Abstract
Purpose
The purpose of this study was to evaluate the long-term clinical outcome and toxicity of induction chemotherapy (IC) followed by concomitant chemoradiotherapy (CCRT) compared with CCRT alone for the treatment of children and adolescent locoregionally advanced nasopharyngeal carcinoma (LACANPC).
Materials and Methods
A total of 194 locoregionally advanced nasopharyngeal carcinoma patients youngerthan 21 years who received CCRT with or without IC before were included in the study population. Overall survival (OS) rate, progression-free survival (PFS) rate, locoregional recurrence-free survival (LRFS) rate, and distant metastasis-free survival (DMFS) rate were assessed by the Kaplan-Meier method and a log-rank test. Treatment toxicities were clarified and compared between two groups.
Results
One hundred and thiry of 194 patients received IC+CCRT. Patients who were younger and with more advanced TNM stage were more likely to receive IC+CCRT and intensive modulated radiotherapy. The addition of IC before CCRT failed to improve survival significantly. The matched analysis identified 43 well-balanced patients in both two groups. With a median follow-up of 51.5 months, no differences were found between the IC+CCRT group and the CCRT group in 5-year OS (83.7% vs. 74.6%, p=0.153), PFS (79.2% vs. 73.4%, p=0.355), LRFS (97.7% vs. 88.2%, p=0.083), and DMFS (81.6% vs. 81.6%, p=0.860). N3 was an independent prognostic factor predicting poorer OS, PFS, and DMFS. The addition of IC was associated with increased rates of grade 3 to 4 neutropenia.
Conclusion
This study failed to demonstrate that adding IC before CCRT could provide a significant additional survival benefit for LACANPC patients. Further investigations are warranted.
Keywords: Nasopharyngeal carcinoma, Children and adolescents, Chemoradiotherapy, Induction chemotherapy, Survival
Introduction
Nasopharyngeal carcinoma (NPC), a malignancy arising from nasopharynx epithelium, is endemic in southern China and Southeast Asia [1]. Although most patients with NPC present in the fourth to fifth decade, there is an additional minor peak appearing at 10-20 years of age [2]. Compared with NPC in adults, NPC in children and adolescents (CANPC) has a closer association with Epstein-Barr virus infection, which is more commonly present as advanced locoregional disease and has a high rate of distant metastasis [3]. As the optimal treatment modality for juvenile patients has not yet been established, the primary treatment strategy is concomitant chemoradiotherapy (CCRT) with or without additional cycles of chemotherapy, which is extrapolated from guidelines tailored for adult patients. With the use of CCRT with or without additional cycles of chemotherapy, CANPC showed better survival than in adults [4]. Distant metastasis is now the main source of treatment failure in CANPC [5,6].
Induction chemotherapy (IC) has been considered to show good compliance and improve distant control in adult NPC. Recently, a phase III study conducted by Sun et al. [7] in adult NPC comparing IC+CCRT with CCRT alone demonstrated that patients receiving IC+CCRT had improved failure-free survival. An individual patient data network metaanalysis [8] also showed that the addition of IC to CCRT achieved the highest effect on distant control. Some studies documented the efficacy and safety of IC followed by CCRT in juvenile NPC with the goal of limiting the high incidence of distant metastasis and improve the outcome [9-11]. Varan et al. [12] retrospectively analyzed 10 patients (aged < 21 years) with NPC who received four cycles of IC before radiotherapy and demonstrated that neoadjuvant chemotherapy before radiotherapy is safe for use in pediatric and young adult nasopharyngeal carcinoma [12].
So far, randomized studies regarding IC followed by CCRT compared with CCRT alone in childhood and young adult NPC patients are deficient. Therefore, we conducted the present observational study to examine the long-term clinical outcome and toxicity of IC followed by CCRT compared with CCRT alone for the treatment of children and adolescent locoregionally advanced nasopharyngeal carcinoma.
Materials and Methods
1. Patients
Patients, who were 21 years or younger, that were biopsy diagnosed with World Health Organization types II-III NPC at our institution between November 1989 and April 2015 were identified; there was a total of 461 patients. All the patients were re-evaluated using the seventh American Joint Committee on Cancer staging system. Fig. 1 shows the summary of the inclusion and exclusion criteria of patients in this study. Patients were excluded if they had no available follow-up information, incomplete treatment, distant metastatic disease, clinical stage I-II, had not received CCRT, or received adjuvant chemotherapy. Ultimately, 194 patients who received CCRT with or without IC were included in the study population, and of these, 64 patients were treated with CCRT alone.
Fig. 1.
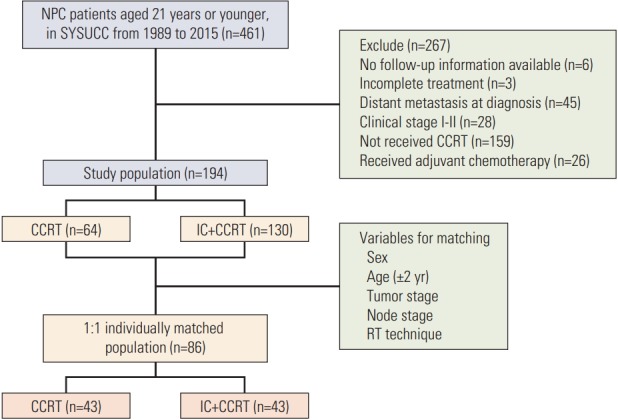
Summary of the inclusion and exclusion criteria. NPC, nasopharyngeal carcinoma; SYSUCC, Sun Yat-Sen University Cancer Center; CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; RT, radiotherapy.
2. Chemotherapy
IC was cisplatin-based chemotherapy, which was cisplatin in combination with fluorouracil or taxane or with both taxane and fluorouracil. Chemotherapy was administered every 3 weeks for two or three cycles. Concurrent chemotherapy with cisplatin was administered either every 3 weeks (80-100 mg/m2) or weekly (30-40 mg/m2) during radiotherapy.
3. Radiotherapy
All patients received definitive radiotherapy in the form of two-dimensional conventional radiotherapy (2D-CRT) or intensity-modulated radiotherapy (IMRT). All patients were treated with one fraction daily for 5 days per week. Uniform radiotherapy protocols for 2D-CRT and IMRT used at Sun Yat-Sen University Cancer Center (SYSUCC) were described in previous studies [13-16]. The accumulated radiation doses for 2D-CRT were 64-80 Gy to the primary tumor at 2 Gy per fraction, 60-64 Gy to the involved areas of the neck, and 50 Gy to the uninvolved areas. The prescribed IMRT radiation doses were 64-70 Gy to the planning target volume (PTV) of the gross tumor volumes of the nasopharynx, 60-68 Gy to the PTV of the gross tumor volumes of the positive neck lymph nodes, 56-64 Gy to the PTV of the first clinical tumor volume, and 50-58 Gy to the PTV of the second clinical target volume in 30-33 fractions.
4. Outcome and follow-up
The primary endpoint for the study was overall survival (OS), which is defined as the date of treatment to the date of death from any cause or patient censoring at the date of the final follow-up. The secondary endpoints for the study were progression-free survival (PFS), distant failure-free survival (DMFS), and locoregional failure-free survival (LRFS). PFS was calculated from the date of treatment to the date of locoregional failure, distant failure, or death from any cause, whichever occurred first. DMFS was defined as the time from the date of treatment to the date of the first observation of distant metastasis or until the date of the last follow-up, and LRFS was defined as the time from the date of treatment to the absence of a primary site or neck lymph node relapse or until the date of the last follow-up. Follow-up was measured from the first day of treatment until the day of the last examination or the day of death. During the first 3 years, patients were evaluated every 3 months and then every 6 months thereafter until death. Treatment-related acute toxicities were graded according to the Common Terminology Criteria for Adverse Events ver. 4.0. Acute and late radiationrelated complications were scored according to the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer late radiation morbidity scoring schema. The last follow-up date was April 30, 2017.
5. Statistical analysis
Statistical analyses were performed using SPSS software ver. 22.0 (IBM Corp., Armonk, NY). Fisher exact test and a chi-square test were used to explore the differences between categorical variables, while t tests and Mann-Whitney U tests were used to analyze continuous variables. Actuarial rates were estimated using the Kaplan-Meier method, and the survival curves were compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards model to estimate hazard ratios (HR). All statistical tests were two-sided, and the criterion for statistical significance was set at p=0.05.
6. Ethical statement
This retrospective study was approved by the institutional review board and ethics committee of the SYSUCC. All of the participants provided written informed consent.
Results
1. Patient characteristics and patterns of failure
Table 1 illustrates the pretreatment characteristics of the patients. There were no statistically significant differences in terms of sex and tumor stage among the patients both with and without IC. Patients who were younger and with a more advanced node stage were more likely to received IC+CCRT and IMRT (p < 0.05 for all comparisons).
Table 1.
Baseline patient demographic and clinical characteristics
| IC+CCRT (n=130) | CCRT (n=64) | p-valuea) | |
|---|---|---|---|
| Age, mean (range, yr) | 17.28 (8-21) | 18.08 (9-21) | 0.046 |
| Sex | |||
| Male | 94 (72.3) | 46 (71.9) | 0.950 |
| Female | 36 (28.7) | 18 (28.1) | |
| Tumor stage | |||
| T1 | 0 | 1 (1.6) | 0.006 |
| T2 | 6 (4.6) | 3 (4.7) | |
| T3 | 56 (43.1) | 41 (64.1) | |
| T4 | 68 (52.3) | 19 (29.7) | |
| Node stage | |||
| N0 | 5 (3.8) | 3 (4.7) | 0.005 |
| N1 | 25 (19.2) | 22 (34.4) | |
| N2 | 73 (56.2) | 36 (56.3) | |
| N3 | 27 (20.8) | 3 (4.7) | |
| Clinical stage | |||
| Stage III | 50 (38.5) | 44 (68.8) | < 0.001 |
| Stage IVa | 53 (40.8) | 17 (26.6) | |
| Stage IVb | 27 (20.8) | 3 (4.7) | |
| RT technique | |||
| 2DRT | 21 (16.2) | 27 (42.2) | < 0.001 |
| IMRT | 109 (83.3) | 37 (57.8) |
Values are presented as number (%) unless otherwise indicated. IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; 2DRT, two-dimensional radiotherapy; IMRT, intensity-modulated radiotherapy.
p-values were calculated using a chi-square test (or Fisher exact test).
After a median follow-up of 48.1 months, 15.4% of patients (20 of 130) receiving IC+CCRT and 21.9% of patients (14 of 64) receiving CCRT alone experienced tumor progression (p=0.264). Table 2 shows the detailed patterns of failure. The most common failure was distant failure. There was a significant reduction in locoregional failure (p=0.010) but not in distant recurrence for patients who received IC.
Table 2.
Patterns of failure
| Failure site | A |
B |
||||
|---|---|---|---|---|---|---|
| CCRT (n=64) | IC+CCRT (n=130) | p-valuea) | CCRT (n=43) | IC+CCRT (n=43) | p-valuea) | |
| T | 6 | 2 | 4 | 0 | ||
| N | 3 | 2 | 1 | 1 | ||
| M | 7 | 17 | 7 | 7 | ||
| T+N | 1 | 0 | 0 | 0 | ||
| T+M | 1 | 0 | 1 | 0 | ||
| N+M | 0 | 1 | 0 | 0 | ||
| T+N+M | 0 | 0 | 0 | 0 | ||
| LF | 8 (12.5) | 4 (3.1) | 0.010 | 5 (11.6) | 1 (2.3) | 0.090 |
| DF | 7 (10.9) | 17 (13.1) | 0.67 | 7 (16.3) | 7 (16.3) | > 0.999 |
| Progression | 14 (21.9) | 20 (15.4) | 0.264 | 11 (25.6) | 8 (18.6) | 0.436 |
Values are presented as number (%). CCRT, concomitant chemoradiotherapy; IC, Induction chemotherapy; T, nasopharynx; N, neck; M, distant metastasis; LF, locoregional failure; DF, distant failure.
p-values were calculated using a chi-square test (or Fisher exact test).
2. Survival outcome
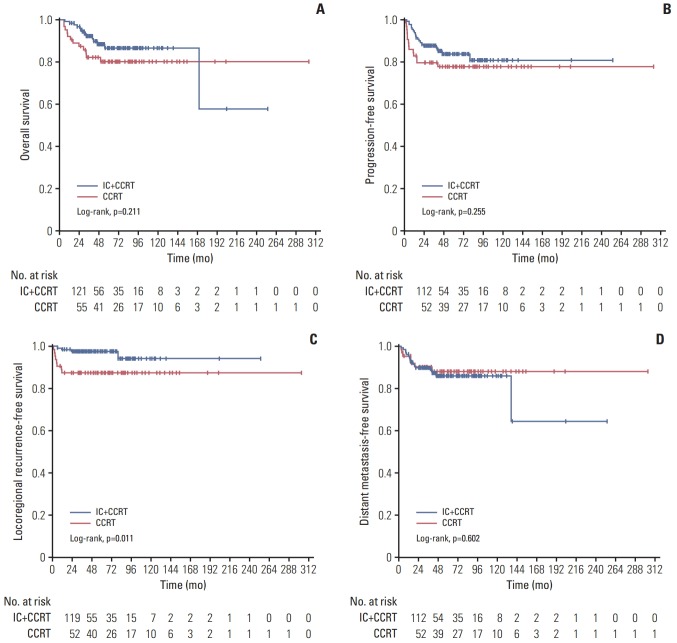
The 5-year OS rate was 86.6% (95% confidence interval [CI], 79.5 to 93.7) in the IC+CCRT group and 80.2% (95% CI, 70.0 to 90.4) in the CCRT group (p=0.211), and the 5-year PFS rate was 83.7% (95% CI, 76.8 to 90.6) for patients in IC+CCRT group compared with 77.7% (95% CI, 67.3 to 88.1) for patients in the CCRT group (p=0.255). The 5-year DMFS rates of the IC+CCRT group versus the CCRT group were 85.9% (95% CI, 79.2 to 92.6) versus 88.0% (95% CI, 79.6 to 96.4) (p=0.602). The 5-year LRFS rates for patients with and without IC were 97.6% (95% CI, 95.1 to 100) and 87.4% (95% CI, 79.2 to 95.6) (p=0.011), which showed that IC significantly improved LRFS (Fig. 2).
Fig. 2.
Comparison of the overall survival (A), progression-free survival (B), locoregional failure (C), and distant metastasise-free survival (D) between patients with (n=130) and without (n=64) induction chemotherapy (IC). CCRT, concurrent chemoradiotherapy.
3. Outcome analysis limited to matching cohorts with same tumor stage, node stage, and radiotherapy technique
To avoid the potential interference of the imbalance of tumor stage, node stage and radiotherapy technique in both groups, 43 patients from the CCRT group matching the 43 patients in the IC+CCRT group were reanalyzed. The matched cohort achieved an adequate balance between the IC+CCRT group and the CCRT group for all variables (Table 3).
Table 3.
Patient characteristics after match
| IC+CCRT (n=43) | CCRT (n=43) | p-valuea) | |
|---|---|---|---|
| Age, mean (range, yr) | 18.23 (14-21) | 18.37 (12-21) | 0.587 |
| Sex | |||
| Male | 33 (74.4) | 33 (74.4) | > 0.999 |
| Female | 10 (23.3) | 10 (23.3) | |
| Tumor stage | |||
| T1 | 0 | 0 | > 0.999 |
| T2 | 1 (2.3) | 1 (2.3) | |
| T3 | 26 (60.5) | 26 (60.5) | |
| T4 | 16 (37.2) | 16 (37.2) | |
| Node stage | |||
| N0 | 2 (4.7) | 2 (4.7) | > 0.999 |
| N1 | 11 (25.6) | 11 (25.6) | |
| N2 | 28 (65.1) | 28 (65.1) | |
| N3 | 2 (4.7) | 2 (4.7) | |
| Clinical stage | |||
| Stage III | 26 (60.5) | 26 (60.5) | > 0.999 |
| Stage IVa | 15 (34.9) | 15 (34.9) | |
| Stage IVb | 2 (4.7) | 2 (4.7) | |
| RT technique | |||
| 2DRT | 13 (30.2) | 13 (30.2) | > 0.999 |
| IMRT | 30 (69.8) | 30 (69.8) |
Values are presented as number (%) unless otherwise indicated. IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; 2DRT, two-dimensional radiotherapy; IMRT, intensity-modulated radiotherapy.
p-values were calculated using a chi-square test (or Fisher exact test).
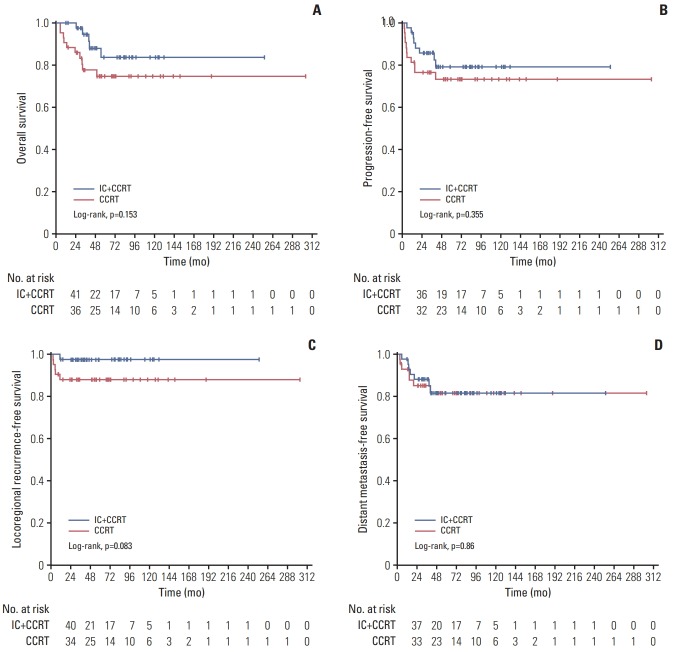
In the matched cohort, with a median follow-up duration of 48.1 months (range, 5.26 to 303.7 months), six patients developed local failure (one from IC+CCRT group and five from CCRT group), 14 exhibited distant metastasis (seven from IC+CCRT group and seven from CCRT group), and 15 patients died (five from IC+CCRT group and 10 from CCRT group). The detailed patterns of failure are listed in Table 2. The 5-year OS rate was 83.7% (95% CI, 70.2 to 97.2) in the IC+CCRT group and 74.6% (95% CI, 60.9 to 88.3) in the CCRT group (p=0.153) (Fig. 3). The 5-year probabilities for PFS in the IC+CCRT group and the CCRT group were 79.2% (95% CI, 66.1 to 92.3) and 73.4% (95% CI, 59.7 to 87.1) (p=0.355), respectively. The 5-year DMFS rates in the IC+CCRT group vs. the CCRT group were 81.6% (95% CI, 69.1 to 94.1) vs. 81.6% (95% CI, 69.1 to 94.1) (p=0.860). The 5-year LRFS rates for patients with and without IC were 97.7% (95% CI, 93.2 to 100) and 88.2% (95% CI, 78.6 to 97.8). Although the CCRT group had about a 10% greater risk of locoregional failure than the IC+CCRT group, the difference was not significant, and the p-value was borderline (p=0.083).
Fig. 3.
Comparison of the overall survival (A), progression-free survival (B), locoregional failure (C), and distant metastasise-free survival (D) in the matched cohort between patients with (n=43) and without (n=43) induction chemotherapy (IC). CCRT, concurrent chemoradiotherapy.
The following factors were included in the multivariate analyses: sex (male vs. female), T stage (T4 vs. T2-3), N stage (N3 vs. N0-2), radiotherapy technique (IMRT vs. 2D-CRT) and IC (yes or not). Multivariate analyses revealed that N3 was an independent prognostic factor predicting poorer OS (HR, 4.958; 95% CI, 1.035 to 23.748; p=0.045), PFS (HR, 5.446; 95% CI, 1.482 to 20.017; p=0.011), and DMFS (HR, 9.501; 95% CI, 2.445 to 36.920; p=0.001). The radiotherapy technique was the only independent prognostic predictor of LRRFS (HR, 0.174; 95% CI, 0.031 to 0.962; p=0.045).
4. Treatment compliance and toxicities in the matched cohort
In pre-matched cohort, only one patient dropped out during IC because of grade 2 transaminase increase and regional lymph node progression. After matching, all patients completed planned radiotherapy, while all the patients in the IC+CCRT group received 2-4 cycles of IC. In terms of concurrent chemotherapy, 71 patients received triweekly cisplatin, and among these patients, 38 (88.3%) and 33 (76.7%) patients were from the IC+CCRT group and CCRT group, respectively. The remaining 14 patients received weekly cisplatin. In the IC+CCRT group and CCRT group, 40 (93.0%) and 39 (90.7%) patients received at least two cycles of triweekly cisplatin or five cycles of weekly cisplatin, respectively. Six patients dropped out from treatment due to toxicities. S1 Table lists the specifics of the concurrent chemotherapy in both groups.
The most common side effects included myelosuppression, radiotherapy-related dermatitis and mucositis. In terms of myelosuppression, 20.9% of patients suffered from grade 3 leucopenia, and no patients developed grade 4 leucopenia. Furthermore, 19.8% and 4.7% of patients suffered from neutropenia and anemia, respectively. No patients underwent moderate to severe thrombocytopenia. The IC+CCRT group had significantly greater rates of grade 3 to 4 neutropenia. With the exceptions of leucopenia, neutropenia, anemia and mucositis, other treatment-related toxicities were mild in both groups. Table 4 lists the distribution of all grades of acute adverse effects.
Table 4.
Cumulative adverse events during treatment by maximum grade per patient during treatment
| Adverse event | Toxic effect |
p-valuea) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC+CCRT (n=43) |
CCRT (n=62) |
All grades | Grade 3-4 | |||||||||
| All grades | 1 | 2 | 3 | 4 | All grades | 1 | 2 | 3 | 4 | |||
| Leucopenia | 39 (90.7) | 8 | 19 | 12 | 0 | 40 (93.0) | 18 | 16 | 6 | 0 | 1 | 0.112 |
| Neutropenia | 36 (83.7) | 7 | 15 | 12 | 2 | 22 (51.2) | 11 | 8 | 3 | 0 | 0.001 | 0.003 |
| Anemia | 32 (74.4) | 21 | 8 | 2 | 1 | 17 (39.5) | 12 | 4 | 1 | 0 | 0.001 | 0.609 |
| Thrombocytopenia | 12 (27.9) | 8 | 4 | 0 | 0 | 9 (20.9) | 6 | 3 | 0 | 0 | 0.451 | - |
| AST increased | 8 (18.6) | 8 | 0 | 0 | 0 | 2 (4.7) | 2 | 0 | 0 | 0 | 0.044 | - |
| ALT increased | 21 (48.8) | 18 | 3 | 0 | 0 | 8 (18.6) | 8 | 0 | 0 | 0 | 0.003 | - |
| BUN | 5 (11.6) | 5 | 0 | 0 | 0 | 1 (2.3) | 1 | 0 | 0 | 0 | 0.204 | - |
| CRE | 4 (9.3) | 4 | 0 | 0 | 0 | 1 (2.3) | 1 | 0 | 0 | 0 | 0.357 | - |
| Mucositis | 38 (88.4) | 14 | 17 | 7 | 0 | 42 (97.7) | 13 | 23 | 6 | 0 | 0.204 | 0.763 |
| Dermatitis | 33 (76.7) | 25 | 7 | 1 | 0 | 34 (79.1) | 24 | 9 | 1 | 0 | 0.795 | > 0.999 |
| Vomiting | 28 (65.1) | 17 | 9 | 2 | 0 | 32 (74.4) | 21 | 9 | 2 | 0 | 0.348 | > 0.999 |
Values are presented as number (%). IC, induction chemotherapy; CCRT, concurrent chemoradiotherapy; AST, aspartate amino transferase; ALT, alanine amino transferase; BUN, blood urea nitrogen; CRE, creatinine.
p-values were calculated using a chi-square test (or Fisher exact test).
To the date of the final follow-up, 71 patients survived, and 38 of these patients were from IC+CCRT group, and the remaining received CCRT. In terms of late toxicities, 38 patients (53.5%) suffered different levels of hearing impairment, which was the most common late toxicity. Radiationinduced brain injuries were observed in nine patients including temporal brain lobes injury in three patients (4.2%) and cranial nerve palsy in six patients (8.5%). Grade 2-4 xerostomia, hearing loss and trismus were observed in 11, 24, and three patients, respectively (Table 5). No significant difference was found between both groups.
Table 5.
Late toxicities in patients treated with IC+CCRT versus CCRT
| Late toxicity | IC+CCRT (n=38) | CCRT (n=33) | p-valuea) |
|---|---|---|---|
| Xerostomiab) | 4 (10.5) | 7 (21.2) | 0.215 |
| Hearing lossb) | 14 (36.8) | 10 (30.3) | 0.561 |
| Skin dystrophy | 1 (2.6) | 1 (3.0) | > 0.999 |
| Neck fibrosis | 9 (23.7) | 6 (18.2) | 0.571 |
| Trismusb) | 3 (8.1) | 2 (6.1) | > 0.999 |
| Radiation encephalopathy | 1 (2.6) | 2 (6.1) | 0.901 |
| Cranial nerve palsy | 2 (5.2) | 4 (12.1) | 0.543 |
| Memory impairmentc) | 7 (18.4) | 5 (15.2) | 0.714 |
Values are presented as number (%). IC, induction chemotherapy; CCRT, concomitant chemoradiotherapy.
p-values were calculated using a chi-square test (or Fisher exact test),
Grade 2-4 toxicities,
Grade 2-3 toxicities.
Discussion
CANPC shows better survival in adults [4]. The combination of chemotherapy and radiotherapy has been accepted as the optimal treatment for CANPC patients in recent literature [17,18] reporting a 5-year OS and PFS of 41-91% and 47%-85%, respectively [5,6,19-22]. The treatment outcome of patients in our study showed a 5-year OS of approximately 80%, which is comparable to previous data. Currently, the primary treatment failure pattern of CANPC is distant metastasis [5,6]. The relatively short time to systemic failure, in the absence of locoregional failure, might suggest the presence of occult systemic disease at the time of diagnosis and the need for a more effective treatment strategy in locoregionally advanced disease. Increasing evidence to incorporate IC into the treatment of patients with the locoregionally advanced disease has been reported. IC followed by CCRT has been accepted as a useful regimen to significantly improve distant control in adult NPC [7]. Though IC was used as part of treatment in several studies, it was difficult to differentiate how much IC contributed to the long-term outcome. Due to the small size of pediatric and adolescents NPC cohort, no prospective trial has been carried out to test the efficacy of IC by directly comparing the treatment outcome of CCRT with or without IC. The role of IC in CANPC remains controversial.
In our study, we first compared the long-term outcomes of children and adolescent patients with locoregionally advanced NPC that received CCRT with or without IC. Individually matching was performed to balance the baseline characteristics between the groups. In both the pre-matched and the matched cohorts, we compared 5-year OS, PFS, LRFS, and DMFS between IC+CCRT and CCRT and failed to demonstrate a survival benefit in locally advanced CANPC patients. IC showed a trend of improving local control, and adding IC improved the rate of LRFS from 88.2% of patients in the CCRT alone group to 97.7% of patients in IC+CCRT group (p=0.083) in this study. Distant metastasis remains a major obstacle in the cure of CANPC, and N3 node stage was the only independent factor predicting poorer OS, PFS, and DMFS.
We have reviewed previous studies about the use of IC followed by CCRT in children and adolescent NPC, which reported the survival rates vary between 55% and 90% for OS and between 60.6% and 77% for disease-free survival and event-free survival [12,18,20,23-25]. Guo et al. [6] retrospectively reviewed 95 locoregionally advanced NPC child and adolescent patients treated with IC before radiotherapy with or without concurrent chemotherapy/adjuvant chemotherapy and reported that the 4-year OS and PFS were 90.8% and 79.1%, respectively. Yan et al. [5] report a retrospective analysis of 185 CANPC cases in an endemic area of which 83.4% patients received IC+CCRT, and the 5-year OS was 78%. The survival rates of these two studies were in accordance with the data in this study. Recently, increasing evidence to incorporate IC into the treatment has been reported. The Italian Rare Tumors in Pediatric Age Project, which uses a prospective protocol of three courses of cisplatin/5-fluorouracil IC followed by CCRT in CANPC, reported that the 5-year OS and PFS rates were 80.9% and 79.3%, respectively [26]. This result was very close to the survival rate that we reported in the IC+CCRT group of the matched cohort. An international randomized phase 2 study comparing two regimens of IC followed by CCRT reported that the estimated 3-year OS rate was 78.0% for the cisplatin and 5-fluorouracil (PF) group and 85.7% for the docetaxel with PF group [9], which is a lower survival rate than in our study probably because of the relatively greater proportion of stage IV disease (approximately 55%) compared to our study (39.6%).
There is no other published direct comparison of the treatment outcome between IC+CCRT and CCRT in CANPC. Experience in adult NPC demonstrated that adding IC could significantly improve failure-free survival in locoregionally advanced nasopharyngeal carcinoma. However, the analysis of the survival outcome in this study showed no significant difference between the two groups, while it seemed to have a trend that with a larger size of study population, adding IC could increase the rate of survival significantly. One plausible explanation for these negative results in this study is that the greater incidence of toxicities caused by adding IC may result in radiotherapy interruption(s) and chemotherapy dose reduction. Another potential explanation for the lack of a benefit is that, in this study, only 12 patients received positron emission tomography and computed tomography (PET/CT), which can detect more distant metastases than conventional staging in patients with NPC [27] during diagnosis. All the patients who developed distant metastases after treatment did not received PET/CT. Additionally, patients with high risk of distant recurrence may benefit from IC; however, the high risk factors of distant metastases have not been identified in children and adolescents NPC.
All the patients in our study completed planned radiotherapy. During CCRT, 93.0% of patients in the IC+CCRT group and 90.7% of patients in CCRT group completed at least two cycles of triweekly cisplatin or five cycles of weekly cisplatin. Toxicities were the main reasons for discontinuation of concurrent cisplatin. As expected, more adverse events were found in IC+CCRT group involving the blood/lymphatic system because of the increased cycles of chemotherapy. Apart from this, including radiotherapy-related acute toxicities, the incidence and severity of adverse events were similar in the two treatment groups. These results showed the feasibility of these two regimens. With better outcomes and longer survival, the incidence of radiation-induced late complications has drawn more attention. The study from Cheuk et al. [23] reported a 15-year cumulative incidence of any morbidity to be 84% (53% for hearing loss, 43% for hypothyroidism, and 14% for growth hormone deficiency). The prospective clinical trial [26] referenced above reported a 65% incidence of all types of morbidity. Consistent with previous studies, we demonstrated that hearing loss and xerostomia were the most common morbidities. No difference was found between the two groups. In the view of similar survival benefit and toxicities, IC followed by CCRT as a primary treatment should be used with more caution, and more evidence is needed to guide the use of IC in locoregionally advanced CANPC patients. Future studies should focus on selecting patients who may benefit from IC and adjusting radiation dose according to how well the tumor responds to IC so to lessen the side effects of the radiation.
However, several limitations of our study should be addressed. Firstly, the retrospective nature of the study certainly served as a fundamental pitfall of the current study. Secondly, for the reason of low incidence of CANPC, the number of patients that can be included is relatively limited, which might make the results of the study underpowered, and selection bias might exist. Thirdly, IC regimens in this study were heterogeneous. These limitations make us more cautious when interpreting the result of this study. Although this study failed to demonstrate that adding IC before CCRT could provide a significant additional survival benefit in locally advanced CANPC patients, it seemed to have a trend that with a larger size of study population, adding IC could increase the rate of survival significantly. In addition, in adult NPC, a phase III study demonstrated that adding IC before CCRT had improved failure-free survival [7]. Furthermore, radiotherapy dose can be adaptation according to IC response to minimize late effects in CANPC. However, besides survival and toxicities, medical and time cost incurred in an effort to control or alleviate the symptoms of either complication or cancer progression in each group is also an important issue we should concern. For example, treatment of myelosuppression and adding IC do cost more. And the duration of IC+CCRT regimen is about 1.5 months longer than treating with CCRT. Therefore, multicenter prospective studies with the emphasis on survival, medical and time cost and quality of life measurement in refined high-risk populations are warranted to validate the benefit of IC with CCRT in the treatment of locoregionally advanced CANPC.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2016YFC0902000), the National Natural Science Foundation of China (No. 81425018, No.81672863, No. 81072226, No. 81201629), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the National Key Basic Research Program of China (No.2013CB910304), the Special Support Plan of Guangdong Province (No.2014TX01R145), the Sci-Tech Project Foundation of Guangdong Province (No.2014A020212103, No.2011B080701034), the Health & Medical Collaborative Innovation Project of Guangzhou City (No. 201400000001), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No.2014BAI09B10), the Sun Yat-sen University Clinical Research 5010 Program, and the Fundamental Research Funds for the Central Universities.
Footnotes
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–54. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Levine PH, Connelly RR, Easton JM. Demographic patterns for nasopharyngeal carcinoma in the United States. Int J Cancer. 1980;26:741–8. doi: 10.1002/ijc.2910260607. [DOI] [PubMed] [Google Scholar]
- 3.Ingersoll L, Woo SY, Donaldson S, Giesler J, Maor MH, Goffinet D, et al. Nasopharyngeal carcinoma in the young: a combined M.D. Anderson and Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19:881–7. doi: 10.1016/0360-3016(90)90008-8. [DOI] [PubMed] [Google Scholar]
- 4.Downing NL, Wolden S, Wong P, Petrik DW, Hara W, Le QT. Comparison of treatment results between adult and juvenile nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1064–70. doi: 10.1016/j.ijrobp.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Yan Z, Xia L, Huang Y, Chen P, Jiang L, Zhang B. Nasopharyngeal carcinoma in children and adolescents in an endemic area: a report of 185 cases. Int J Pediatr Otorhinolaryngol. 2013;77:1454–60. doi: 10.1016/j.ijporl.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Cui X, Lin S, Lin J, Pan J. Locoregionally advanced nasopharyngeal carcinoma in childhood and adolescence: Analysis of 95 patients treated with combined chemotherapy and intensity-modulated radiotherapy. Head Neck. 2016;38 Suppl 1:E665–72. doi: 10.1002/hed.24066. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–20. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 8.Ribassin-Majed L, Marguet S, Lee AW, Ng WT, Ma J, Chan AT, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol. 2017;35:498–505. doi: 10.1200/JCO.2016.67.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casanova M, Ozyar E, Patte C, Orbach D, Ferrari A, Veyrat-Follet C, et al. International randomized phase 2 study on the addition of docetaxel to the combination of cisplatin and 5-fluorouracil in the induction treatment for nasopharyngeal carcinoma in children and adolescents. Cancer Chemother Pharmacol. 2016;77:289–98. doi: 10.1007/s00280-015-2933-2. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Galindo C, Krailo MD, Krasin MJ, McCarville MB, Hicks J, Pashankar F, et al. Treatment of childhood nasopharyngeal carcinoma (cNPC) with neoadjuvant chemotherapy (NAC) and concomitant chemoradiotherapy (CCRT): results of the Children’s Oncology Group ARAR0331 study. J Clin Oncol. 2016;34(15 Suppl):Abstr. doi: 10.1200/JCO.19.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaghloul MS, Eldebawy E, Ahmed S, Ammar H, Khalil E, Abdelrahman H, et al. Does primary tumor volume predict the outcome of pediatric nasopharyngeal carcinoma?: A prospective single-arm study using neoadjuvant chemotherapy and concomitant chemotherapy with intensity modulated radiotherapy. Asia Pac J Clin Oncol. 2016;12:143–50. doi: 10.1111/ajco.12460. [DOI] [PubMed] [Google Scholar]
- 12.Varan A, Ozyar E, Corapcioglu F, Koksal Y, Aydin B, Yazici N, et al. Pediatric and young adult nasopharyngeal carcinoma patients treated with preradiation cisplatin and docetaxel chemotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1116–20. doi: 10.1016/j.ijrobp.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–83. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Mai HQ, Hong MH, Cui NJ, Lu TX, Lu LX, et al. Is the 1997 AJCC staging system for nasopharyngeal carcinoma prognostically useful for Chinese patient populations? Int J Radiat Oncol Biol Phys. 2001;50:1181–9. doi: 10.1016/s0360-3016(01)01537-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Han F, Lu LX, Huang SM, Lin CG, Deng XW, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23(11 Suppl):1532–7. [PubMed] [Google Scholar]
- 16.Ma J, Liu L, Tang L, Zong J, Lin A, Lu T, et al. Retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: prognostic value and staging categories. Clin Cancer Res. 2007;13:1445–52. doi: 10.1158/1078-0432.CCR-06-2059. [DOI] [PubMed] [Google Scholar]
- 17.Ozyar E, Selek U, Laskar S, Uzel O, Anacak Y, Ben-Arush M, et al. Treatment results of 165 pediatric patients with nonmetastatic nasopharyngeal carcinoma: a Rare Cancer Network study. Radiother Oncol. 2006;81:39–46. doi: 10.1016/j.radonc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Bakkal BH, Kaya B, Berberoglu S, Aksu G, Sayin MY, Altundag MB, et al. The efficiency of different chemoradiotherapy regimens in patients with paediatric nasopharynx cancer: review of 46 cases. Int J Clin Pract. 2007;61:52–61. doi: 10.1111/j.1742-1241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu S, Chang H, Sun X, Zhen Z, Sun F, Zhu J, et al. Long-term outcomes of nasopharyngeal carcinoma in 148 children and adolescents. Medicine (Baltimore) 2016;95:e3445. doi: 10.1097/MD.0000000000003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orbach D, Brisse H, Helfre S, Klijanienko J, Bours D, Mosseri V, et al. Radiation and chemotherapy combination for nasopharyngeal carcinoma in children: Radiotherapy dose adaptation after chemotherapy response to minimize late effects. Pediatr Blood Cancer. 2008;50:849–53. doi: 10.1002/pbc.21372. [DOI] [PubMed] [Google Scholar]
- 21.Tao CJ, Liu X, Tang LL, Mao YP, Chen L, Li WF, et al. Longterm outcome and late toxicities of simultaneous integrated boost-intensity modulated radiotherapy in pediatric and adolescent nasopharyngeal carcinoma. Chin J Cancer. 2013;32:525–32. doi: 10.5732/cjc.013.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zubizarreta PA, D'Antonio G, Raslawski E, Gallo G, Preciado MV, Casak SJ, et al. Nasopharyngeal carcinoma in childhood and adolescence: a single-institution experience with combined therapy. Cancer. 2000;89:690–5. [PubMed] [Google Scholar]
- 23.Cheuk DK, Billups CA, Martin MG, Roland CR, Ribeiro RC, Krasin MJ, et al. Prognostic factors and long-term outcomes of childhood nasopharyngeal carcinoma. Cancer. 2011;117:197–206. doi: 10.1002/cncr.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Galindo C, Wofford M, Castleberry RP, Swanson GP, London WB, Fontanesi J, et al. Preradiation chemotherapy with methotrexate, cisplatin, 5-fluorouracil, and leucovorin for pediatric nasopharyngeal carcinoma. Cancer. 2005;103:850–7. doi: 10.1002/cncr.20823. [DOI] [PubMed] [Google Scholar]
- 25.Cannon T, Zanation AM, Lai V, Weissler MC. Nasopharyngeal carcinoma in young patients: a systematic review of racial demographics. Laryngoscope. 2006;116:1021–6. doi: 10.1097/01.mlg.0000217243.08756.0c. [DOI] [PubMed] [Google Scholar]
- 26.Casanova M, Bisogno G, Gandola L, Cecchetto G, Di Cataldo A, Basso E, et al. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian Rare Tumors in Pediatric Age (TREP) project. Cancer. 2012;118:2718–25. doi: 10.1002/cncr.26528. [DOI] [PubMed] [Google Scholar]
- 27.Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31:2861–9. doi: 10.1200/JCO.2012.46.0816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.