Abstract
AIM
To compare the thickness of the peripapillary retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GCIPL) among patients with various forms of optic neuritis (ON) and to identify whether any particular parameters or their thinning pattern can be used to distinguish the type of ON.
METHODS
This prospective study was conducted at the Department of Ophthalmology, Faculty of Medicine, Siriraj Hospital, Thailand, between January, 2015 and December, 2016. We enlisted patients over 18 years of age with history of ON and categorized patients into 4 groups: 1) aquaporin 4 antibodies (AQP4-IgG) positive; 2) multiple sclerosis (MS); 3) myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) positive; 4) idiopathic-ON patients. Healthy controls were also included during the same study period. All patients underwent complete ophthalmological examination and spectral domain optical coherence tomography (OCT) imaging to analyze RNFL and GCIPL thickness after at least 3mo since the last episode of acute ON. The generalized estimating equation (GEE) models were used to compare the data amongst ON groups.
RESULTS
Among 87 previous ON eyes from 57 patients (43 AQP4-IgG+ON, 17 MS-ON, 8 MOG-IgG+ON, and 19 idiopathic-ON), mean logMAR visual acuity of AQP4-IgG+ON, MS-ON, MOG-IgG+ON, and idiopathic-ON groups was 0.76±0.88, 0.12±0.25, 0.39±0.31, and 0.75±1.08, respectively. Average, superior, and inferior RNFL were significantly reduced in AQP4-IgG+ON, MOG-IgG+ON and idiopathic-ON eyes, relative to those of MS-ON. Differences were not statistically significant for RNFL or GCIPL between the AQP4-IgG+ON and MOG-IgG+ON groups, whereas visual acuity in MOG-IgG+ON was slightly, but not significantly, better (0.39 vs 0.76). Although RNFL thickness in MOG-IgG+ON was significantly reduced as compared to MS-ON, mean visual acuity and GCIPL were not different.
CONCLUSION
Thinning of superior and inferior quadrants of RNFL are more commonly seen in MOG-IgG+ON and AQP4-IgG+ON. Long term visual acuity in MOG-IgG+ON is often better than AQP4-IgG+ON, whereas the structural change from OCT is comparable.
Keywords: optical coherence tomography, neuromyelitis optica, multiple sclerosis, myelin oligodendrocyte glycoprotein antibody, optic neuritis
INTRODUCTION
Optic neuritis (ON) is an inflammatory condition of the optic nerve commonly seen in central nervous system (CNS) demyelinating diseases such as multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD) and recently recognized in myelin oligodendrocyte glycoprotein antibodies (MOG-IgG) related diseases. However, these causes may not be identifiable, especially in Asian populations. Several weeks after an ON attack, optic nerve fibers are lost, resulting to the thinning of the retinal nerve fiber and ganglion cell layers. Recent studies reported that the thinning quickly aggravates within the first 3mo from the onset of ON attack and gradually continues until 6mo[1]–[3]. Damage to the optic nerve and its fibers can be measured objectively by optical coherence tomography (OCT) as a thickening of the retinal nerve fiber layer (RNFL) around optic nerve head and the ganglion cell-inner plexiform layers (GCIPL) at macula area (ganglion cell complex)[4]–[5].
Previous studies reported that ON in NMOSD resulted to a more severe RNFL and ganglion cell layer thinning than in MS, and suggested that OCT might be useful in differentiating NMOSD from MS[6]–[9]. However, comparative studies of the OCT in various types of CNS-demyelinating diseases are limited. Moreover, there are now only a limited number of published studies regarding OCT in MOG-IgG-positive patients.
The objective of this study was to identify whether any particular OCT parameters or their thinning pattern can be used to distinguish the type of ON.
SUBJECTS AND METHODS
Eligible Patients
This prospective study was conducted at the Department of Ophthalmology, Faculty of Medicine, Siriraj Hospital, Thailand, between January, 2015 and December, 2016. We enrolled patients >18 years of age with a history of acute ON but were without an acute episode for at least the previous 3mo. Diagnosis of ON was based on a history of subacute onset of decreased vision, a relative afferent pupillary defect when unilateral, and gadolinium enhancement of the optic nerve on fat-suppressed magnetic resonance imaging (MRI) at onset. Exclusion criteria were patients with other optic nerve disorders (ischemic optic neuropathy, glaucoma, neuroretinitis, perineuritis, ON related with CNS infection/toxic/malignancy), macula diseases, pathologic myopia with spherical equivalent of the refractive error >6.0 diopters. This study was approved by Siriraj Institutional Review Board and was conducted in accordance with the Declaration of Helsinki for biomedical research. All participants gave their written consent.
We categorized patients with CNS demyelinating related ON as the follows. 1) AQP4-IgG+ON was defined as eyes with a history of ON from patients whose blood tested seropositive for aquaporin 4 antibody (AQP4-IgG; diagnosed as NMOSD with AQP4[10]). All serum samples were analyzed at Tohoku University to detect AQP4 and MOG antibodies using cell-based assays[11]–[12]. 2) MS-ON was defined as eyes with a history of ON from patients who fulfilled the revised 2010 McDonald Criteria for diagnosis of MS[13]. 3) MOG-IgG+ON was defined as eyes with a history of ON from patients that tested seropositive for MOG-IgG. MOG-IgG were measured using a cell-based assay[11]–[12]. Serum samples were obtained during attacks of disease. 4) Idiopathic-ON was defined as eyes with a history of ON from patients that did not meet the above definitions.
All MS and MOG-IgG-positive-ON patients tested seronegative for AQP4-IgG. All patients with idiopathic-ON were tested for AQP4-IgG, MOG-IgG, lumber puncture for cerebrospinal fluid studies, blood test for other possible causes (VDRL, anti-HIV, ANA, RF, anti-Ro/La, thyroid function test) and results were negative.
Healthy controls were age-matched. They had no any ocular or systemic diseases which might affect RNFL thickness, including diabetes mellitus, glaucoma, age-related macular degeneration, optic neuropathy and ocular surgery or trauma. Eyes without a history of ON from patients with CNS demyelinating diseases were defined as non-ON eyes and considered as baseline on each type of ON (zero episode of ON). Visual field test results of all non-ON eyes included in this study were normal.
Ophthalmological Examinations and Optical Coherence Tomography Protocol
All participants underwent complete ophthalmological examination, visual field testing, and spectral domain-OCT imaging. Best-corrected visual acuity (VA) was tested by using the ETDRS chart. Visual field was measured by using the Humphrey 750i Visual Field Analyzer (Carl Zeiss Meditec), 24-2 or 30-2 SITA-standard programs. Testing results with false-positive, false-negative, and fixation loss scores <25% were selected in the study. Visual field testing cannot be done in some patients with severe visual loss or physical limitation.
Peripapillary RNFL and GCIPL thicknesses were measured by using the Cirrus HD-OCT 5000 (SW Ver: 6.0.0.599, Carl Zeiss Meditec), protocol of optic disc cube scan of 200×200 and macular cube scan of 512×128 centered on the fovea. The numeric values for all parameters are shaded as white, green, yellow, or red, with the yellow and red representing, <5% and <1%, respectively compared to the normative database. Only high-quality images with a signal strength index of at least 6/10 were included.
Statistical Analysis
Data analysis was performed using the statistical package IBM-SPSS 20.0. We used the generalized estimating equation (GEE) models, accounting for intrasubject inter-eye dependencies, to compare the data amongst ON groups, and Pearson Chi-Square test to compare thinning of RNFL among ON groups. Unpaired t-test was used to compare mean age and mean age at onset, and Fisher's exact test for sex.
RESULTS
Fifty-seven patients (25 AQP4-IgG+, 12 MS, 6 MOG-IgG+, 14 idiopathic-ON) and 30 healthy controls were registered, all of Asian descent. The demographic and clinical features of previous ON subjects and healthy controls are presented in Table 1. There were no significant differences in age at time of examination, age at onset, and sex ratio among the groups. In AQP4-IgG+ON group, OCT could not be evaluated in 3 eyes that had severe visual impairment (no light perception in 3 eyes). OCT could not be obtained in 2 eyes and 1 eye in MOG-IgG+ and idiopathic ON respectively, because patients had failed to do follow-up checkups 3mo after the ON attack. Finally, a total of 87 ON eyes (43 AQP4-IgG+ON, 17 MS-ON, 8 MOG-IgG+ON, and 19 idiopathic-ON eyes) were utilized in the analysis.
Table 1. Demographic data of patients with ON.
| Characteristics | AQP4-IgG+ | MS | MOG-IgG+ | Idiopathic-ON | HC |
| Patients | 25 | 12 | 6 | 14 | 30 |
| Sex (F/M) | 24/1 | 11/1 | 4/2 | 13/1 | 28/2 |
| Age (y) | 43.3±13.5 (19-76) | 40.8±13.3 (24-61) | 38.3±14.9 (24-58) | 44.3±6.2 (33-54) | 42.9±1.7 (40-46) |
| Age at onset (y) | 34.5±13.8 (17-71) | 32.1±10.1 (17-50) | 33.8±16.3 (18-54) | 39.9±8.9 (21-54) | - |
| Disease duration (y) | 9.6±7.4 | 8±4 | 4.4±2.7 | 4±6.8 | - |
| No. of ON attacks | 2 (1-5) | 1(1-5) | 2.5 (1-3) | 1 (1-2) | - |
| History of bilateral ON, n (%) | 13 (52) | 4 (33) | 3 (50) | 4 (29) | - |
| ON eyes, n (%) | 46 (92) | 17 (71) | 10 (83) | 20 (71) | - |
MS: Multiple sclerosis; ON: Optic neuritis; HC: Healthy controls.
mean±SD
Because of the severe visual loss or physical limitation in some cases, high quality visual field tests could be obtained in 13 MS-ON, 8 MOG-IgG+ON, 13 idiopathic-ON, and only 29 AQP4-IgG+ON eyes, as follows: mean deviation of visual field: -6.06±5.34, -10.54±5.66, -5.8±5.36, -9.45±9.04, and -0.75±1.13 in healthy controls.
Previous Optic Neuritis vs Healthy Controls
VA in AQP4-IgG+ON, MOG-IgG+ON and idiopathic-ON groups exhibited severe reductions relative to healthy control values (Table 2). In all previous ON groups, average and all quadrants of peripapillary RNFL were significantly less than healthy controls (P<0.001 for all, except P=0.034 for nasal quadrant in MS-ON). In comparison with healthy controls, OCT showed an average RNFL thickness loss of 36.2 µm in AQP4-IgG+ON, 22.3 µm in MS-ON, 44.1 µm in MOG-IgG+ON and 33.7 µm in idiopathic-ON. Average and all sectors of macular GCIPL thickness from any previous ON groups were significantly different than those in healthy controls (P<0.001 for all).
Table 2. Summary of VA and OCT results.
| Parameters | ON (n=87) |
HC (n=30) | |||
| AQP4-IgG+ON (n=43) | MS-ON (n=17) | MOG-IgG+ON (n=8) | Idiopathic-ON (n=19) | ||
| VA (logMAR) | 0.76±0.88 | 0.12±0.25 | 0.39±0.31 | 0.75±1.08 | 0.04±0.06 |
| RNFL (µm) | |||||
| Average | 65±11 | 79±9 | 57±13 | 68±14 | 101±8 |
| Superior | 77±20 | 102±14 | 65±19 | 81±23 | 127±15 |
| Nasal | 57±10 | 65±10 | 55±6 | 59±10 | 73±11 |
| Inferior | 76±23 | 103±19 | 64±25 | 79±22 | 132±14 |
| Temporal | 51±12 | 52±14 | 46±10 | 53±19 | 74±7 |
| GCIPL (µm) | n=32 | n=17 | n=6 | n=17 | n=30 |
| Average | 57±7 | 68±9 | 58±11 | 57±10 | 85±4 |
| Superior | 56±8 | 68±9 | 59±10 | 59±11 | 86±4 |
| Superonasal | 56±7 | 69±14 | 56±14 | 57±12 | 88±4 |
| Inferonasal | 56±8 | 68±14 | 56±12 | 57±10 | 86±4 |
| Inferior | 58±7 | 68±10 | 60±11 | 57±10 | 83±4 |
| Inferotemporal | 60±10 | 68±12 | 58±10 | 57±10 | 84±4 |
| Superotemporal | 58±11 | 67±12 | 57±10 | 56±11 | 85±5 |
ON: Optic neuritis; MS: Multiple sclerosis; RNFL: Retinal nerve fiber layer; GCIPL: Ganglion cell-inner plexiform layers; HC: Healthy controls; VA: Visual acuity.
mean±SD
Visual Acuity
VA for individual patients did not differ significantly among AQP4-IgG+ON, MOG-IgG+ON and idiopathic-ON groups. Indeed, mean logMAR VA was 0.76±0.88 in AQP4-IgG+ON (correlated to acuity on a Snellen test of 20/115), 0.39±0.31 in MOG-IgG+ON (20/49), and 0.75±1.08 in idiopathic-ON (20/112). Visual impairment was less severe in MS-ON with a mean VA of 0.12±0.25 (20/26) (Table 2). VA was significantly lower in AQP4-IgG+ON relative to that of MS-ON eyes (P<0.001). However, differences in VA was not significant between MOG-IgG+ and MS-ON groups (P=0.106; Table 3).
Table 3. Comparisons of visual function, RNFL thickness, and GCIPL thickness among each cause of ON (GEE).
| Parameters | AQP4-IgG+ON vs MS-ON | AQP4-IgG+ON vs MOG-IgG+ON | MS-ON vs MOG-IgG+ON | AQP4-IgG+ON vs idiopathic-ON | MS-ON vs idiopathic-ON | MOG-IgG+ON vs idiopathic-ON |
| VA (logMAR) | <0.001a | 0.144 | 0.106 | 1.0 | 0.346 | 1.0 |
| RNFL | ||||||
| Average | <0.001a | 0.661 | <0.001a | 1.0 | 0.026 | 0.357 |
| Superior | <0.001a | 0.590 | <0.001a | 1.0 | 0.007a | 0.366 |
| Nasal | 0.053 | 1.0 | 0.007a | 1.0 | 0.635 | 1.0 |
| Inferior | <0.001a | 1.0 | <0.001a | 1.0 | 0.002a | 0.794 |
| Temporal | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| GCIPL | ||||||
| Average | <0.001a | 1.0 | 0.232 | 1.0 | 0.004a | 1.0 |
| Superior | <0.001a | 1.0 | 0.263 | 1.0 | 0.038 | 1.0 |
| Superonasal | <0.001a | 1.0 | 0.257 | 1.0 | 0.008a | 1.0 |
| Inferonasal | <0.001a | 1.0 | 0.171 | 1.0 | 0.004a | 1.0 |
| Inferior | 0.004a | 1.0 | 0.813 | 1.0 | 0.007a | 1.0 |
| Infero-temporal | 0.008a | 1.0 | 0.264 | 1.0 | 0.014 | 1.0 |
| Supero-temporal | 0.007a | 1.0 | 0.176 | 1.0 | 0.007a | 1.0 |
VA: Visual acuity; RNFL: Retinal nerve fiber layer; GCIPL: Ganglion cell-inner plexiform layers. aP<0.01.
Retinal Nerve Fiber Layer Measurement
Mean of average RNFL thickness was 65±11 µm in AQP4-IgG+ON, 79±9 µm in MS-ON, 57±13 µm in MOG-IgG+ON, 68±14 µm in idiopathic-ON. Average peripapillary RNFL thicknesses were lower in AQP4-IgG+ON, MOG-IgG+ON, and idiopathic-ON eyes, when compared with those of MS-ON eyes (P<0.001, P<0.001, and P=0.026 respectively). RNFL thickness from superior and inferior quadrants of AQP4-IgG+ON, MOG-IgG+ON, and idiopathic-ON eyes were significantly lower than those of MS-ON eyes. RNFL did not differ remarkably between the AQP4-IgG+ON and MOG-IgG+ON groups (P=0.661; Table 3).
Macular Ganglion Cell-inner Plexiform Layer Measurement
Average and all sectors of GCIPL of AQP4-IgG+ON, and idiopathic-ON eyes were evidently reduced than those of MS-ON eyes. GCIPL thickness did not differ significantly (P=1.0) between AQP4-IgG+ON and MOG-IgG+ON groups in concert with measurements from RNFL. Similar to VA test, GCIPL was not significantly different between MOG-IgG+ON and MS-ON groups (P=0.232; Table 3).
Color Scale Referenced to a Normative Database
According to the color scale for a normative database in the OCT peripapillary RNFL printout (measurements in red are considered RNFL thinning), MS-ON eyes showed thin superior and inferior quadrants in only 29.4% (5/17) and 17.6% (3/17) respectively, whereas non-MS-ON (AQP4-IgG+ON, MOG-IgG+ON, and idiopathic-ON) showed greater thinning in 77.1% (54/70) and 75.7% (53/70) (Table 4).
Table 4. Thinning of peripapillary RNFL (referenced to a normative database).
| RNFL thinning areas | AQP4-IgG+ON (n=43) | MS-ON (n=17) | MOG-IgG+ON (n=8) | Idiopathic-ON (n=19) | Pb |
Pb |
|||
| MS-ON vs AQP4-IgG+ON | MS-ON vs MOG-IgG+ON | MS-ON vs idiopathic ON | AQP4-IgG+ON vs MOG-IgG+ON | ||||||
| Average | 40 (93) | 8 (47.1) | 7 (87.5) | 15 (78.9) | 0.001a | <0.001a | 0.054 | 0.047 | 0.594 |
| Superior | 35 (81.4) | 5 (29.4) | 7 (87.5) | 12 (63.2) | 0.001a | <0.001a | 0.007a | 0.043 | 0.677 |
| Nasal | 3 (7) | 0 | 0 | 0 | 0.365 | 0.264 | - | - | 0.441 |
| Inferior | 33 (76.7) | 3 (17.6) | 7 (87.5) | 13 (68.4) | <0.001a | <0.001a | 0.001a | 0.002a | 0.497 |
| Temporal | 18 (41.9) | 8 (47.1) | 4 (50) | 10 (52.6) | 0.875 | 0.714 | 0.891 | 0.738 | 0.67 |
aP<0.01; bPearson Chi-square test.
n (%)
Number of Episodes of Optic Neuritis and Optical Coherence Tomography Measures
Eyes without a history of ON from patients with CNS demyelinating diseases were defined as non-ON eyes. AQP4-IgG+-non-ON (n=13), MS-non-ON (n=23), MOG-IgG+-non-ON (n=1) and idiopathic-non-ON (n=8) were considered as baseline on each type of ON (zero episode of ON). In MOG-IgG+ON, the episodes of ON attacks were 1, 2, and 3 in 1, 5 and 2 eyes, respectively.
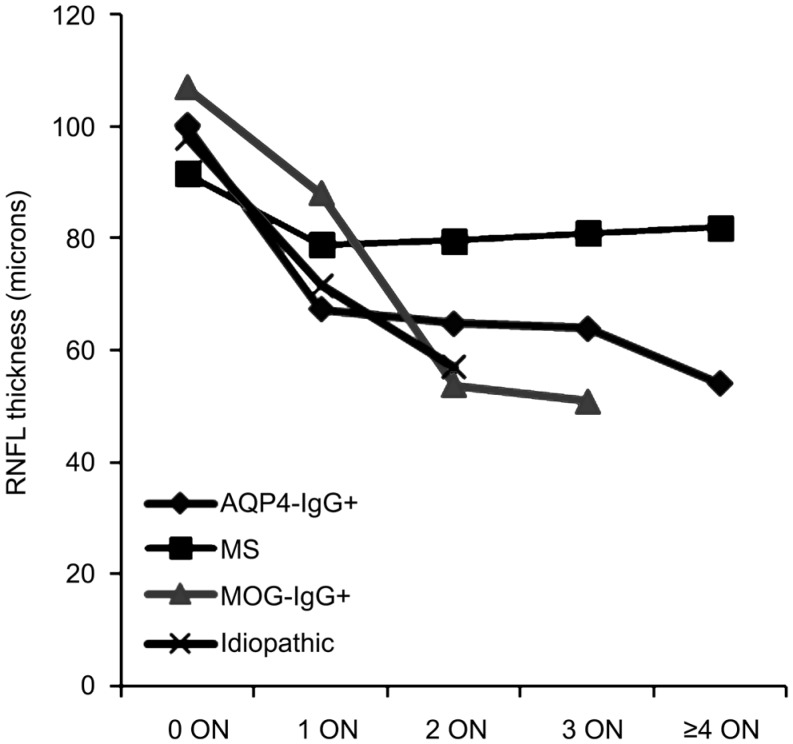
After the first episode of ON, average RNFL thickness, 100 µm decreased by 33 to 67 µm, in AQP4-IgG+ON, 13 µm (92 to 79 µm) in MS-ON, 19 µm (107 to 88 µm) in MOG-IgG+ON and 26 µm (98 to 72 µm) in idiopathic-ON when compared with those of non-ON groups. Average GCIPL thickness decreased 26 µm (84 to 58 µm), 10 µm (77 to 67 µm), 8 µm (86 to 78 µm) and 23 µm (82 to 59 µm) in AQP4-IgG+ON, MS-ON, MOG-IgG+ON and idiopathic-ON, respectively. After subsequent ON attacks, RNFL thickness in the AQP4-IgG+ON, MOG-IgG+ON, and idiopathic-ON groups tended to lessen, but differences were not statistically significant (Table 5, Figure 1). No significant decrease in GCIPL thickness was found in any ON groups after subsequent ON attacks.
Table 5. No. of episodes of ON and the worsening of VA, RNFL, and GCIPL.
| No. of episodes of ON | AQP4-IgG+ (non-ON=13, ON=43) | MS (non-ON=23, ON=17) | MOG-IgG+ (non-ON=1, ON=8) | Idiopathic-ON (non-ON=8, ON=19) |
| VA (logMAR) | ||||
| 0 | 0.09±0.09 | 0.05±0.07 | 0.1 | 0.04±0.04 |
| 1 | 0.69±0.78 | 0.12±0.28 | 0 | 0.81±1.15 |
| 2 | 0.7±0.96 | 0.11±0.12 | 0.5±0.31 | 0.56±0.98 |
| 3 | 0.78±1 | - | 0.32±0.25 | - |
| ≥4 | 1.5±1.32 | 0.12 | - | - |
| Average RNFL thickness (µm) | ||||
| 0 | 100±6 | 92±10 | 107 | 98±10 |
| 1 | 67±13 | 79±10 | 88 | 72±13 |
| 2 | 65±8 | 80±6 | 54±4 | 57±7 |
| 3 | 64±11 | - | 51±3 | - |
| ≥4 | 54±3 | 82 | - | - |
| Average GCIPL thickness (µm) | Non-ON=10, ON=32 | Non-ON=23, ON=17 | Non-ON=1, ON=6 | Non-ON=8, ON=17 |
| 0 | 84±5 | 77±6 | 86 | 82±4 |
| 1 | 58±8 | 67±10 | 78 | 59±10 |
| 2 | 55±3 | 72±5 | 54±5 | 50±1 |
| 3 | 59±6 | - | - | - |
| ≥4 | 60 | 64 | - | - |
ON: Optic neuritis; VA: Visual acuity; RNFL: Retinal nerve fiber layer; GCIPL: Ganglion cell-inner plexiform layers.
mean±SD
Figure 1. Change of average RNFL thickness after each episode of ON.
DISCUSSION
Several previous studies reported NMOSD caused more severe RNFL thinning at 55-83 µm than in MS at 74-95 µm[6],[14]–[23]. Our study demonstrated RNFL thickness in MS-ON were impaired less than in AQP4-IgG+NMOSD-ON, MOG-IgG+ON and idiopathic-ON groups. We found the mean RNFL thickness was 65±11 µm in AQP4-IgG+ON, 79±9 µm in MS-ON, 57±13 µm in MOG-IgG+ON, and 68±14 µm in idiopathic-ON. These findings are in accordance with those of the previous reports and add more data to literature on OCT in MOG-IgG+ autoimmunity.
Few studies have reported macular GCIPL measurements in previous ON eyes. Our study found that GCIPL thickness from all macular sectors of all ON groups was significantly different and less than those from healthy controls (P<0.001). This may reflect the high sensitivity of GCIPL measurements in distinguishing between previous ON and normal eyes.
Differences in macular GCIPL measurements between MS-ON and AQP4-IgG+ON remain debatable. Some studies reported greater GCIPL loss in AQP4-IgG+ON while others reported no difference[8],[22]–[23]. We confirmed the smaller loss in MS-ON compared with that in AQP4-IgG+ON.
Currently it is debated whether MOG-IgG-associated disorders should be classified as NMOSD or as opticospinal MS, or as a unique disease entity[24]–[25]. MOG-IgG have been detected in a proportion of AQP4-IgG negative NMOSD patients[11],[26]–[28]. However, the clinical features, histopathology of lesions, and evidence from immunological studies of MOG-IgG+ patients have been shown to differ from those of AQP4-IgG+ patients[24]–[25],[29]–[31].
We reported for OCT findings in MOG-IgG+ON an average RNFL thickness of 57 µm, average GCIPL thickness of 58 µm, correlated with logMAR VA of 0.39 (Snellen acuity 20/49) and mean deviation of visual field of -10.54. There was no any significant difference in OCT findings, including all quadrants of RNFL and all segments of GCIPL, between MOG-IgG+ON and AQP4-IgG+ON. RNFL of MOG-IgG+ON were obviously thinner when compared with that of MS-ON. Differences were significant at average, superior nasal and inferior RNFL quadrants (P<0.001, P<0.001, P=0.007 and P<0.001), respectively. Surprisingly there was no significant different in GCIPL between MOG-IgG+ON and MS-ON.
There are relatively few studies of OCT findings in MOG-IgG+ON and their results are inconsistent. One study reported no significant difference in OCT between MOG-IgG+ON and MS-ON, but did not show the comparison between MOG-IgG+ON and AQP4-IgG+ON[32]. One study, published in 2017, reported that RNFL was better preserved in eyes of patients with MOG-IgG compared to those with AQP4-IgG[33]. However, a larger study on a Caucasian population reported severe RNFL and GCIPL thinning in MOG-IgG+ON were comparable to AQP4-IgG+ON[34] and in general accord with the findings of our study other than race of patients.
Some previous studies observed that MOG-IgG+ON patients have better visual recovery from relapses than AQP4-IgG+ON patients[11]–[12],[33]–[35]. We found that VA in MOG-IgG+ON was slightly, but not significantly, better than that for AQP4-IgG+ON, whereas the structural change from OCT was comparable. In this study, 7 of 8 MOG-IgG+ON experienced recurrent ON attacks which can result in the significant thinning of RNFL. However, severe thinning can still be observed after classifying it by the number of ON episode, particularly in recurrent ON (Figure 1).
According to the results, wherein MOG-IgG+ON causes visual impairment and GCIPL thinning (but not RNFL) is comparable to MS-ON, may imply that MOG-IgG+ON preserve macular ganglion cells and central vision, which is in contrast to AQP4-IgG+ON.
According to the correlation between number of episodes of ON and OCT thinning, Ratchford et al[7] estimated a single episode of ON caused a 31 µm decrease in RNFL thickness in patients with NMOSD and a reduction of 10 µm in MS. Moreover, subsequent episodes of ON in the same eye were estimated to each cause an additional loss of 10 µm of RNFL thickness in NMOSD in comparison with a non-significant change in the MS group[7]. Our study demonstrated that after the first episode of ON, average RNFL thickness decreased 33 µm in AQP4-IgG+ON, 13 µm in MS-ON, 19 µm in MOG-IgG+ON, and 26 µm in idiopathic-ON when compared with those baselines of non-ON groups. However, after subsequent ON attacks, RNFL thickness in the AQP4-IgG+ON, MOG-IgG+ON, and idiopathic-ON groups showed a trend towards further thinning, but each episode of ON associated with an additional loss of <5 µm in RNFL thickness. These are the varying results between our study and Ratchford et al's[7].
We also observed that the cases with history of single ON episode, OCT in MS-ON was significantly better compared with AQP4-IgG+ON than with MOG-IgG+ON. However, in a recurring second or more episodes, OCT findings in MOG-IgG+ON were similar to AQP4-IgG+ON and apparently reduced its comparability with MS-ON.
Some studies found that temporal quadrant of peripapillary RNFL are preferentially affected in MS-ON, whereas AQP4-IgG+ON affected RNFL in all quadrants especially the superior and inferior[14],[17]–[18],[36]. Our study enlisted nearly all types of common inflammatory demyelinating ON and found that superior and inferior RNFL were two best parameters for distinguishing the type of ON (Tables 3, 4).
Long term treatments differ among MS and other demyelinating diseases. Hence any decision on how a patient should be treated with disease-modifying therapies for MS or immunosuppressive agents for other types of ON is very important[34]. Some MS medications can activate attack of NMOSD[37]–[42]. Thus, OCT may have an important role in making a treatment decision.
Idiopathic-ON group in our study, which may also be referred to as unclear diagnostic group, showed poor visual outcome and thinning of inner retinal structure that is comparable to AQP4-IgG+ON group. These results may not be applicable to patients with Caucasian descent, wherein possibility to develop MS is high[43]. However, previous ON patients with unfavorable visual outcome and remarkably superior/inferior RNFL thinning should recognize other possible causes other than MS.
Our study registered a relatively large number of patients, relative to the low prevalence of ON in human populations, especially those with AQP4-IgG. This study is one of only a few that reports on OCT findings in MOG-IgG+ON, especially amongst Asians. Our study included almost all types of common inflammatory demyelinating ON found in clinical practices. We also evaluated both RNFL and GCIPL in various aspects such as the assessment of OCT findings separated by quadrants and sectors, the use of a color scale referenced to a normative database, OCT findings correlated with the number of ON episodes.
Limitations include the followings. First, MOG-IgG+ON is a rare disease and a reliable test for detecting MOG-IgG antibodies has recently become available. This study includes the small number of the MOG-IgG+ON patients that may limit the significance of the analysis. Second, the difference amongst the number of eyes in each group may interfere with the statistical analysis. Third, although the use of GEE models, adjusting within patient inter-eye dependence, is suitable, evaluation of both eyes of the patients may add statistical bias to the study.
In summary, we demonstrate that OCT imaging for measurement of RNFL thickness seems to be better than GCIPL, residual VA and mean deviation of visual field for differentiating the types of ON. RNFL in MS-ON is better preserved when compared to that in others. Thinning of superior and inferior quadrants of RNFL are more commonly seen in non-MS previous ON. Long term VA in MOG-IgG+ON is often better than AQP4-IgG+ON, whereas the structural change from OCT is comparable.
Acknowledgments
We would like to thank Dr. William Beamish and Dr. Daisy J. Gonzales in reviewing of this manuscript.
Authors' contributions: Mekhasingharak N initiated the project, designed the data collection tools, monitored the data collection for the whole trial, wrote the statistical analysis plan, cleaned and analyzed the data, as well as drafted and revised the manuscript. Chirapapaisan N initiated the project, designed the data collection tools, monitored the data collection for the whole trial, revised the manuscript, approved the final manuscript prior to journal submission, and supervised the study. Laowanapiban P initiated the project, designed the data collection tools, and revised the manuscript. Siritho S initiated the project, revised the manuscript, and supervised the study. Satukijchai C, Prayoonwiwat N and Jitprapaikulsan J initiated the project and revised the manuscript.
Conflicts of Interest: Mekhasingharak N, None; Laowanapiban P, None; Siritho S has received funding for travel and speaker honoraria from Merck Serono, Pacific Healthcare (Thailand), Menarini (Thailand), Biogen Idec, UCB (Thailand) and Novartis; Satukijchai C has received funding for travel from Merck Serono, Biogen Idec, UCB (Thailand) and Novartis; Prayoonwiwat N has received funding for travel and speaker honoraria from Bayer Schering Pharma, Eisai Inc, Pfizer Pharmaceutical Company Limited, Novartis, Sanofi-Aventis; Jitprapaikulsan J, None; Chirapapaisan N, None; Siriraj Neuroimmunology Research Group, None.
REFERENCES
- 1.Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14(7):893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- 2.Behbehani R, Al-Moosa A, Sriraman D, Alroughani R. Ganglion cell analysis in acute optic neuritis. Mult Scler Relat Disord. 2016;5:66–69. doi: 10.1016/j.msard.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(Pt 2):521–533. doi: 10.1093/brain/awr264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aref AA, Budenz DL. Spectral domain optical coherence tomography in the diagnosis and management of glaucoma. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S15–S27. doi: 10.3928/15428877-20101031-01. [DOI] [PubMed] [Google Scholar]
- 5.Mwanza JC, Chang RT, Budenz DL, Durbin MK, Gendy MG, Shi W, Feuer WJ. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–5730. doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naismith RT, Tutlam NT, Xu J, Klawiter EC, Shepherd J, Trinkaus K, Song SK, Cross AH. Optical coherence tomography differs in neuromyelitis optica compared with multiple sclerosis. Neurology. 2009;72(12):1077–1082. doi: 10.1212/01.wnl.0000345042.53843.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, Calabresi PA, Kerr DA. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73(4):302–308. doi: 10.1212/WNL.0b013e3181af78b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian G, Li Z, Zhao G, Feng C, Li M, Huang Y, Sun X. Evaluation of retinal nerve fiber layer and ganglion cell complex in patients with optic neuritis or neuromyelitis optica spectrum disorders using optical coherence tomography in a Chinese cohort. J Ophthalmol. 2015;2015:832784. doi: 10.1155/2015/832784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petzold A, Wattjes MP, Costello F, Flores-Rivera J, Fraser CL, Fujihara K, Leavitt J, Marignier R, Paul F, Schippling S, Sindic C, Villoslada P, Weinshenker B, Plant GT. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447–458. doi: 10.1038/nrneurol.2014.108. [DOI] [PubMed] [Google Scholar]
- 10.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, Nakashima I, Apostolos-Pereira SL, Talim N, Simm RF, Lino AM, Misu T, Leite MI, Aoki M, Fujihara K. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. doi: 10.1212/WNL.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akaishi T, Sato DK, Nakashima I, Takeshita T, Takahashi T, Doi H, Kurosawa K, Kaneko K, Kuroda H, Nishiyama S, Misu T, Nakazawa T, Fujihara K, Aoki M. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry. 2016;87(4):446–448. doi: 10.1136/jnnp-2014-310206. [DOI] [PubMed] [Google Scholar]
- 13.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merle H, Olindo S, Donnio A, Richer R, Smadja D, Cabre P. Retinal peripapillary nerve fiber layer thickness in neuromyelitis optica. Invest Ophthalmol Vis Sci. 2008;49(10):4412–4417. doi: 10.1167/iovs.08-1815. [DOI] [PubMed] [Google Scholar]
- 15.de Seze J, Blanc F, Jeanjean L, Zephir H, Labauge P, Bouyon M, Ballonzoli L, Castelnovo G, Fleury M, Defoort S, Vermersch P, Speeg C. Optical coherence tomography in neuromyelitis optica. Arch Neurol. 2008;65(7):920–923. doi: 10.1001/archneur.65.7.920. [DOI] [PubMed] [Google Scholar]
- 16.Green AJ, Cree BA. Distinctive retinal nerve fibre layer and vascular changes in neuromyelitis optica following optic neuritis. J Neurol Neurosurg Psychiatry. 2009;80(9):1002–1005. doi: 10.1136/jnnp.2008.166207. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Nakazawa T, Doi H, Hariya T, Omodaka K, Misu T, Takahashi T, Fujihara K, Nishida K. Early high-dose intravenous methylprednisolone is effective in preserving retinal nerve fiber layer thickness in patients with neuromyelitis optica. Graefes Arch Clin Exp Ophthalmol. 2010;248(12):1777–1785. doi: 10.1007/s00417-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 18.Petzold A, de Boer JF, Schippling S, Vermersch P, Kardon R, Green A, Calabresi PA, Polman C. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9(9):921–932. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 19.Schneider E, Zimmermann H, Oberwahrenbrock T, Kaufhold F, Kadas EM, Petzold A, Bilger F, Borisow N, Jarius S, Wildemann B, Ruprecht K, Brandt AU, Paul F. Optical coherence tomography reveals distinct patterns of retinal damage in neuromyelitis optica and multiple sclerosis. PLoS One. 2013;8(6):e66151. doi: 10.1371/journal.pone.0066151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange AP, Sadjadi R, Zhu F, Alkabie S, Costello F, Traboulsee AL. Spectral-domain optical coherence tomography of retinal nerve fiber layer thickness in NMO patients. J Neuroophthalmol. 2013;33(3):213–219. doi: 10.1097/WNO.0b013e31829c510e. [DOI] [PubMed] [Google Scholar]
- 21.Park KA, Kim J, Oh SY. Analysis of spectral domain optical coherence tomography measurements in optic neuritis: differences in neuromyelitis optica, multiple sclerosis, isolated optic neuritis and normal healthy controls. Acta Ophthalmol. 2014;92(1):e57–e65. doi: 10.1111/aos.12215. [DOI] [PubMed] [Google Scholar]
- 22.Outteryck O, Majed B, Defoort-Dhellemmes S, Vermersch P, Zephir H. A comparative optical coherence tomography study in neuromyelitis optica spectrum disorder and multiple sclerosis. Mult Scler. 2015;21(14):1781–1793. doi: 10.1177/1352458515578888. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler. 2015;21(6):678–688. doi: 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamvil SS, Slavin AJ. Does MOG Ig-positive AQP4-seronegative opticospinal inflammatory disease justify a diagnosis of NMO spectrum disorder? Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e62. doi: 10.1212/NXI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters P, Woodhall M, O'Connor KC, Reindl M, Lang B, Sato DK, Jurynczyk M, Tackley G, Rocha J, Takahashi T, Misu T, Nakashima I, Palace J, Fujihara K, Leite MI, Vincent A. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e89. doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Probstel AK, Rudolf G, Dornmair K, Collongues N, Chanson JB, Sanderson NS, Lindberg RL, Kappos L, de Seze J, Derfuss T. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflammation. 2015;12:46. doi: 10.1186/s12974-015-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, Shima T, Mukaino A, Yoshimura S, Miyazaki T, Shiraishi H, Kawakami A, Tsujino A. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. 2015;5(4):e007766. doi: 10.1136/bmjopen-2015-007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spadaro M, Gerdes LA, Mayer MC, Ertl-Wagner B, Laurent S, Krumbholz M, Breithaupt C, Hogen T, Straube A, Giese A, Hohlfeld R, Lassmann H, Meinl E, Kumpfel T. Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol. 2015;2(3):295–301. doi: 10.1002/acn3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation. 2011;8:184. doi: 10.1186/1742-2094-8-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarius S, Metz I, Konig FB, Ruprecht K, Reindl M, Paul F, Bruck W, Wildemann B. Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ‘pattern II multiple sclerosis’ and brain biopsy findings in a MOG-IgG-positive case. Mult Scler. 2016;22(12):1541–1549. doi: 10.1177/1352458515622986. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Lapiscina EH, Sepulveda M, Torres-Torres R, Alba-Arbalat S, Llufriu S, Blanco Y, Guerrero-Zamora AM, Sola-Valls N, Ortiz-Perez S, Villoslada P, Sanchez-Dalmau B, Saiz A. Usefulness of optical coherence tomography to distinguish optic neuritis associated with AQP4 or MOG in neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. 2016;9(5):436–440. doi: 10.1177/1756285616655264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiebel-Kalish H, Lotan I, Brody J, Chodick G, Bialer O, Marignier R, Bach M, Hellmann MA. Retinal nerve fiber layer may be better preserved in MOG-IgG versus AQP4-IgG optic neuritis: a cohort study. PLoS One. 2017;12(1):e0170847. doi: 10.1371/journal.pone.0170847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation. 2016;13(1):282. doi: 10.1186/s12974-016-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Hernandez E, Sepulveda M, Rostasy K, Hoftberger R, Graus F, Harvey RJ, Saiz A, Dalmau J. Antibodies to aquaporin 4, myelin-oligodendrocyte glycoprotein, and the glycine receptor alpha1 subunit in patients with isolated optic neuritis. JAMA Neurol. 2015;72(2):187–193. doi: 10.1001/jamaneurol.2014.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bock M, Brandt AU, Dorr J, Kraft H, Weinges-Evers N, Gaede G, Pfueller CF, Herges K, Radbruch H, Ohlraun S, Bellmann-Strobl J, Kuchenbecker J, Zipp F, Paul F. Patterns of retinal nerve fiber layer loss in multiple sclerosis patients with or without optic neuritis and glaucoma patients. Clin Neurol Neurosurg. 2010;112(8):647–652. doi: 10.1016/j.clineuro.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B, Lebrun C, Moreau T, Vermersch P, Fontaine B, Lyon-Caen O, Gout O. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler. 2007;13(2):256–259. doi: 10.1177/1352458506070732. [DOI] [PubMed] [Google Scholar]
- 38.Uzawa A, Mori M, Hayakawa S, Masuda S, Kuwabara S. Different responses to interferon beta-1b treatment in patients with neuromyelitis optica and multiple sclerosis. Eur J Neurol. 2010;17(5):672–676. doi: 10.1111/j.1468-1331.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 39.Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler. 2012;18(1):113–115. doi: 10.1177/1352458511431973. [DOI] [PubMed] [Google Scholar]
- 40.Kleiter I, Hellwig K, Berthele A, Kumpfel T, Linker RA, Hartung HP, Paul F, Aktas O, Neuromyelitis Optica Study Group Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69(2):239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 41.Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler. 2012;18(1):108–112. doi: 10.1177/1352458511421185. [DOI] [PubMed] [Google Scholar]
- 42.Ayzenberg I, Schollhammer J, Hoepner R, et al. Efficacy of glatiramer acetate in neuromyelitis optica spectrum disorder: a multicenter retrospective study. J Neurol. 2016;263(3):575–582. doi: 10.1007/s00415-015-7991-1. [DOI] [PubMed] [Google Scholar]
- 43.Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727–732. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]