Abstract
Introduction
Stakeholder engagement is an essential component of HIV clinical trials. We define stakeholder engagement as an input by individuals or groups with an interest in HIV clinical trials to inform the design or conduct of said trials. Despite its value, stakeholder engagement to inform HIV clinical trials has not been rigorously examined. The purpose of our systematic review is to examine stakeholder engagement for HIV clinical trials and compare it to the recommendations of the UNAIDS/AVAC Good Participatory Practice (GPP) guidelines.
Methods
We used the PRISMA checklist and identified English language studies describing stakeholder engagement to inform HIV clinical trials. Four databases (PubMed, Ovid, CINAHL and Web of Science) and six journals were searched, with additional studies identified using handsearching and expert input. Two independent reviewers examined citations, abstracts and full texts. Data were extracted on country, engagement methods, stakeholder types and purpose of stakeholder engagement. Based on the GPP guidelines, we examined how frequently stakeholder engagement was conducted to inform clinical trial research question development, protocol development, recruitment, enrolment, follow‐up, results and dissemination.
Results and discussion
Of the 917 citations identified, 108 studies were included in the analysis. Forty‐eight studies (44.4%) described stakeholder engagement in high‐income countries, thirty (27.8%) in middle‐income countries and nine (8.3%) in low‐income countries. Fourteen methods for stakeholder engagement were identified, including individual (e.g. interviews) and group (e.g. community advisory boards) strategies. Thirty‐five types of stakeholders were engaged, with approximately half of the studies (60; 55.6%) engaging HIV‐affected community stakeholders (e.g. people living with HIV, at‐risk or related populations of interest). We observed greater frequency of stakeholder engagement to inform protocol development (49 studies; 45.4%) and trial recruitment (47 studies; 43.5%). Fewer studies described stakeholder engagement to inform post‐trial processes related to trial results (3; 2.8%) and dissemination (11; 10.2%).
Conclusions
Our findings identify important directions for future stakeholder engagement research and suggestions for policy. Most notably, we found that stakeholder engagement was more frequently conducted to inform early stages of HIV clinical trials compared to later stages. In order to meet recommendations established in the GPP guidelines, greater stakeholder engagement across all clinical trial stages is needed.
Keywords: stakeholder engagement, community, HIV clinical trials, reporting quality, systematic review, advisory mechanisms
1. Introduction
Engaging stakeholders in the clinical trial research process has been well established as a method to improve research implementation, procedures, and outcomes 1, 2. Stakeholders can be defined broadly as any individual or group who can have an impact on or is affected by a clinical trial 3. Some examples of stakeholders include trial participants, members of local communities in which a trial is conducted, governmental organizations and funders who shape the research process. Strong stakeholder engagement can potentially result in trials that more effectively address stakeholders’ needs and perspectives 4, as well as improve health equity, access and participant welfare 5. Stakeholder engagement is particularly important in HIV clinical trials, which require careful consideration of the unique physical, psychological and social vulnerabilities associated with HIV infection 6 and subsequent ethical obligations towards trial participants 7. In addition, despite the disproportionate impact of the HIV epidemic on minority communities, these populations are underrepresented in HIV research 8. These factors make stakeholder engagement critical for building effective and sustainable collaborations.
The field of HIV research has championed innovative stakeholder engagement efforts, spurred partly by the activism of those living with HIV. Following the efforts of the ACT‐UP movement, the National Institutes of Health (NIH) established community advisory boards (CABs) in the 1980s to help design and implement research within the NIH trials network, making CABs one of the earliest mandated forms of stakeholder engagement in HIV trials in the United States 9. The first CABs in low‐ and middle‐income countries were established in the late 1990s 10. Since these early efforts, further advancements in stakeholder engagement have included the development of guidance documents such as Principles of Community Engagement by the Centers for Disease Control and Prevention 11, as well as guidelines specific to the conduct of HIV research, including: Respect, Protect, Fulfill, a guidance document for researchers involving men who have sex with men (MSM) in the HIV research process 12; the Stakeholder Engagement Toolkit for HIV Prevention Trials 13; Recommendations for Community Engagement in HIV/AIDS Research, developed by community stakeholders partnering with the NIH 14; and the Good Participatory Practice (GPP) guidelines for stakeholder engagement in biomedical HIV prevention trials 3. Developed jointly by UNAIDS and the AIDS Vaccine Advocacy Coalition (AVAC) in 2007, the GPP guidelines established a framework for effective stakeholder engagement in HIV clinical trials that are applicable to a broad range of stakeholders and use of an array of engagement methods 3. These guidelines were revised in 2011 based on extensive consultation and feedback with global stakeholders. The GPP guidelines recommend stakeholder engagement as a continual process throughout the stages of a clinical trial: research question development, protocol development, recruitment, enrolment, follow‐up, trial results and dissemination.
Although the importance of stakeholder engagement for HIV clinical trials is widely recognized, little is known about how engagement strategies are being implemented in this field. Existing literature is limited to examining the historical development of stakeholder engagement 15, exploring single sites of stakeholder engagement 16 and reviewing implementation challenges 17. The purpose of our systematic review is to examine stakeholder engagement for HIV clinical trials and compare it to GPP benchmarks. More data on how stakeholder engagement is being conducted in‐practice could help inform GPP guidelines and local engagement strategies for specific HIV trials. Five primary research questions are used to guide our inquiry: (1) What are the geographical locations in which stakeholder engagement is conducted for HIV clinical trials? (2) What methods of stakeholder engagement have been used to inform HIV clinical trials? (3) What types of stakeholders have been engaged? (4) For what purpose has stakeholder engagement been undertaken in relation to informing HIV clinical trials? (5) What is the quality of reporting on stakeholder engagement for HIV clinical trials? By examining how stakeholder engagement for HIV clinical trials has been conducted and reported, our review aims to provide a better understanding of patterns and gaps in existing engagement efforts, pointing to opportunities for improvement in accordance with the recommendations established by the GPP guidelines.
2. Methods
2.1. Search strategy
We used the PRISMA checklist for reporting systematic review findings (Figure 1). We searched English language studies published before 9 August 2017. Search terms included variations to capture the concept of stakeholder engagement (community engage* OR community consult* OR participatory OR community advis* OR stakeholder*) in combination with the terms HIV and clinical trial*. We searched four databases: PubMed, OVID, CINAHL, and Web of Science. To supplement database results, we additionally searched six HIV journals using their respective journal search functions: Lancet HIV, Journal of the International AIDS Society, AIDS, Journal of Acquired Immune Deficiency Syndromes, AIDS Research and Human Retroviruses and International Journal of STD & AIDS. Finally, studies’ reference lists were handsearched for additional articles to include. We also contacted three individuals with relevant expertise to recommend additional references for inclusion. These individuals were experts in the field of stakeholder engagement for HIV clinical trials and/or principal investigators on NIH‐funded projects examining the conduct of HIV research.
Figure 1.
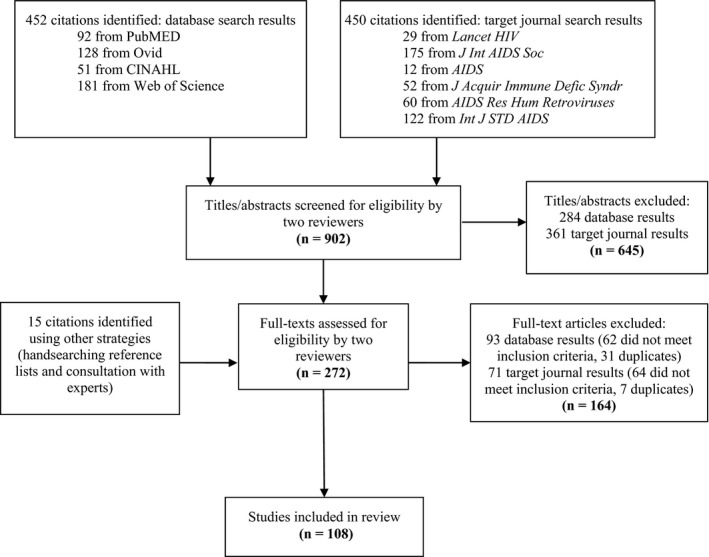
Search and selection strategy results for a systematic review of stakeholder engagement for HIV clinical trials.
2.2. Study selection
To be selected for review, a study had to describe some form of stakeholder engagement undertaken for informing the design or conduct of an HIV clinical trial. Two reviewers independently screened all titles and abstracts returned from searches. Disagreements were resolved through discussion with a third reviewer. The full texts of selected abstracts were then read in full independently by two reviewers for final inclusion and again compared for agreement, with discrepancies resolved by third reviewer. Duplicates were removed and reasons for excluding abstracts and full texts were recorded at each selection stage. A two‐reviewer selection process was similarly applied to studies identified via reference list searching and expert input.
As the purpose of our review was to provide an overview of stakeholder engagement for HIV clinical trials, we used a broad definition of stakeholder engagement. Adapting descriptions of stakeholders and advisory mechanisms for HIV prevention trials outlined in the GPP guidelines 3, we defined stakeholder engagement as any input sought from an individual or group with a stake in HIV clinical trials to inform the design or conduct of said trials. Our definition of clinical trials follows the NIH definition, which encompasses interventions in both biomedical and behavioural outcomes 18. Using this definition allowed us to include studies describing stakeholder engagement to inform both biomedical HIV‐related trials (e.g. vaccine and microbicide trials) and behavioural trials (e.g. trials of behavioural interventions for HIV prevention). Regardless of whether the trial was biomedical or behavioural, it had to be related explicitly to HIV in order to be included in the review; for example, we did not include behavioural trials for prevention of sexually transmitted infections in general.
Recognizing that stakeholder engagement takes place along a continuum from minimal to substantial involvement 2, we did not limit selection of studies based on the extent of stakeholder engagement in the studies identified. We also did not limit inclusion of studies solely to stakeholder engagement efforts undertaken for a current HIV clinical trial; studies describing stakeholder engagement to inform future and/or hypothetical HIV clinical trials (i.e. the field of HIV clinical trial research in general) were also included. Studies were excluded on the basis of not involving stakeholder engagement to inform HIV clinical trials as per our set definitions. We excluded editorials and reviews.
2.3. Data extraction and analysis
A data extraction chart was developed to record four characteristics of studies included in the review: the geographical location of engagement activities, the methods used for stakeholder engagement, the types of stakeholders engaged and the purpose of stakeholder engagement. Geographical location was extracted based on the country where stakeholder engagement was conducted using World Bank classifications (high‐, middle‐ or low‐income countries) 19. We did not extract data on the location of the clinical trial given our focus on stakeholder engagement. The purpose of stakeholder engagement refers to the reason that it was undertaken relative to informing the conduct of an HIV clinical trial. Our choice to extract descriptions of purpose rather than outcome was again due to our selection strategy: since we included studies of stakeholder engagement to inform future/hypothetical HIV clinical trials, it was not always possible to extract data on the impact or outcomes of stakeholder engagement. However, all studies described (in varying levels of detail) the purpose for which stakeholder engagement was undertaken relative to inform HIV clinical trials.
Following data extraction, a series of analytic codes were developed to categorize the purpose of stakeholder engagement in each study by the stage of the HIV clinical trial research process that stakeholder engagement was undertaken to inform. Broad coding categories were initially developed based on the seven stages of an HIV clinical trial process as outlined in the GPP guidelines: research question development, protocol development, trial recruitment, participant enrolment, follow‐up, results and dissemination 3. We then used an open coding process to develop and apply thematic codes to each study based on the various reasons for which stakeholder engagement was undertaken to inform any given stage in the HIV clinical trial research process. Coding was conducted exhaustively to categorize the potentially multiple reasons for conducting stakeholder engagement in any one study. For example, if a study included stakeholder engagement to both enhance the ethical conduct of the trial as well as develop effective recruitment strategies, the study would receive both codes.
Data analysis involved comparing our extracted and coded data to three benchmarks outlined in the GPP guidelines 3. First, given that GPP guidelines recommend use of an array of stakeholder advisory mechanisms beyond the clinical trial CABs, we identified and categorized all stakeholder engagement methods used and calculated the number of studies using each method. We did not assess the extent of stakeholder engagement in each study because there are no standardized metrics 20. Second, GPP guidelines stress the identification of relevant trial stakeholders, noting a distinction between community stakeholders (i.e. stakeholders representing the interests of persons participating in the trial and/or affected by the trial) and other stakeholders with interests in HIV clinical trials more broadly (i.e. funders, government representatives). As such, our analysis aimed to identify and categorize the types of stakeholders presently engaged in HIV clinical trials, again calculating the number of studies engaging each stakeholder type. Finally, given that GPP guidelines recommend stakeholder engagement throughout the entire clinical trial, we categorized purpose of engagement according to the seven stages of the HIV clinical trial process.
2.4. Quality of reporting on stakeholder engagement
To assess reporting quality, we adapted the short form of a checklist on Guidance for Reporting Involvement of Patients and the Public (GRIPP) in health research 21 into a reporting quality assessment tool. All studies were assessed by one reviewer per study for inclusion of the following information: (1) description of stakeholder engagement purpose; (2) explanation for choice of stakeholder engagement method; (3) description of the development of stakeholder engagement methods used; (4) the number of stakeholders engaged; (5) the results of stakeholder engagement; (6) the impact of stakeholder engagement on trial design/conduct; and (7) discussion of limitations to the stakeholder engagement method used. In each study, these seven reporting details were assessed as being either present or absent. Analysis of reporting quality was conducted for all 108 studies overall, as well as by the type of trial that engagement was conducted to inform (i.e. behavioural prevention trials, biomedical prevention trials, treatment trials or combination/trial type not specified). Additionally, in accordance with the Sex and Gender Equity in Research (SAGER) guidelines 22, we assessed two indicators of the extent to which studies reported on stakeholders’ sex and/or gender (depending on which variable was relevant to the study): the number of stakeholders engaged by sex and/or gender category, and reporting of stakeholder results disaggregated by sex and/or gender.
3. Results
As illustrated in Figure 1, a total of 452 titles and abstracts were returned for screening from our four database searches. Of these, 168 full texts were assessed, resulting in 75 studies included for review from the database search strategy. Our target journal search produced 402 titles and abstracts for screening, of which 89 full texts were assessed and 18 studies were retained for review. Our additional search strategies (handsearching of reference lists and inquiry with field experts) yielded an additional 15 studies for inclusion. In total, 108 studies were selected for final inclusion and data extraction. The oldest study included in our review was published in 1988 23, and the most recent was published July 2017 24. Of the 108 studies in our review, 11 studies conducted stakeholder engagement to inform behavioural HIV prevention trials, 70 for biomedical HIV prevention trials and 10 for HIV treatment trials. In the remaining 17 studies, stakeholder engagement was conducted to inform either a combination of HIV‐related trial types or an unspecified type of HIV clinical trial (i.e. HIV clinical trials in general, without specifying whether prevention or treatment, behavioural or biomedical) (see Appendix 1).
Analysis of the data extracted from these 108 studies was guided by our five research questions on the characteristics and reporting of stakeholder engagement to inform HIV clinical trials. The following subsections present in detail the results of each of these five analyses. In terms of the geographical location of stakeholder engagement, the majority of studies in our review described engagement conducted in high‐income countries. Engagement methods used included both individual and group methods. We identified a wide array of stakeholders engaged, ranging from stakeholders directly involved in clinical trial processes to stakeholders on the periphery of HIV clinical trials. Engagement was found to be undertaken much more often for informing earlier stages of HIV clinical trials as compared to later stages. Finally, we found that reporting on the results of stakeholder engagement and limitations associated with engagement are the main gaps in the quality of reporting.
3.1. Location of stakeholder engagement
Of the 108 studies, 48 studies (44.4%) conducted stakeholder engagement in high‐income countries 5, 23, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70. In contrast, fewer studies conducted stakeholder engagement in middle‐ (30 studies; 27.8%) 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 and low‐income (nine studies; 8.3%) 101, 102, 103, 104, 105, 106, 107, 108, 109 countries. The location of stakeholder engagement could not be discerned in six studies (5.6%) 110, 111, 112, 113, 114, 115, and fifteen studies (13.9%) 16, 24, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128 conducted stakeholder engagement in multiple countries at different income levels.
3.2. Methods of stakeholder engagement for HIV clinical trials
In addition to CABs, we identified 13 other methods of conducting stakeholder engagement across the studies in our review, for a total of 14 distinct methods (Table 1). Methods were separated into five individual methods (i.e. methods involving input or feedback by one stakeholder at a time, such as interviews) and nine group methods (i.e. methods involving input or feedback in a collective format, such as focus groups).
Table 1.
Methods of stakeholder engagement (n = 108)
| Methods of stakeholder engagement | Method description | Studies using each method | Number of studies using each methoda (%) | ||
|---|---|---|---|---|---|
| Individual engagement methods | Stakeholder interviews | Interviews conducted with individuals identified as key stakeholders | 5, 16, 26, 27, 28, 32, 33, 44, 49, 54, 56, 58, 62, 68, 69, 72, 76, 79, 82, 86, 88, 89, 90, 91, 93, 95, 96, 97, 98, 100, 101, 102, 106, 108, 109, 110, 111, 113, 116, 117, 124, 125, 128 | 43 (39.8%) | 75 (69.4%) |
| Surveys/questionnaires | Surveys or questionnaires about stakeholder perspectives administered by mail, online or in‐person | 24, 26, 30, 31, 43, 48, 49, 52, 53, 56, 57, 58, 62, 63, 64, 65, 66, 67, 80, 81, 83, 87, 114, 123 | 24 (22.2%) | ||
|
Individual stakeholder consultations |
Consultations on trial issues/processes sought with specific key informants | 30, 34, 40, 45, 50, 72, 103, 104, 105, 118, 119, 120, 121 | 13 (12.0%) | ||
| Cognitive mapping | Mixed‐methods approach involving stakeholder interviews, map sketching and observational techniques | 77 | 1 (0.9%) | ||
| Concept mapping | Mixed‐methods approach involving initial stakeholder idea generation and subsequent stakeholder‐led categorization and ranking of submitted ideas. | 35 | 1 (0.9%) | ||
| Group engagement methods | Focus group discussions | Multiple stakeholders led in a group discussion by a facilitator | 5, 16, 27, 32, 33, 37, 38, 39, 45, 46, 47, 50, 55, 60, 71, 74, 79, 82, 85, 89, 90, 94, 96, 98, 100, 101, 106, 107, 108, 109, 116, 125, 128 | 33 (30.6%) | 66 (61.1%) |
|
Community advisory boards/groups |
A formally established group of stakeholders representing community interests and providing a link between trial researchers and the broader community | 23, 25, 26, 28, 29, 30, 34, 36, 37, 42, 50, 51, 75, 80, 84, 92, 102, 112, 115, 118, 119, 120, 121, 122 | 24 (22.2%) | ||
|
Community forums or meetings |
Public or invitational meetings held to inform the community about trial issues/processes and obtain feedback from community members | 30, 34, 61, 70, 75, 92, 99, 103, 108, 112, 118, 121, 122, 126, 127 | 15 (13.9%) | ||
| Stakeholder workshops/education sessions | Events where stakeholders are convened to solve specific trial‐related problem(s) and/or build capacity to understand trial issues/processes | 26, 30, 41, 75, 78, 102, 108, 115, 118, 126 | 10 (9.3%) | ||
| Community working groups | Group of stakeholders convened to solve or advise on trial‐related problems | 59, 75, 120 | 3 (2.8%) | ||
| Media outreach campaigns | Informing the broader community about trial issues/processes through mass media and inviting commentary/feedback from stakeholders reached through media messaging | 34, 73, 92 | 3 (2.8%) | ||
| Crowdsourcing | Having a group participate in solving a problem and then sharing the solution with the public | 72 | 1 (0.9%) | ||
| Participatory mapping | Community members collaborate with field workers to develop a map depicting local knowledge and community needs | 102 | 1 (0.9%) | ||
| Dramatic performances | Skits or plays performed to inform about trial‐related issues/processes and prompt feedback from the audience | 112 | 1 (0.9%) | ||
For totals and percentages of overall individual versus group methods, studies that used multiple types of individual or group methods were only counted once for each of the two method categories.
As shown in Table 1, individual methods appeared in 75 (69.4%) studies and group methods were used in 66 studies (61.1%). The most frequently used method for stakeholder engagement was stakeholder interviews, followed by focus group discussions. CABs were used as often as surveys/questionnaires. Five methods were used by only one study each: concept mapping 35, cognitive mapping 77, crowdsourcing 72 (having a group participate in solving a problem and then sharing the solution with the public), participatory mapping 102 and dramatic performances 112. All five of these studies were published from the year 2005 onward, suggesting more recent diversification of stakeholder engagement methods. Additionally, many studies used a combination of both individual and group methods for stakeholder engagement; for example, 19 studies (17.6%) paired focus group discussions with stakeholder interviews 5, 16, 27, 32, 33, 79, 82, 89, 90, 96, 98, 100, 101, 106, 108, 109, 116, 125, 128.
3.3. Types of stakeholders engaged for HIV clinical trial research
Table 2 presents our analysis of the types of stakeholders engaged throughout all studies reviewed. We identified 35 unique types of stakeholders, which can be grouped into eight subcategories under three broader categories: trial‐related stakeholders, community stakeholders and broader stakeholders. Similar to the categories of stakeholders identified in the GPP guidelines as being relevant to HIV clinical trial research 3, we found that the types of stakeholders engaged ranged from individuals or groups in close proximity to the trial (e.g. trial participants themselves) to broader stakeholders who hold an interest in HIV trial outcomes more generally (e.g. policymakers).
Table 2.
Types of Stakeholders Engaged (n = 108)
| Types of stakeholders engaged | Studies engaging each stakeholder type | Number of studies engaging each stakeholder typea (%) | |||
|---|---|---|---|---|---|
| Trial‐related stakeholders | Participant trial‐related stakeholders | Trial participants (past or current) | 16, 30, 31, 36, 49, 56, 62, 70, 74, 79, 80, 81, 83, 86, 87, 91, 98, 101, 103, 106, 108, 112, 113, 116, 118, 125, 128 | 27 (25.0%) | 29 (26.9%) |
| Partners of trial participants | 75, 79, 106 | 3 (2.8%) | |||
| Potential trial participants (not further specified) | 81, 94 | 2 (1.9%) | |||
| Non‐participant trial‐related stakeholders | Community advisory board/group members (not further specified) | 28, 30, 35, 37, 71, 74, 77, 78, 79, 84, 85, 88, 89, 92, 98, 101, 108, 115, 116, 118, 119, 120, 124, 127 | 24 (22.2%) | 36 (33.3%) | |
| Trial research team members (e.g. site staff, recruitment officers) | 16, 30, 56, 58, 71, 74, 76, 78, 88, 89, 98, 101, 102, 103, 108, 112, 113, 114, 116, 124, 127, 128 | 22 (20.4%) | |||
| Trial sponsors | 61, 78, 83, 88 | 4 (3.7%) | |||
| Community stakeholders | HIV‐affected community stakeholders | Populations of interest (e.g. based on race, sexual orientation, occupation, geographical location, risk status) | 16, 28, 33, 37, 38, 39, 41, 42, 43, 44, 45, 47, 48, 50, 52, 54, 55, 63, 64, 65, 66, 67, 68, 69, 72, 77, 85, 90, 93, 95, 96, 97, 100, 102, 109, 117, 121, 123, 125, 128 | 40 (37.0%) | 60 (55.6%) |
| People involved in HIV advocacy (e.g. community outreach) | 16, 23, 25, 28, 30, 33, 34, 36, 51, 57, 61, 78, 96, 100, 105, 118, 127 | 17 (15.7%) | |||
| People living with HIV (not further specified) | 5, 26, 27, 32, 36, 53, 57, 60, 109 | 9 (8.3%) | |||
| Partners of people living with HIV | 101 | 1 (0.9%) | |||
| Family members/guardians of people living with HIV | 23, 104 | 2 (1.9%) | |||
| Local community stakeholders | Community leaders (e.g. political, traditional, religious) | 5, 16, 23, 29, 32, 36, 45, 60, 75, 82, 93, 96, 98, 100, 102, 105, 106, 109, 112, 121, 122 | 21 (19.4%) | 41 (38.0%) | |
| Community stakeholders/representatives (not further specified) | 29, 30, 56, 59, 70, 92, 98, 108, 112, 120, 122, 125, 128 | 13 (12.0%) | |||
| General community members (general public) | 26, 30, 46, 48, 72, 73, 82, 92, 99, 107, 115, 121 | 12 (11.1%) | |||
| Local media representatives | 29, 79, 88 | 3 (2.8%) | |||
| School teachers/principals | 75, 85 | 2 (1.9%) | |||
| Food/recreation facility owners/managers | 102 | 1 (0.9%) | |||
| Organizational community stakeholders | Non‐governmental organizations | 16, 29, 75, 76, 88, 92, 102, 106, 110, 111, 121, 122 | 12 (11.1%) | 20 (18.5%) | |
| Community‐based organizations/groups serving people living with HIV | 5, 16, 26, 32, 36, 79, 82, 111, 118 | 9 (8.3%) | |||
| Community‐based organizations (not further specified) | 29, 36, 75, 79, 85, 102, 121, 122 | 8 (7.4%) | |||
| Human rights groups | 111 | 1 (0.9%) | |||
| Broader stakeholders | Healthcare stakeholders | Healthcare providers | 27, 29, 33, 34, 36, 42, 57, 60, 75, 82, 93, 96, 100, 106, 109, 118, 121, 126, 128 | 19 (17.6%) | 23 (21.3%) |
| Healthcare facility managers/staff | 23, 75, 85, 93, 94, 126 | 6 (5.6%) | |||
| Drug industry representatives | 61 | 1 (0.9%) | |||
| Research stakeholders | IRB/Ethics Committee Membersb | 27, 30, 70, 83, 88 | 5 (4.6%) | 13 (12.0%) | |
| HIV researchers | 24, 61, 76, 118 | 4 (3.7%) | |||
| Clinical researchers | 70, 94 | 2 (1.9%) | |||
| Ethics experts (not further specified) | 70, 120 | 2 (1.9%) | |||
| Survey design experts | 72 | 1 (0.9%) | |||
| Research advocates | 70 | 1 (0.9%) | |||
| Women's health researchers | 34 | 1 (0.9%) | |||
| Anthropologists | 72 | 1 (0.9%) | |||
| Governmental stakeholders | Government health research organizations | 30, 35, 40, 61, 127 | 5 (4.6%) | 12 (11.1%) | |
| Government health officials | 40, 61, 70, 102, 118 | 5 (4.6%) | |||
| Policymakers and government representatives (not further specified) | 29, 76, 88, 122 | 4 (3.7%) | |||
For totals and percentages of subcategories of stakeholders, studies that engaged multiple types of stakeholders within the same subcategory were only counted once per subcategory.
Engagement of IRB/Ethics Committee Members refers to engagement efforts outside of the standard IRB/Ethics review process.
As shown in Table 2, 29 studies included participant trial‐related stakeholders in their engagement efforts and 36 studies engaged non‐participant trial‐related stakeholders, the latter of which included community advisory board/group members, trial staff, and trial funders. Collectively these stakeholders are in closest proximity to the HIV clinical trial research process.
The community stakeholders category presented in Table 2 comprises stakeholders drawn from communities (socially or geographically defined) in which HIV clinical trials may be embedded. Approximately half of all studies included HIV‐affected community stakeholders in their engagement efforts, a subcategory of community stakeholders which encompassed key populations of interest, people involved in HIV advocacy, people living with HIV, and partners and family members of people living with HIV. The most frequently engaged type of stakeholder was populations of interest (40 studies; 37.0%), defined on the basis of socio‐demographic characteristics, occupation, relationship status, HIV risk status or other factors that made a population of particular interest to an HIV clinical trial. In contrast to this targeted approach in defining stakeholders, members of the general public were engaged in just 12 studies (11.1%). Thirteen studies (12%) described engagement conducted with community stakeholders or community representatives without specifying further as to what aspect of the community these stakeholders represent 29, 30, 56, 59, 70, 92, 98, 108, 112, 120, 122, 125, 128.
Stakeholder types encompassed by the broader stakeholders category in Table 2 differ substantially from lay community members. Studies engaging broader stakeholders sought input from medical, academic and governmental experts, including three types of healthcare stakeholders (23 studies), eight types of research stakeholders (13 studies) and three types of governmental stakeholders (12 studies). It is important to note that while these are not the most frequently engaged stakeholders in HIV clinical trials, limiting investigation of engagement to ‘community’ members/representatives would fail to capture the involvement of these groups.
3.4. Purpose of stakeholder engagement for HIV clinical trials
Table 3 presents the results of our coding for the purpose of stakeholder engagement in the studies reviewed, organized by the seven stages of HIV clinical trial research.
Table 3.
Purpose of stakeholder engagement (n = 108)
| Purpose of stakeholder engagement, by research stage | Studies using stakeholder engagement for each purpose | Number of studies using each purposea (%) | ||
|---|---|---|---|---|
| Research question development | Understanding stakeholder perspectives on trial feasibility/acceptability | 5, 27, 30, 32, 82, 106, 112, 121, 122 | 9 (8.3%) | 15 (13.9%) |
| Setting research priorities/goals | 36, 40, 61, 84, 117, 118 | 6 (5.6%) | ||
| Protocol design | Informing ethical conduct of trial (e.g. participant rights, stopping rules, communication, IRB submission, confidentiality, concepts of fairness) | 23, 36, 67, 68, 70, 76, 78, 88, 94, 99, 115, 117, 120, 123, 125, 127, 128 | 17 (15.7%) | 49 (45.4%) |
| Developing trial tools (e.g. interventions, measurements, training materials, participant education materials) | 24, 25, 29, 35, 36, 37, 38, 39, 42, 72, 74, 103, 106, 107, 119 | 15 (13.9%) | ||
| Developing stakeholder engagement strategies for trial | 16, 50, 57, 59, 70, 75, 76, 102, 110, 111, 113, 116, 122, 124 | 14 (13.0%) | ||
| Developing trial protocol (in general or not further specified) | 29, 34, 36, 51, 109 | 5 (4.6%) | ||
| Selecting trial sites | 34, 37, 105 | 3 (2.8%) | ||
| Determining trial participation incentives/compensation | 25, 94 | 2 (1.9%) | ||
| Securing healthcare services for trial participants | 126 | 1 (0.9%) | ||
| Developing trial site management strategies | 114 | 1 (0.9%) | ||
| Recruitment | Understanding factors affecting trial recruitment (e.g. attitudes about trial participation) | 31, 33, 43, 45, 46, 48, 52, 53, 54, 55, 58, 60, 62, 63, 64, 65, 66, 69, 85, 87, 89, 90, 95, 97, 98, 100, 106, 109, 110 | 29 (26.9%) | 47 (43.5%) |
| Building community education/awareness to enhance recruitment and/or community support for trial | 26, 29, 30, 41, 73, 75, 92, 112, 115, 121, 122 | 11 (10.2%) | ||
| Developing trial recruitment strategies | 29, 34, 47, 50, 68, 77, 93 | 7 (6.5%) | ||
| Building credibility for trial among community to enhance recruitment | 37 | 1 (0.9%) | ||
| Enrolment | Enhancing the informed consent process | 49, 53, 56, 71, 80, 81, 96, 99, 101, 108, 112, 120, 128 | 13 (12.0%) | 13 (12.0%) |
| Follow‐up | Developing retention strategies | 34, 36, 50, 77, 93, 104 | 6 (5.6%) | 17 (15.7%) |
| Understanding factors affecting trial adherence/retention | 28, 31, 44, 79, 91 | 5 (4.6%) | ||
| Addressing participants’ concerns as they arise in trial | 75, 108, 118, 122 | 4 (3.7%) | ||
| Understanding participants’ expectations about the trial | 86 | 1 (0.9%) | ||
| Building community education/awareness to enhance retention | 115 | 1 (0.9%) | ||
| Results | Developing post‐trial processes (e.g. post‐trial access to medication) | 62, 83 | 2 (1.9%) | 3 (2.8%) |
| Reviewing/interpreting trial results | 29 | 1 (0.9%) | ||
| Dissemination | Communicating results to broader stakeholders | 23, 25, 36, 110, 117, 118, 122 | 7 (6.5%) | 11 (10.2%) |
| Communicating results to trial participants | 62, 75, 86, 110, 118, 122 | 6 (5.6%) | ||
| Developing academic products based on trial results | 29 | 1 (0.9%) | ||
For totals and percentages by research stage, studies that conducted stakeholder engagement for multiple purposes within the same research stage were only counted once per research stage.
We identified 25 distinct purposes for which stakeholder engagement was undertaken. The most frequently reported purpose for stakeholder engagement was for understanding factors affecting trial recruitment (29 studies). This includes studies that examined how stakeholders’ attitudes about HIV trial participation may impact recruitment; for example, examining how stakeholders’ perceptions of early trial termination might affect willingness to participate in future vaccine trials 33, 62. Additional examples of studies using stakeholder engagement for this purpose include studies investigating barriers and facilitators to trial participation among specific populations 55, 87. The second and third most frequent purpose for conducting stakeholder engagement was to inform the ethical conduct of the trial (16 studies) and to develop trial tools (15 studies) respectively. In informing the ethical conduct of trials, stakeholders were engaged for providing input on ethics‐related concerns, either in terms of the overall trial process 70, 76, 88, 99 or in relation to particular aspects of the trial; for example, trial stopping rules 78, trial communication strategies 123 and concepts of fairness in the research relationship 94.
By examining the purpose of stakeholder engagement by research stage in Table 3, we observed that stakeholder engagement was conducted more often to inform the earlier stages of trials. More studies described undertaking stakeholder engagement to inform the trial protocol development stage (49 studies; 45.4%) than any other research stage. Nearly the same volume of studies (47; 43.5%) undertook stakeholder engagement to inform trial recruitment. Stakeholder engagement to inform the final two stages of the research process was described least frequently, with just three studies engaging stakeholders to inform the trial results stage and eleven to inform dissemination of trial results. This disparity in studies conducting stakeholder engagement for purposes across the seven stages of research is more clearly visualized by Figure 2.
Figure 2.
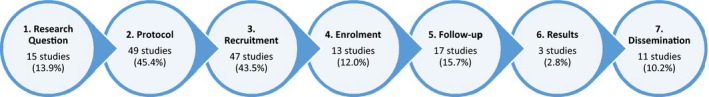
Summary of the purpose of stakeholder engagement by clinical trial research stage.
3.5. Quality of stakeholder engagement reporting
Table 4 summarizes the results of our assessment of reporting quality, indicating the number of studies meeting seven criteria adapted from the GRIPP2 checklist to improve reporting of stakeholder involvement in health research 21. We also disaggregated our analysis of stakeholder engagement reporting quality by the type of HIV‐related trial that the stakeholder engagement was meant to inform (see Appendix 1).
Table 4.
Quality of stakeholder engagement reporting (n = 108 studies)
| Reporting quality criteria | Studies meeting reporting quality criteria | Number of studies (%) | |
|---|---|---|---|
| Aim | Describes the purpose of stakeholder engagement | 5, 16, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128 | 108 (100%) |
| Methods | Explains reasons for choice of stakeholder engagement method(s) | 23, 27, 29, 35, 36, 38, 39, 41, 63, 69, 72, 73, 77, 78, 81, 86, 88, 92, 99, 101, 102, 104, 105, 106, 115, 116, 120, 121, 122, 124 | 30 (27.8%) |
| Describes development of engagement method(s) used | 5, 16, 23, 26, 27, 29, 30, 32, 33, 34, 35, 36, 38, 39, 40, 41, 43, 44, 45, 46, 47, 48, 49, 52, 53, 54, 55, 56, 57, 58, 59, 60, 62, 63, 64, 66, 69, 70, 72, 73, 75, 77, 78, 79, 80, 81, 82, 83, 85, 86, 87, 88, 89, 90, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 108, 109, 110, 111, 114, 116, 119, 120, 122, 124, 125, 126, 128 | 82 (75.9%) | |
| Reports the number of all stakeholders engaged | 16, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 35, 36, 38, 39, 40, 43, 44, 45, 46, 47, 48, 49, 52, 53, 54, 55, 56, 57, 59, 60, 62, 63, 64, 65, 66, 67, 68, 69, 71, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 106, 108, 109, 110, 111, 113, 114, 116, 123, 124, 125, 127, 128 | 82 (75.9%) | |
| Results | Describes results of stakeholder engagement | 5, 16, 23, 24, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 38, 39, 40, 41, 43, 44, 45, 46, 48, 49, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 104, 105, 106, 108, 109, 110, 111, 113, 114, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128 | 97 (89.8%) |
| Outcomesa | Discusses impact of stakeholder engagement on HIV clinical trial (where applicable) | 23, 25, 29, 30, 31, 34, 36, 39, 41, 47, 49, 50, 61, 72, 73, 75, 78, 92, 103, 104, 105, 108, 109, 115, 118, 119, 120, 121, 122 | 29 (70%)a |
| Reflections | Discusses limitations of stakeholder engagement | 5, 16, 24, 26, 27, 28, 29, 31, 32, 33, 34, 35, 36, 41, 43, 44, 45, 46, 48, 52, 53, 54, 55, 56, 58, 60, 62, 63, 64, 68, 71, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 87, 88, 90, 91, 93, 94, 95, 96, 98, 99, 100, 101, 102, 110, 111, 114, 116, 120, 123, 125, 127 | 62 (57.4%) |
Reporting on outcomes was assessed only among 41 studies that were not related to future/hypothetical trials.
While all 108 studies included in the review described at least one purpose for conducting stakeholder engagement, Table 4 demonstrates that most studies also provided details on the development of the engagement methods, the number of stakeholders engaged and the results of stakeholder engagement. ‘Results of stakeholder engagement’ refers to reporting the information obtained through a study's engagement method, such as reporting findings from focus group discussions 27, 71, 101. This differs from reporting the outcome of stakeholder engagement, which we defined as reporting on the impact that stakeholder engagement made on the design or conduct of an HIV clinical trial, such as describing how the results of crowdsourcing were subsequently used to develop a clinical trial's intervention 72. We found that stakeholder engagement outcomes were assessable among 41 studies (67 studies included in the review conducted stakeholder engagement to inform future/hypothetical trials); however, among these 41 studies, only 29 (70%) met the reporting criteria, meaning 30% of studies with the opportunity to report on stakeholder engagement outcomes did not do so. Additionally, of all 108 studies reviewed, 60 (55.6%) reported the number of engaged stakeholders by sex and/or gender category; however, only four studies reported stakeholder engagement results disaggregated by sex and/or gender category 38, 52, 74, 87.
4. Discussion
This systematic review described stakeholder engagement for HIV clinical trials and compared this engagement to GPP recommendations. Our review suggests critical gaps in stakeholder engagement that should be examined and addressed in the field of HIV clinical trial research.
First, we found more of the studies included in our review conducted stakeholder engagement in HICs compared to LMICs. This finding is consistent with a review of clinical trial priority setting processes 129. One potential explanation for these results may be that there is a greater proportion of HIV clinical trials conducted in HIC settings, as a review of infectious disease trials registered with ClinicalTrials.gov found that the greatest proportion of all registered HIV trials were located in North America and Europe 130. In addition, conducting stakeholder engagement in LMICs may be hindered by limited resources, communication barriers, and mistrust of research 16. However, while stakeholder engagement may be challenging to conduct in LMICs, these are also the contexts in which stakeholder engagement may be most important 131. Our results demonstrate a need for more evidence to inform HIV clinical trials in LMICs.
Second, our data suggest that while many methods are used, most stakeholder engagement is conducted using researcher‐driven, top‐down methods. This often involves formal social science methods such as in‐depth interviews or focus group discussions. It is unclear how effective these methods are for fostering meaningful partnerships and continuous dialogue as the GPP guidelines recommend 3. Additionally, the extent to which top‐down engagement methods can inform the design and conduct of HIV clinical trials depends entirely on trial researchers. Thus, while the GPP guidelines recommend that trial researchers carefully consider and select from the range of possible advisory mechanisms 3, the reliance on top‐down, expert‐driven stakeholder engagement suggests the need for these and other guidance documents to consider innovative, bottom‐up engagement strategies. For example, crowdsourcing approaches that allow community members a more participatory role in informing HIV clinical trials could supplement existing stakeholder engagement strategies 132. Engagement methods that follow a participatory model can help to achieve more meaningful inclusion of stakeholders and greater opportunities to change the status quo 2.
Third, we found that stakeholder engagement was predominately conducted to inform early trial stages. These findings are comparable to those of studies examining stakeholder engagement in other fields of health research 133, 134. Both of these studies emphasize the importance of engaging stakeholders throughout all stages of the research, a recommendation also posed by the GPP guidelines for HIV clinical trials 3. In order to meet these benchmarks for GPP, our results suggest that greater efforts are particularly needed to engage stakeholders in the later stages of HIV clinical trial research. Future research should examine innovative methods to foster opportunities for stakeholder contributions at these points in the research process. Additionally, while multiple guidance documents exist to promote meaningful and effective stakeholder engagement 3, 11, 12, 13, 14, HIV clinical trial teams should consider how to tailor these recommendations so that engagement efforts account for the specificities of the type of trial being conducted as well as for local contexts (e.g. social, political). These efforts by HIV researchers could help to establish models for stakeholder engagement in clinical trial research more broadly.
Our findings should be considered alongside broader factors that inform the engagement process and researcher‐stakeholder relationship in HIV clinical research. The extent to which stakeholders are engaged is shaped not only by the clinical trial team, but also by the structural contexts within which clinical trial research is embedded. As noted by others 15, it is important to consider how funders and corporate interests influence stakeholder engagement. For example, the funding of many HIV trials by high‐income countries may inadvertently assert norms and activities (e.g. community advisory boards) that are not locally driven. The impact of global resource disparities on stakeholder engagement should also be considered, particularly for the potential to reproduce inequalities in terms of which stakeholders are engaged 16. Thus, while the results of our review help to make visible some of the gaps in current stakeholder engagement for HIV clinical trials, more research is needed to account for why these gaps occur and how best to address these gaps as a product of broader structural contexts.
There are several important limitations to this review. First, we did not assess quality of engagement. However, there is a notable lack of quality measurement tools for stakeholder engagement 20, as well as disagreement regarding whether and how to determine what level of engagement is appropriate 135. Second, our review does not examine the outcomes of stakeholder engagement; however, only 41 studies (38%) in our review provided information on engagement outcomes. Future reviews should focus on systematically assessing engagement outcomes in relation to methods used and stakeholders engaged. Third, our finding that fewer studies conducted stakeholder engagement in LMICs may be attributable in part to our search strategy being limited to English language studies only. Manuscript selection bias (i.e. the overrepresentation of scientific publications from HICs) may also play a role 136. Fourth, the extent to which this review can provide an overview of stakeholder engagement for HIV clinical trials is necessarily dependent on the extent to which these activities are reported. It is possible that more engagement takes place “behind the scenes” of clinical trial research without making its way into published accounts of trial results. Improved reporting standards in accordance with guidance documents such as those used in our analysis of reporting quality 21 may help to provide further evidence for all research teams seeking to enhance their own engagement approaches, regardless of HIV trial type.
5. Conclusions
The results of this systematic review of stakeholder engagement for HIV clinical trials have implications for research and policy. First, our finding of fewer studies conducting stakeholder engagement in LMICs suggests the need for further reporting on stakeholder engagement in these settings 131. Additional resources and regulations to support and sustain stakeholder engagement in these settings may be necessary to address potential barriers to engagement. Second, despite engagement recommendations outlined in comprehensive guidelines 3 and funding allocated on the part of national and international funding bodies to support engagement activities 20, our findings suggest that stakeholder engagement is not being conducted evenly to inform all stages of the HIV clinical trial process. More research is needed in order to understand barriers and facilitators to involving stakeholders in the later stages of HIV clinical trial research specifically, as well as which methods of engagement would be most conducive to involving stakeholders in trial results and dissemination processes. Funders should additionally consider adding specifications to stakeholder engagement requirements to help address this gap, such as requiring clinical trial researchers to include detailed engagement plans for each stage of the trial process. Future research could then examine whether and how stakeholder engagement changes over time in response to such efforts. Finally, to address gaps identified in reporting quality, HIV research journals should consider implementing policies about reporting stakeholder engagement. Checklists for reporting on stakeholder engagement 21, 133 may help to promote greater transparency as to what engagement efforts are undertaken in trials and how this engagement shapes the research process. This information will be particularly valuable for undertaking future research to evaluate the quality of stakeholder engagement.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
S.D., J.T. and R.S. conceived of the idea for this review. S.D. designed the review protocol, and S.D. and M.B conducted the search. S.D., Y.Z., M.B. and T.V read and selected the citations, abstracts and full texts. S.D., Y.Z. and T.V extracted the data. S.D. with assistance from M.B. and T.V., as well as in consultation with S.R. and J.T conducted the coding and analysis. S.D., M.B. and T.V., with substantial contributions and edits provided by Y.Z., S.R. and J.T, prepared the manuscript. All authors reviewed, provided feedback and approved the final manuscript.
Authors’ information
The authors are part of a working group examining the social and ethical aspects of research on curing HIV (searcHIV). More information about our working group is available at: http://searchiv.web.unc.edu/
Supporting information
Appendix S1. Quality of Reporting on Stakeholder Engagement, by Type of HIV Clinical Trial.
Acknowledgements
The authors thank the searcHIV research team, the Department of Social Medicine and the Center for Bioethics at UNC Chapel Hill. The authors sincerely thank Dr Kathleen MacQueen for her expert assistance in identifying additional studies for inclusion in the review. This research was supported by the National Institutes of Health (NIAID R01A108366, 5P30AI050410).
Day S., Blumberg M., Vu T., Zhao Y., Rennie S. and Tucker J. D. Stakeholder engagement to inform HIV clinical trials: a systematic review of the evidence. J Int AIDS Soc. 2018; 21(S7):e25174
References
- 1. Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community‐based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. [DOI] [PubMed] [Google Scholar]
- 2. Goodman MS, Sanders Thompson VL. The science of stakeholder engagement in research: classification, implementation, and evaluation. Transl Behav Med. 2017;7(3):486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS/AIDS Vaccine Advocacy Coalition . Good participatory practice: guidelines for biomedical HIV prevention trials. Geneva: UNAIDS; 2011. [Google Scholar]
- 4. Brizay U, Golob L, Globerman J, Gogolishvili D, Bird M, Rios‐Ellis B, et al. Community‐academic partnerships in HIV‐related research: a systematic literature review of theory and practice. J Int AIDS Soc. 2015;18:19354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corbie‐Smith G, Isler MR, Miles MS, Banks B. Community‐based HIV clinical trials: an integrated approach in underserved, rural, minority communities. Prog Community Health Partnersh. 2012;6(2):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Santis J. Exploring the concepts of vulnerability and resilience in the context of HIV infection. Res Theory Nurs Pract. 2008;22(4):273–87. [DOI] [PubMed] [Google Scholar]
- 7. Rennie S, Sugarman J. Developing ethics guidance for HIV prevention research: the HPTN approach. J Med Ethics. 2010;36(12):810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castillo‐Mancilla JR, Cohn SE, Krishnan S, Cespedes M, Floris‐Moore M, Schulte G, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. 2014;15(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddy P, Buchanan D, Sifunda S, James S, Naidoo N. The role of community advisory boards in health research: divergent views in the South African experience. SAHARA J. 2010;7(3):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International AIDS Vaccine Initiative . Guidance tool for community advisory boards. New York: International AIDS Vaccine Initiative; 2012. [Google Scholar]
- 11. Centers for Disease Control and Prevention . Principles of community engagement. 2nd ed [Cited August 8, 2018]. Available from: http://www.atsdr.cdc.gov/communityengagement/pdf/PCE_Report_508_FINAL.pdf National Institutes of Health Research; 2011. [Google Scholar]
- 12. amfAR, International AIDS Vaccine Initiative (IAVI), Johns Hopkins University – Center for Public Health and Human Rights (JHU‐CPHHR), United Nations Development Programme (UNDP) . Respect, protect, fulfill: best practices guidance in conducting HIV research with gay, bisexual, and other men who have sex with men (MSM) in rights constrained environments. [Cited August 8, 2018]. Available from: https://www.avac.org/sites/default/files/resource-files/RespectProtectFulfill2015.pdf 2015. [Google Scholar]
- 13. Macqueen KM, Harlan SV, Selvin KW, Hannah S, Bass E, Moffett J. Stakeholder engagement toolkit for HIV prevention trials. Durham: FHI; 2012. [Google Scholar]
- 14. HIV/AIDS Network Coordination, Community Partners – National Institutes of Health . Recommendations for community engagement in HIV/AIDS research. [Cited August 8, 2018]. Available from: https://www.hanc.info/cp/resources/Documents/Recommendations%202014%20FINAL%206-5-14%20rc.pdf National Institutes of Health; 2014. [Google Scholar]
- 15. Lo YR, Chu C, Ananworanich J, Excler JL, Tucker JD. Stakeholder engagement in HIV cure research: lessons learned from other HIV interventions and the way forward. AIDS Patient Care STDS. 2015;29(7):389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newman PA, Rubincam C, Slack C, Essack Z, Chakrapani V, Chuang DM, et al. Towards a science of community stakeholder engagement in biomedical HIV prevention trials: an embedded four‐country case study. PLoS ONE. 2015;10(8):e0135937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kagee A, De Wet A, Kafaar Z, Lesch A, Swartz L, Newman PA. Caveats and pitfalls associated with researching community engagement in the context of HIV vaccine trials. J Health Psychol. 2017; Dec 1:1359105317745367. doi: 10.1177/1359105317745367. [DOI] [PubMed] [Google Scholar]
- 18. National Institutes of Health . NIH's definition of a clinical trial 2017. [cited February 1, 2018]. Available from: https://grants.nih.gov/policy/clinical-trials/definition.htm
- 19. World Bank . World Bank Country and Lending Groups 2018. [cited February 1, 2018]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 20. MacQueen KM, Bhan A, Frohlich J, Holzer J, Sugarman J. Evaluating community engagement in global health research: the need for metrics. BMC Med Ethics. 2015;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valdiserri RO, Tama GM, Ho M. The role of community advisory committees in clinical trials of anti‐HIV agents. Irb. 1988;10(4):5–7. [PubMed] [Google Scholar]
- 24. Stalter RM, Tharaldson J, Owen DH, Okumu E, Moench T, Mack N, et al. Attitudes and perceptions towards novel objective measures of ARV‐based vaginal ring use: results from a global stakeholder survey. PLoS ONE. 2017;12(7):e0180963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gamble T, Branson B, Donnell D, Hall HI, King G, Cutler B, et al. Design of the HPTN 065 (TLC‐Plus) study: a study to evaluate the feasibility of an enhanced test, link‐to‐care, plus treat approach for HIV prevention in the United States. Clin Trials 2017;14(4):322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vines AI, Melvin CL, Hunter JC, Carlisle VA. Project ACCRUE: exploring options to increase awareness of AIDS malignancy consortium clinical trials in North Carolina. N C Med J. 2017;78(2):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rhodes E, Andreae M, Bourgiose T, Indyk D, Rhodes R, Sacks H. Stakeholders’ views on barriers to research on controversial controlled substances. J Clin Ethics. 2016;27(4):308–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia J, Parker C, Parker RG, Wilson PA, Philbin M, Hirsch JS. Psychosocial implications of homophobia and HIV stigma in social support networks: insights for high‐impact HIV prevention among black men who have sex with men. Health Educ Behav. 2016;43(2):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Isler MR, Miles MS, Banks B, Perreras L, Muhammad M, Parker D, et al. Across the miles: process and impacts of collaboration with a rural community advisory board in HIV research. Prog Community Health Partnersh. 2015;9(1):41–8. [DOI] [PubMed] [Google Scholar]
- 30. Dawson L, Garner S, Anude C, Ndebele P, Karuna S, Holt R, et al. Testing the waters: ethical considerations for including PrEP in a phase IIb HIV vaccine efficacy trial. Clin Trials. 2015;12(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuchs JD, Sobieszczyk ME, Madenwald T, Grove D, Karuna ST, Andrasik M, et al. Intentions to use pre‐exposure prophylaxis among current phase 2B preventive HIV‐1 vaccine efficacy trial participants. J Acquir Immune Defic Syndr. 2013;63(3):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Isler MR, Miles MS, Banks B, Corbie‐Smith G. Acceptability of a mobile health unit for rural HIV clinical trial enrollment and participation. AIDS Behav. 2012;16(7):1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman PA, Logie C, James L, Charles T, Maxwell J, Salam K, et al. “Speaking the dialect”: understanding public discourse in the aftermath of an HIV vaccine trial shutdown. Am J Public Health. 2011;101(9):1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falcon R, Bridge DA, Currier J, Squires K, Hagins D, Schaible D, et al. Recruitment and retention of diverse populations in antiretroviral clinical trials: practical applications from the gender, race and clinical experience study. J Womens Health. 2011;20(7):1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kagan JM, Kane M, Quinlan KM, Rosas S, Trochim WM. Developing a conceptual framework for an evaluation system for the NIAID HIV/AIDS clinical trials networks. Health Res Policy Syst. 2009;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. NIMH Multisite HIV/STD Prevention Trial for African American Couples Group . The role of Community Advisory Boards (CABs) in Project Eban. J Acquir Immune Defic Syndr. 2008;49 Suppl 1:S68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. NIMH Multisite HIV/STD Prevention Trial for African American Couples Group . Methodological overview of an African American couple‐based HIV/STD prevention trial. J Acquir Immune Defic Syndr. 2008;49 Suppl 1:S3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. NIMH Multisite HIV/STD Prevention Trial for African American Couples Group . Formative study to develop the Eban treatment and comparison interventions for couples. J Acquir Immune Defic Syndr. 2008;49 Suppl 1:S42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sormanti M, Pereira L, El‐Bassel N, Witte S, Gilbert L. The role of community consultants in designing an HIV prevention intervention. AIDS Educ Prev. 2001;13(4):311–28. [DOI] [PubMed] [Google Scholar]
- 40. Uyei J, Li L, Braithwaite RS. HIV and alcohol research priorities of city, state, and federal policymakers: results of a delphi study. Am J Public Health. 2015;105(9):e23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leonard NR, Banfield A, Riedel M, Ritchie AS, Mildvan D, Arredondo G, et al. Description of an efficacious behavioral peer‐driven intervention to reduce racial/ethnic disparities in AIDS clinical trials. Health Educ Res. 2013;28(4):574–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naar‐King S, Outlaw AY, Sarr M, Parsons JT, Belzer M, Macdonell K, et al. Motivational Enhancement System for Adherence (MESA): pilot randomized trial of a brief computer‐delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38(6):638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR 3rd. Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sex Transm Dis. 1998;25(6):296–302. [DOI] [PubMed] [Google Scholar]
- 44. Bentley ME, Morrow KM, Fullem A, Chesney MA, Horton SD, Rosenberg Z, et al. Acceptability of a novel vaginal microbicide during a safety trial among low‐risk women. Fam Plann Perspect. 2000;32(4):184–8. [PubMed] [Google Scholar]
- 45. Andrasik MP, Chandler C, Powell B, Humes D, Wakefield S, Kripke K, et al. Bridging the divide: HIV prevention research and Black men who have sex with men. Am J Public Health. 2014;104(4):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brooks RA, Newman PA, Duan N, Ortiz DJ. HIV vaccine trial preparedness among Spanish‐speaking Latinos in the US. AIDS Care. 2007;19(1):52–8. [DOI] [PubMed] [Google Scholar]
- 47. Witte SS, El‐Bassel N, Gilbert L, Wu E, Chang M, Steinglass P. Recruitment of minority women and their main sexual partners in an HIV/STI prevention trial. J Womens Health. 2004;13(10):1137–47. [DOI] [PubMed] [Google Scholar]
- 48. Allen MA, Liang TS, La Salvia T, Tjugum B, Gulakowski RJ, Murguia M. Assessing the attitudes, knowledge, and awareness of HIV vaccine research among adults in the United States. J Acquir Immune Defic Syndr. 2005;40(5):617–24. [DOI] [PubMed] [Google Scholar]
- 49. Coletti AS, Heagerty P, Sheon AR, Gross M, Koblin BA, Metzger DS, et al. Randomized, controlled evaluation of a prototype informed consent process for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2003;32(2):161–9. [DOI] [PubMed] [Google Scholar]
- 50. Siskind RL, Andrasik M, Karuna ST, Broder GB, Collins C, Liu A, et al. Engaging transgender people in NIH‐Funded HIV/AIDS clinical trials research. J Acquir Immune Defic Syndr. 2016;72 Suppl 3:S243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keefer MC, Wolff M, Gorse GJ, Graham BS, Corey L, Clements‐Mann ML, et al. Safety profile of phase I and II preventive HIV type 1 envelope vaccination: experience of the NIAID AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1997;13(14):1163–77. [DOI] [PubMed] [Google Scholar]
- 52. Priddy FH, Cheng AC, Salazar LF, Frew PM. Racial and ethnic differences in knowledge and willingness to participate in HIV vaccine trials in an urban population in the Southeastern US. Int J STD AIDS. 2006;17(2):99–102. [DOI] [PubMed] [Google Scholar]
- 53. Schrooten W, Borchert M, Dreezen C, Baratta C, Smets E, Kosmidis J, et al. Participants in HIV clinical trials in Europe. Int J STD AIDS. 2001;12(2):94–9. [DOI] [PubMed] [Google Scholar]
- 54. Van de Ven P, Mao L, Crawford J, Prestage G, Grulich A, Kaldor J, et al. Willingness to participate in HIV vaccine trials among HIV‐negative gay men in Sydney, Australia. Int J STD AIDS. 2005;16(4):314–7. [DOI] [PubMed] [Google Scholar]
- 55. Andrasik MP, Yoon R, Mooney J, Broder G, Bolton M, Votto T, et al. Exploring barriers and facilitators to participation of male‐to‐female transgender persons in preventive HIV vaccine clinical trials. Prev Sci. 2014;15(3):268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corneli A, Namey E, Mueller MP, Tharaldson J, Sortijas S, Grey T, et al. Evidence‐based strategies for shortening informed consent forms in clinical research. J Empir Res Hum Res Ethics. 2017;12(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cox LE, Rouff JR, Svendsen KH, Markowitz M, Abrams DI. Community advisory boards: their role in AIDS clinical trials. Terry Beirn Community Programs for Clinical Research on AIDS. Health Soc Work. 1998;23(4):290–7. [DOI] [PubMed] [Google Scholar]
- 58. Magnus M, Franks J, Griffith S, Arnold MP, Goodman K, Wheeler DP. Engaging, recruiting, and retaining black men who have sex with men in research studies: don't underestimate the importance of staffing–lessons learned from HPTN 061, the BROTHERS study. J Public Health Manag Pract. 2014;20(6):E1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Isler MR, Brown AL, Eley N, Mathews A, Batten K, Rogers R, et al. Curriculum development to increase minority research literacy for HIV prevention research: a CBPR approach. Prog Community Health Partnersh. 2014;8(4):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. King WD, Wyatt GE, Liu H, Williams JK, DiNardo AD, Mitsuyasu RT. Pilot assessment of HIV gene therapy‐hematopoietic stem cell clinical trial acceptability among minority patients and their advisors. J Natl Med Assoc. 2010;102(12):1123–8. [DOI] [PubMed] [Google Scholar]
- 61. Miller V. The forum for collaborative HIV research: a model for an integrated and inclusive approach to clinical research and drug development. Clin Pharmacol Ther. 2009;86(3):332–5. [DOI] [PubMed] [Google Scholar]
- 62. Newman PA, Yim S, Daley A, Walisser R, Halpenny R, Cunningham W, et al. “Once Bitten, Twice Shy”: participant perspectives in the aftermath of an early HIV vaccine trial termination. Vaccine. 2011;29(3):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hennessy M, MacQueen K, McKirnan DJ, Buchbinder S, Judson F, Douglas JM Jr, et al. A factorial survey study to assess the acceptability of HIV vaccine trial designs. Control Clin Trials. 1996;17(3):209–20. [DOI] [PubMed] [Google Scholar]
- 64. Bartholow BN, MacQueen KM, Douglas JM Jr, Buchbinder S, McKirnan D, Judson FN. Assessment of the changing willingness to participate in phase III HIV vaccine trials among men who have sex with men. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):108–15. [DOI] [PubMed] [Google Scholar]
- 65. MacQueen KM, Buchbinder S, Douglas JM, Judson FN, McKirnan DJ, Bartholow B. The decision to enroll in HIV vaccine efficacy trials: concerns elicited from gay men at increased risk for HIV infection. AIDS Res Hum Retroviruses. 1994;10 Suppl 2:S261–4. [PubMed] [Google Scholar]
- 66. Scheer S, Douglas JM Jr, Vittinghoff E, Bartholow BN, McKirnan D, Judson FN, et al. Feasibility and suitability of targeting young gay men for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(2):172–8. [DOI] [PubMed] [Google Scholar]
- 67. MacQueen KM, Buchbinder SP, Douglas JM, Judson FN, McKirnan DJ, Bartholow BN. Required HIV antibody testing, social risk, and HIV‐vaccine efficacy trials. AIDS Public Policy J. 1996;11(2):104–12. [PubMed] [Google Scholar]
- 68. Kegeles SM, Johnson MO, Strauss RP, Ralston B, Hays RB, Metzger DS, et al. How should HIV vaccine efficacy trials be conducted? Diverse U.S. communities speak out. AIDS education and prevention : official publication of the International Society for AIDS. Education. 2006;18(6):560–72. [DOI] [PubMed] [Google Scholar]
- 69. Strauss RP, Sengupta S, Kegeles S, McLellan E, Metzger D, Eyre S, et al. Willingness to volunteer in future preventive HIV vaccine trials: issues and perspectives from three U.S. communities. J Acquir Immune Defic Syndr. 2001;26(1):63–71. [DOI] [PubMed] [Google Scholar]
- 70. Garner SA, Anude CJ, Adams E, Dawson L. Ethical considerations in HIV prevention and vaccine research in resource‐limited settings. J Acquir Immune Defic Syndr. 2014;67(1):77–83. [DOI] [PubMed] [Google Scholar]
- 71. Slack C, Thabethe S, Lindegger G, Matandika L, Newman PA, Kerr P, et al. I've gone through this my own self, so I practice what I preach. J Empir Res Hum Res Ethics. 2016;11(4):322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang W, Han L, Best J, Zhang Y, Mollan K, Kim J, et al. Crowdsourcing HIV test promotion videos: a noninferiority randomized controlled trial in China. Clin Infect Dis. 2016;62(11):1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Medeossi BJ, Stadler J, Delany‐Moretlwe S. ‘I heard about this study on the radio’: using community radio to strengthen Good Participatory Practice in HIV prevention trials. BMC Public Health. 2014;14:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gafos M, Mzimela MA, Ndlovu HB, McCormack S, McGrath N. How effective is effective enough? Opinions of potential end‐users of microbicides from a rural South African community. AIDS Care. 2013;25(5):573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ramjee G, Coumi N, Dladla‐Qwabe N, Ganesh S, Gappoo S, Govinden R, et al. Experiences in conducting multiple community‐based HIV prevention trials among women in KwaZulu‐Natal, South Africa. AIDS Res Ther. 2010;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Allman D, Ditmore MH, Kaplan K. Improving ethical and participatory practice for marginalized populations in biomedical HIV prevention trials: lessons from Thailand. PLoS ONE. 2014;9(6):e100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stadler J, Dugmore C, Venables E, MacPhail C, Delany‐Moretlwe S. Cognitive mapping: using local knowledge for planning health research. BMC Med Res Methodol. 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. White R, Chileshe M, Dawson L, Donnell D, Hillier S, Morar N, et al. Fostering community understanding of sufficient benefit and early stopping for a phase 2B HIV prevention clinical trial in Africa. Clin Trials. 2011;8(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE‐C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014;17 3 Suppl 2:19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sugarman J, Corneli A, Donnell D, Liu TY, Rose S, Celentano D, et al. Are there adverse consequences of quizzing during informed consent for HIV research? J Med Ethics. 2011;37(11):693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lindegger G, Milford C, Slack C, Quayle M, Xaba X, Vardas E. Beyond the checklist: assessing understanding for HIV vaccine trial participation in South Africa. J Acquir Immune Defic Syndr. 2006;43(5):560–6. [DOI] [PubMed] [Google Scholar]
- 82. Kebaabetswe P, Ndase P, Mujugira A, Sekoto T, Ntshimane M, Owor A, et al. Perceptions of couple HIV counseling and testing in Botswana: a stakeholder analysis. Patient Educ Couns. 2010;79(1):120–3. [DOI] [PubMed] [Google Scholar]
- 83. Dainesi SM, Goldbaum M. Post‐trial access to study medication: a Brazilian e‐survey with major stakeholders in clinical research. J Med Ethics. 2012;38(12):757–62. [DOI] [PubMed] [Google Scholar]
- 84. Kilmarx PH, van de Wijgert JH, Chaikummao S, Jones HE, Limpakarnjanarat K, Friedland BA, et al. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a phase II clinical trial. J Acquir Immune Defic Syndr. 2006;43(3):327–34. [DOI] [PubMed] [Google Scholar]
- 85. Jaspan HB, Soka NF, Mathews C, Flisher AJ, Mark D, Middelkoop K, et al. A qualitative assessment of perspectives on the inclusion of adolescents in HIV vaccine trials in South Africa. Int J STD AIDS. 2010;21(3):172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Khowsroy K, Dhitavat J, Sabmee Y, Laowarakul P, Wattanakitwichai J, Auetian J, et al. Expectation of volunteers towards the vaccine efficacy of the prime‐boost HIV vaccine phase III trial during unblinding. AIDS Res Hum Retroviruses. 2014;30(11):1041–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Volk JE, Hessol NA, Gray GE, Kublin JG, Churchyard GJ, Mlisana K, et al. The HVTN503/Phambili HIV vaccine trial: a comparison of younger and older participants. Int J STD AIDS. 2014;25(5):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Essack Z, Koen J, Barsdorf N, Slack C, Quayle M, Milford C, et al. Stakeholder perspectives on ethical challenges in HIV vaccine trials in South Africa. Dev World Bioeth. 2010;10(1):11–21. [DOI] [PubMed] [Google Scholar]
- 89. Sifunda S, Reddy P, Naidoo N, James S, Buchanan D. Recruiting and educating participants for enrollment in HIV‐vaccine research: ethical implications of the results of an empirical investigation. Public Health Ethics. 2014;7(1):78–85. [Google Scholar]
- 90. Lesch A, Kafaar Z, Kagee A, Swartz L. Community members’ perceptions of enablers and inhibitors to participation in HIV vaccine trials. S Afr J Psychol. 2006;36(4):734–61. [Google Scholar]
- 91. Corneli A, Perry B, McKenna K, Agot K, Ahmed K, Taylor J, et al. Participants’ explanations for nonadherence in the FEM‐PrEP clinical trial. J Acquir Immune Defic Syndr. 2016;71(4):452–61. [DOI] [PubMed] [Google Scholar]
- 92. Sahay S, Kumar M, Srikrishnan AK, Ramanathan V, Mehendale S. Experiences in recruiting volunteers through community based initiatives in phase‐1 vaccine trials in India. Hum Vaccin Immunother. 2014;10(2):485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Were E, Wools‐Kaloustian K, Baliddawa J, Ayuo PO, Sidle J, Fife K. Stakeholders perception of HIV sero‐discordant couples in western Kenya. East Afr Med J. 2008;85(7):326–33. [DOI] [PubMed] [Google Scholar]
- 94. Shaffer DN, Yebei VN, Ballidawa JB, Sidle JE, Greene JY, Meslin EM, et al. Equitable treatment for HIV/AIDS clinical trial participants: a focus group study of patients, clinician researchers, and administrators in western Kenya. J Med Ethics. 2006;32(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Farquhar C, John‐Stewart GC, John FN, Kabura MN, Kiarie JN. Pediatric HIV type 1 vaccine trial acceptability among mothers in Kenya. AIDS Res Hum Retroviruses. 2006;22(6):491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chakrapani V, Newman PA, Singhal N, Nelson R, Shunmugam M. “If It's Not Working, Why Would They Be Testing It?”: mental models of HIV vaccine trials and preventive misconception among men who have sex with men in India. BMC Public Health. 2013;13:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Doshi M, Avery L, Kaddu RP, Gichuhi M, Gakii G, du Plessis E, et al. Contextualizing willingness to participate: recommendations for engagement, recruitment & enrolment of Kenyan MSM in future HIV prevention trials. BMC Public Health. 2017;17(1):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nyblade L, Singh S, Ashburn K, Brady L, Olenja J. “Once I begin to participate, people will run away from me”: understanding stigma as a barrier to HIV vaccine research participation in Kenya. Vaccine. 2011;29(48):8924–8. [DOI] [PubMed] [Google Scholar]
- 99. Vreeman R, Kamaara E, Kamanda A, Ayuku D, Nyandiko W, Atwoli L, et al. A qualitative study using traditional community assemblies to investigate community perspectives on informed consent and research participation in western Kenya. BMC Medical Ethics. 2012;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chakrapani V, Newman PA, Singhal N, Jerajani J, Shunmugam M. Willingness to participate in HIV vaccine trials among men who have sex with men in Chennai and Mumbai, India: a social ecological approach. PLoS ONE. 2012;7(12):e51080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ssali A, Poland F, Seeley J. Exploring informed consent in HIV clinical trials: a case study in Uganda. Heliyon. 2016;2(11):e00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shagi C, Vallely A, Kasindi S, Chiduo B, Desmond N, Soteli S, et al. A model for community representation and participation in HIV prevention trials among women who engage in transactional sex in Africa. AIDS Care. 2008;20(9):1039–49. [DOI] [PubMed] [Google Scholar]
- 103. Psaros C, Haberer JE, Katabira E, Ronald A, Tumwesigye E, Campbell JD, et al. An intervention to support HIV preexposure prophylaxis adherence in HIV‐serodiscordant couples in Uganda. J Acquir Immune Defic Syndr. 2014;66(5):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bwakura‐Dangarembizi M, Musesengwa R, Nathoo KJ, Takaidza P, Mhute T, Vhembo T. Ethical and legal constraints to children's participation in research in Zimbabwe: experiences from the multicenter pediatric HIV ARROW trial. BMC Med Ethics. 2012;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chingono A, Lane T, Chitumba A, Kulich M, Morin S. Balancing science and community concerns in resource‐limited settings: project Accept in rural Zimbabwe. Clin Trials. 2008;5(3):273–6. [DOI] [PubMed] [Google Scholar]
- 106. Woodsong C, Alleman P. Sexual pleasure, gender power and microbicide acceptability in Zimbabwe and Malawi. AIDS Educ Prev. 2008;20(2):171–87. [DOI] [PubMed] [Google Scholar]
- 107. Nakimuli‐Mpungu E, Wamala K, Okello J, Alderman S, Odokonyero R, Mojtabai R, et al. Group support psychotherapy for depression treatment in people with HIV/AIDS in northern Uganda: a single‐centre randomised controlled trial. Lancet HIV. 2015;2(5):e190–9. [DOI] [PubMed] [Google Scholar]
- 108. Vallely A, Lees S, Shagi C, Kasindi S, Soteli S, Kavit N, et al. How informed is consent in vulnerable populations? Experience using a continuous consent process during the MDP301 vaginal microbicide trial in Mwanza, Tanzania. BMC Medical Ethics. 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Corneli AL, Piwoz EG, Bentley ME, Moses A, Nkhoma JR, Tohill BC, et al. Involving communities in the design of clinical trial protocols: the BAN Study in Lilongwe. Malawi. Contemp Clin Trials. 2007;28(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Essack Z, Koen J, Slack C, Lindegger G, Newman PA. Civil society perspectives on negative biomedical HIV prevention trial results and implications for future trials. AIDS Care. 2012;24(10):1249–54. [DOI] [PubMed] [Google Scholar]
- 111. Koen J, Essack Z, Slack C, Lindegger G, Newman PA. ‘It looks like you just want them when things get rough’: civil society perspectives on negative trial results and stakeholder engagement in HIV prevention trials. Dev World Bioeth. 2013;13(3):138–48. [DOI] [PubMed] [Google Scholar]
- 112. Woodsong C, Karim QA. A model designed to enhance informed consent: experiences from the HIV prevention trials network. Am J Public Health. 2005;95(3):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. South A, Hanley B, Gafos M, Cromarty B, Stephens R, Sturgeon K, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: findings from ten case studies. Trials. 2016;17:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rosas SR, Cope MT, Villa C, Motevalli M, Utech J, Schouten JT. Assessing the challenges of multi‐scope clinical research sites: an example from NIH HIV/AIDS clinical trials networks. J Eval Clin Pract. 2014;20(2):149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kublin JG, Morgan CA, Day TA, Gilbert PB, Self SG, McElrath MJ, et al. HIV vaccine trials network: activities and achievements of the first decade and beyond. Clin Investig. 2012;2(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Morin SF, Morfit S, Maiorana A, Aramrattana A, Goicochea P, Mutsambi JM, et al. Building community partnerships: case studies of Community Advisory Boards at research sites in Peru, Zimbabwe, and Thailand. Clin Trials. 2008;5(2):147–56. [DOI] [PubMed] [Google Scholar]
- 117. Cochrane G, Robin E, Hanlin R, Castle‐Clarke S, MacLure C, Parks S, et al. The international AIDS vaccine initiative's capacity building activities in East Africa: evaluating progress and impacts in Kenya, Uganda and Rwanda. Rand Health Q. 2016;5(3):3. [PMC free article] [PubMed] [Google Scholar]
- 118. Ndase P, Celum C, Campbell J, Bukusi E, Kiarie J, Katabira E, et al. Successful discontinuation of the placebo arm and provision of an effective HIV prevention product after a positive interim efficacy result: the partners PrEP study experience. J Acquir Immune Defic Syndr. 2014;66(2):206–12. [DOI] [PubMed] [Google Scholar]
- 119. Woodsong C, Mutsambi JM, Ntshele S, Modikoe P. Community and research staff collaboration for development of materials to inform microbicide study participants in Africa. J Int AIDS Soc. 2014;17 3 Suppl 2:19156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. NIMH Collaborative HIV/STD Prevention Trial Group . Ethical issues in the NIMH Collaborative HIV/STD Prevention Trial. Aids. 2007;21 Suppl 2:S69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ramjee G, Kapiga S, Weiss S, Peterson L, Leburg C, Kelly C, et al. The value of site preparedness studies for future implementation of phase 2/IIb/III HIV prevention trials: experience from the HPTN 055 study. J Acquir Immune Defic Syndr. 2008;47(1):93–100. [DOI] [PubMed] [Google Scholar]
- 122. Mack N, Kirkendale S, Omullo P, Odhiambo J, Ratlhagana M, Masaki M, et al. Implementing good participatory practice guidelines in the FEM‐PrEP Preexposure Prophylaxis Trial for HIV Prevention among African Women: a focus on local stakeholder involvement. Open Access J Clin Trials. 2013;5:127–35. [Google Scholar]
- 123. Ditmore MH, Allman D. ‘Who is Helsinki?’ Sex workers advise improving communication for good participatory practice in clinical trials. Health Educ Res. 2011;26(3):466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Morin SF, Maiorana A, Koester KA, Sheon NM, Richards TA. Community consultation in HIV prevention research: a study of community advisory boards at 6 research sites. J Acquir Immune Defic Syndr. 2003;33(4):513–20. [DOI] [PubMed] [Google Scholar]
- 125. MacQueen K, Namey E, Chilongozi D, Mtweve S, Mlingo M, Morar N, et al. Community perspectives on care options for HIV prevention trial participants. AIDS Care. 2007;19(4):554–60. [DOI] [PubMed] [Google Scholar]
- 126. MacQueen KM, McLoughlin K, Alleman P, Burke HM, Mack N. Partnering for care in HIV prevention trials. J Empir Res Hum Res Ethics. 2008;3(4):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. MacQueen KM, Alleman P. International perspectives on the collection, storage, and testing of human biospecimens in HIV research. Irb. 2008;30(4):9. [PMC free article] [PubMed] [Google Scholar]
- 128. Woodsong C, Macqueen K, Namey E, Sahay S, Morar N, Mlingo M, et al. Women's autonomy and informed consent in microbicides clinical trials. J Empir Res Hum Res Ethics. 2006;1(3):11–26. [DOI] [PubMed] [Google Scholar]
- 129. Tomlinson M, Chopra M, Hoosain N, Rudan I. A review of selected research priority setting processes at national level in low and middle income countries: towards fair and legitimate priority setting. Health Res Policy Syst. 2011;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Goswami ND, Pfeiffer CD, Horton JR, Chiswell K, Tasneem A, Tsalik EL. The state of infectious diseases clinical trials: a systematic review of ClinicalTrials.gov. PLoS ONE. 2013;8(10):e77086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Newman PA, Rubincam C. Advancing community stakeholder engagement in biomedical HIV prevention trials: principles, practices and evidence. Expert Rev Vaccines. 2014;13(12):1553–62. [DOI] [PubMed] [Google Scholar]
- 132. Mathews A, Farley S, Blumberg M, Knight K, Hightow‐Weidman L, Muessig K, et al. HIV cure research community engagement in North Carolina: a mixed‐methods evaluation of a crowdsourcing contest. J Virus Erad. 2017;3(4):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Concannon TW, Fuster M, Saunders T, Patel K, Wong JB, Leslie LK, et al. A systematic review of stakeholder engagement in comparative effectiveness and patient‐centered outcomes research. J Gen Intern Med. 2014;29(12):1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Forsythe LP, Ellis LE, Edmundson L, Sabharwal R, Rein A, Konopka K, et al. Patient and stakeholder engagement in the PCORI pilot projects: description and lessons learned. J Gen Intern Med. 2016;31(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bowen DJ, Hyams T, Goodman M, West KM, Harris‐Wai J, Yu JH. Systematic review of quantitative measures of stakeholder engagement. Clin Transl Sci. 2017;10(5):314–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yousefi‐Nooraie R, Shakiba B, Mortaz‐Hejri S. Country development and manuscript selection bias: a review of published studies. BMC Med Res Methodol. 2006;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Quality of Reporting on Stakeholder Engagement, by Type of HIV Clinical Trial.


