Abstract
Purpose
Adenoid cystic carcinoma (ACC) of the trachea and bronchus is a rare tumor. Although MYB-NFIB oncogene fusion and Notch1 mutation have been identified in ACC, little is known about the expression and clinical significance of Notch1 and its target gene fatty acid binding protein 7 (FABP7) in tracheobronchial ACC.
Materials and Methods
Primary tracheobronchial ACC that were resected between 1998 and 2014 were identified through the pathology and oncology database from five thoracic oncology centers in China. A tissue array was constructed from the patients’ samples and the expressions of Notch1 and FABP7 were evaluated by immunohistochemistry. The association between the expression of both markers and survival was determined.
Results
Overexpression of Notch1 and FABP7, detected in 37.8% and 38.3% of 368 patients with tracheobronchial ACC, respectively, was an independent prognostic indicator for recurrencefree survival (RFS) by multivariable Cox proportional hazard model (p=0.032 and p=0.048, respectively). Overexpression of Notch1, but not of FABP7, predicted overall survival (OS) (p=0.018). When categorized into four groups according to coexpression of Notch1 and FABP7, patients with overexpression of both Notch1 and FABP7 belonged to the group with the shortest RFS and OS (p=0.01 and p=0.048, respectively).
Conclusion
Expression of Notch1 and FABP7, and coexpression of Notch1 and FABP7, is strongly associated with poor survival in resected tracheobronchial ACC. These data are consistent with the hypothesis that poor differentiation of tracheobronchial ACC correlates with the activation of Notch signaling.
Keywords: Adenoid cystic carcinoma, Prognosis, Bronchi, Trachea, Notch1, FABP7
Introduction
Adenoid cystic carcinoma (ACC) of the trachea and bronchus is a rare type of cancer [1]. This histology exhibits perineural invasion and the rate of local recurrence and late metastasis is relatively high. Tubular, cribriform, and solid subtypes were three main histological growth patterns [2]. Surgical resection is the main treatment for patients with ACC. The 5-year survival rate of patients with resected tracheobronchial ACC were 52% [3,4]. Complete resection is achieved in approximately 50% of patients due to mucosal invasion [5]. Radiotherapy is recommended for patients with positive resection margin [6]. Tracheobronchial ACC is rather prone to recur locally or distantly after the surgical resection [7]. However, the prognostic factors of tracheobronchial ACC remain unknown.
A diagnostic feature of ACC is a t(6;9) rearrangement that translocates MYB to the NFIB locus [8-12]. Most ACCs overexpress MYB, only 30% harbor fusion transcript [13]. ACCs harbor activating Notch1 mutations [14]. MYB coordinates with Notch in ACC [8]. Notch activation by gain-of-function mutation underlies the switch to poorly differentiated histology and worse clinical outcome. Fatty acid binding protein 7 (FABP7) is a target gene of Notch and related to poor prognosis in ACC of the salivary glands [15,16]. We assume that ACCs with high expression of Notch1 and FABP7 have an a ggressive phenotype and poor prognosis. In order to test this hypothesis, we investigated the expression of both Notch1 and FABP7 of resected tracheobronchial ACC in a large retrospective study. The aim of this study is to investigate the association between expression of Notch1 and FABP7 and survival.
Materials and Methods
1. Patients selection
The multicenter study of rare thoracic cancer is a cooperative multicenter group composed of five hospitals in China (The First Affiliated Hospital of Guangzhou Medical University, The First Affiliated Hospital of Tsinghua University, Sun-Yet Sen University Cancer Center, The First Affiliated Hospital, Sun-Yet Sen University, and Guangdong General Hospital). All resected tracheobronchial ACC at the participating institutions from January 1998 to July 2014 were registered. Microscopic involvement of tracheal or bronchial margin was accepted if the airway was normal on gross inspection and no further length of airway could be resected. Radiotherapy of 54 Gy was administered 6 weeks after surgical resection. Patients who died of postoperative complications during the 30 days following the surgery, had history of primary ACC at other sites or secondary tumor were excluded.
2. Clinicopathological data
We analyzed the association between clinicopathological characteristics and patterns of recurrence and overall survival. Clinicopathological variables included age, sex, location of the tumor, histologic subtype, tumor size, lymph node involvement, resection margin, and postoperative treatment.
3. Immunohistochemical assay
After dewaxing in xylene and rehydrating stepwise in ethanol, sections were subjected to heat-induced antigen retrieval. Endogenous peroxidase activity and nonspecific binding were blocked with 3% H2O2 and nonimmune sera, respectively. Sections were then incubated with primary antibodies overnight at 4°C. The primary antibodies were used as below: cleaved Notch1 (Val1744) antibody from Cell Signaling (Danvers, MA) and FABP7 antibody from Abcam (Ab32423, Cambridge, MA). The next day, primary antibody was detected by Streptavidin-Biotin kit (Maixin Biotechnology, Fuzhou, China). Immunolabeled sections were visualized with 3,3´-diaminobenzidine, counterstained with hematoxylin, dehydrated, mounted and observed by means of a DM2000 microscope. Images were analyzed with Image J software. The analysis was performed by two independent pathologists (Y.G. and X.F). Scoring of Notch1 and FABP7 was calculated according to the staining intensity (0, no staining; 1+, weak staining; 2+, moderate staining; and 3+, strong staining) and the percentage of tumor cells showing staining (< 25%, 25%-50%, and > 50%). To determine the cut-off point for immunohistochemical staining of Notch1 and FABP7, the cohort was divided into "training set" and "validation set." Cut-off points were determined based on the results from the training set and were then proved in validation set. H-Score > 150 was considered high expression of Notch1 and FABP7. This method has been described by Hilsenbeck et al. [17] to reduce the risk of type 1 error associated with multiple testing for optimal cut-off points.
4. Fluorescent in-situ hybridization
MYB/NFIB rearrangements were detected by fluorescent in-situ hybridization. In nuclei containing the MYB-NFIB fusion, green and red signals from the MYB and NFIB genes overlap in a red/green (yellow) signal. Details are available in the supplementary data.
5. Statistical analysis
The expressions of Notch1 and FABP7 were categorized into either "low" or "high" scores according to the criteria described above. Survival data were collected from a systematic follow-up database. Chi-squared tests were used to evaluate the association between expression of Notch1 and FABP7 and clinicopathological parameters. Recurrence-free survival (RFS) was defined as the time period from the date of surgery until recurrence. The overall survival (OS) was calculated from the date of diagnosis to the date of death caused by ACC or December, 2015, the follow-up cut-off date. Patients who died of causes other than ACC and patients who still alive at the time of the follow-up cut-off were censored. Survival curves were estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazard regression model was used for univariate and multivariate analyses of survival. Significant variables (p < 0.1) in univariate analysis were included in multivariate analysis. All the statistical tests were two-sided. p-value ≤ 0.05 was regarded as significant. SPSS software ver. 19.0 (SPSS Inc., Chicago, IL) was used for statistical analyses.
6. Ethical statement
Ethical approval was obtained from each participating institution through institutional review board (IRB). Written informed consent for tumor sample collection and analysis was obtained from all patients.
Results
1. Patients
Patients with tracheobronchial ACC who received surgical resection have been retrospectively reviewed in five thoracic cancer center. Four hundred eleven medical records were reviewed. Twenty-three patients were excluded due to history of primary ACC at other sites or secondary tumor and 20 patients were excluded due to either the unavailability of an formalin fixed paraffin embedded material from our archives or the quality of the material. A total of 368 patients between January 1998 to July 2014 were included in this study. Demographics and clinical features are shown in S1 Table. The median age at presentation was 50.2 years (range, 17 to 82 years). Mean tumor size was 3.2 cm (range, 1.6 to 7.8 cm). Three hundred fifty-six patients (96.7%) were symptomatic. The mean duration of symptoms was 11.9 months. Pathological features are described in S2 Table. The types of resection for primary tracheobronchial ACC are shown in S3 Table. Sixty-one point four percent (226/368) of resected specimens had positive microscopic margins. Selective sampling lymph node metastases were found in 22.0% (81/368) of patients, most from peritracheal and subcarinal stations; nodal biopsies were not obtained in 30.7% (113/368) of resected patients. One hundred forty patients (38.0%) had no postoperative treatment. Two hundred twenty-eight patients (62.0%) received postoperative therapy. One hundred eighty-three patients (49.7%) received postoperative radiotherapy alone and 45 patients (12.3%) received the postoperative sequential radiotherapy followed by chemotherapy. MYB-NFIB gene fusion was identified in 186 patients (50.5%) (S4 Fig.).
2. Association of clinicopathological parameters with expression of Notch1 and FABP7
Representative immunostaining of Notch1 and FABP7 in tracheobronchial ACC tissues are shown in Fig. 1. Activated Notch1 were nuclear stained in cancer cells. The staining patterns of FABP7 were most nuclear and cytoplasm in cancer cells. No expression was observed in normal bronchial epithelium. Among 368 patients who had pathological slides for central review, 139 patients (37.8%) showed Notch1 overexpression and 141 patients (38.3%) showed FABP7 overexpression. Associations between expression of Notch1 and FABP7 and clinicopathological characteristics (age, sex, tumor size, lymph node involvement, histologic subtype, tracheobronchial location, and MYB-NFIB gene fusion) are shown in Table 1. Notch1 expression was significantly associated with solid pattern (p=0.006), lymph node involvement (p=0.027) and large tumor size (p=0.01). FABP7 expression was associated with solid pattern (p=0.012) and lymph node involvement (p=0.034). Both Notch1 (p=0.016) and FABP7 (p=0.013) expressions were associated with MYB-NFIB gene fusion.
Fig. 1.
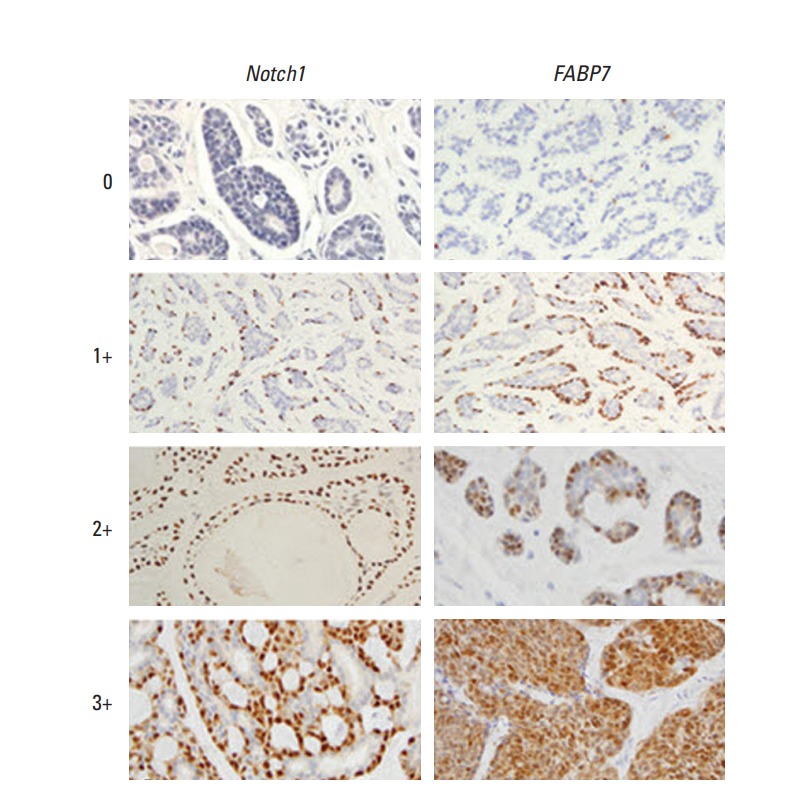
Representative immunohistochemical staining intensity of Notch1 (left column) and FABP7 (right column) in patients with tracheobronchial adenoid cystic carcinoma. 0, no staining; 1+, mild; 2+, moderate; 3+, strong intensity of staining (×200).
Table 1.
Association between Notch1 and FABP7 expression and clinicopathological characteristics in 368 patients with resected tracheobronchial adenoid cystic carcinoma
| Characteristic |
Notch1 expression |
FABP7 expression |
||||
|---|---|---|---|---|---|---|
| Low | High | p-value | Low | High | p-value | |
| Age (yr) | ||||||
| < 50 | 126 | 66 | 0.381 | 129 | 63 | 0.533 |
| ≥ 50 | 103 | 73 | 98 | 78 | ||
| Sex | ||||||
| Male | 128 | 75 | 0.863 | 123 | 80 | 0.367 |
| Female | 101 | 64 | 104 | 61 | ||
| Histologic subtype | ||||||
| Cribriform/Tubular | 207 | 87 | 0.006 | 200 | 94 | 0.012 |
| Solid | 22 | 52 | 27 | 47 | ||
| Margin status | ||||||
| Negative | 127 | 69 | 0.472 | 119 | 77 | 0.639 |
| Positive | 102 | 70 | 108 | 64 | ||
| Lymph node involvement | ||||||
| Negative | 122 | 52 | 0.027 | 114 | 60 | 0.034 |
| Positive | 33 | 48 | 35 | 46 | ||
| No biopsy | 74 | 39 | 78 | 35 | ||
| Location | ||||||
| Trachea | 100 | 66 | 0.176 | 105 | 61 | 0.062 |
| Bronchus | 129 | 73 | 122 | 80 | ||
| Tumor size (cm) | ||||||
| < 3 | 172 | 89 | 0.010 | 159 | 102 | 0.468 |
| ≥ 3 | 57 | 50 | 68 | 39 | ||
| MYB-NFIB gene fusion | ||||||
| Yes | 70 | 116 | 0.016 | 65 | 121 | 0.013 |
| No | 159 | 23 | 162 | 20 | ||
3. Expression of Notch1 and FABP7 as prognostic factors in patients with tracheobronchial ACC
With a median follow-up period of 65 months (range, 18 to 156 months), the OS for all resected ACC patients was 72.0% at 5 years and 45.6% at 10 years. The 5-year and 10-year RFS was 43.2% and 12.1%.
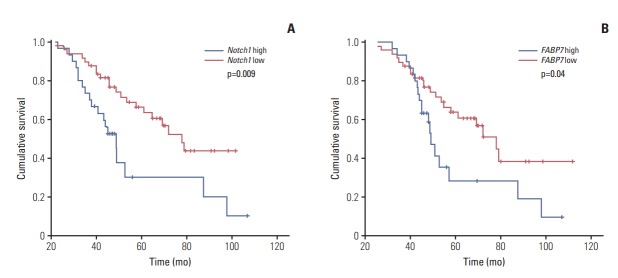
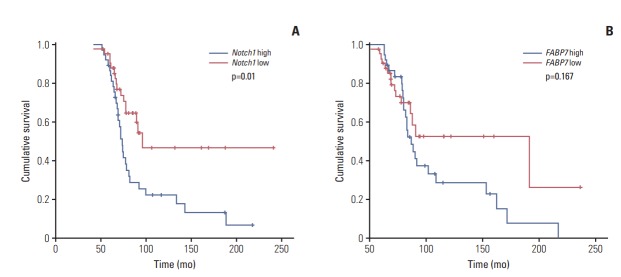
Kaplan-Meier survival analysis was performed to evaluated the prognostic value of individual marker in the whole cohort. Univariable analysis for all other variable is shown in Tables 2 and 3. The results show that Notch1 expression had significant impact on both RFS (p=0.009; hazard ratio [HR], 1.90; 95% confidence interval [CI], 1.68 to 2.25) (Fig. 2A) and OS (p=0.01; HR, 1.67; 95% CI, 1.38 to 2.12) (Fig. 3A). FABP7 showed a trend towards poor RFS (p=0.04; HR, 1.37; 95% CI, 1.11 to 1.63) (Fig. 2B). No statistical relationship was found between OS and FABP7 (p=0.167) (Fig. 3B). There was no significant difference of OS between tracheobronchial ACC patients with or without MYB-NFIB rearrangement (p=0.109) (S5 Fig.).
Table 2.
Univariate and multivariate analysis of recurrence-free survival in 368 patients with resected tracheobronchial adenoid cystic carcinoma
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | ||||||
| < 50 | 1.00 | 0.435 | ||||
| ≥ 50 | 1.18 | 0.74-1.55 | ||||
| Sex | ||||||
| Male | 1.00 | 0.746 | ||||
| Female | 0.97 | 0.75-1.24 | ||||
| Tracheobronchial location | ||||||
| Trachea | 1.00 | 0.722 | ||||
| Bronchus | 0.96 | 0.61-1.53 | ||||
| Lymph node involvement | ||||||
| Negative | 1.00 | 0.062 | ||||
| Positive | 1.09 | 0.89-1.45 | ||||
| Tumor size (cm) | ||||||
| < 3 | 1.00 | 0.013 | 1.00 | 0.118 | ||
| ≥ 3 | 1.67 | 1.44-1.88 | 1.18 | 0.97-1.43 | ||
| Margin status | ||||||
| Negative | 1.00 | 0.003 | 1.00 | 0.007 | ||
| Positive | 2.35 | 1.91-2.65 | 1.90 | 1.63-2.21 | ||
| Histologic subtype | ||||||
| Cribriform/Tubular | 1.00 | 0.001 | 1.00 | 0.008 | ||
| Solid | 2.36 | 1.63-2.99 | 1.92 | 1.70-2.13 | ||
| Postoperative therapy | ||||||
| Yes | 1.00 | 0.003 | 1.00 | 0.010 | ||
| No | 2.02 | 1.52-2.78 | 1.83 | 1.47-2.19 | ||
| Notch1 expression | ||||||
| Low | 1.00 | 0.009 | 1.00 | 0.032 | ||
| High | 1.90 | 1.68-2.25 | 1.39 | 1.16-1.76 | ||
| FABP7 expression | ||||||
| Low | 1.00 | 0.040 | 1.00 | 0.048 | ||
| High | 1.37 | 1.11-1.63 | 1.34 | 1.11-1.62 | ||
| Notch1/FABP7 expression | ||||||
| Notch1lowFABP7low | 1.00 | 0.010 | 1.00 | 0.036 | ||
| Notch1highFABP7low | 1.15 | 0.67-1.55 | 1.08 | 0.66-1.56 | ||
| Notch1lowFABP7high | 1.28 | 0.96-1.74 | 1.19 | 0.67-1.89 | ||
| Notch1highFABP7high | 1.85 | 1.28-2.62 | 1.37 | 1.12-1.89 | ||
HR, hazard ratio; CI, confidence interval.
Table 3.
Univariate and multivariate analysis of overall survival in 368 patients with resected tracheobronchial adenoid cystic carcinoma
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | ||||||
| < 50 | 1.00 | 0.440 | ||||
| ≥ 50 | 1.22 | 0.74-1.56 | ||||
| Sex | ||||||
| Male | 1.00 | 0.638 | ||||
| Female | 0.95 | 0.73-1.18 | ||||
| Tracheobronchial location | ||||||
| Trachea | 1.00 | 0.431 | ||||
| Bronchus | 0.92 | 0.71-1.60 | ||||
| Lymph node involvement | ||||||
| Negative | 1.00 | 0.591 | ||||
| Positive | 1.16 | 0.99-1.29 | ||||
| Tumor size (cm) | ||||||
| < 3 | 1.00 | 0.323 | ||||
| ≥ 3 | 1.29 | 0.83-1.79 | ||||
| Margin status | ||||||
| Negative | 1.00 | 0.007 | 1.00 | 0.026 | ||
| Positive | 1.91 | 1.70-2.19 | 1.52 | 1.32-1.79 | ||
| Histologic subtype | ||||||
| Cribriform/Tubular | 1.00 | 0.002 | 1.00 | 0.023 | ||
| Solid | 2.38 | 1.92-2.82 | 1.45 | 1.18-1.97 | ||
| Postoperative therapy | ||||||
| Yes | 1.00 | 0.005 | 1.00 | 0.021 | ||
| No | 2.15 | 1.87-2.45 | 1.66 | 1.37-1.93 | ||
| Notch1 expression | ||||||
| Low | 1.00 | 0.010 | 1.00 | 0.018 | ||
| High | 1.67 | 1.38-2.12 | 1.59 | 1.27-1.90 | ||
| FABP7 expression | ||||||
| Low | 1.00 | 0.167 | 1.00 | 0.375 | ||
| High | 1.10 | 0.93-1.37 | 1.16 | 0.83-1.48 | ||
| Notch1/FABP7 expression | ||||||
| Notch1lowFABP7low | 1.00 | 0.048 | 1.00 | 0.164 | ||
| Notch1highFABP7low | 1.16 | 0.88-1.63 | 1.07 | 0.67-1.58 | ||
| Notch1lowFABP7high | 1.22 | 0.86-1.79 | 1.13 | 0.85-1.71 | ||
| Notch1highFABP7high | 1.47 | 1.15-1.94 | 1.32 | 1.09-2.15 | ||
HR, hazard ratio; CI, confidence interval.
Fig. 2.
Kaplan-Meier analysis of recurrence-free survival (RFS) in patients with tracheobronchial adenoid cystic carcinoma. (A) RFS in patients with Notch1 low versus high expression. (B) RFS in patients with FABP7 low versus high expression.
Fig. 3.
Kaplan-Meier analysis of overall survival (OS) in patients with tracheobronchial adenoid cystic carcinoma. (A) OS in patients with Notch1 low versus high expression. (B) OS in patients with FABP7 low versus high expression.
Variables with p-value of 0.1 or less were entered in Cox regression model for multivariable analysis. Both Notch1 and FABP7 were independent predictors of survival. For RFS, the five variables that remained independently substantially associated with RFS were positive resection margin (p=0.007), solid pattern (p=0.008), without postoperative therapy (p=0.010), Notch1 overexpression (p=0.032), and FABP7 overexpression (p=0.048) (Table 2). In multivariate analysis for OS, the four variables that remained independently substantially associated with OS were positive resection margin (p=0.026), solid pattern (p=0.023), without postoperative therapy (p=0.021), and Notch1 overexpression (p=0.018).
4. Prognostic prediction using combined Notch1 and FABP7 staining
We divided 368 patients into four groups according to the expression of Notch1 and FABP7: Notch1highFABP7high (n=100), Notch1lowFABP7low (n=89), Notch1highFABP7low (n=96), and Notch1lowFABP7high (n=83). Survival curves were generated by Kaplan-Meier method and differences between these four groups were compared by log-rank test. The results showed that patients with high expression of both Notch1 and FABP7 had significantly shorter RFS (p=0.01) (Fig. 4) and OS (p=0.048) (Fig. 5). In multivariate analysis, patients with high expression of both Notch1 and FABP7 (Notch1highFABP7high) had significantly shorter RFS (p=0.036; HR, 1.37; 95% CI, 1.12 to 1.89) (Table 2), but not OS (p=0.164; HR, 1.32; 95% CI, 1.12 to 1.89) (Table 3).
Fig. 4.
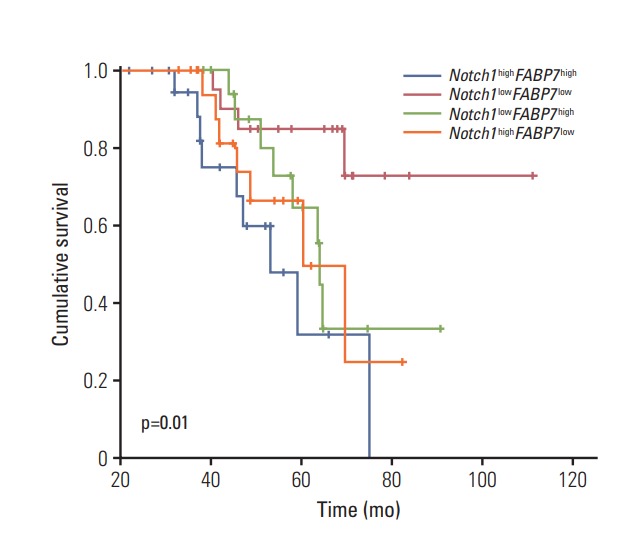
Kaplan-Meier analysis of recurrence-free survival in patients with tracheobronchial adenoid cystic carcinoma stratified according to the expression of both Notch1 and FABP7.
Fig. 5.
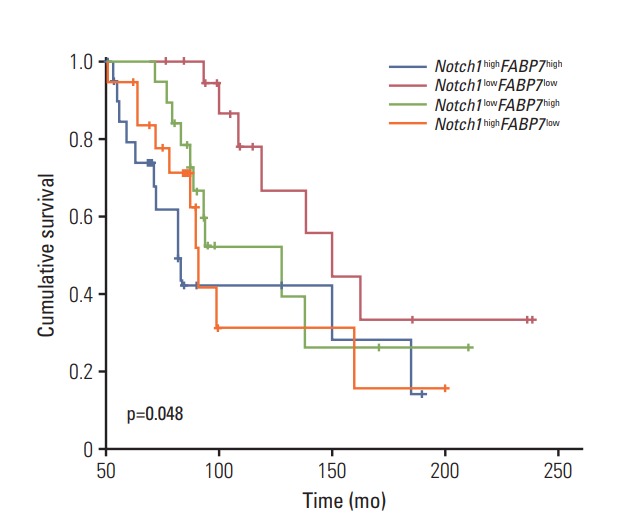
Kaplan-Meier analysis of overall survival in patients with tracheobronchial adenoid cystic carcinoma stratified according to the expression of both Notch1 and FABP7.
Discussion
Primary tumor of the upper airway is relatively rare. ACC is the second most frequent histologic type of tracheal tumor. ACC often develops in a relatively major airway between the trachea and the lobular bronchus [18]. Several poor prognostic factors of recurrence and metastasis have been reported. However, these prognostic factors remain uncertain [19,20].
In this study, we investigated the prognostic significance of Notch1 and FABP7 expression in patients with resected tracheobronchial ACC. Our results showed that the expression of Notch1 and FABP7 was significantly associated with shorter RFS in primary tracheobronchial ACC. In multivariable analysis, Notch1 was an independent factor for both worse RFS and both OS. FABP7 was only associated with shorter RFS. Overexpression of both Notch1 and FABP7 were associated with the worst outcome. Absence of expression of Notch1 and FABP7 identified a best prognostic group, whereas expression of one of these two markers conferred an intermediate prognosis.
In this study analyzing 368 patients with primary tracheobronchial ACC, 139 patients (37.8%) showed Notch1 overexpression and 141 patients (38.3%) showed FABP7 overexpression. The solid subtype was the most undifferentiated form in ACC [2]. Our study showed that Notch1 and FABP7 overexpression was associated with more solid subtype.
The Notch pathway is involved in maintenance of stem cells, cell proliferation, and angiogenesis [21]. Whole exome sequencing of ACC samples showed Notch pathway alterations occurred in 11% to 29% of patients [22,23]. Ferrarotto et al. [24] found that majority of Notch1 mutations in ACC were activating. The association between Notch1 mutation and solid subtype suggests that Notch1 drives the prometastatic phenotype in ACC [25-27]. Canonical Notch signaling relies on a series of ligand-dependent proteolytic cleavages, and releases the Notch intracellular domain (NICD), which translocates to the nucleus and regulates transcription of targeted genes. Immunohistochemical staining of cleaved Notch1 was sensitive to identify Notch1 activation [24].
Notch1 and SOX10 is essential for proliferation in ACC [28]. Knockdown of Notch1, SOX10, and downstream effector FABP7 inhibited tumor formation, and induced cell death. FABP7 belongs to a large family of hydrophobic proteins [29]. High expression of FABP7 were observed in glioblastoma, breast cancer and renal cancer, and its expression was significantly associated with poor survival. FABP7 is regulated differently by homeobox 1 (EN1) and MYB and was correlated with poor prognosis in salivary ACC. ACC with solid subtype had strong positive immunostaining of FABP7 [16].
Current study shows the significant benefit of both RFS and OS from postoperative therapy by multivariate analysis. A high proportion (61.4%) of resected specimens had positive microscopic margins. Forty-eight point seven percent patients received postoperative radiotherapy and 12.3% patients received postoperative radiotherapy followed by chemotherapy. Both high expression of Notch1 and FABP7 showed a trend towards poor RFS. Combinatorial high expression of Notch1 and FABP7 was able to distinguish patients with worse prognosis regarding recurrence. Therefore, Notch1 and FABP7 may be useful for identifying patients with a high risk of recurrence who may benefit from postoperative therapy. Our findings clearly indicate Notch1 and FABP7 are independent prognostic factors of survival time and clinical indication of postoperative therapy especially radiotherapy in ACC of trachea and bronchus.
In conclusion, this study demonstrated the expression of Notch1 and FABP7 in a fraction of primary ACC of trachea and bronchus. Patients with high expression of Notch1 and FABP7 showed a significantly increased risk of recurrence and disease related mortality. Further prospective study with internal and external validation is necessary.
Acknowledgments
This study was funded by National Natural Science Foundation of China (No.81301999 and No. 81672293 to Dr. Xie).
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
References
- 1.Xu LT, Sun ZF, Li ZJ, Wu LH, Zhang ZY, Yu XQ. Clinical and pathologic characteristics in patients with tracheobronchial tumor: report of 50 patients. Ann Thorac Surg. 1987;43:276–8. doi: 10.1016/s0003-4975(10)60611-x. [DOI] [PubMed] [Google Scholar]
- 2.Nomori H, Kaseda S, Kobayashi K, Ishihara T, Yanai N, Torikata C. Adenoid cystic carcinoma of the trachea and mainstem bronchus: a clinical, histopathologic, and immunohistochemical study. J Thorac Cardiovasc Surg. 1988;96:271–7. [PubMed] [Google Scholar]
- 3.Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg. 1990;49:69–77. doi: 10.1016/0003-4975(90)90358-d. [DOI] [PubMed] [Google Scholar]
- 4.Li N, Xu L, Zhao H, El-Naggar AK, Sturgis EM. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer. 2012;118:3945–53. doi: 10.1002/cncr.26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maziak DE, Todd TR, Keshavjee SH, Winton TL, Van Nostrand P, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg. 1996;112:1522–31. doi: 10.1016/S0022-5223(96)70011-9. [DOI] [PubMed] [Google Scholar]
- 6.Gaissert HA. Primary tracheal tumors. Chest Surg Clin N Am. 2003;13:247–56. doi: 10.1016/s1052-3359(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 7.Prommegger R, Salzer GM. Long-term results of surgery for adenoid cystic carcinoma of the trachea and bronchi. Eur J Surg Oncol. 1998;24:440–4. doi: 10.1016/s0748-7983(98)92465-9. [DOI] [PubMed] [Google Scholar]
- 8.Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48:265–72. doi: 10.1038/ng.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden AC, Greipp PT, Knutson DL, Kloft-Nelson SM, Jenkins SM, Marks RS, et al. Histopathologic and cytogenetic features of pulmonary adenoid cystic carcinoma. J Thorac Oncol. 2015;10:1570–5. doi: 10.1097/JTO.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 10.Mitani Y, Rao PH, Futreal PA, Roberts DB, Stephens PJ, Zhao YJ, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17:7003–14. doi: 10.1158/1078-0432.CCR-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.West RB, Kong C, Clarke N, Gilks T, Lipsick JS, Cao H, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Pathol. 2011;35:92–9. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci U S A. 2009;106:18740–4. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–31. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho AS, Kannan K, Roy DM, Morris LG, Ganly I, Katabi N, et al. The mutational landscape of adenoid cystic carcinoma. Nat Genet. 2013;45:791–8. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 16.Phuchareon J, Overdevest JB, McCormick F, Eisele DW, van Zante A, Tetsu O. Fatty acid binding protein 7 is a molecular marker in adenoid cystic carcinoma of the salivary glands: implications for clinical significance. Transl Oncol. 2014;7:780–7. doi: 10.1016/j.tranon.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilsenbeck SG, Clark GM, McGuire WL. Why do so many prognostic factors fail to pan out? Breast Cancer Res Treat. 1992;22:197–206. doi: 10.1007/BF01840833. [DOI] [PubMed] [Google Scholar]
- 18.Gaissert HA, Grillo HC, Shadmehr MB, Wright CD, Gokhale M, Wain JC, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78:1889–96. doi: 10.1016/j.athoracsur.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 19.Azar T, Abdul-Karim FW, Tucker HM. Adenoid cystic carcinoma of the trachea. Laryngoscope. 1998;108:1297–300. doi: 10.1097/00005537-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lin CM, Li AF, Wu LH, Wu YC, Lin FC, Wang LS. Adenoid cystic carcinoma of the trachea and bronchus: a clinicopathologic study with DNA flow cytometric analysis and oncogene expression. Eur J Cardiothorac Surg. 2002;22:621–5. doi: 10.1016/s1010-7940(02)00406-2. [DOI] [PubMed] [Google Scholar]
- 21.Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL. Notch and epithelial-mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle. 2004;3:718–21. [PubMed] [Google Scholar]
- 22.Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123:2965–8. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JS, Wang K, Rand JV, Sheehan CE, Jennings TA, Al-Rohil RN, et al. Comprehensive genomic profiling of relapsed and metastatic adenoid cystic carcinomas by next-generation sequencing reveals potential new routes to targeted therapies. Am J Surg Pathol. 2014;38:235–8. doi: 10.1097/PAS.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 24.Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to Notch1 inhibitors. J Clin Oncol. 2017;35:352–60. doi: 10.1200/JCO.2016.67.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Weert S, van der Waal I, Witte BI, Leemans CR, Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015;51:71–6. doi: 10.1016/j.oraloncology.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Kluk MJ, Ashworth T, Wang H, Knoechel B, Mason EF, Morgan EA, et al. Gauging NOTCH1 activation in cancer using immunohistochemistry. PLoS One. 2013;8:e67306. doi: 10.1371/journal.pone.0067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell D, Hanna EY, Miele L, Roberts D, Weber RS, El-Naggar AK. Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann Diagn Pathol. 2014;18:10–3. doi: 10.1016/j.anndiagpath.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panaccione A, Chang MT, Carbone BE, Guo Y, Moskaluk CA, Virk RK, et al. NOTCH1 and SOX10 are essential for proliferation and radiation resistance of cancer stem-like cells in adenoid cystic carcinoma. Clin Cancer Res. 2016;22:2083–95. doi: 10.1158/1078-0432.CCR-15-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285:32679–83. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.