Abstract
Purpose
Local recurrence is the most common cause of failure in retroperitoneal soft tissue sarcoma patients after surgical resection. Postoperative radiotherapy (PORT) is infrequently used due to its high complication risk. We investigated the efficacy of PORT using modern techniques in patients with retroperitoneal soft tissue sarcoma.
Materials and Methods
Eighty patients, who underwent surgical resection for non-metastatic primary retroperitoneal soft tissue sarcoma at the Yonsei Cancer Center between 1994 and 2015, were retrospectively reviewed. Thirty-eight (47.5%) patients received PORT: three-dimensional conformal radiotherapy in 29 and intensity-modulated radiotherapy in nine patients. Local failure-free survival (LFFS), overall survival (OS), and RT-related toxicities were investigated.
Results
Median follow-up was 37.1 months (range, 5.8–207.9). Treatment failure occurred in 47 (58.8%) patients including local recurrence in 33 (41.3%), distant metastasis in eight (10%), and both occurred in six (7.5%) patients. The 2-year and 5-year LFFS rates were 63.9% and 47.9%, respectively. The 2-year and 5-year OS rates were 87.5% and 71.1%. The 5-year LFFS rate was significantly higher in PORT group than in no-PORT group (74.2% vs. 24.3%, p<0.001). In multivariate analysis, PORT was the only independent prognostic factor for LFFS. However, there was no significant correlation between RT dose and LFFS. OS showed no significant difference between the two groups. Grade ≤2 acute toxicities were observed in 63% of patients, but no acute toxicity ≥grade 3 was observed.
Conclusion
PORT using modern technique markedly reduced local recurrence in retroperitoneal sarcoma patients, with low toxicity. The optimal RT technique, in terms of RT dose and target volume, should be further investigated.
Keywords: Retroperitoneal sarcoma, postoperative radiotherapy, local recurrence, toxicity
INTRODUCTION
Retroperitoneal soft tissue sarcomas are relatively uncommon, accounting for 10–15% of all soft tissue sarcomas and 30% of all malignant retroperitoneal tumors.1 Surgical resection is the standard curative treatment for patients with localized disease. However, retroperitoneal sarcoma is often diagnosed in the advanced stage, as it is frequently asymptomatic. Moreover, it makes complete excision difficult. Complete resection with negative margins is essential to increase survival, even if resection of adjacent organs cannot be avoided.2,3,4 The rate of complete resection ranges from 41.8% to 76%.4,5,6 Even after complete resection, local recurrence remains the most common cause of failure and is ultimately fatal, with a mortality rate of 20–75%.7
To improve local control as well as survival, several neoadjuvant or adjuvant treatments have been investigated. The rationale for applying perioperative radiotherapy (RT) in addition to surgery comes from small randomized controlled trials and several retrospective studies that provide evidence for improved local control with RT in soft tissue sarcoma without affecting survival.8,9 Postoperative RT (PORT) is clinically used infrequently, since it has higher complication rate compared to preoperative RT.10 However, RT techniques used in previous studies were relatively old-fashioned, mostly composed of two-dimensional techniques using anterioposterior (AP)/posterioanterior (PA) beams. Currently, three-dimensional-conformal RT (3D-CRT) and intensity-modulated RT (IMRT) are used, which facilitate normal organ sparing. It is necessary to reexamine the role of PORT in the modern era, where more sophisticated techniques and conformal treatment planning are possible. In this study, we investigated the efficacy of PORT using modern techniques in patients with retroperitoneal soft tissue sarcoma.
MATERIALS AND METHODS
Patient characteristics
One hundred patients, who underwent surgical resection for primary retroperitoneal soft tissue sarcoma between 1994 and 2015 at Yonsei Cancer Center, were retrospectively reviewed. We included only patients who were treated after 1994, as 3D-CRT has been employed since 1994 at our institution. Twenty patients were excluded from this analysis due to initial stage IV (11 patients), no follow-up after surgery (six patients), or double primary malignancy (three patients). Finally, a total of 80 patients were included in our analysis, and their demographic characteristics, clinicopathological parameters, and treatment variables were obtained from electronic medical records.
Pre and postoperative imaging studies included computed tomography (CT) and/or magnetic resonance imaging (MRI). Tumor staging was performed in accordance with the seventh edition of American Joint Committee on Cancer staging system for soft tissue sarcoma. Histopathologic subtypes were subdivided into leiomyosarcoma, liposarcoma, undifferentiated pleomorphic sarcoma, and others, due to the small number of other rare sarcoma subtypes. Histologic grading was performed according to Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system. Tumor size was defined as the maximum dimension on cross-sectional imaging for a solitary mass, and as the sum of all maximum dimensions for more than one mass. This study was approved by Institutional Review Board of Yonsei University Health System (IRB-4-2017-1060). Patients' records/information were anonymized and de-identified prior to analysis, and informed consent was not obtained from each participant.
Treatment
All patients underwent surgical resection as initial treatment. Surgical resection was intended to achieve a negative margin, even if resection of adjacent organs was needed. Surgical margins were categorized as R0 (histologically negative margin), R1 (macroscopically negative, but microscopically positive margin), and R2 (macroscopically positive margin).
PORT was performed in 38 patients (47.5%) using 3D-CRT in 29 patients and IMRT in 9 patients, with a conventional fractionation schedule (1.8–2.6 Gy per fraction, once per day, 5 days per week). IMRT was delivered with helical TomoTherapy (Tomotherapy, Inc., Madison, WI, USA) or Volumetric Arc-Therapy. Tumor bed was delineated using MRI or CT fusion on either MIM software (MIM Software, Cleveland, OH, USA) or Pinnacle Radiotherapy Planning System (Phillips Medical System, Andover, MA, USA). CT simulation and reproducible patient immobilization were essential. A four-dimensional CT (4D-CT) scan was acquired for the assessment of respiratory movement.
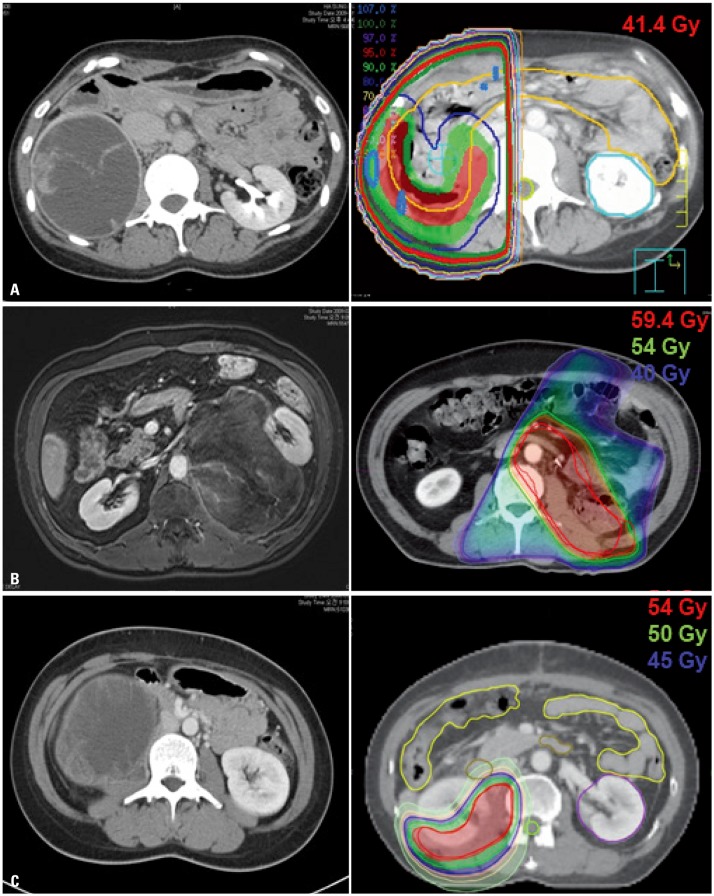
Clinical target volume (CTV) was determined by allowing a margin of at least 2 cm to tumor bed, considering the postoperative anatomic change individually. We did not take into account preoperative tumor volume. Internal target volume (ITV) was delineated, taking account of respiratory movement if 4D-CT was used. Planning target volume included CTV or ITV, plus a 3 to 5-mm margin. Cone-down was performed in 3D-CRT planning, and final target volume at the time of cone-down was defined as tumor bed plus a 0.5-cm margin. In IMRT planning, simultaneous integrated boost (SIB) technique was used instead of cone-down. Radiation dose for initial target volume was considered to be 45 Gy for microscopic tumor control, which could be lowered individually considering normal tissue tolerance dose. Using either cone-down for 3D-CRT or SIB technique for IMRT, the total dose of radiation was approximately 54 Gy. Intended prescribed radiation dose was 60 Gy or more, in the case of R1 or R2 resection. The most frequent dose-limiting organ was small bowel, especially duodenum, which was restricted to ≤45 Gy. We were not concerned about the dose to kidney near tumor bed, if patients had another healthy kidney or if kidney was removed during surgical resection. RT plan was designed to be as conformal as possible, using multiple beam ports in 3D-CRT and intensity modulation in IMRT. Examples of RT plans at our institution are shown in Fig. 1.
Fig. 1. Examples of radiotherapy (RT) plan of (A) two-dimensional RT, (B) three-dimensional conformal RT, and (C) intensity-modulated RT.
Adjuvant chemotherapy was administered to 23 patients (28.8%), depending on the clinician's discretion. All patients who were treated after year 2000 were administered doxorubicin/ifosfamide, while three patients who were treated before 2000 received other regimens. The use of adjuvant chemotherapy was slightly higher in no-PORT group than in PORT group, without statistical significance (21.1%, PORT and 35.7%, no-PORT; p=0.230).
Follow-up
All patients were examined weekly during RT to monitor treatment-related toxicity and general condition. Imaging studies were conducted at 1 and 3 months after completion of the planned treatment. If there was no evidence of recurrence for 1 year, patients were followed at 6-month intervals for the next 2 years and annually thereafter. Treatment-related toxicities were recorded according to Common Toxicity Criteria for Adverse Events (version 4.0). Recurrences were defined as either histologic confirmation of disease recurrence or, if pathological confirmation was not performed, conclusive imaging demonstrating recurrence. Local failure, defined as recurrence in retroperitoneal space, was divided into infield and outfield recurrence, depending on the location of tumor in relation to RT target volume. Distant metastasis was defined as tumor recurrence outside of retroperitoneal space (i.e., chest, abdomen, peritoneum, brain, and elsewhere).
Statistical analysis
The primary endpoint was local failure-free survival (LFFS), and secondary endpoints were overall survival (OS) and RT-related toxicities. Survival duration was calculated from the date of diagnosis to corresponding event (local failure or death). Local failure was classified based on the location of recurrent tumor: infield in event of tumor recurrence within RT target volume, marginal in event of recurrence inside initial target volume, but outside of cone-down volume, and outfield in event of recurrence outside RT target volume. We performed Pearson's chi-square test or Fisher's exact test, as appropriate, for categorical variables, and independent t-tests for continuous variables to evaluate differences between the two groups (PORT and no-PORT). Kaplan-Meier method with log-rank test and Cox regression were used to analyze survival outcomes between groups. Stepwise Cox proportional hazards regression was used to perform multivariable analysis on prognostic factors for LFFS and OS (inclusion criteria p<0.05, exclusion criteria p>0.1). All statistical tests were two-sided with significance defined as p<0.05. Data were analyzed using IBM SPSS software version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient and treatment characteristics
Patient and tumor characteristics are shown in Table 1. Patients' ages ranged from 20 to 81 years, with a median age of 55 years. The most common histologic subtype was liposarcoma (52.5%), followed by leiomyosarcoma (22.5%), undifferentiated pleomorphic sarcoma (11.2%), and others (13.8%). Median tumor size was 13 cm (range, 2.7–40 cm). FNCLCC grade was reported in 52 patients (65%), with grade 1 in 17 patients (21.3%), grade 2 in 18 patients (22.4%), and grade 3 in 17 patients (21.3%). Surgical resection margin was R0 in 45 patients (56.2%), R1 in 24 patients (30%), and R2 in 11 patients (13.8%). After comparing the two groups (PORT vs. no-PORT), we found no significant differences in patient characteristics, although resection margin showed a marginal difference: rate of R1 or R2 resection was higher in PORT group (p=0.053). There were more patients with FNCLCC grade 2 or 3 included in PORT group than in no-PORT group, but this was not significant (50% vs. 38%, p=0.432).
Table 1. Patient Characteristics.
| No-PORT (n=42) | PORT (n=38) | Total (n=80) | p value | |
|---|---|---|---|---|
| Age (yr) | 53.7±15.3 | 52.7±15.4 | 53.2±15.3 | 0.782 |
| Sex | 0.111 | |||
| Male | 24 (57.1) | 14 (36.8) | 38 (47.5) | |
| Female | 18 (42.9) | 24 (63.2) | 42 (52.5) | |
| ECOG | 0.624 | |||
| 0 | 18 (42.9) | 16 (42.1) | 34 (42.5) | |
| 1 | 24 (57.1) | 22 (57.9) | 45 (56.2) | |
| Pathology | 0.096 | |||
| Liposarcoma | 27 (64.3) | 15 (39.5) | 42 (52.5) | |
| Leiomyosarcoma | 6 (14.3) | 12 (31.6) | 18 (22.5) | |
| UPS | 3 (7.1) | 6 (15.8) | 9 (11.2) | |
| Other | 6 (14.3) | 5 (13.2) | 11 (13.8) | |
| FNCLCC grade | 0.432 | |||
| 1 | 11 (26.3) | 6 (15.8) | 17 (21.3) | |
| 2 | 8 (19.0) | 10 (26.3) | 18 (22.4) | |
| 3 | 8 (19.0) | 9 (23.7) | 17 (21.3) | |
| Unknown | 15 (35.7) | 13 (34.2) | 28 (35.0) | |
| Size | 0.599 | |||
| <10 cm | 10 (23.8) | 12 (31.6) | 22 (27.5) | |
| ≥10 cm | 32 (76.2) | 26 (68.4) | 58 (72.5) | |
| Stage | 0.358 | |||
| IA | 2 (4.8) | 3 (7.9) | 5 (6.2) | |
| IB | 22 (52.4) | 12 (31.6) | 34 (42.5) | |
| IIA | 0 (0.0) | 1 (2.6) | 1 (1.2) | |
| IIB | 7 (16.7) | 9 (23.7) | 16 (20.0) | |
| III | 11 (26.2) | 13 (34.2) | 24 (30.0) | |
| Resection margin | 0.053 | |||
| R0 | 29 (69.0) | 16 (42.1) | 45 (56.2) | |
| R1 | 9 (21.4) | 15 (39.5) | 24 (30.0) | |
| R2 | 4 (9.5) | 7 (18.4) | 11 (13.8) | |
| Adjuvant chemotherapy | 0.230 | |||
| No | 27 (64.3) | 30 (78.9) | 57 (71.2) | |
| Yes | 15 (35.7) | 8 (21.1) | 23 (28.8) |
PORT, postoperative radiotherapy; ECOG, Eastern Cooperative Oncology Group; UPS, undifferentiated pleomorphic sarcoma; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer.
Data are presented as mean±SD or number (%).
PORT was delivered with a median dose of 54 Gy (range, 36.0–66.9 Gy) at a median 1.8 Gy per fraction (range, 1.8–2.6 Gy). Radiation dose tended to be higher in patients with R1 or R2 resection than in those with R0 resection (Table 2). In PORT group, 57.1% of patients with R2 resection received >59.4 Gy of radiation, while half of the patients with R0 or R1 resection received <50.4 Gy of radiation. Median doses of the two RT techniques were the same (54 Gy), with a range of 36.0–66.0 Gy for 3D-CRT and 45.0–66.9 Gy for IMRT.
Table 2. Postoperative Radiation Dose according to Resection Margin Status.
| ≤45 Gy | 45–50.4 Gy | 50.4–54 Gy | 54–59.4 Gy | >59.4 Gy | Total | |
|---|---|---|---|---|---|---|
| R0 | 6 (37.5) | 2 (12.5) | 5 (31.2) | 1 (6.3) | 2 (12.5) | 16 |
| R1 | 3 (20.0) | 4 (26.6) | 1 (6.7) | 6 (40.0) | 1 (6.7) | 15 |
| R2 | 1 (14.3) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 4 (57.1) | 7 |
| Total | 10 | 6 | 8 | 7 | 7 | 38 |
Data are presented as number (%).
Local control and survival outcomes
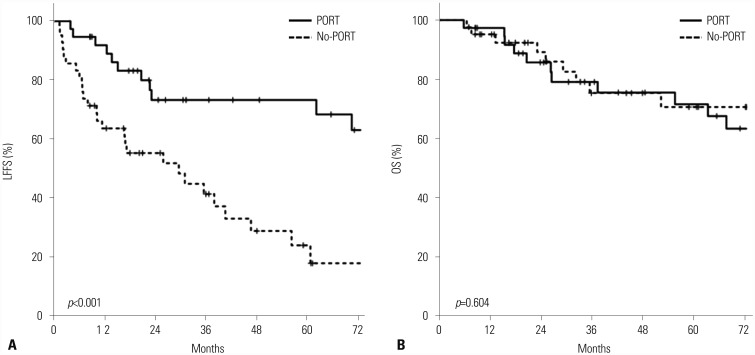
Median follow-up was 37.1 months (range, 2.5–207.9 months) for the entire cohort. The 2-year and 5-year LFFS rates were 63.9% and 47.9%, respectively. The 2-year and 5-year OS rates were 87.5% and 71.1%, respectively. In univariate analysis, tumor stage [hazard ratio (HR) 1.53, 95% confidence interval (CI) 1.07–2.19; p=0.021], FNCLCC grade (HR 2.31, 95% CI 1.28–4.16; p=0.005), and use of PORT (HR 0.272, 95% CI 0.13–0.57; p=0.001) were significantly associated with LFFS. Among these, PORT was the only powerful prognostic factor associated with LFFS after adjusting for confounding factors in multivariate analysis (HR 0.179, 95% CI 0.07–0.49; p=0.001). LFFS was significantly better in PORT group; the 5-year LFFS rates were 74.2% for PORT group and 24.3% for no-PORT group (Fig. 2A). However, there was no significant correlation between RT dose and LFFS. In univariate analysis for OS, tumor stage (HR 2.22, 95% CI 1.37–3.58; p=0.001) and FNCLCC grade (HR 6.48, 95% CI 2.05–20.52; p=0.001) were significantly associated with OS, but the use of PORT was not prognostic for OS. The 5-year OS rate was 71.6% for PORT group and 70.6% for no-PORT group (p=0.604) (Fig. 2B). There was no significant prognostic factor for OS in multivariate analysis. In particular, the use of chemotherapy was not beneficial in our analysis.
Fig. 2. Kaplan-Meire curves for (A) LFFS and (B) OS. LFFS, local failure-free survival; OS, overall survival.
Patterns of failure
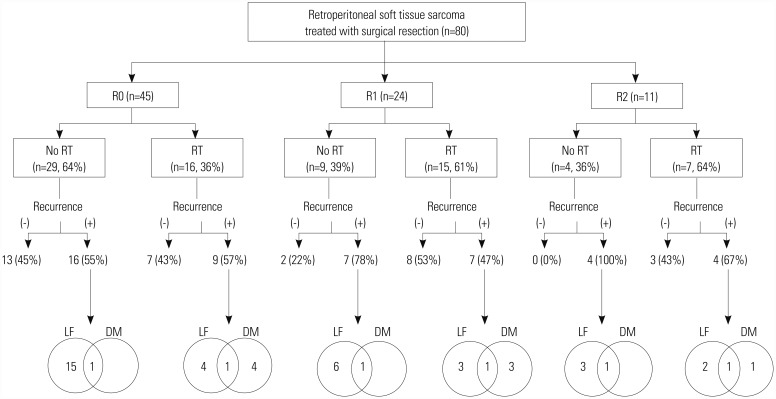
Treatment failure occurred in 47 (58.8%) of 80 patients. The most common pattern of failure was local recurrence. Local recurrence occurred in 33 patients (41.3%), distant metastasis in eight patients (10%), and both in six patients (7.5%). Lung was the predominant site of distant metastasis, which was observed in six patients, followed by peritoneal seeding in three patients and liver metastasis in two patients. The patterns of failure by resection margin are summarized in Fig. 3. Patients who did not receive PORT, despite R1 or R2 resection, experienced more recurrences compared to those treated with PORT: 78% versus 47% in patients with R1 resection and 100% versus 67% in those with R2 resection for no-PORT and PORT groups, respectively. In regard to local recurrence, the difference became more apparent across all resection margin statuses. Local recurrence occurred in 55% of no-PORT group and 32% of PORT group in R0 patients, and 78% of no-PORT group and 27% of PORT group in R1 patients. All R2 patients who did not receive PORT had local recurrence, while 43% of PORT group had local recurrence.
Fig. 3. Pattern of failures according to resection margin and use of postoperative RT. RT, radiotherapy; LF, local failure; DM, distant metastasis.
Of the 12 patients who experienced local recurrence after PORT, eight had infield recurrence, two had marginal recurrence, and two had outfield recurrence. There was no significant correlation between resection margin status and the pattern of local recurrence; infield recurrence occurred in three patients, each whom achieved R0 and R1 resection and two patients who achieved R2 resection; marginal recurrence occurred in one patient, each of whom achieved R0 and R2 resection; and outfield recurrence occurred in one patient, each of whom achieved R0 and R1 resection. We also analyzed whether radiation dose was related to recurrence pattern. Median radiation dose was 45 Gy (range, 45–57 Gy) in patients with infield recurrence, while it was 50.4 Gy and 65 Gy for the two patients with marginal recurrence and 54 Gy and 59.4 Gy for the two patients with outfield recurrence.
Acute and late toxicities
Radiation-related acute toxicities were observed in 24 of 38 patients who received PORT (63%). Grade ≤2 enteritis and anorexia were observed in 13 patients and seven patients, respectively. Four patients experienced grade ≤2 nausea/vomiting, but there was no acute toxicity ≥grade 3 observed. In regard to late complications, there were only two patients who had late complication. One patient who received 65 Gy in 25 fractions to residual tumor mass at right psoas muscle experienced neuropathy extending to right leg, although residual tumor was well-controlled until the last follow-up (over 9 years). In another patient who had neuropathy with right leg tingling sensation, it was difficult to distinguish radiation toxicity from surgical morbidity since it occurred after surgical resection and persisted.
DISCUSSION
We found that PORT significantly improved LFFS in patients who received surgical resection for retroperitoneal soft tissue sarcoma. The benefit was more prominent in patients with R1 and R2 resection, but PORT significantly improved local tumor control even in patients with R0 resection. Moreover, there were no RT-related toxicities ≥grade 3. Using modern RT techniques, including 3D-CRT and IMRT, we could perform PORT safely without increasing complications.
The effects of PORT on local control have been demonstrated in three prospective studies conducted in extremity sarcomas, which are the most common type of soft tissue sarcoma.8,9,11 However, there has been no prospective study investigating the effect of PORT in retroperitoneal soft tissue sarcomas, so it is still subject to debate. Through several retrospective studies, the role of preoperative and postoperative RT has been investigated. 5,7,12,13,14,15 Sampath, et al.13 reported the results from a multiinstitutional study of 251 patients, which revealed a significant improvement in LFFS in PORT arm over surgery alone; the 5-year LFFS was 64% in no-RT group and 79% in RT group. Another retrospective study from Scandinavian Sarcoma Group demonstrated a statistically significant difference in 5-year LRFS between RT group (77%) and no-RT group (39%) (p<0.001).16
Our finding that RT acts as a significant prognostic factor for local control concurs with the findings of these previous studies. In our study, the 5-year LFFS was 74% in PORT group and 24% in no-PORT group (p=0.001), and PORT was the strongest prognostic factor for LFFS in multivariate analysis, overtaking both FNCLCC grade and tumor stage. On the contrary, Choi, et al.17 showed that PORT had no benefit on disease-specific survival, based on analysis of a relatively large number of patients using propensity-scoring matching from Surveillance, Epidemiology, and End Results (SEER) database. However, this study included patients who had been treated with older techniques and lacked details about surgical margin, pathological risk factors, and RT technique.
Although PORT significantly improved LFFS, the benefit did not lead to improved OS in our study. In case of local recurrence, which was the most common cause of failure, salvage treatment was actively performed, this may have diluted the difference in OS. Of the 33 patients who had only local recurrence, 24 had not undergone PORT. Of these, 10 patients underwent surgery followed by PORT as salvage treatment and only two died after 5 and 42 months, with the 5-year OS rate of 67.5%. Moreover, five patients received salvage RT without surgery, of whom only one died after 13 months and others were alive until last follow-up at 12, 62, 71, and 108 months. This suggests that salvage surgery followed by PORT could be an effective treatment even if relapsed.
The optimal timing of RT remains a controversy. The rationale for preoperative radiation is that treatment volume is less extensive than with PORT, as only clinically and radiographically demonstrated tumor is included in target volume, and tumor often displaces bowel out of radiation field, so as to allow a lower dose to the normal tissue.18,19 Moreover, resection could also be less extensive than if surgery was performed after RT. However, we have to consider that radiation beam arrangement prior to the development of 3D-CRT consisted of AP/PA beam, such that PORT contained a relatively large amount of bowel in RT field compared to preoperative RT. Current technologies overcome this disadvantage and give a higher dose to target volume. One advantage of PORT is that the pathologist has an intact tumor specimen for determination of histologic grade and type, which will allow the clinical team to better determine the proper postoperative treatment. Additionally, there is no delay of surgery, which can be an emotional advantage for some patients. Several studies have investigated whether preoperative or postoperative RT provides better outcomes.20,21,22,23,24 Zlotecki, et al.24 suggested that preoperative RT may be preferred, with similar clinical outcomes and less risk of complications than PORT. However, this study has several limitations; most patients were treated before 1990, when CT-based treatment planning became standard, and patients who received preoperative RT were primarily seen after 1994. On the contrary, the largest study of a nationwide clinical oncology database revealed that perioperative RT, whether preoperative or postoperative, was associated with improved OS compared with surgery alone.25 It is hard to conclude whether preoperative and postoperative RT is preferable based on previous studies, as they are all retrospective studies including a relatively small number of patients and show conflicting results. The ongoing randomized European Organization for Research and Treatment of Cancer trial investigating 50.4 Gy of preoperative RT and surgery versus surgery alone for retroperitoneal sarcoma may provide additional insight into this issue (EORTC 62092-22092; STRASS trial; NCT01344018).
In practice, many clinicians dislike the use of PORT due to its higher complication rate compared with preoperative RT. Overall, the rate of acute PORT-induced enteritis is reported to be 30–80%, and grade 3 or 4 enteritis is present in approximately 6–80% of patients.24,26 However, most studies do not reflect the latest advances in RT technique, since they include a large number of patients treated before 3D-CRT was commonly used. In our study, acute toxicities were reported in 63% of patients, but no grade ≥3 toxicities were observed. Late toxicity was observed in only one patient who received high dose (>65 Gy) of radiation. A study from M.D. Anderson Cancer Center, which investigated 121 patients treated between 1965 and 2012, reported a 10-year actuarial complication rate of 5%, and all complications occurred in patients treated between 1965 and 1985.10
Although most studies have shown that RT is helpful for local tumor control, there is a lack of consensus on the optimal radiation dose and target volume. Most institutions treat patients individually according to their institutional protocols. The postoperative radiation dose reported in previous studies is approximately 45–55 Gy.15,16,26 According to the treatment guideline for preoperative RT for retroperitoneal sarcoma published in 2015,27 50 Gy is recommended as a reasonable dose based on panel discussion, due to lack of data. However, there is no consensus guideline on an optimal radiation dose for PORT. Some small retrospective studies have investigated an optimal radiation dose. Fein, et al.28 recommended a radiation dose ≥55.2 Gy, based on the higher local recurrence rate in patients receiving radiation <55.2 Gy. On the other hand, Ballo, et al.29 reported that a higher dose of radiation (>60 Gy) increased toxicities, but not local control. In our study, it was difficult to draw a solid conclusion about the optimal radiation dose, due to the small number of patients included. Our results suggest that a radiation dose >45 Gy is necessary to achieve local control, considering that most of the infield recurrences occurred in patients who received ≤45 Gy to tumor bed, even in R0 patients, while patients who received radiation >45 Gy had marginal or outfield recurrences only. Among nine patients who received PORT for R2 resection, five received ≤54 Gy and four received ≥65 Gy. Four of the five patients who received ≤54 Gy had local recurrence (infield in three patients, marginal recurrence in one patient), while only one marginal recurrence occurred among those who received ≥65 Gy. For those who received ≥65 Gy, no grade ≥3 toxicities were observed, and only one patient experienced neuropathy as late complication, without any tumor recurrence. This may suggest that a radiation dose ≥65 Gy is necessary for patients with R2 resection. To clarify the optimal dose, further well-designed large studies are needed.
This study has several limitations driven from its retrospective nature. First, there are inherent selection biases, as with any retrospective study. Second, the number of patients included in this study was relatively small due to the low incidence of retroperitoneal soft tissue sarcoma, and the treatment spanned several decades, which can affect the outcomes. However, our study's strength is the exclusive inclusion of patients with primary retroperitoneal sarcoma treated with surgical resection and RT using a modern technique as primary treatment in a single institution.
In conclusion, PORT markedly reduced local recurrence in retroperitoneal sarcoma patients. We suggest that PORT can be a safe treatment option in surgically resected retroperitoneal sarcoma patients without increasing toxicity, if a conformal and safe treatment plan is performed using modern RT techniques. The optimal RT technique in terms of timing, dose, and target volume should be further investigated.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Clark JA, Tepper JE. Role of radiation therapy in retroperitoneal sarcomas. Oncology (Williston Park) 1996;10:1867–1872. [PubMed] [Google Scholar]
- 2.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. doi: 10.1097/01.sla.0000086542.11899.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storm FK, Mahvi DM. Diagnosis and management of retroperitoneal soft-tissue sarcoma. Ann Surg. 1991;214:2–10. doi: 10.1097/00000658-199107000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoeckle E, Coindre JM, Bonvalot S, Kantor G, Terrier P, Bonichon F, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Toulmonde M, Bonvalot S, Méeus P, Stoeckle E, Riou O, Isambert N, et al. Retroperitoneal sarcomas: patterns of care at diagnosis, prognostic factors and focus on main histological subtypes: a multicenter analysis of the French Sarcoma Group. Ann Oncol. 2014;25:735–742. doi: 10.1093/annonc/mdt577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van De Voorde L, Delrue L, van Eijkeren M, De Meerleer G. Radiotherapy and surgery-an indispensable duo in the treatment of retroperitoneal sarcoma. Cancer. 2011;117:4355–4364. doi: 10.1002/cncr.26071. [DOI] [PubMed] [Google Scholar]
- 8.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 10.Bishop AJ, Zagars GK, Torres KE, Hunt KK, Cormier JN, Feig BW, et al. Combined modality management of retroperitoneal sarcomas: a single-institution series of 121 patients. Int J Radiat Oncol Biol Phys. 2015;93:158–165. doi: 10.1016/j.ijrobp.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heslin MJ, Lewis JJ, Nadler E, Newman E, Woodruff JM, Casper ES, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 13.Sampath S, Hitchcock YJ, Shrieve DC, Randall RL, Schultheiss TE, Wong JY. Radiotherapy and extent of surgical resection in retroperitoneal soft-tissue sarcoma: multi-institutional analysis of 261 patients. J Surg Oncol. 2010;101:345–350. doi: 10.1002/jso.21474. [DOI] [PubMed] [Google Scholar]
- 14.Pierie JP, Betensky RA, Choudry U, Willett CG, Souba WW, Ott MJ. Outcomes in a series of 103 retroperitoneal sarcomas. Eur J Surg Oncol. 2006;32:1235–1241. doi: 10.1016/j.ejso.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Le Péchoux C, Musat E, Baey C, Al Mokhles H, Terrier P, Domont J, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol. 2013;24:832–837. doi: 10.1093/annonc/mds516. [DOI] [PubMed] [Google Scholar]
- 16.Trovik LH, Ovrebo K, Almquist M, Haugland HK, Rissler P, Eide J, et al. Adjuvant radiotherapy in retroperitoneal sarcomas. A Scandinavian Sarcoma Group study of 97 patients. Acta Oncol. 2014;53:1165–1172. doi: 10.3109/0284186X.2014.921723. [DOI] [PubMed] [Google Scholar]
- 17.Choi AH, Barnholtz-Sloan JS, Kim JA. Effect of radiation therapy on survival in surgically resected retroperitoneal sarcoma: a propensity score-adjusted SEER analysis. Ann Oncol. 2012;23:2449–2457. doi: 10.1093/annonc/mdr616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng CW, Fiveash JB, Popple RA, Arnoletti JP, Russo SM, Urist MM, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 20.Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys. 2003;56:482–488. doi: 10.1016/s0360-3016(02)04510-8. [DOI] [PubMed] [Google Scholar]
- 21.Cheng EY, Dusenbery KE, Winters MR, Thompson RC. Soft tissue sarcomas: preoperative versus postoperative radiotherapy. J Surg Oncol. 1996;61:90–99. doi: 10.1002/(SICI)1096-9098(199602)61:2<90::AID-JSO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Sampath S, Schultheiss TE, Hitchcock YJ, Randall RL, Shrieve DC, Wong JY. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int J Radiat Oncol Biol Phys. 2011;81:498–505. doi: 10.1016/j.ijrobp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Pollack A, Zagars GK, Goswitz MS, Pollock RA, Feig BW, Pisters PW. Preoperative vs. postoperative radiotherapy in the treatment of soft tissue sarcomas: a matter of presentation. Int J Radiat Oncol Biol Phys. 1998;42:563–572. doi: 10.1016/s0360-3016(98)00277-6. [DOI] [PubMed] [Google Scholar]
- 24.Zlotecki RA, Katz TS, Morris CG, Lind DS, Hochwald SN. Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol. 2005;28:310–316. doi: 10.1097/01.coc.0000158441.96455.31. [DOI] [PubMed] [Google Scholar]
- 25.Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966–975. doi: 10.1016/S1470-2045(16)30050-X. [DOI] [PubMed] [Google Scholar]
- 26.Gilbeau L, Kantor G, Stoeckle E, Lagarde P, Thomas L, Kind M, et al. Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol. 2002;65:137–143. doi: 10.1016/s0167-8140(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 27.Baldini EH, Wang D, Haas RL, Catton CN, Indelicato DJ, Kirsch DG, et al. Treatment guidelines for preoperative radiation therapy for retroperitoneal sarcoma: preliminary consensus of an international expert panel. Int J Radiat Oncol Biol Phys. 2015;92:602–612. doi: 10.1016/j.ijrobp.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Fein DA, Corn BW, Lanciano RM, Herbert SH, Hoffman JP, Coia LR. Management of retroperitoneal sarcomas: does dose escalation impact on locoregional control? Int J Radiat Oncol Biol Phys. 1995;31:129–134. doi: 10.1016/0360-3016(94)E0302-Z. [DOI] [PubMed] [Google Scholar]
- 29.Ballo MT, Zagars GK, Pollock RE, Benjamin RS, Feig BW, Cormier JN, et al. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys. 2007;67:158–163. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]