Abstract
Purpose
The purpose of this study was to demonstrate the existence of a bimodal survival pattern in metastatic uveal melanoma. Secondary aims were to identify the characteristics and prognostic factors associated with long-term survival and to develop a clinical decision tree.
Materials and Methods
The medical records of 99 metastatic uveal melanoma patients were retrospectively reviewed. Patients were classified as either short (≤ 12 months) or long-term survivors (> 12 months) based on a graphical interpretation of the survival curve after diagnosis of the first metastatic lesion. Ophthalmic and oncological characteristicswere assessed in both groups.
Results
Of the 99 patients, 62 (62.6%) were classified as short-term survivors, and 37 (37.4%) as long-term survivors. The multivariate analysis identified the following predictors of long-term survival: age ≤ 65 years (p=0.012) and unaltered serum lactate dehydrogenase levels (p=0.018); additionally, the size (smaller vs. larger) of the largest liver metastasis showed a trend towards significance (p=0.063). Based on the variables significantly associated with long-term survival, we developed a decision tree to facilitate clinical decision-making.
Conclusion
The findings of this study demonstrate the existence of a bimodal survival pattern in patients with metastatic uveal melanoma. The presence of certain clinical characteristics at diagnosis of distant disease is associated with long-term survival. A decision tree was developed to facilitate clinical decision-making and to counsel patients about the expected course of disease.
Keywords: Uveal melanoma, Neoplasm metastasis, Long-term survivors, Decision trees
Introduction
Malignant uveal melanoma is the most common primary intraocular cancer in adults [1]. The main aim of treatment is to eliminate the tumor and prevent metastatic dissemination. Local recurrence can be effectively prevented through enucleation or eye-conserving therapies [2,3]. Dissemination is common in uveal melanoma: even when the primary tumor is completely eradicated, approximately 50% of patients will develop metastasis, most commonly in the liver (89% of cases) [4]. Once metastasis occurs, the prognosis is very poor, with a median survival of only 6 months and an 80% mortality rate within the first year [5,6]. Even with recent advances in the management of this disease, the survival rate has remained unchanged for 40 years, and no existing treatment has yet been proven to extend survival [7-9].
Although long-term survival is uncommon in metastatic disease, clinical practice and a few reports suggest that a subset of patients achieve long-term survival [5,10-13]. However, the characteristics associated with long-term survival are not well understood. Gragoudas et al. [5] first used the term “long survivors” to describe a small subset of patients who survive for more than 1 year. The Collaborative Ocular Melanoma Study, which evaluated the largest patient cohort reported to date, found that patients who survived > 6 months after diagnosis of dissemination were significantly younger than patients with the earliest death [11]. Similarly, Rietschel et al. [12] found that 22% of 119 metastatic patients remained alive at 4 years.
Given this context, the aim of the present study was to identify the bimodal survival distribution pattern (long and short) in a single-center cohort of metastatic uveal melanoma patients. We also evaluated a broad range of variables in an effort to determine the predictors of both short and long-term survival. Finally, based on those findings, we developed a clinical decision tree to help clinicians estimate the expected survival outcomes.
Materials and Methods
1. Patients and clinical assessments
This was a retrospective, consecutive case series. From May 1996 through May 2014, 99 patients with metastatic uveal melanoma were diagnosed at the ocular oncology unit at Bellvitge University Hospital (Barcelona, Spain).
The diagnosis of metastatic melanoma was based on standard clinical and imaging findings, progression, and the absence of other cancers. A fine-needle aspiration biopsy was performed only in cases of diagnostic uncertainty.
The outcome of interest was overall survival after diagnosis of metastatic disease. Survival time (months) was calculated from the date of the first metastasis until death.
The clinical information was extracted from medical records and classified into five categories, as follows: demographic data, primary tumor characteristics, features of first metastatic lesion, patient outcomes after metastasis, and metastatic treatment.
Tumor size was assessed according to the seventh edition of the American Joint Committee on Cancer tumor staging criteria [14]. The following serum liver function tests at diagnosis of metastatic spread were registered: aspartate transaminase, alanine transaminase, gamma glutamyl transpeptidase (GGT), lactate dehydrogenase (LDH), and alkaline phosphatase. To facilitate analysis, biochemical data were categorized as either normal or elevated according to our laboratory reference levels.
2. Statistical analysis
The survival analysis was performed from the date of first metastatic diagnosis. The Kaplan-Meier method was used to determine the cumulative probability of survival during follow-up. Long- and short-term survival groups were identified by graphical interpretation of the survival curve. This was subsequently analyzed as a qualitative dichotomous variable.
After defining the two different survival groups, we performed a bivariate analysis of the factors associated with long or short survival (chi-square test or Fisher exact test for categorical variables; Student's t test or Mann-Whitney's U test for quantitative variables).
To identify independent predictors of long-term survival, a multivariate analysis was performed using a logistic regression model. Those variables that were significantly associated (p < 0.05) with survival on the bivariate analysis were subsequently analyzed to identify the combination of factors most closely related to long-term survival. Effect estimates were expressed as odds ratios (OR) with 95% confidence intervals (95% CI).
The results were analyzed using IBM SPSS software, ver. 20.0 (IBM Corp., Armonk, NY). p-values < 0.05 were considered statistically significant.
3. Decision tree
A regression tree analysis was performed [15]. This estimates a regression relationship by binary recursive partitioning in a conditional inference framework and identifies, in a hierarchical order, the variables providing discriminative information and the best cutoff points for continuous variables.
4. Ethical statement
The study was approved by the Institutional Review Board of Bellvitge University Hospital with a waiver of informed consent (IRB No. PR357/13) and performed in accordance with the principles of the Declaration of Helsinki.
Results
1. Patient characteristics
A total of 99 consecutive patients (50 male and 49 female) diagnosed with metastatic uveal melanoma were evaluated. Histopathological confirmation was available in 38 patients (38.4%). The mean age at distant metastasis was 60.7 years (standard deviation, 12.8; range, 22 to 85 years). The median disease-free interval (DFI) was 37.7 months (interquartile range [IQR], 34). The most common site of initial metastasis was the liver (92.9%). First-line chemotherapy was performed in 52.7% of patients. At the end of follow-up, most patients (96/99) had died of metastatic uveal melanoma. The median overall survival from metastatic diagnosis was 8 months (IQR, 14).
2. Definition of long-term and short-term survivors
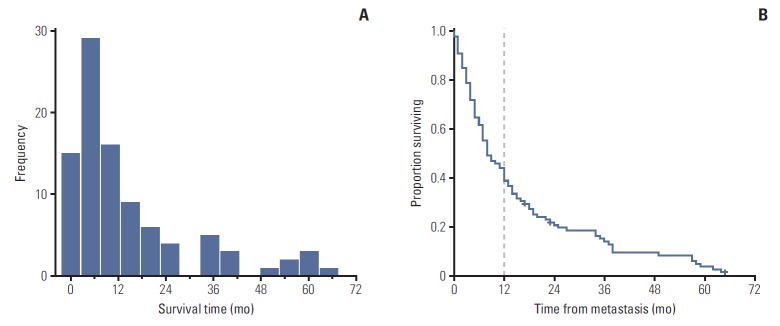
The graphical interpretation of overall survival revealed two well-differentiated trends (Fig. 1). The histogram showed that most patients died within the first 2-6 months after detection of the first metastatic lesion. After month 12, survival duration was more widely distributed until the maximum survival time at month 64. The Kaplan-Meier survival analysis showed a biphasic pattern: the slope of the survival curve was initially steep but flattened out after month 12, when a notable minority of patients appeared to have a more indolent course.
Fig. 1.
Definition of long (> 12 mo) and short (≤ 12 mo) survival patterns from graphs showing the overall survival distribution of 99 patients with metastatic uveal melanoma. (A) The histogram provides a quick visual summary of the survival frequencies in our cohort. A left-skewed, one-peaked distribution is shown with most subjects (> 60%) located within the first 12 months of survival. From this point, a minority of patients appeared to present a more indolent course, as evidenced by the elongated and scattered survival pattern in the remaining patients. (B) The Kaplan-Meier curve for overall survival is marked with a dashed line to indicate the approximate time point of slope change. Note the sharp decline in the survival curve during the first 12 months and its subsequent stabilization (flattening slope) beyond this point.
Considering the graphical evaluation of this curve in the context of published data that estimates a median survival of 6 months in patients with metastatic uveal melanoma [5,7,16], we assumed the existence of a well-differentiated bimodal metastatic survival pattern consisting of long-survivors (> 12 months) and short-survivors (≤ 12 months) (Fig. 2).
Fig. 2.
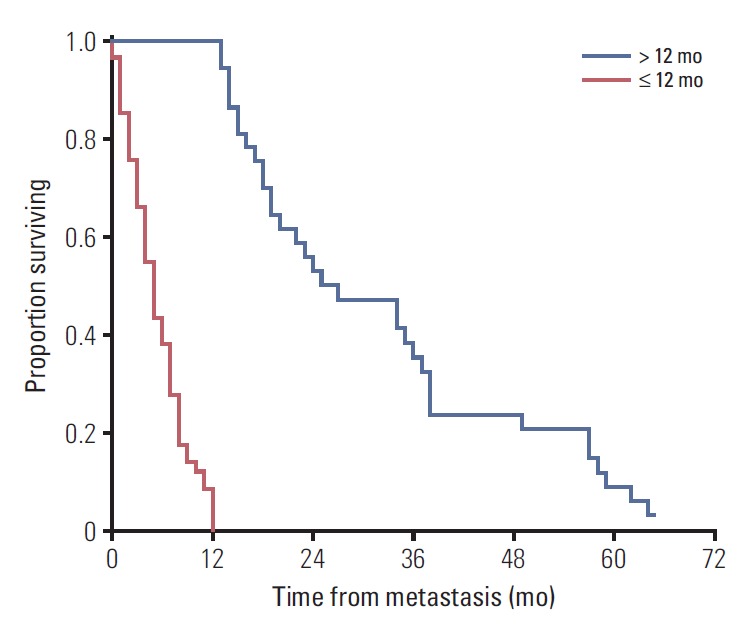
Kaplan-Meier plot for overall survival comparing survival patterns. Patients were divided into long-term survivors (> 12 mo, blue line) and short-term survivors (≤ 12 mo, red line). Note the wide dispersion of data among the long-term survivors over time. Estimated median survival time for long survivors was 27 months (95% confidence interval, 14.6 to 39.2) versus 5 months for short-term survivors (95% confidence interval, 3.9 to 6.1). Comparison of survival rates between groups were performed by log-rank test (p < 0.001).
Of the 99 patients, 37 (37.4%) were classified as long-term survivors (> 12 months), with a median survival of 24.5 months (IQR, 21), while 62 patients (62.6%) were classified as short-term survivors (median survival, 5 months; IQR, 6). Three patients remained alive at the end of the study surviving > 12 months and were therefore included in the long-term survivor group.
3. Subgroup characteristics
The clinical characteristics of the long and short survivors are shown in Table 1. The bivariate analysis demonstrated statistically significant differences between the survival groups (Table 2). Overall, patients with prolonged survival were significantly more likely to be younger (≤ 65 years) and to have a longer DFI (> 40 months). None of the primary tumor-related variables—including ciliary body involvement and larger tumor size—were significant.
Table 1.
Baseline demographic and clinical characteristics for all patients shown according to subgroups
| All patients (n=99) | Short-term survival (n=62) | Long-term survival (n=37) | p-value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age at diagnosis of UM, mean±SD (yr) | 57.33±13.55 | 58.89±15.08 | 55.24±10.28 | 0.157 |
| ≤ 65 yr | 71 (71.7) | 39 (62.9) | 32 (86.5) | 0.012* |
| Female sex | 50 (50.5) | 32 (51.6) | 18 (48.6) | 0.775 |
| Other lifetime primary cancer | 12 (12.1) | 9 (14.5) | 3 (8.1) | 0.347 |
| Uveal melanoma characteristic | ||||
| Origin of uveal melanoma | ||||
| Choroid | 90 (90.9) | 57 (91.9) | 33 (89.2) | 0.560 |
| Ciliary body | 9 (9.1) | 5 (8.1) | 4 (10.8) | |
| Largest basal tumor diameter, median (IQR, mm) | 15.83 (3.48) | 15.71 (3.35) | 16.01 (3.71) | 0.735 |
| Tumor thickness, median (IQR, mm) | 7.3 (4.0) | 7.3 (4.4) | 7.25 (4.5) | 0.277 |
| T, tumor size category (n=97) | ||||
| T1 | 2 (2.1) | 0 | 2 (5.6) | 0.259 |
| T2 | 25 (25.8) | 15 (24.6) | 10 (27.8) | |
| T3 | 44 (45.4) | 30 (49.2) | 14 (38.9) | |
| T4 | 26 (26.8) | 16 (26.2) | 10 (27.8) | |
| Anatomic staging, TNM (n=97) | ||||
| I | 2 (2.1) | 0 | 2 (5.6) | 0.340 |
| IIa | 24 (24.7) | 14 (23.0) | 10 (27.8) | |
| IIb | 35 (36.1) | 24 (39.3) | 11 (30.6) | |
| IIIa | 31 (32.0) | 21 (34.4) | 10 (27.8) | |
| IIIb | 2 (2.1) | 1 (1.6) | 1 (2.8) | |
| IV | 3 (3.1) | 1 (1.6) | 2 (5.6) | |
| Distance to optic disc, median (IQR, mm) | 4.50 (5.3) | 4.25 (5.4) | 4.5 (5.1) | 0.538 |
| Distance to fovea, median (IQR, mm) | 4.25 (6.1) | 4.75 (8.0) | 3 (5.3) | 0.828 |
| Rupture of Bruch's membrane | 27 (28.4) | 18 (30.0) | 9 (25.7) | 0.655 |
| Orange pigment | 20 (21.1) | 7 (11.9) | 13 (36.1) | 0.005* |
| Histopathology (cell type) (n=47) | ||||
| Spindle cell | 5 (10.6) | 3 (10.0) | 2 (11.8) | 0.978 |
| Epithelioid | 22 (46.8) | 14 (46.7) | 8 (47.1) | |
| Mixed | 20 (42.6) | 13 (43.3) | 7 (41.2) | |
| Chromosome 3 monosomy (n=14) | 8 (57.1) | 3 (37.5) | 5 (83.3) | 0.138 |
| Primary treatment | ||||
| Brachytherapy | 53 (53.5) | 32 (51.6) | 21 (56.8) | 0.860 |
| Transscleral resection | 5 (5.0) | 3 (4.8) | 2 (5.4) | |
| Endoresection | 4 (4.0) | 2 (3.2) | 2 (5.4) | |
| Enucleation | 35 (35.4) | 24 (38.7) | 11 (29.7) | |
| No treatment | 2 (2.0) | 1 (1.6) | 1 (2.7) | |
| Local recurrence | 15 (15.2) | 9 (14.5) | 6 (16.2) | 0.819 |
| Months to recurrence (n=15), median (IQR) | 21 (40.0) | 15 (36.0) | 28 (49.0) | 0.953 |
| Characteristics of the first uveal melanoma metastasis | ||||
| Age at metastatic diagnosis (≤ 65 yr) | 60 (60.6) | 31 (50.0) | 29 (78.4) | 0.005* |
| Metastasis-free interval > 40 mo | 30 (30.3) | 14 (22.6) | 16 (43.2) | 0.030* |
| Liver metastasis | 92 (92.9) | 59 (95.2) | 33 (89.2) | 0.419 |
| No. of liver metastases (n=84), median (IQR) | 4 (5.0) | 4.5 (4.0) | 4 (4.0) | 0.143 |
| 1 Lesion | 9 (10.7) | 3 (5.8) | 6 (18.8) | 0.078 |
| Largest diameter of the largest liver metastasis, median (IQR, mm) | 22 (22.0) | 26 (20.0) | 18 (16.0) | 0.001* |
| Diagnosis of metastasis | ||||
| Surveillance | 68 (70.8) | 38 (63.3) | 30 (83.3) | 0.037* |
| Symptom-prompted | 31 (29.2) | 24 (36.7) | 7 (16.7) | |
| Method of diagnosis | ||||
| Imaging | 81 (84.4) | 53 (88.3) | 28 (77.8) | 0.073 |
| Biopsy | 11 (11.5) | 4 (6.7) | 7 (19.4) | |
| Biochemical | 3 (3.1) | 3 (5.0) | 0 | |
| Physical exam | 1 (1.0) | 0 | 1 (2.8) | |
| PET-positive (n=38) | 27 (71.1) | 15 (78.9) | 12 (63.2) | 0.283 |
| Patient characteristics at first metastatic diagnosis | ||||
| Performance status, ECOG | ||||
| 0 | 59 (62.8) | 30 (51.7) | 29 (80.6) | 0.036* |
| 1 | 25 (26.6) | 19 (32.8) | 6 (16.7) | |
| 2 | 9 (9.6) | 8 (13.8) | 1 (2.8) | |
| 3 | 1 (1.1) | 1 (1.7) | 0 | |
| Absence of symptoms | 60 (63.8) | 31 (53.4) | 29 (80.6) | 0.008* |
| M-stage (AJCC) (n=91) | ||||
| 1a | 60 (65.9) | 34 (61.8) | 26 (72.2) | 0.406 |
| 1b | 25 (27.5) | 16 (29.1) | 9 (25.0) | |
| 1c | 6 (6.6) | 5 (9.1) | 1 (2.8) | |
| Lactate dehydrogenase, unaltered | 43 (52.4) | 18 (37.5) | 25 (73.5) | 0.001* |
| Aspartate transaminase, unaltered | 57 (68.7) | 26 (53.1) | 31 (91.2) | < 0.001* |
| Alanine transaminase, unaltered | 61 (72.6) | 30 (60.0) | 31 (91.2) | 0.002* |
| Gamma glutamyl transpeptidase, unaltered | 48 (57.8) | 19 (38.8) | 29 (85.3) | < 0.001* |
| Bilirubin, unaltered | 77 (91.7) | 44 (88.0) | 33 (97.1) | 0.233 |
| Alkaline phosphatase, unaltered | 62 (75.6) | 30 (61.2) | 32 (97.0) | < 0.001* |
| Hemoglobin (g/L) | ||||
| 120-160 | 73 (85.9) | 41 (80.4) | 32 (94.1) | 0.163 |
| > 160 | 3 (3.5) | 2 (3.9) | 1 (2.9) | |
| First metastatic treatment | ||||
| Surgery | 18 (19.8) | 4 (7.1) | 14 (40.0) | < 0.001* |
| Radiotherapy | 11 (12.1) | 5 (8.9) | 6 (17.1) | 0.324 |
| First-line chemotherapy | 48 (52.7) | 26 (46.4) | 22 (62.9) | 0.127 |
| First-line response (n=46) | ||||
| Complete response | 12 (26.1) | 3 (12.0) | 9 (42.9) | 0.054 |
| Partial response | 2 (4.3) | 1 (4.0) | 1 (4.8) | |
| Progressive disease | 32 (69.6) | 21 (84.0) | 11 (52.4) | |
| Second-line chemotherapy | 16 (17.6) | 6 (10.7) | 10 (28.6) | 0.029* |
| Second-line response (n=16) | ||||
| Complete response | 2 (12.5) | 0 | 2 (20.0) | 0.501 |
| Progressive disease | 14 (87.5) | 6 (100) | 8 (80.0) |
Values are presented as number (%) unless otherwise indicated. UM, uveal melanoma; SD, standard deviation; IQR, interquartile range; TNM, tumor, node and metastasis; PET, positron emission tomography; ECOG, Eastern Cooperative Oncology Group; AJCC, American Joint Committee on Cancer.
Significant values with p < 0.05.
Table 2.
Univariate analysis (simple logistic regression model) of predictors of long-term survival
| Odds ratio | 95% Confidence interval | p-value | |
|---|---|---|---|
| Age ≤ 65 at metastatic diagnosis | 3.63 | 1.43-9.17 | 0.005 |
| Metastasis-free interval > 40 mo | 2.61 | 1.08-6.31 | 0.03 |
| Orange pigment over melanoma | 4.20 | 1.48-11.9 | 0.005 |
| Smaller largest diameter of the largest liver metastasis | 0.97 | 0.94-0.99 | 0.034 |
| Metastasis diagnosis by surveillance testing | 2.90 | 1.04-8.04 | 0.037 |
| Lower ECOG performance status | 0.34 | 0.15-0.74 | 0.007 |
| Asymptomatic patient | 3.61 | 1.36-9.55 | 0.008 |
| Unaltered lactate dehydrogenase | 4.63 | 1.77-12.05 | 0.001 |
| Unaltered aspartate transaminase | 9.17 | 2.46-34.48 | < 0.001 |
| Unaltered alanine transaminase | 6.90 | 1.85-25.64 | 0.002 |
| Unaltered alkaline phosphatase | 20.41 | 2.55-166.67 | < 0.001 |
| Unaltered gamma glutamyl transpeptidase | 9.17 | 3.02-27.78 | < 0.001 |
| Surgery of first metastasis | 8.67 | 2.56-29.41 | < 0.001 |
ECOG, Eastern Cooperative Oncology Group.
In terms of the characteristics of the metastatic lesions, the presence of a smaller maximum diameter of the liver lesion and surveillance-based (vs. symptomatic) diagnosis were both associated with long-term survival. No between-groups differences in extra-hepatic metastatic locations were observed. Other variables associated with long-term survival at metastatic diagnosis included: a lower Eastern Cooperative Oncology Group performance status; asymptomatic status at metastasis diagnosis; and unaltered liver enzymes. The only treatment significantly associated with long-term survival was surgery. There were no significant gender-based differences between the groups.
4. Multivariate analysis
The odds of becoming a long-term survivor were 5 times higher in subjects aged < 65 years at first metastatic diagnosis (OR, 4.98; 95% CI, 1.42 to 17.50; p=0.012), and 4.4 times higher in patients with normal initial serum LDH values (OR, 4.43; 95% CI, 1.31 to 15.01; p=0.017). Additionally, there was a trend towards significance for the diameter of the largest liver metastasis, with an inverse relationship between the diameter and survival: the smaller the size, the greater the likelihood of long-term survival (OR, 0.96; 95% CI, 0.92 to 1.00; p=0.063) (Table 3). Serum GGT levels were also significant on the univariate analysis and positively correlated with LDH levels (Pearson coefficient > 0.7).
Table 3.
Statistically significant predictors of long-term survival identified on the final multivariate-adjusted logistic regression model
| Odds ratio | 95% Confidence interval | p-value | |
|---|---|---|---|
| Age at first metastatic diagnosis ≤ 65 yr | 5.14 | 1.44-18.31 | 0.012 |
| Normal lactate dehydrogenase level | 4.38 | 1.29-14.88 | 0.018 |
| Diameter of the largest liver metastasis (millimeter reduction) | 0.96 | 0.92-1.00 | 0.063 |
5. Decision tree
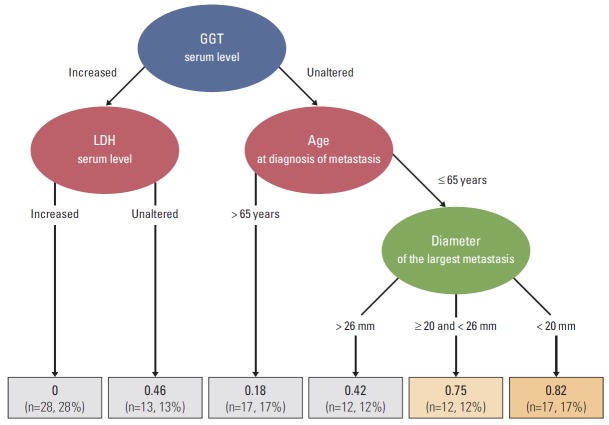
The decision tree was based on the four main variables associated with long-term survival, ordered by their relative importance according to the model. The four variables were: GGT, LDH, age at metastatic diagnosis, and largest diameter of the largest liver metastasis. The terminal nodes categorized the study sample into six prognostic groups according to the probability of achieving long-term survival (Fig. 3).
Fig. 3.
Decision tree model depicting the prognostic factors associated with long-term (> 12 mo) survival at diagnosis of the first uveal melanoma metastasis. The regression tree representation corresponds to a binary recursive partition of the feature space among the four main variables associated with long-term survival, ordered by the relative importance of each feature given by the model, as follows: categorized gamma-glutamyl transferase (GGT), categorized lactate dehydrogenase (LDH), age at metastatic diagnosis, and the largest diameter of the largest liver metastasis. The terminal nodes divided the study sample into six prognostic groups according to the probability (in bold) of long-term survival. The fraction (%) of long-surviving patients is displayed for each terminal node. The orange color shows the most favorable combination of prognostic factors for long-term survival. The characteristics associated with the most favorable outcome at first metastatic diagnosis (82% chance of long-term survival) were as follows: unaltered serum GGT, age ≤ 65 years, and diameter of the largest liver metastasis < 20 mm. By contrast, the combination of elevated LDH and GGT levels at metastatic diagnosis yielded the worst outcomes (long-term survival probability close to 0%).
Discussion
Metastatic uveal melanoma usually leads to rapid death, with most patients surviving less than 12 months [5,7,16,17]. Notwithstanding these poor outcomes, several studies have found that a small subset of patients survive more than one year [5,11-13]. However, the characteristics associated with long-term survival remain poorly understood. In the present study, we classified patients into two groups (long- and short-term survivors) and then compared the two groups to identify the differentiating characteristics. We found the following clinical features were independently predictive of long-term survival at metastatic diagnosis: age ≤ 65 years and unaltered serum LDH levels; additionally, the size (smaller vs. larger) of the largest liver metastasis showed a trend towards significance. Based on these variables, we developed a clinical decision tree.
The two distinct survival patterns that we found in this study led us to hypothesize that metastatic disease may actually have two distinct biological behaviors. In fact, recent advances in the molecular biology of uveal melanoma now make it possible to classify these tumors, based on their distinct gene expression profiles, into low-risk (class I) or high-risk (class II) of metastasizing [18]. However, the molecular pathways underlying long or short survival in metastatic patients remain unknown. Nevertheless, these pathobiological mechanisms have already begun to be elucidated in other types of cancers such as metastatic cutaneous melanoma: one study found that subjects could be stratified into better or worse survival groups according to a set of microRNAs [19]. Consequently, it seems clear that future research into the molecular basis of metastatic uveal melanoma will likely play an important role in improving our understanding of the disease. However, given the current state of knowledge, we must—for the present—rely primarily on clinical parameters for prognosis, as we did in this study.
In our series of 99 patients, the liver was the site of first metastasis in more than 90% of subjects, a finding that is consistent with previous reports [20]. We were able to identify a clear subgroup of long-term survivors who accounted for more than one-third (37.4%) of the sample. Remarkably, none of the primary tumor characteristics that increase the risk of metastatic spread [21] were predictive of survival outcomes in our study. We found significant differences between long and short survivors in several variables related to the onset of metastatic disease. Long-term survival was associated with age ≤ 65 years, longer DFI, absence of symptoms, initial diagnosis by surveillance testing, smaller dimension of the metastatic liver lesion, better performance status, unaltered liver function tests, and a previous history of primary liver surgery or second-line chemotherapy. Buzzacco et al. [13] analyzed the characteristics of nine patients who survived more than 24 months, with findings that were largely consistent with our results. Notably, some non-randomized studies have found that surgical resection of liver metastases prolongs survival in a select subset of patients [22,23]. However, given that no treatment for metastatic uveal melanoma has ever been proven to change survival outcomes, surgery may be a confounder for long-term survival because only the best candidates undergo this procedure. In our series, the patients who underwent surgery were precisely those who also presented other features associated with prolonged survival such as younger age, better performance status, and less bulky hepatic disease.
Ipilimumab, nivolumab, and pembrolizumab antibodies have demonstrated clinical benefit in the treatment of advanced cutaneous melanoma. However, the efficacy of immunotherapy in metastatic uveal melanoma is limited [24-26]. The immune privileged role of the eye influences uveal melanoma cells that use similar mechanisms to escape immune surveillance [27].
Rietschel et al. [12] evaluated overall survival in a series of metastatic uveal melanoma patients, finding that five independent variables were correlated with prolonged survival: lung/soft tissue as the only site of first metastasis; treatment with surgery or intrahepatic therapy; female sex; age < 60 years; and longer DFI. By contrast, we evaluated independent factors that were associated with long-term survival versus short-term survival. Based on our multivariate analysis, the two strongest predictors of long-term survival at the time of metastatic diagnosis were age ≤ 65 years and unaltered serum LDH levels. In addition, we found that the smaller size of the largest metastatic lesion (an indicator of the metastatic tumor burden) approached statistical significance. The only variable significantly associated with prolonged survival in both our study and that performed by Rietschel et al. [12] was age. Interestingly, those authors found higher survival rates than we did (22% of patients alive at 4 years versus 7% in our sample). It seems probable that these differences between the two studies are due to the fact that 91% of patients in our study had liver metastases—which confers a worse prognosis than other metastatic sites—versus only 60%.
In our cohort, serum LDH and GGT were the liver tests most strongly associated with survival. Given the close correlation between these two liver markers, we only included serum LDH in the multivariate analysis because this is considered a better biomarker of cancer metabolism and it is also a predictor of survival in cutaneous melanoma [28]. Our results corroborate previous findings demonstrating that the presence of normal serum LDH levels upon detection of liver metastasis is an independent predictor of long-term survival [17,29].
An important strength of the current study is the development of a straightforward clinical decision tree model based on compelling predictive data for classifying patients according to their relative probability of long-term survival. Indeed, perhaps the main value of this study is that these findings allow clinicians to predict survival outcomes based on clinical features. This provides crucial information to avoid patient mismanagement. This model may be valuable as a tool for counseling patients with regards to survival prognosis and in stratifying subjects for clinical trials to avoid survival bias. Another benefit is that the decision tree can be easily and quickly applied in daily clinical practice to estimate survival in metastatic patients. This can best be seen with an example. Take the case of a 50-year-old patient recently diagnosed with liver metastasis secondary to uveal melanoma, with unaltered GGT serum levels, and a liver lesion with a maximum diameter of < 20 mm. In this case, the decision tree suggests that this patient has an 82% chance of becoming a long-term survivor.
The main limitation of this study is its retrospective design, which prevented us from obtaining complete data for all subjects; nevertheless, only eight of the initial 107 patients were excluded due to missing data. The relatively small number of cases prevented us from validating the study, thus a future external validation is anticipated. In addition, despite the important role of molecular biology in uveal melanoma, we did not completely assess genetic factors.
In conclusion, this report describes the existence of two well-differentiated survival patterns in metastatic uveal melanoma: long-term (> 12 months) and short-term (≤ 12 months) survival. Importantly, even when the primary uveal melanoma presented poor prognostic factors suggestive of metastatic dissemination, these factors were not significantly associated with differences in survival. The only characteristics associated with long-term survival were those present at the time of distant metastasis: age ≤ 65 years, unaltered serum LDH level, and smaller diameter of the largest liver metastasis. The value of this study and the decision tree model is that patients can be categorized according to estimated survival time using common clinical factors and thus counselled more effectively. This information may be useful to offer personalized prognosis and to stratify patients in future clinical trials.
Acknowledgments
This study was supported in part by a grant of the Spanish Ministry of Health, Instituto de Salud Carlos III, AES 2015 Proyectos de Salud-ISCIII (PI15/01461).
The authors wish to thank Bradley Londres, biomedical editor, for his invaluable assistance in editing and improving this manuscript.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2014;134:2961–71. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Ocular Melanoma Study Group The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–93. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 3.Cho Y, Chang JS, Yoon JS, Lee SC, Kim YB, Kim JH, et al. Ruthenium-106 brachytherapy with or without additional local therapy shows favorable outcome for variable-sized choroidal melanomas in Korean patients. Cancer Res Treat. 2018;50:138–47. doi: 10.4143/crt.2016.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwala SS, Eggermont AM, O'Day S, Zager JS. Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer. 2014;120:781–9. doi: 10.1002/cncr.28480. [DOI] [PubMed] [Google Scholar]
- 5.Gragoudas ES, Egan KM, Seddon JM, Glynn RJ, Walsh SM, Finn SM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–9. doi: 10.1016/s0161-6420(91)32285-1. [DOI] [PubMed] [Google Scholar]
- 6.Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology. 2005;112:1784–9. doi: 10.1016/j.ophtha.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–5. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Woodman SE. Metastatic uveal melanoma: biology and emerging treatments. Cancer J. 2012;18:148–52. doi: 10.1097/PPO.0b013e31824bd256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buder K, Gesierich A, Gelbrich G, Goebeler M. Systemic treatment of metastatic uveal melanoma: review of literature and future perspectives. Cancer Med. 2013;2:674–86. doi: 10.1002/cam4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duh EJ, Schachat AP, Albert DM, Patel SM. Long-term survival in a patient with uveal melanoma and liver metastasis. Arch Ophthalmol. 2004;122:285–7. doi: 10.1001/archopht.122.2.285. [DOI] [PubMed] [Google Scholar]
- 11.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–43. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 12.Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076–80. doi: 10.1200/JCO.2005.02.6534. [DOI] [PubMed] [Google Scholar]
- 13.Buzzacco DM, Abdel-Rahman MH, Park S, Davidorf F, Olencki T, Cebulla CM. Long-term survivors with metastatic uveal melanoma. Open Ophthalmol J. 2012;6:49–53. doi: 10.2174/1874364101206010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edge SB, Byrd SR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York: Springer; 2010. pp. 547–59. [Google Scholar]
- 15.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15:651–74. [Google Scholar]
- 16.Kath R, Hayungs J, Bornfeld N, Sauerwein W, Hoffken K, Seeber S. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72:2219–23. doi: 10.1002/1097-0142(19931001)72:7<2219::aid-cncr2820720725>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Eskelin S, Pyrhonen S, Hahka-Kemppinen M, Tuomaala S, Kivela T. A prognostic model and staging for metastatic uveal melanoma. Cancer. 2003;97:465–75. doi: 10.1002/cncr.11113. [DOI] [PubMed] [Google Scholar]
- 18.Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121:1281–8. doi: 10.1016/j.ophtha.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura MF, Belitskaya-Levy I, Rose AE, Zakrzewski J, Gaziel A, Hanniford D, et al. Melanoma MicroRNA signature predicts post-recurrence survival. Clin Cancer Res. 2010;16:1577–86. doi: 10.1158/1078-0432.CCR-09-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damato B. Developments in the management of uveal melanoma. Clin Exp Ophthalmol. 2004;32:639–47. doi: 10.1111/j.1442-9071.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 21.Singh AD, Shields CL, Shields JA. Prognostic factors in uveal melanoma. Melanoma Res. 2001;11:255–63. doi: 10.1097/00008390-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hsueh EC, Essner R, Foshag LJ, Ye X, Wang HJ, Morton DL. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100:122–9. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042–6. doi: 10.1136/bjo.2008.153684. [DOI] [PubMed] [Google Scholar]
- 24.Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–53. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101:38–44. doi: 10.1136/bjophthalmol-2016-309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piulats JM, De La Cruz-Merino L, Curiel García MT, Berrocal A, Alonso-Carrión L, Espinosa E, et al. Phase II multicenter, single arm, open label study of nivolumab (NIVO) in combination with ipilimumab (IPI) as first line in adult patients (pts) with metastatic uveal melanoma (MUM): GEM1402 NCT-02626962. J Clin Oncol. 2017;35(Suppl):Abstr 9533. [Google Scholar]
- 27.Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med. 2016;4:172. doi: 10.21037/atm.2016.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedikian AY, Legha SS, Mavligit G, Carrasco CH, Khorana S, Plager C, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76:1665–70. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]