Abstract
Purpose
Previous reports have shown that hyperglycemia-induced inhibition of transient receptor potential vanilloid sub type 4 (TRPV4), a transient receptor potential ion channel, affects the severity of hearing impairment (HI). In this study, we explored the role of TRPV4 in HI using HEI-OC1 cells exposed to high glucose (HG).
Materials and Methods
HEI-OC1 cells were cultured in a HG environment (25 mM D-glucose) for 48 hours, and qRT-PCR and Western blotting were used to analyze the expression of TRPV4 at the mRNA and protein level. TRPV4 agonist (GSK1016790A) or antagonist (HC-067047) in cultured HEI-OC1 cells was used to obtain abnormal TRPV4 expression. Functional TRPV4 activity was assessed in cultured HEI-OC1 cells using the MTT assay and a cell death detection ELISA.
Results
TRPV4 agonists exerted protective effects against HG-induced HI, as evidenced by increased MTT levels and inhibition of apoptosis in HEI-OC1 cells. TRPV4 overexpression significantly increased protein levels of phosphorylated p38 mitogen-activated protein kinase (p-p38 MAPK), while TRPV4 antagonists had the opposite effect. Our results indicated that TRPV4 is a hyperglycemia-related factor that can inhibit cell proliferation and promote cell apoptosis by activating the MAPK signaling pathway in HEI-OC1 cells.
Conclusion
Our results show that the overexpression of TRPV4 can attenuate cell death in HEI-OC1 cells exposed to HG.
Keywords: TRPV4, high glucose, hearing impairment, HEI-OC1 cells
INTRODUCTION
Hearing impairment (HI) is one of the most highly prevalent chronic diseases1 and represents a huge burden on society and on individuals in terms of reduced emotional, social, mental, and physical health.2,3,4 HI has many causes, the most common of which is presbycusis, followed by noise exposure, ototoxic drugs, and viral infections.5,6 In addition to these factors, accumulating evidence suggests that HI is yet another disabling complication of diabetes.7 We believe that, as time goes on, hyperglycemia may impair cochlear microvascular circulation and affect the steady state of hearing cells, thereby reducing patient hearing.8
Hearing is a complex system that depends on various tissue and cell types in the inner ear. Damage to cochlear hair cells is the main reason for threshold elevation in hearing loss.9 Previous studies have shown that abnormalities of homeostasis and apoptosis of cochlear hair cells are important contributing factors leading to HI.10 The way that auditory cell apoptosis affects HI has been studied for a long time.11 However, the mechanisms of cochlear hair cell apoptosis during the progression of HI remain poorly understood.
As a member of the TRP super family, transient receptor potential vanilloid sub type 4 (TRPV4) is a receptor-activated nonselective cation channel that is endogenously expressed in many tissue and cell types, such as skin,12 cortical astrocytes,13 keratinocyte cell lines,14 and tracheal epithelial cells,15 including cochlear hair cells.16 Previous studies have found that TRPV4 is linked to the development of diabetes.17 In addition, multiple studies have found that damage to TRPV4 can cause hearing delay, which makes the cochlea vulnerable to noise injury. It has been suggested that TRPV4 is associated with hearing loss.18,19 TRPV4 expression was down-regulated in high glucose (HG)-cultured retinal microvascular endothelial cells20; however, the potential role of TRPV4 expression in HG-cultured cochlear hair cells has not been investigated. In this study, we sought to detect the expression of TRPV4 and discover its role in HG-mediated HI.
In mammals, mitogen-activated protein kinase (MAPK) signaling cascades regulate important cellular processes, including gene expression, cell proliferation, cell survival and death, and cell motility.21 Recently, research has shown that TRPV4 channels, as TRP cation channel subfamily V members, are involved in MAPK activation in dorsal root ganglion neuropathic pain.22 Research by Jie, et al.21 has shown that the TRPV4 gene contains two conserved MAPK-binding sites that confer responsiveness to calcineurin-MAPK signaling, thus making MAPK and its downstream signals promising targets for therapeutic intervention. However, the specific mechanism whereby MAPK signaling pathways affect HI has not been fully understood.
Therefore, in this study, our aim was to confirm the expression levels of TRPV4 in HG-induced cochlear hair cells. Our experimental results show that the ectopic expression of TRPV4 in cochlear hair cells inhibits cell proliferation and promotes cell apoptosis in vitro. In addition, we also demonstrate that the MAPK signaling pathway is a direct target of TRPV4 and show that TRPV4 activates the MAPK signaling pathway. These findings show that TRPV4 could have potential implications for therapeutic approaches to HI as a complication of diabetes.
MATERIALS AND METHODS
Cell culture and transfection
The cochlear auditory cell line HEI-OC1 was purchased from the House Ear Institute (Los Angeles, CA, USA). HEI-OC1 cells were cultured in DMEM medium containing 10% fetal bovine serum at 33℃ under 10% CO2 (permissive conditions). Glucose was supplemented in the culture medium to induce a diabetes cell model; an appropriate concentration of mannitol was supplemented in the control culture medium. After 72 hours of culture, cells were divided into three groups: a euglycemic control group (5 mM D-glucose), a hyperglycemia group (25 mM D-glucose), and an osmotic control group (5 mM D-glucose+20 mM mannitol). HEI-OC1 cells were transfected with pcDNA3.1 empty vector, pcDNA3.1-TRPV4 vector, TRPV4-siRNA, and scrambled siRNA control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Drug treatment
The TRPV4 antagonist HC-067047 and agonist GSK1016790A were purchased from Calbiochem (Merck, Darmstadt, Germany). Following pre-culture, HEI-OC1 cells were stimulated either with GSK1016790A (100 nM23; Sigma-Aldrich, St. Louis, MO, USA) or HC067047 (1 µM24; Maybridge Ltd., Cambridge, UK) for 24 hours. The p38 inhibitor SB203580 (10 µM; Tocris Bioscience, Bristol, UK) was given to the experimental groups at the recommended concentrations.25
RNA extraction and RT-PCR
Total RNA was isolated from HEI-OC1 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. For reverse transcription (RT), the oligonucleotide sequences of primers specific for TRPV4 were 5′-GATGGGCGAC CAAATCTGC-3′ and 5′-GAGGACTCATATAGGGTGGACTC-3′. Aliquots of the reaction mixture were used as templates under the following conditions: initial denaturation at 95℃ for 10 min, followed by 40 cycles of 95℃ for 15 s, 60℃ for 1 min, and 72℃ for 45 s. PCR experiments were performed in triplicate. As a control, β-actin expression was analyzed (forward, 5′-ACCGAGCGCG GCTACA-3′ and reverse, 5′-CAGCCGTGGCCATCTCTT-3′).
Western blot assays
Cultured cells were washed twice with PBS and lysed on ice using ice-cold RIPA buffer for 20 minutes. The proteins were then subjected to SDS-PAGE and transferred to a PVDF membrane. The PVDF membrane was blocked in a 5% ECL priming blocker for 1 hour and incubated overnight at 4℃ with primary antibody: rabbit anti-TRPV4 (1:800, Abcam, Cambridge, UK), rabbit anti-p38 polyclonal antibody (1:200, CST, Beverly, MA, USA), rabbit anti-Pp38 polyclonal antibody (1:1000, CST), and anti-β-actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing three times with TBST, the membrane was placed in 1:5000 diluted secondary antibody (sc-2054, Santa Cruz Biotechnology) and incubated for 1 hour at room temperature. The immunoreaction products were visualized using the chemiluminescence-emanating ChemiDocTM MP Imaging System (Bio-Rad, Hercules, CA, USA).
Cell proliferation assay
HEI-OC1 cells were cultured in a 96-well plate at a concentration of 2×105 cells/well. 20 µL of a 5 mg/mL MTT solution was added to each well, and the plate was incubated at 33℃ for 4 hours under 10% CO2 and 95% air. The supernatants were removed and 250 µL of DMSO added. An automatic microplate reader was used to read the absorbance of each well at 540 nm.
Apoptosis assay
To measure the influence of ectopic expression of TRPV4 protein on cell apoptosis, we using the cell death detection ELISA-Plus (Roche, Indianapolis, IN, USA) to analyze the percentage of apoptotic cells, according to the manufacturer's protocol. The assay system quantifies the amount of histonecoupled DNA fragments by measuring absorbance at 405 nm.
ELISA analysis
An ELISA was used to analyze p38 concentrations in the culture medium according to the manufacturer's protocol. Absorbance was measured at 450 nm using a 680XR Microplate reader (Bio-Rad). All of the samples were analyzed in duplicate.
Statistical analysis
All experiments were independently repeated at least three times. Data are presented as means±SEM. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was tested with a one-way ANOVA test or Student's t-test. p values <0.05 were considered to indicate statistically significant differences.
RESULTS
HGs reduce the expression of TRPV4 in HEI-OC1 cells
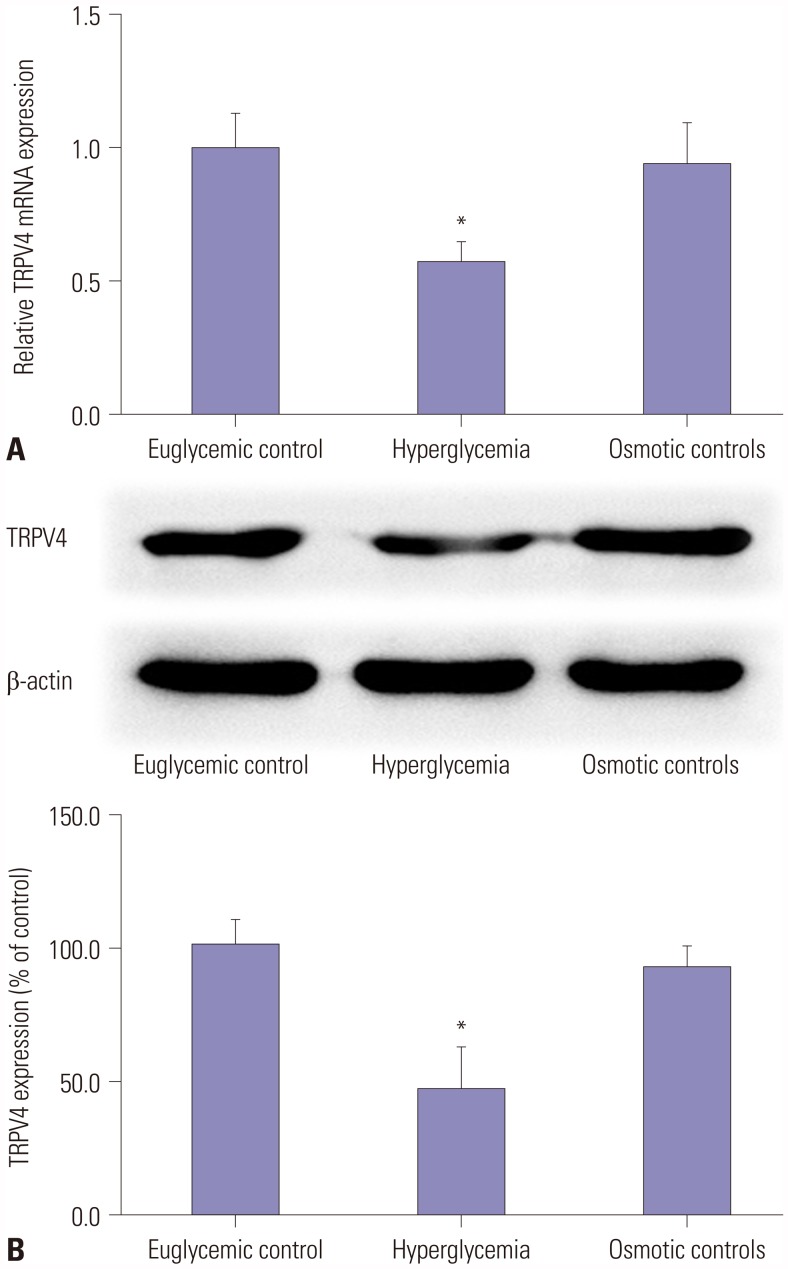
To investigate the role of TRPV4 in HEI-OC1 cells cultured in HG, qRT-PCR and Western blotting were used to detect the expression of TRPV4 at the mRNA level and the protein level, respectively. As shown in Fig. 1A, TRPV4 expression was significantly reduced in HEI-OC1 cells following culture in HG for 48 h, compared with the euglycemic and osmotic controls. Western blotting shows that the expression of the TRPV4 protein was decreased compared with the controls (Fig. 1B).
Fig. 1. HG reduces the expression of TRPV4 in HEI-OC1 cells. HEI-OC1 cells were cultured under euglycemic, hyperglycemia, and osmotic control conditions for 48 h, and qRT-PCR (A) and Western blot (B) were used to analyze the expression of TRPV4 mRNA and protein. Results are representative of three independent experiments. *Indicates differences from controls at p<0.05. TRPV4, transient receptor potential vanilloid sub type 4; HG, high glucose.
Expression of TRPV4 in agonist and inhibitor stimulated HEI-OC1 cells
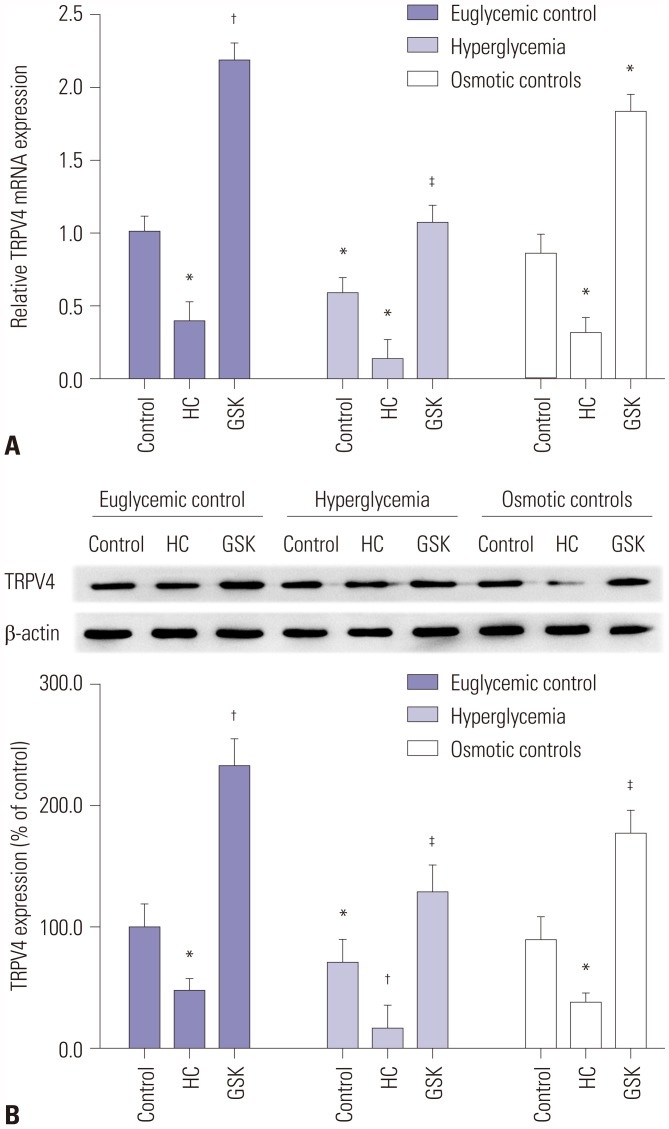
To investigate whether TRPV4 expression is affected by pharmacological agonists or inhibitors, HEI-OC1 cells were treated with 100 nM of a TRPV4 agonist or 1 µM of a TRPV4 antagonist. The control group was given normal saline in an equal quantity. After stimulation of HEI-OC1 cells with the TRPV4 antagonist HC-067047, mRNA expression was significantly decreased. Conversely, stimulation with the TRPV4 agonist GSK1016790A significantly promoted mRNA expression (Fig. 2A). Western blotting showed that the expression of TRPV4 protein was similarly affected compared with the controls (Fig. 2B). Moreover, our results suggested that TRPV4 expression significant decreased in HEI-OC1 cells treated with HG, compared with HEI-OC1 cells treated with TRPV4 agonist in the euglycemic condition. While the HG condition induced HEI-OC1 cell death, we believe that TRPV4 agonist may rescue HEI-OC1 cell death from HG status.
Fig. 2. The effect of reagents on the expression of TRPV4. (A) The relative expression of TRPV4 mRNA in high glucose cultured HEI-OC1 cells after treatment with HC-067047 (HC) or GSK1016790A (GSK), compared with the untreated control, was determined by qRT-PCR. (B) Western blotting was used to analyze the expression of TRPV4 protein in HEI-OC1 cells after treatment with HC-067047 or GSK1016790A. Data are representative of three experiments. *Indicates differences from euglycemic controls at p<0.05, †Indicates differences from euglycemic controls at p<0.01, ‡Indicates differences from untreated controls at p<0.05, respectively. TRPV4, transient receptor potential vanilloid sub type 4.
Effects of HG on cell proliferation and apoptosis in HEI-OC1 cells
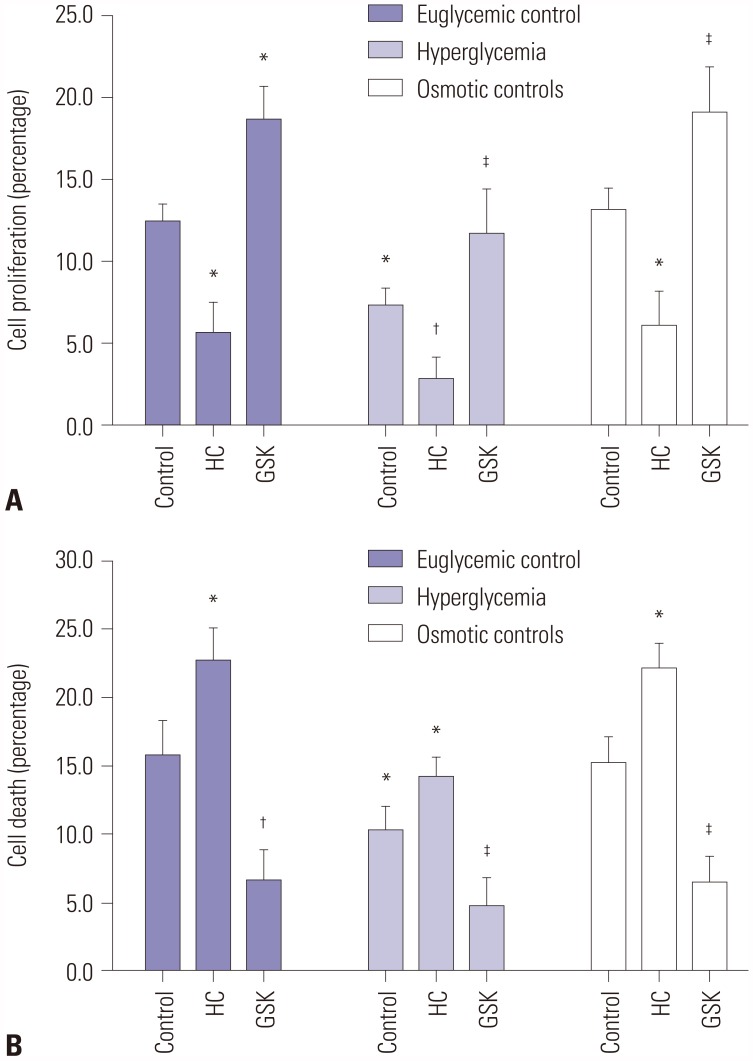
To identify the mechanisms of TRPV4 action on cellular behavior, we first examined the effects of HG on cell proliferation in HEI-OC1 cells. Cell proliferation was measured using the MTT-8 assay in HEI-OC1 cells. Overexpression of TRPV4 significantly increased cell proliferation following 48 h of exposure to HG. Conversely, in the HG condition, down-regulated TRPV4 inhibited cell proliferation in comparison with the control group (Fig. 3A). The cell death detection ELISA-Plus cell apoptosis assay showed that overexpression of TRPV4 inhibits cell apoptosis. Conversely, down-regulated TRPV4 promoted cell apoptosis in comparison with the corresponding control group (Fig. 3B). To further confirm the effects of TRPV4 on HEI-OC1 cell proliferation and apoptosis, TRPV4 overexpression and silence HEI-OC1 cell were constructed (Supplementary Fig. 1, only online). Moreover, we found that TRPV4 overexpression induced HEI-OC1 cell proliferation and impeded cell apoptosis, whereas TRPV4 depletion had the opposite effects (Supplementary Fig. 1, only online).
Fig. 3. TRPV4 directly affects proliferation and apoptosis in HEI-OC1 cells. (A) The effect of TRPV4 on cell proliferation of HEI-OC1 cells was detected using the MTT assay. (B) The effect of TRPV4 on cell apoptosis of HEI-OC1 cells was detected using the cell death detection ELISA-Plus apoptosis assay. Data are representative of three experiments. *indicates differences from euglycemic controls at p<0.05, †Indicates differences from euglycemic controls at p<0.01, ‡Indicates differences from untreated controls at p<0.05, respectively. TRPV4, transient receptor potential vanilloid sub type 4; HC, TRPV4 antagonist HC-067047; GSK, TRPV4 agonist GSK1016790A.
Overexpression of TRPV4 promotes activation of MAPK pathways
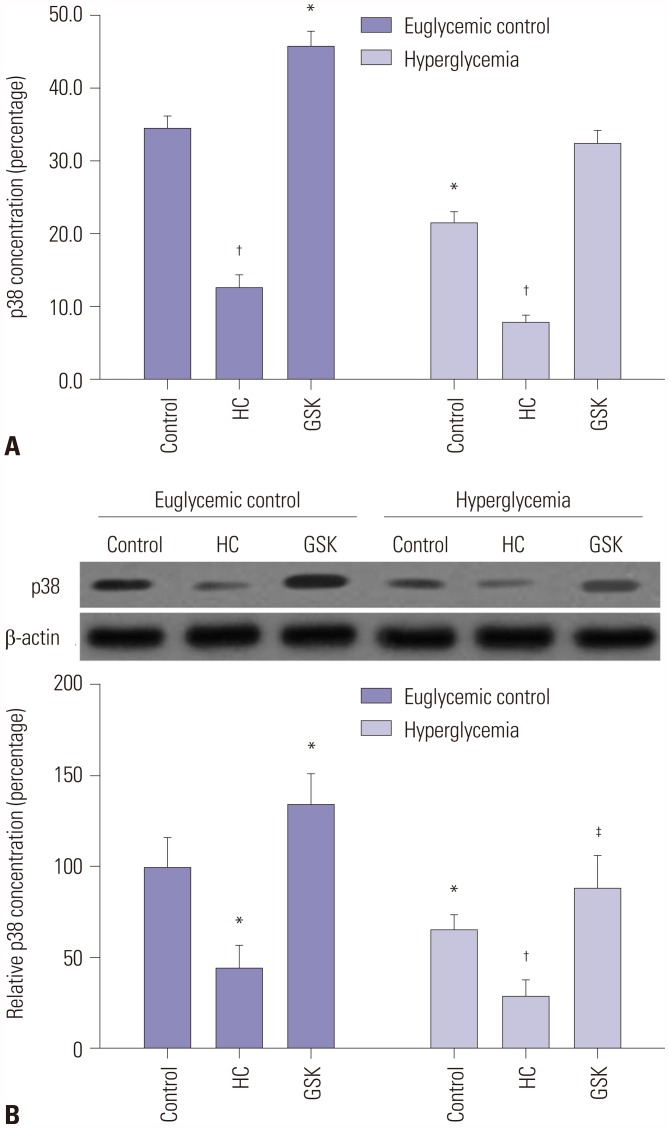
In order to verify the relationships between TRPV4 and MAPK pathways, we studied the effect of HG on the phosphorylation of p38 MAPK in HEI-OC1 cells. HEI-OC1 cells were incubated in a HG environment for 24 hours, and an anti-p-p38 polyclonal antibody was used to quantify the phosphorylated form of the p38 protein. The results showed that overexpression of TRPV4 significantly increased the concentrations of p-p38 in the HEI-OC1 cell culture medium (Fig. 4A). In addition, we also found that the expression of TRPV4 could positively regulate the protein expression of p-p38 (Fig. 4B). This result indicated that TRPV4 is involved in the activation of the MAPK pathway.
Fig. 4. Overexpression of TRPV4 triggered activation of the MAPK signaling pathway. (A) After pretreatment with the TRPV4 agonist GSK1016790A (GSK) or the TRPV4 antagonist HC067047 (HC), the p38 concentration in the culture medium of HEI-OC1 cells was assessed by ELISA. (B) The protein expression of p38 was analyzed by Western blotting. Data represent means±SD from three independent experiments. *Indicates differences from euglycemic controls at p<0.05, †Indicates differences from euglycemic controls at p<0.01, ‡Indicates differences from untreated controls at p<0.05, respectively. TRPV4, transient receptor potential vanilloid sub type 4; MSPK, mitogen-activated protein kinase.
TRPV4 modulates HEI-OC1 cell proliferation and apoptosis by targeting MAPK signaling pathways
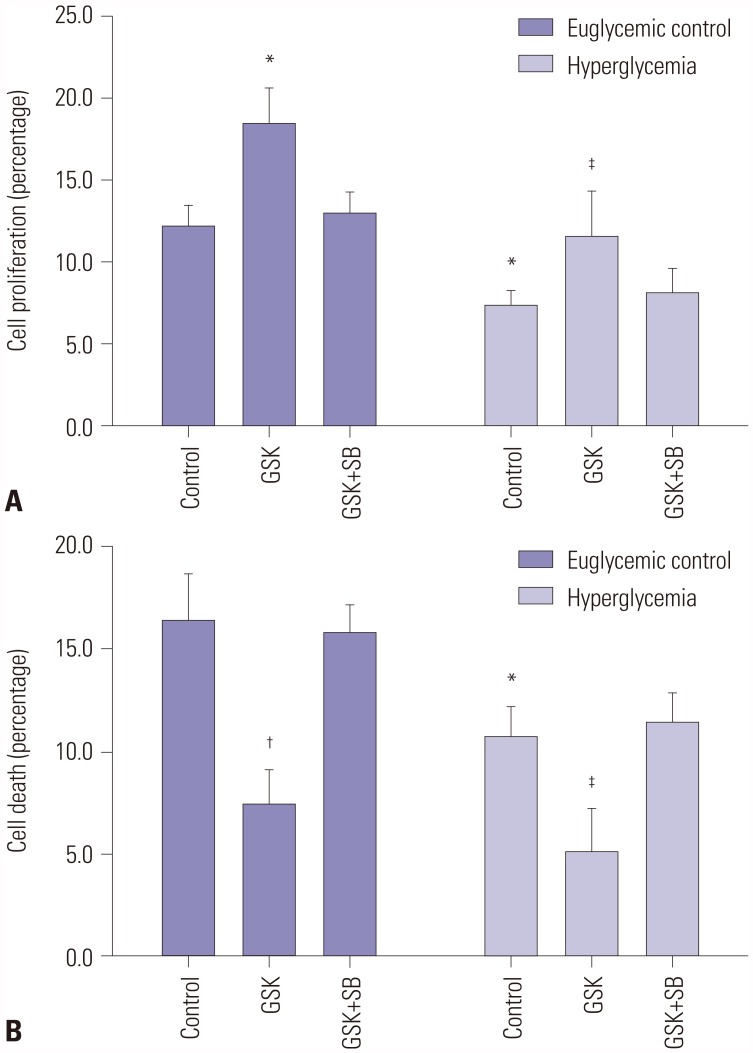
To further clarify the effect of MAPK on TRPV4-modulated HEI-OC1 cell growth and apoptosis, the MAPK signaling inhibitor SB203580 was used. After a series of functional restoration assays, we found that the decrease in HEI-OC1 cell proliferation (Fig. 5A) induced by TRPV4 up-regulation was dramatically attenuated after stimulation with SB203580. Moreover, disruption of MAPK signaling by SB203580 treatment also significantly increased the apoptosis rate (Fig. 5B). These results indicate that TRPV4 may promote cochlear hair cell proliferation and repress apoptosis by modulating MAPK signaling.
Fig. 5. TRPV4 regulates cochlear hair cell development by targeting MAPK signaling pathways. After pretreatment with the TRPV4 agonist GSK1016790A (GSK) or a combination of GSK1016790A and the p38 inhibitor SB203580 (GSK+SB), the effect of TRPV4 on cell proliferation of HEI-OC1 cells was detected using the MTT assay, and the effect of TRPV4 on cell apoptosis of HEI-OC1 cells was detected using the cell death detection ELISA-Plus apoptosis assay. (A) Inhibition of MAPK signaling rescued cell proliferation induced by TRPV4 in HEI-OC1 cells. (B) Disruption of MAPK signaling promoted cell apoptosis induced by TRPV4 in HEI-OC1 cells. Data are represented as means±SD from three independent experiments. *Indicates differences from euglycemic controls at p<0.05, †Indicates differences from euglycemic controls at p<0.01, ‡Indicates differences from untreated controls at p<0.05, respectively. TRPV4, transient receptor potential vanilloid sub type 4; MSPK, mitogenactivated protein kinase.
DISCUSSION
The calcium-permeable cation channel protein TRPV4 is known to function as a Ca2+-permeable TRP channel in cells and is involved in a variety of different physiological processes. TRPV4 has been reported to be expressed in the inner-ear hair cells.26 However, previous published reports on TRPV4 expression in hearing tissues/cells were discordant. TRPV4 family member expression has been reported in cochlear hair cells.27,28,29 A study by Zanini and Göpfert30 indicated that TRP family members are associated with hearing impairment; however, no TRPV4 transcripts were detected in HEI-OC1 cells. Therefore, in this study, the expression of TRPV4 in HEI-OC1 cells was investigated. Our results showed that TRPV4 was expressed in HEI-OC1 cells.
As an intrinsic constituent of receptor-operated cation entry, TRPV4 may participate in numerous physiological functions.20,31,32 Increasing evidence suggests that TRPV4 may be involved in the development of HG-induced HI.27 Therefore, in our study, we demonstrated that HG influences the expression of TRPV4 in HEI-OC1 cells, inducing a significant down-regulation thereof, consistent with the results of previous in vivo studies, which indicated significantly decreased expression of TRPV4 following diabetes.33,34 However, the exact functions and mechanisms of TRPV4 in cochlear hair cells are largely unknown. We found that the mRNA and protein levels of TRPV4 were much lower in HG-cultured HEI-OC1 cells, compared with controls. Our findings are consistent with previous reports, and allow us to speculate that the high-expression of TRPV4 may be conducive to improving the hearing of diabetic patients.
Studies have indicated that TRPV4 promotes activation of MAPK signaling. MAPK is an important mediator of signal transduction from the cell surface to the nucleus. Recently, many studies have confirmed that the MAPK signaling pathway plays a crucial role in cell development, including effects on proliferation and survival.11 The significance of MAPK and its therapeutic value as a target for hearing loss therapy have been investigated in several reports,35,36 in which MAPK status was found to be positively correlated with oxidative stress, cell injury, and inflammation.37,38 Our work broadens our understanding of the roles that TRPV4 plays in HEI-OC1, showing that it targets MAPK signaling pathways and that it can be a target for therapy for hearing damage caused by diabetes.
In conclusion, this study showed that TRPV4 expression is lower in a diabetic hearing cell model, consistent with previous studies. We also found that, in the diabetic cell model, overexpression of TRPV4 induces cell proliferation and inhibits apoptosis by activation of MAPK signaling pathways associated with cochlear hair cell growth. Thus, increased TRPV4 expression in diabetic patients may decrease cochlear hair cell amplification. Therefore, our study may provide a therapeutic strategy for improving HI in diabetic patients.
ACKNOWLEDGEMENTS
Financial support was provided by the National Natural Science Foundation of China (NSFC) (NO. 81670924) and the Science and Technology Development Fund Projects of the Forth Military Medical University (2016XC095).
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIAL
TRPV4 involved in HG-induced HEI-OC1 cell proliferation and apoptosis. (A) The relative expression of TRPV4 mRNA in HG cultured HEI-OC1 cells after transfection with pcDNA3.1 empty vector, pcDNA3.1-TRPV4 vector, TRPV4-siRNA, or scrambled siRNA control was determined by qRT-PCR. (B) Western blotting was used to analyze the expression of TRPV4 protein. (C) The effect of TRPV4 on cell proliferation of HEI-OC1 cells was detected using the MTT assay. (D) The effect of TRPV4 on cell apoptosis of HEI-OC1 cells was detected using the cell death detection ELISA-Plus apoptosis assay. Data are representative of three experiments. *Indicates differences from controls at p<0.05. TRPV4, transient receptor potential vanilloid sub type 4; HG, high glucose.
References
- 1.Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309:2322–2324. doi: 10.1001/jama.2013.5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia EM, Wang JJ, Rochtchina E, Cumming RR, Newall P, Mitchell P. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28:187–195. doi: 10.1097/AUD.0b013e31803126b6. [DOI] [PubMed] [Google Scholar]
- 3.Gopinath B, Wang JJ, Schneider J, Burlutsky G, Snowdon J, Mc-Mahon CM, et al. Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc. 2009;57:1306–1308. doi: 10.1111/j.1532-5415.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 4.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003;289:1976–1985. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]
- 6.Hong JW, Jeon JH, Ku CR, Noh JH, Yoo HJ, Kim DJ. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010–2012 Korea National Health and Nutrition Examination Survey (observational study) Medicine (Baltimore) 2015;94:e611. doi: 10.1097/MD.0000000000000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose K. Hearing loss and diabetes: you might not know what you're missing. Ann Intern Med. 2008;149:54–55. doi: 10.7326/0003-4819-149-1-200807010-00232. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132:934–938. doi: 10.1001/archotol.132.9.934. [DOI] [PubMed] [Google Scholar]
- 9.Liberman MC, Liberman LD, Maison SF. Chronic conductive hearing loss leads to cochlear degeneration. PLoS One. 2015;10:e0142341. doi: 10.1371/journal.pone.0142341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue T, Wei L, Zha DJ, Qiu JH, Chen FQ, Qiao L, et al. miR-29b overexpression induces cochlear hair cell apoptosis through the regulation of SIRT1/PGC-1α signaling: Implications for age-related hearing loss. Int J Mol Med. 2016;38:1387–1394. doi: 10.3892/ijmm.2016.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biol Reprod. 2010;83:807–817. doi: 10.1095/biolreprod.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, et al. Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium. 2008;44:374–385. doi: 10.1016/j.ceca.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, et al. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci. 2005;118(Pt 11):2435–2440. doi: 10.1242/jcs.02372. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A. 2008;105:12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Park C, Kim SJ, Kim HJ, Oh GS, Shen A, et al. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp Mol Med. 2013;45:e12. doi: 10.1038/emm.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, et al. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension. 2013;62:134–139. doi: 10.1161/HYPERTENSIONAHA.113.01500. [DOI] [PubMed] [Google Scholar]
- 18.Oonk AM, Ekker MS, Huygen PL, Kunst HP, Kremer H, Schelhaas JJ, et al. Intrafamilial variable hearing loss in TRPV4 induced spinal muscular atrophy. Ann Otol Rhinol Laryngol. 2014;123:859–865. doi: 10.1177/0003489414539130. [DOI] [PubMed] [Google Scholar]
- 19.Sexton JE, Desmonds T, Quick K, Taylor R, Abramowitz J, Forge A, et al. The contribution of TRPC1, TRPC3, TRPC5 and TRPC6 to touch and hearing. Neurosci Lett. 2016;610:36–42. doi: 10.1016/j.neulet.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaghan K, McNaughten J, McGahon MK, Kelly C, Kyle D, Yong PH, et al. Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS One. 2015;10:e0128359. doi: 10.1371/journal.pone.0128359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jie P, Hong Z, Tian Y, Li Y, Lin L, Zhou L, et al. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015;6:e1775. doi: 10.1038/cddis.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu YJ, Zhang X, Fan ZZ, Huai J, Teng YB, Zhang Y, et al. Effect of TRPV4-p38 MAPK pathway on neuropathic pain in rats with chronic compression of the dorsal root ganglion. Biomed Res Int. 2016;2016:6978923. doi: 10.1155/2016/6978923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihara H, Suzuki N, Boudaka AA, Muhammad JS, Tominaga M, Tabuchi Y, et al. Transient receptor potential vanilloid 4-dependent calcium influx and ATP release in mouse and rat gastric epithelia. World J Gastroenterol. 2016;22:5512–5519. doi: 10.3748/wjg.v22.i24.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrill L, Vizzard MA. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am J Physiol Regul Integr Comp Physiol. 2014;307:R471–R480. doi: 10.1152/ajpregu.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong Z, Jie P, Tian Y, Chen T, Chen L, Chen L. Transient receptor potential vanilloid 4-induced modulation of voltage-gated sodium channels in hippocampal neurons. Mol Neurobiol. 2016;53:759–768. doi: 10.1007/s12035-014-9038-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 27.Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci Lett. 2005;382:304–308. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Corey DP, García-Añoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 29.Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:120068. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanini D, Göpfert MC. TRPs in hearing. Handb Exp Pharmacol. 2014;223:899–916. doi: 10.1007/978-3-319-05161-1_7. [DOI] [PubMed] [Google Scholar]
- 31.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, et al. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol. 2013;305:C704–C715. doi: 10.1152/ajpcell.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Bhaskaran A, Scott H, Ardekani S, Xu J, Mohideen U, et al. Rho/ROCK-mediated retinal endothelial stiffening impairs TRPV4 signaling and promotes diabetic retinal inflammation. Invest Ophthalmol Vis Sci. 2016;57:3220. [Google Scholar]
- 33.Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zsombok A, Derbenev AV. TRP channels as therapeutic targets in diabetes and obesity. Pharmaceuticals (Basel) 2016;9:pii: E50. doi: 10.3390/ph9030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshraghi AA, Hoosien G, Ramsay S, Dinh CT, Bas E, Balkany TJ, et al. Inhibition of the JNK signal cascade conserves hearing against electrode insertion trauma-induced loss. Cochlear Implants Int. 2010;11(Suppl 1):104–109. doi: 10.1179/146701010x12671177544104. [DOI] [PubMed] [Google Scholar]
- 37.Van De Water TR, Lallemend F, Eshraghi AA, Ahsan S, He J, Guzman J, et al. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004;25:627–632. doi: 10.1097/00129492-200407000-00035. [DOI] [PubMed] [Google Scholar]
- 38.Tadros SF, D'Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13:1303–1321. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TRPV4 involved in HG-induced HEI-OC1 cell proliferation and apoptosis. (A) The relative expression of TRPV4 mRNA in HG cultured HEI-OC1 cells after transfection with pcDNA3.1 empty vector, pcDNA3.1-TRPV4 vector, TRPV4-siRNA, or scrambled siRNA control was determined by qRT-PCR. (B) Western blotting was used to analyze the expression of TRPV4 protein. (C) The effect of TRPV4 on cell proliferation of HEI-OC1 cells was detected using the MTT assay. (D) The effect of TRPV4 on cell apoptosis of HEI-OC1 cells was detected using the cell death detection ELISA-Plus apoptosis assay. Data are representative of three experiments. *Indicates differences from controls at p<0.05. TRPV4, transient receptor potential vanilloid sub type 4; HG, high glucose.