooled analysis of 3,159 patients. Onco Targets Ther. 2018;11:383–393. doi: 10.2147/OTT.S153682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La SH, Kim SJ, Kang HG, Lee HW, Chun KH. Ablation of human telomerase reverse transcriptase (hTERT) induces cellular senescence in gastric cancer through a galectin-3 dependent mechanism. Oncotarget. 2016;7:57117–57130. doi: 10.18632/oncotarget.10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Wang YG, Lee HW, Kang HG, La SH, Choi IJ, et al. Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget. 2014;5:3386–3398. doi: 10.18632/oncotarget.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szász AM, Lánczky A, Nagy Á, Förster S, Hark K, Green JE, et al. Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget. 2016;7:49322–49333. doi: 10.18632/oncotarget.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 23.Dai C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: from proteomic stability to oncogenesis. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160525. doi: 10.1098/rstb.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang RE. Targeting heat shock proteins 70/90 and proteasome for cancer therapy. Curr Med Chem. 2011;18:4250–4264. doi: 10.2174/092986711797189574. [DOI] [PubMed] [Google Scholar]
- 25.Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332:275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Feldman RA, Radhakrishnan VM, Carey S, Martinez JD. Hsf1 is required for the nuclear translocation of p53 tumor suppressor. Neoplasia. 2008;10:1138–1145. doi: 10.1593/neo.08430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma A, Meena AS, Bhat MK. Hyperthermia-associated carboplatin resistance: differential role of p53, HSF1 and Hsp70 in hepatoma cells. Cancer Sci. 2010;101:1186–1193. doi: 10.1111/j.1349-7006.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cigliano A, Wang C, Pilo MG, Szydlowska M, Brozzetti S, Latte G, et al. Inhibition of HSF1 suppresses the growth of hepatocarcinoma cell lines in vitro and AKT-driven hepatocarcinogenesis in mice. Oncotarget. 2017;8:54149–54159. doi: 10.18632/oncotarget.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gökmen-Polar Y, Badve S. Upregulation of HSF1 in estrogen receptor positive breast cancer. Oncotarget. 2016;7:84239–84245. doi: 10.18632/oncotarget.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi A, Ciafrè S, Balsamo M, Pierimarchi P, Santoro MG. Targeting the heat shock factor 1 by RNA interference: a potent tool to enhance hyperthermochemotherapy efficacy in cervical cancer. Cancer Res. 2006;66:7678–7685. doi: 10.1158/0008-5472.CAN-05-4282. [DOI] [PubMed] [Google Scholar]
- 31.Cen H, Zheng S, Fang YM, Tang XP, Dong Q. Induction of HSF1 expression is associated with sporadic colorectal cancer. World J Gastroenterol. 2004;10:3122–3126. doi: 10.3748/wjg.v10.i21.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]