Abstract
The modulation of an animal’s behavior through external sensory stimuli, previous experience and its internal state is crucial to survive in a constantly changing environment. In most insects, octopamine (OA) and its precursor tyramine (TA) modulate a variety of physiological processes and behaviors by shifting the organism from a relaxed or dormant condition to a responsive, excited and alerted state. Even though OA/TA neurons of the central brain are described on single cell level in Drosophila melanogaster, the periphery was largely omitted from anatomical studies. Given that OA/TA is involved in behaviors like feeding, flying and locomotion, which highly depend on a variety of peripheral organs, it is necessary to study the peripheral connections of these neurons to get a complete picture of the OA/TA circuitry. We here describe the anatomy of this aminergic system in relation to peripheral tissues of the entire fly. OA/TA neurons arborize onto skeletal muscles all over the body and innervate reproductive organs, the heart, the corpora allata, and sensory organs in the antennae, legs, wings and halteres underlining their relevance in modulating complex behaviors.
Introduction
The adrenergic system of mammals influences various aspects of the animal’s life. Its transmitters/hormones, adrenaline and noradrenaline, modulate a variety of physiological processes and behaviors. They are secreted into the bloodstream by the adrenal glands in response to stress. In addition, they are synthesized and released by axonal terminals in the central nervous system (CNS) as well as sympathetic fibers of the autonomic nervous system. Adrenaline and noradrenaline have been described as modulators to shift the organism from a relaxed or dormant state to a responsive, excited and alerted state1. Stressful stimuli induce a metabolic and behavioral adaptation, leading to enhanced energy supply, increased muscle performance, increased sensory perception and a matched behavior. This so-called “fight or flight” response can be seen in vertebrates and invertebrates. In insects, the stress response is mediated - among others - by octopamine (OA) and its precursor tyramine (TA)2–4. TA is synthesized from tyrosine by the action of a tyrosine decarboxylase enzyme (Tdc) and functions as an independent neurotransmitter/-modulator as well as the intermediate step in OA synthesis. For this, TA is catalyzed by the tyramine-ß-hydroxylase (TßH). Similar to the vertebrate adrenergic system, OA and TA act through specific G-protein coupled receptors. Besides structural similarities between OA/TA and adrenaline/noradrenaline and the corresponding receptors, functional similarities are illustrated by the action of these transmitters/hormones in the regulation of physiological processes and behaviors. OA and TA are known to modulate muscle performance, glycogenolysis, fat metabolism, heart rate, and respiration in insects (reviewed by5).
While the role of TA as an independent signaling molecule was underestimated for a long time, OA has been extensively studied and was shown to have effects on almost every organ, sensory modality and behavior in a great variety of insects. The most intensively studied peripheral organs regarding the modulatory role of OA are muscles6–10. Here, OA is thought to not exclusively modulate muscle performance or motor activity. OA rather modulates muscle action according to metabolic and physiological processes, for example by promoting energy mobilization directly from the fat body, or indirectly by promoting the release of adipokinetic homones (AKH) from neuroendocrine cells in the corpora cardiaca (CC, a homolog of the vertebrate anterior pituitary gland and an analog of mammalian pancreatic alpha cells)11–14. In addition to the impact of OA/TA on muscles, fat body and AKH cells, OA is shown to modulate the heart, trachea and air sacs, gut, hemocytes, salivary glands, Malpighian tubules and ovaries in insects, mainly to induce a general stress or arousal state. However, in total OA seems to modulate a vast number of behaviors, which are not necessarily coupled to stress responses. The OA/TA system is shown to also act on inter alia (i.a.) learning and memory, sleep, feeding, flight, locomotion, and aggression5,8,10,12,13,15–40.
As mentioned above, OA and TA act as neurotransmitters and neuromodulators, allowing them to act in a paracrine, endocrine or autocrine fashion. In the fruit fly Drosophila, huge efforts were made to describe OA/TA neurons (OANs/TANs) in the brain and ventral nervous system (VNS) down to the single cell level8,17,41–45. Nevertheless, although our knowledge about physiological processes and behaviors modulated by the OA/TA system in the brain is rich, less is known about how OA and TA reach all its target organs and tissues in the periphery (exceptions: reproductive organs41,45–48 and muscles8,49–52).
Here we use the genetically tractable fruit fly Drosophila melanogaster to describe the arborizations of Tdc2-Gal4-positive, and therefore OANs and TANs in the periphery, as the Drosophila Tdc2 gene is expressed neurally45. We found that OANs/TANs are widespread distributed throughout the fly’s body with innervations in the skeletal muscles, reproductive organs, corpora allata, antennae, legs, wings, halteres and the heart. This diverse innervation pattern reflects the modulatory role of OA/TA in many different behaviors and physiological processes. Our results provide, for the very first time, a complete and comprehensive map of the OA/TA circuitry in the entire insect body. This map allows assumptions about the type of OA/TA signaling (paracrine or endocrine) to a specific organ and, at the same time, it provides a deeper understanding to what extend the OA/TA-dependent activity of peripheral organs is altered, for example by genetically manipulating Tdc2-Gal4-positive neurons in the brain and VNS.
Results
The OANs/TANs of the brain and ventral nervous system (VNS) are described in detail even on single cell level in Drosophila10,42–44,49. In contrast little is known about their peripheral arborizations. We used the well-characterized Tdc2-Gal4 line to deepen our knowledge about the OA/TA system in the entire body of Drosophila43–46,53. Nearly all Tdc2-Gal4-positive cells in the brain are stained by a Tdc2 antibody53. In the VNS all of the Tdc2-Gal4-positive cells were labeled by a TβH antibody and therefore have to be Tdc2-positive44. We here expressed myristoylated GFP, enhanced by GFP antibody staining, to label the membranes of Tdc2-Gal4-positive neurons from the soma to its fine endings in the periphery. The peripheral organs, tissues and cells are visualized by fluorescent markers for cell bodies (DAPI binds to DNA), muscles (Phalloidin binds F-actin) and antibodies against the synaptic protein Bruchpilot and the cell adhesion molecule Fasciclin 2 (Fig. 1).
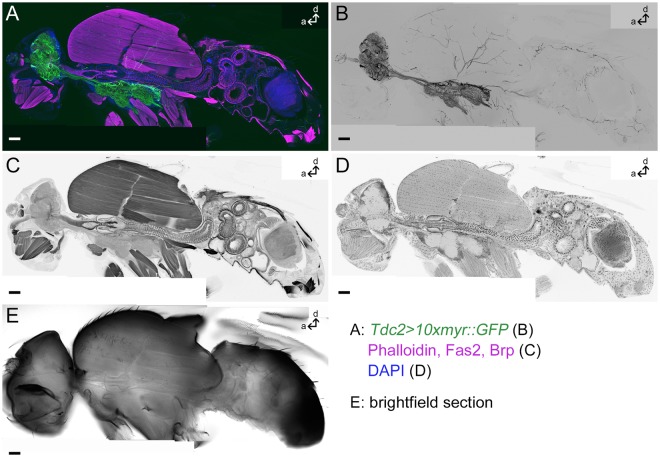
Figure 1.
Tdc2-Gal4-positive arborizations in a whole fly section. Projection of one medial sagittal agarose section of 80 µm thickness labeled by anti-GFP to visualize membranes of Tdc2-Gal4-positive neurons (green in (A), black in (B)); Phalloidin, anti-Fasciclin2 (Fas2) and anti-Bruchpilot (Brp) to visualize muscles, cells and synapses, respectively (magenta in (A), black in (C)) and DAPI to mark cell bodies (blue in (A), black in (D)). (E) A single optical section showing the bright-field picture. Scale bars = 50 µm.
Tdc2-Gal4-positive arborizations in the head
Tdc2-Gal4-positive neurons (Tdc2Ns) project through all peripheral nerves of the brain: antennal nerve (AN), ocellar nerve (OCN), pharyngeal nerve (PhN) and accessory PhN (APhN), maxillary-labial nerve (MxLbN), corpora cardiaca nerve (NCC), and the cervical connective (CV) (Fig. 2). Tdc2Ns in the antennal nerve are connected to the antennal lobe and the antennal mechanosensory and motor center (AMMC), respectively, and give rise to staining in the pedicel- the Johnston’s organ (JO)-, funiculus and arista of the antenna (Fig. 2A–D). While no cell bodies are visible in the third to fifth segment of the antennae, the JO contains stained cell bodies indicating that the Tdc2-Gal4 line includes mechanosensory neurons (Fig. 2C).
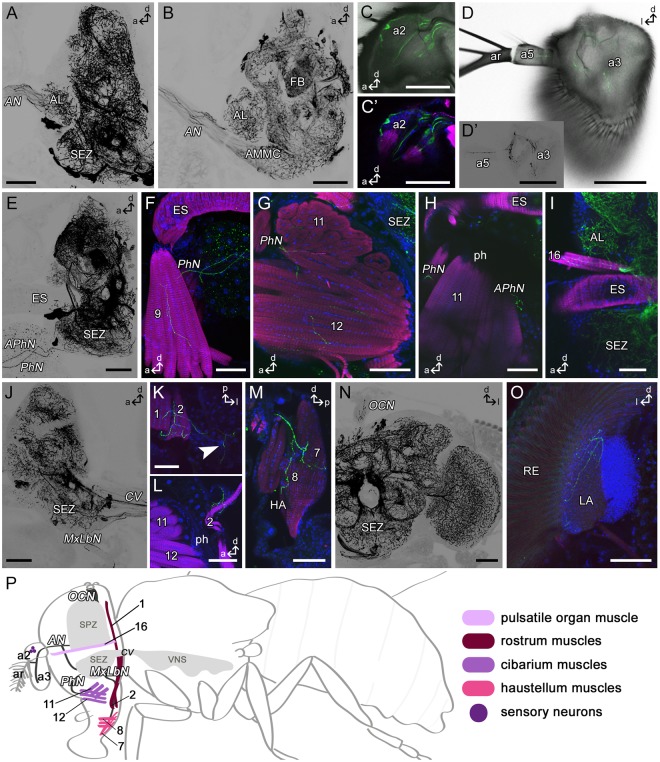
Figure 2.
Tdc2-Gal4-positive arborizations in the fly’s head. Projections of sagittal (A–C,E–J,L-M) or frontal (D,N-O) or horizontal (K) optical sections visualizing the arborization pattern of Tdc2-Gal4-positive neurons (Tdc2N; black or green) in the head. (A–D) Tdc2Ns run through the antennal nerve (AN) and project in antennal segments a2, a3 and a5. Mechanosensory neurons of the Johnston’s organ are visible (C). (E–H) Efferent Tdc2Ns of the pharyngeal (PhN) and accessory pharyngeal nerve (APhN). (F,G) Cells of the PhN innervate muscles 9, 11 and 12. (H) Bouton-like structures of APhN neurons beside the pharynx (ph). (I) Innervation of muscle 16. (J–M) Tdc2Ns of the maxillary-labial nerve (MxLbN) project along muscle 1 and 2 (K,L) in the haustellum (HA) and seem to innervate muscles 7 and 8 (M). Arborizations from the MxLbN reach the lateral brain (arrowhead in K). (N) Tdc2Ns arborize in the ocellar nerve (OCN). (O) Ramifications in the lateral lamina (LA) close to the retina (RE). (P) Schematic drawing of a fly visualizing peripheral nerves (dark grey), muscles of the head and proboscis (rose to pink) and sensory neurons (purple) shown in A-N. a, anterior; AL, antennal lobe; AMMC, antennal mechanosensory and motor center; ar, arista; CV, cervical connective; d, dorsal; ES, esophagus; FB, fan-shaped body; l, lateral; p, posterior; SEZ, subesophageal zone. Scale bars = 50 µm.
The Tdc2-Gal4-positive efferent nerves of the subesophageal zone (SEZ) arborize in the rostrum of the proboscis mainly onto muscles (Fig. 2E–M). Cells leaving the brain via the PhN innervate muscles 9, 10, 11 and 12 (nomenclature after54; Fig. 2F,G). Cells projecting towards the APhN build bouton like structures beside muscle 11 ventral to the pharynx (ph; Fig. 2H). Cells of the MxLbN arborize along muscles 1 and 2 (Fig. 2J–L). It seems as if also muscles 7 and 8 of the haustellum are innervated (Fig. 2M). We only observed this staining in two different specimens. Due to our cutting technique we probably lost these parts of the haustellum frequently. In addition to the innervation of the proboscis muscles we observed arborizations in the ventrolateral head arising from the MxLbN (arrowhead Fig. 2K). The ocellar nerve, which connects the ocellar ganglion with the brain, contains fibers arising from the brain (Fig. 2N). The central brain and optic lobes were shown to contain a dense network of OANs/TANs42,43. In addition, we identified arborizations in the distal part of the lamina by Tdc2Ns (Fig. 2O). Muscle 16, which is located dorsal to the esophagus, is innervated via ascending Tdc2Ns from the thorax (Fig. 2I).
Tdc2-Gal4-positive arborizations in the thorax
OANs/TANs form connections between the head and thorax via the CV and NCC (Fig. 3A). Tdc2Ns running through the NCC arborize close to the corpora allata (CA; Fig. 3B) and anterior stomatogastric ganglion, while no staining is visible in the corpora cardiaca (CC; asterisk Fig. 3B). The CV connects the brain and VNS and contains many Tdc2Ns (Fig. 3A). All peripheral nerves of the thoracic ganglion seem to contain Tdc2-Gal4-positive axons. Most prominent are the paired leg nerves of each thoracic neuromere (LN1-3 Fig. 3C; ProLN, MesoLN, MetaLN after55), the paired wing (WN Fig. 3K; ADMN after55) and posterior dorsal mesothoracic nerves (PDMN; Fig. 4A) of the mesothoracic neuromere and paired haltere nerves of the metathoracic neuromere (HN Fig. 3N; DMetaN after55). Interestingly, all these nerves, with the exception of PDMN, seem to contain efferent Tdc2Ns innervating mainly muscles as well as afferent Tdc2-Gal4-positive sensory neurons.
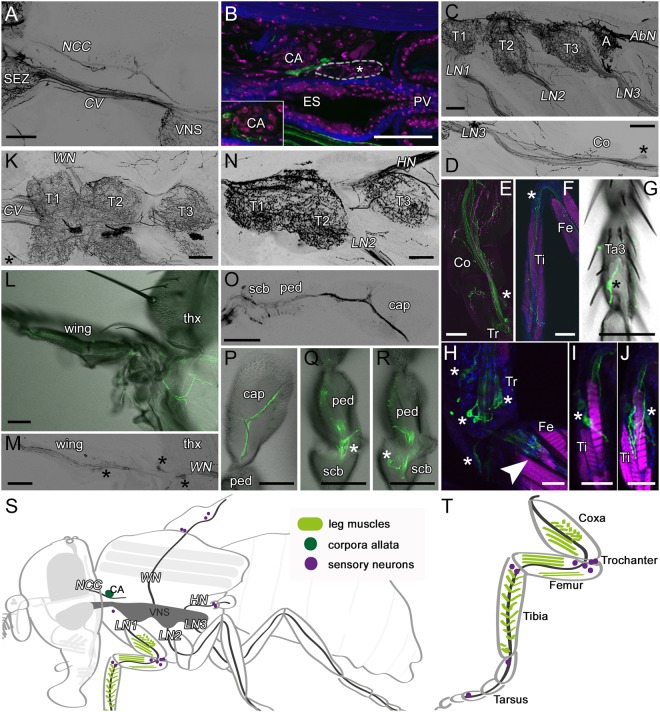
Figure 3.
Tdc2-Gal4-positive arborizations in the thorax. Projections of optical sections visualizing the arborization pattern of Tdc2-Gal4-positive (Tdc2Ns; A–N,Q,R) and Tdc2-positive (O,P) neurons (black or green) in the thorax. (A) Tdc2Ns run through the cervical connective (CV) and corpora cardiaca nerve (NCC). (B) Tdc2Ns arborize close to the corpora allata (CA) and the anterior stomatogastric ganglion (white-rimmed). (C–F) Tdc2Ns project along the legs and innervate leg muscles. (G) An afferent sensory neuron in the third segment of the tarsus (Ta). (H–J) Cell bodies of sensory neurons (asterisks) of the trochanter (Tr; H) and tibia (Ti; I,J). (H) Neurons of the chordotonal organ in the femur (Fe; arrowhead). (K–M) Tdc2Ns project along the wing nerve (WN). (K) Innervation of the thoracic chordotonal organ (asterisk). (L,M) Tdc2Ns run along the L1 wing vein. Cell bodies of sensory neurons are visible (asterisks). (N–R) Tdc2Ns in the haltere nerve (HN). (O,P) anti-Tdc2-positive cells project to the distal part of the capitellum (cap). (Q,R) Sensory neurons of the pedicellus (ped) and scabellum (scb) are labeled by Tdc2-Gal4 and Tdc2 antibody (O). (S,T) Schematic drawing of a fly and one leg visualizing the VNS and peripheral nerves (dark grey), CA (dark green), leg muscles (light green) and sensory neurons (purple) shown in A–R. (A–J,N–R) sagittal sections; (K) horizontal sections; (L,M) frontal sections. A, abdominal segment; Co, coxa; ES, esophagus; LN, leg nerve; PV, proventriculus; SEZ, subesophageal zone; T1–3, thorax segment1–3; thx, thorax. Scale bars: A–G,K–R = 50 µm; H–J = 25 µm.
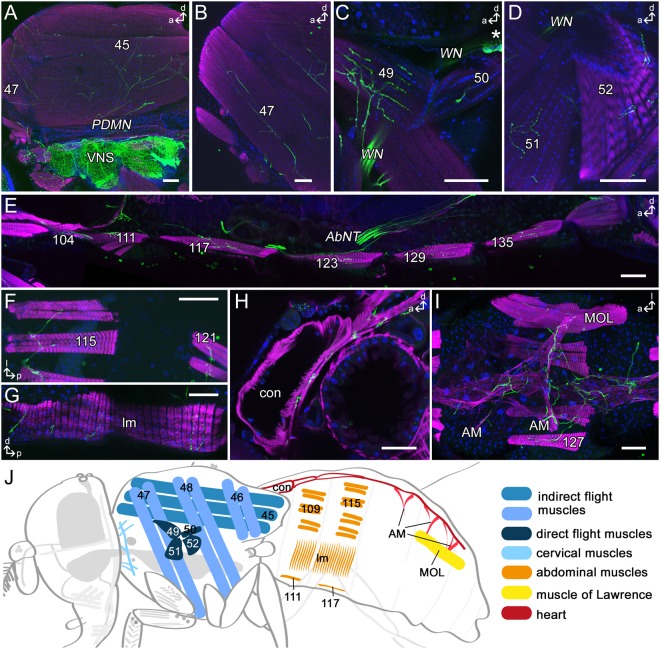
Figure 4.
Tdc2-Gal4-positive innervation of skeletal muscles. Projections of optical sections visualizing the innervation pattern of Tdc2-Gal4-positive neurons (Tdc2Ns; black or green) on skeletal muscles of thorax and abdomen (numbered after54). (A–D) Innervation of the indirect flight muscles (A,B) and direct flight muscles (C,D). (E–H) Tdc2Ns innervate the ventral (E), dorsal (F) and lateral (G) abdominal body wall muscles. (H,I) The longitudinal (H) as well as the alary muscles (I) of the heart are innervated by Tdc2Ns. (I) In males arborizations on the muscle of Lawrence (MOL) in segment 5 are visible. (J) Schematic drawing of a male fly visualizing flight and neck muscles (blue), abdominal skeletal muscles (ocher), male specific MOL (yellow) and the heart (red) shown in A–I. (A–E,G,H) Sagittal sections; (F,I) dorsal sections. a, anterior; AbNT, abdominal nerve trunk; AM, alary muscle; con, conical chamber; d, dorsal; l, lateral; lm, lateral muscles; p, posterior; PDMN, posterior dorsal mesothoracic nerve; VNS, ventral nervous system; WN, wing nerve. Scale bars = 50 µm.
The efferent Tdc2Ns in LN1-3 arborize on the leg muscles down to the tibia (Fig. 3E,F), while afferent fibers originate from sensory neurons of all leg segments (asterisks Fig. 3D–J), including i.a. mechanosensory neurons of the chordotonal organ of the femur (arrowhead Fig. 3H) and campaniform sensilla of the tarsus (asterisk Fig. 3G). The WN contains Tdc2Ns arborizing on indirect and direct flight muscles (Fig. 4B–D) and afferent axons from sensory neurons of the proximal wing (asterisks Figs 3M, 4C). Moreover, efferent Tdc2Ns running to the PDMN innervate all six longitudinal indirect flight muscles (45a-f; Fig. 4A) and the posterior dorsal-ventral indirect flight muscles (46a-b). Tdc2Ns project along the L1 wing vein (Fig. 3L). Tdc2-positive cells innervating the haltere project to the most distal tip of the capitellum (cap; Fig. 3O,P), while Tdc2-Gal4 labeling is very weak in the distal parts of the cap. Tdc2-Gal4 includes sensory neurons of campaniform sensilla of the pedicellus (ped; Fig. 3Q,R) and scabellum (scb; Fig. 3R). Additionally, it seems that Tdc2-Gal4 labels sensory neurons of the chordotonal organs of the halteres and wings.
Tdc2-Gal4-positive arborizations in the abdomen
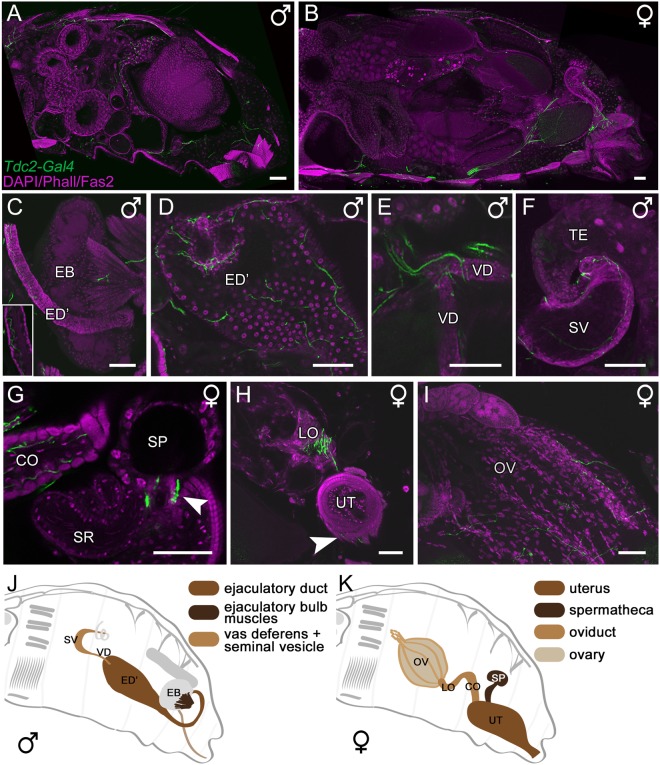
Tdc2Ns innervate all ventral (111, 117, 123, 129, 135; Fig. 4E) and dorsal skeletal muscles (109, 115, 121, 127, 133; Fig. 4F) of abdominal segments 2–6 as well as lateral muscles (Fig. 4G). Additionally, Tdc2-Gal4-positive ramifications on the male specific “muscle of Lawrence” in segment 5 are visible (Fig. 4I). Beside the body wall muscles, the ventral longitudinal and the alary muscles of the heart are innervated (Fig. 4H,I). Tdc2Ns running along the abdominal nerve trunk (AbNT) innervate the female and male reproductive organs, respectively (Fig. 5). In males, as described before46 the anterior ejaculatory duct, the vas deferens and seminal vesicle are innervated, while the ejaculatory bulb itself is not innervated but its muscles (Fig. 5C–F). The innervations of the female oviducts, uterus and spermathecal duct have been described in previous publications41,45–49 (Fig. 5G–I).
Figure 5.
Tdc2-Gal4-positive innervation of the reproductive organs. Projections of optical sections visualizing the innervation pattern of Tdc2-Gal4-positive neurons (Tdc2Ns; green) of reproductive organs visualized by DAPI, Phalloidin and Fasciclin2 staining (magenta). (A,B) Tdc2N arborization pattern in a sagittal section of a male (A) and female (B) abdomen, respectively. (C–F) The anterior ejaculatory duct (ED’), muscles of the ejaculatory bulb (EB), the vas deferens (VD) and seminal vesicle (SV) are innervated by Tdc2Ns. (G–I) Tdc2Ns arborize onto the common and lateral oviduct (CO, LO), spermathecal duct (arrowhead in G), uterus (UT) muscles (arrowhead in H) and the ovaries (OV). (J) Schematic drawing of a male fly visualizing the ED’ (brown), EB muscles (dark brown) and the VD and SV (light brown) shown in A,C-F. (K) Schematic drawing of a female fly visualizing the UT (brown), spermatheca (SP, dark brown), CO and LO (light brown) and the OV (grey) shown in B,G-I. SR, seminal receptacle; TE, testes. Scale bars = 50 µm.
Discussion
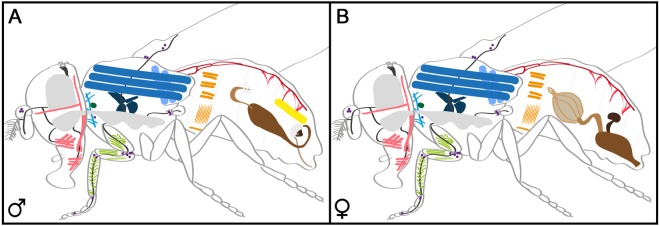
Here we show a comprehensive description of the innervation of OANs/TANs in the periphery of Drosophila. For this, we used the Tdc2-Gal4 line, allowing Gal4 expression under the control of a regulatory sequence of the tyrosine decarboxylase enzyme45. As this enzyme is essential for the synthesis of TA from tyrosine, the Tdc2-Gal4-line labels both TANs and OANs. Within the Drosophila brain, Tdc2-Gal4 labels in total about 137 cells, while additional 39 cells are located in the VNS43,44. The small number of Tdc2Ns lead to arborizations in large parts of the central brain, optic lobes and the thoracic and abdominal ganglion42–45. Based on the profound innervation of Tdc2Ns in the brain and VNS, the variety of behaviors modulated by the OA/TA system including learning and memory, feeding, vision, and sleep, are not surprising. Beyond the brain and VNS, OANs and TANs massively innervate regions within the periphery of the fly. Here, we described arborizations on most skeletal muscles, the antennae, wings, halteres and reproductive system and parts of the circulatory system and stomodaeal ganglion (Fig. 6; Table 1).
Figure 6.
Overview of organs, tissues and skeletal muscles innervated by Tdc2Ns. Schematic drawing of a male (A) and female (B) fly showing the internal structures innervated by Tdc2Ns: proboscis/head muscles (rose); CNS and VNS (grey); peripheral nerves targeting the antenna, ocelli, proboscis, wing, leg or haltere (dark grey); neck, direct and indirect flight muscles (blue); corpora allata (dark green); leg muscles (light green); heart and alary muscles (red); abdominal skeletal muscles (ocher); muscle of Lawrence (yellow); reproductive organs (brown). Sensory neurons labeled by the Tdc2-Gal4 line are shown as purple dots.
Table 1.
Organs, tissues, visceral and skeletal muscles innervated by Tdc2-Gal4-positive neurons (Tdc2Ns).
| Organs, tissues, visceral muscles | Tdc2N staining | Fig nr | Skeletal muscles (after54) | Tdc2N staining | Fig nr |
|---|---|---|---|---|---|
| Digestive system | Head | ||||
| Esophagus (ES) | o | 2E,I | 1 | x | 2K |
| Crop | ? | ns | 2 | x | 2K,L |
| Hindgut | ? | ns | 7 | (x) | 2M |
| Circulatory system | 8 | (x) | 2M | ||
| Corpora allata (CA) | o | 3B | 10 (after54) | x | ns |
| Ventral longitudinal muscles of heart | x | 4H,I | 11 | x | 2G |
| 12 | x | 2G | |||
| Alary muscles of heart | x | 4I | 16 | x | 2I |
| Nervous system | Thorax | ||||
| Brain | x | 1A,2 | Leg muscles (after83) | ||
| Ventral nervous system (VNS) | x | 1A,3 | Coxa | ||
| trdm, trlm, trrm | x,x,x | 3E | |||
| Stomodaeal ganglion | x | 3B | Trochanter | ||
| Ocellar nerve (OCN) | x | 2N | fedm, ferm | x,x | 3E,H |
| Sensory organs | Femur | ||||
| Antennae | ltm2, tilm, tidm, tirm | ?,x,x,x | 3F | ||
| Pedicel (a2) | x | 2C | Tibia | ||
| Funiculus (a3) | x | 2D | ltm1, talm, tadm, tarm | x,x,x,x | 3F |
| Arista (a4,a5) | x | 2D | Flight muscles | ||
| Wings | x | 3L | indirect | ||
| Halteres | x | 3O | 45,46,47,48 | x,x,x,x | 4A,B |
| Male reproductive System | direct | ||||
| 49,50,51,52,54, | x,x,x,x,x, | 4C,D | |||
| Muscles of Ejaculatory bulb (EB) | x | 5C | 55,56,57,58 | (x),?,x,? | ns |
| Cervical muscles | |||||
| Anterior ejaculatory duct (ED’) | x | 5D | D 20,21,22,23 | x,?,x,x, | ns |
| L 24; V 25,26,27 | x; x,x,x | ns | |||
| Accessory gland (AG) | ? | ns | Mesothorax muscles | ||
| Vas deferens (VD) | x | 5E | 59,60,61,62 | x,?,?,x | ns |
| Seminal vesicle (SV) | x | 5F | Metathorax muscles | ||
| Female reproductive system | 77,78,79 | ? | ns | ||
| Abdomen | |||||
| Ovary (OV) | x | 5I | Segment 1 | ||
| Lateral oviduct (LO) | x | 5H | D 98,99,100,101,102 | x,x,?,x,x | ns |
| Common oviduct (CO) | x | 5G | L103; V 80,81,104 | x;?,x,x | ns,4E |
| Spermathecal duct (SPd) | x | 5G | Segment 2–6 | ||
| D 109,115,121,127,133 | x,x,x,x,x | 4F | |||
| Uterus (UT) | x | 5H | L 110,116,122,128,134 | x,x,x,x,x | 4G |
| Uterus muscles | x | 5H | V 111,117,123,129,135 (after54) | x,x,x,x,x | 4E |
| Sensory organ (SO) | Tdc2N cell body | ||||
| Chordotonal organ (CO) antennae | x | 2C | |||
| CO thorax | x | 3K | |||
| CO legs | x | 3H | |||
| SO legs | x | 3E–J | |||
| SO wings | x | 3M | |||
| SO halteres | x | 3O-R |
x, staining; o, encircled by staining; (x), not enough samples; ?, ambiguous results or not investigated; D, dorsal; L, lateral; V, ventral; ns, not shown.
Our findings are in line with previous reports focusing on the expression of different OA and TA receptors in the fly56,57. Accordingly, the OA receptor OAMB is expressed in reproductive organs (in both male and female flies) and muscles, which are directly innervated by Tdc2Ns. Additionally, the midgut and trachea contain OA and TA receptors56,57, but do not seem to be innervated by Tdc2Ns, even though axons run in close vicinity to these organs. Likewise, the OA receptor Octß2R is expressed in the fat body, salivary glands and Malpighian tubules, tissues that seem not to be innervated by Tdc2Ns, while the expression of Octß1R and Octß3R is more specific56,57. The three tyramine receptors TyrR, TyrRII and TyrRIII show a broad expression in the periphery, also in tissues not innervated by Tdc2Ns56. The lack of direct innervation of these peripheral tissues might argue for volume transmission or hemolymph released OA/TA from Tdc2Ns. Alternatively, Drosophila TDC1, the product of the non-neurally expressed Tdc gene, is expressed in the gut musculature, rectal papillae, Malpighian tubules and two small clusters in the thoracic nervous system and might be the source for peripheral TA45. Interestingly, TyrR seems to be the only receptor expressed in the heart, suggesting that only TA modulates heart function56. Contrary, OA has a modulatory effect on the heart of other insect species including honeybees, olive fruit flies and cockroaches58. This is also in line with a previous report providing evidence that OA modulates the heart rate of the Drosophila fly and pupa, but not the larva59.
OA-dependent modulation of organs and tissues is mainly elicited through muscle action, especially in terms of its impact on the “fight or flight” response. In line with this, we observed Tdc2-Gal4-positive arborizations on nearly all skeletal muscles and many visceral muscles (Table 1). In both Drosophila and desert locusts OA and TA is expressed in type II terminals of skeletal muscles50. OA has an excitatory effect on Drosophila flight muscles, while TA was shown to inhibit excitatory junction potentials, and thereby reduce muscle contractions and locomotion at least in the larva6,8,60,61. In addition, flies lacking OA show severe deficits in flight initiation and maintenance10,50. Interestingly, in an antagonistic effect to serotonin, OA reduces crop muscle activity presumably via Octß1R, suggesting that OA has different effects on muscle activity dependent on the type of muscle56,62. However, our data do not provide any convincing evidence of a direct innervation of Tdc2Ns of the crop, even though many fibers run in close vicinity, suggesting that OA might target the crop by volume transmission.
Furthermore, OA modulates ovulation and fertilization in insects63–67. Flies lacking OA display a severe egg-laying phenotype. Remarkably, within the female reproductive organ two different OA receptors, OAMB and Octß2R, are necessary. Again, OA has a strong impact on muscle activity within the reproductive system. Octß2R is expressed in the visceral oviduct muscle and elicits muscle relaxation through an increase of intracellular cAMP levels63. Such an OA-dependent modulation appears to be conserved as OA is found in dorsal unpaired median neurons of locusts innervating oviduct muscles through the oviducal nerve68. However, our data suggest that OA-positive fibers not only innervate oviduct muscles, but also enter the organs themselves. The OAMB receptor is expressed in epithelial cells inducing fluid secretion through increasing intracellular Ca2+ levels63. Thus, OA affects different processes within the female reproductive organ due to the expression of different receptors and their coupled signaling pathways, which may be a general mechanism of the OA/TA system to fulfill an extensive modulatory function69.
OA does not exclusively modulate muscle activity, but also sensory neurons of external tissues like the antennae, halteres and wings. OA has also been shown to increase the spontaneous activity of olfactory receptor neurons (ORN)70,71. The modulation of ORNs allows OA to modulate the innate response to attractive stimuli like fruit odors or pheromones72,73. Further, this modulation helps nestmate recognition in ants74. In addition to Tdc2-Gal4-positive arborizations in the funiculus, we found Tdc2-Gal4-positive sensory neurons in the JO, a chordotonal organ sensitive to mechanosensory stimuli and thus important for hearing in insects. In mosquitos, OA modulates auditory frequency tuning and thereby affects mating behavior75. In locusts, OA similarly modulates the response of chordotonal neurons in the legs to encode proprioceptive information76. Our data suggest that chordotonal neurons in the legs, wings, halteres and thorax are included in the Tdc2-Gal4 line suggesting a conserved modulatory role of OA/TA for insect proprioception.
Taken together, our study suggest that the OA/TA system massively modulates various organs and tissues in the periphery of Drosophila. Through distinct receptors and coupled signaling pathways OANs/TANs mainly induce “fight or flight” responses by modulating muscle activity, proprioception, and heart rate. As a result, the innervation pattern in the periphery supports the idea that the OA/TA system is crucial for insects to switch from a dormant to an excited state, by a positive modulation of muscle activity, heart rate and energy supply, and a simultaneous negative modulation of physiological processes like sleep.
Methods
Fly strains and fly rearing
All flies were cultured according to standard methods. In short, vials were kept under constant conditions with 25 °C and 60% humidity in a 12:12 light:dark cycle. Flies carrying the Tdc2-Gal445 (Bloomington Stock Center) and 10xUAS-IVS-myrGFP77, (Bloomington Stock Center) constructs were used for immunohistochemistry. To control for an unspecific expression of the UAS construct, we stained 10xUAS-IVS-myrGFP alone. No GFP staining was detected. Additionally, we performed anti-Tdc2 (pab0822-P; Covalab; 1:200) staining experiments to confirm our Tdc2-Gal4 expression pattern. Indeed, we observed Tdc2 staining in all Tdc2-Gal4-positive peripheral nerves (data not shown).
Immunocytochemistry
To visualize the arborizations of Tdc2-Gal4-positive neurons in the periphery whole body sections, as well as sections of the head, thorax and abdomen, were performed, respectively (see78). In short, the cuticle of 4 to 7 days old flies was opened in phosphate buffered saline (PBS, 0.1 M) to ensure that the fixative can penetrate into the tissue. Whole flies were fixed with 4% paraformaldehyde in PBS for two hours at room temperature and afterwards washed three times with PBS. Subsequently flies were embedded in hot 7% Agarose low EEO (A2114; AppliChem). After hardening, the flies were cut with a vibratome (Leica VT1000S) into 80–100 µm sections. Staining of the sections was continued after washing in PBS containing 0.3% Triton-X100 (PBT) and blocking in 5% normal goat serum in PBT. Rabbit anti-GFP (A6455, Molecular Probes; 1:1000) in combination with mouse anti-Synapsin (3C11;79; 1:50) or mouse anti-Bruchpilot (nc82;80; 1:50) and mouse anti-Fasciclin 2 (1D4; DSHB; 1:100) were used as primary antibodies. After one night at 4 °C the specimens were washed six times in PBT and incubated in secondary antibody solution for a subsequent night at 4 °C. As secondary antibodies goat anti-rabbit Alexa488 (Molecular Probes; 1:200) and goat anti-mouse DyLight649 (Jackson ImmunoResearch; 1:200) were used. 4′,6-Diamidino-2-phenylindol Dihydrochlorid (DAPI; Sigma-Aldrich; 1:1000) and Alexa Fluor 633 Phalloidin (Molecular Probes; 1:400) were used to visualize DNA and actin, respectively.
Confocal microscopy and data processing
Confocal images were taken with a Leica TCS SP8 microscope (Leica Microsystems, Germany) with a 20x high aperture objective. Labelled specimens were scanned with a step size of 1.0 μm to 1.5 μm. Image processing and stitching was performed using Fiji81 and Adobe Photoshop CS6 (Adobe Systems, USA). Adobe Illustrator CS6 was used to draw the scheme of the fly and its peripheral organs and muscles (after54,82). Table 1 summarizes the arborization pattern of the Tdc2Ns. The innervation of organs, tissues and muscles is confirmed by at least two male and two female samples (except for reproductive organs and MOL).
Acknowledgements
We thank the Bloomington stock center for flies, Claudia Groh and the Development Studies Hybridoma Bank for antibodies. We thank Christian Wegener and Charlotte Förster for their support and fruitful discussions, and Christian Wegener for comments on the manuscript. This research was supported by a grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg (to M.S.); SCIENTIA fellowship “Bayerische Gleichstellungsförderung: Programm zur Realisierung der Chancengleichheit für Frauen in Forschung und Lehre” (to M.S.), and by the Deutsche Forschungsgemeinschaft (PA1979/2-1 to D.P., EL784/1-1 to B.e.J.). This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing.
Author Contributions
D.P. and M.S. conceived and designed the experiments. D.P., C.B., F.F., B.e.J. and M.S. performed the experiments. M.S. analyzed the data. D.P. and M.S. wrote the manuscript and M.S. prepared the figures. All authors reviewed the manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldstein DS. Adrenal Responses to Stress. Cell Mol Neurobiol. 2010;30:1433–1440. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson PA, Rillich J. Controlling the decision to fight or flee: the roles of biogenic amines and nitric oxide in the cricket. Curr Zool. 2016;62:265–275. doi: 10.1093/cz/zow028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamo SA. The stress response and immune system share, borrow, and reconfigure their physiological network elements: Evidence from the insects. Horm Behav. 2017;88:25–30. doi: 10.1016/j.yhbeh.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Adamo SA, Linn CE, Hoy RR. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 1995;198:1691–1700. doi: 10.1242/jeb.198.8.1691. [DOI] [PubMed] [Google Scholar]
- 5.Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu. Rev. Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 6.Saraswati S, Fox LE, Soll DR, Wu C-F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- 7.Koon AC, et al. Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 2011;14:190–199. doi: 10.1038/nn.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selcho M, Pauls D, El Jundi B, Stocker RF, Thum AS. The role of octopamine and tyramine in Drosophila larval locomotion. J. Comp. Neurol. 2012;520:3764–3785. doi: 10.1002/cne.23152. [DOI] [PubMed] [Google Scholar]
- 9.Pauls D, et al. Potency of transgenic effectors for neurogenetic manipulation in Drosophila larvae. Genetics. 2015;199:25–37. doi: 10.1534/genetics.114.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brembs B, Christiansen F, Pflüger HJ, Duch C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J. Neurosci. 2007;27:11122–11131. doi: 10.1523/JNEUROSCI.2704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mentel T, et al. Central modulatory neurons control fuel selection in flight muscle of migratory locust. J. Neurosci. 2003;23:1109–1113. doi: 10.1523/JNEUROSCI.23-04-01109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pflüger H-J, Duch C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology (Bethesda) 2011;26:293–303. doi: 10.1152/physiol.00002.2011. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, et al. Octopamine controls starvation resistance, life span and metabolic traits in Drosophila. Sci Rep. 2016;6:35359. doi: 10.1038/srep35359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damrau C, Toshima N, Tanimura T, Brembs B, Colomb J. Octopamine and Tyramine Contribute Separately to the Counter-Regulatory Response to Sugar Deficit in Drosophila. Front Syst Neurosci. 2017;11:100. doi: 10.3389/fnsys.2017.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roeder T. Octopamine in invertebrates. Prog. Neurobiol. 1999;59:533–561. doi: 10.1016/S0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 16.Roeder T, Seifert M, Kähler C, Gewecke M. Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 2003;54:1–13. doi: 10.1002/arch.10102. [DOI] [PubMed] [Google Scholar]
- 17.Selcho M, Pauls D, Huser A, Stocker RF, Thum AS. Characterization of the octopaminergic and tyraminergic neurons in the central brain of Drosophila larvae. J. Comp. Neurol. 2014;522:3485–3500. doi: 10.1002/cne.23616. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson PA, Hofmann HA, Schoch K, Schildberger K. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 2000;43:107–120. doi: 10.1002/(SICI)1097-4695(200005)43:2<107::AID-NEU1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Vergoz V, Roussel E, Sandoz J-C, Giurfa M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE. 2007;2:e288. doi: 10.1371/journal.pone.0000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Certel SJ, Savella MG, Schlegel DCF, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc. Natl. Acad. Sci. USA. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr. Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 24.Mercer AR, Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J. Comp. Physiol. 1982;145:363–368. doi: 10.1007/BF00619340. [DOI] [Google Scholar]
- 25.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a Neural Circuit that Underlies the Effects of Octopamine on Sleep:Wake Behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crocker A, Sehgal A. Octopamine regulates sleep in drosophila through protein kinase A-dependent mechanisms. J. Neurosci. 2008;28:9377–9385. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Branch A, Shen P. Octopamine-mediated circuit mechanism underlying controlled appetite for palatable food in Drosophila. PNAS. 2013;110:15431–15436. doi: 10.1073/pnas.1308816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn H, Kirkhart C, Chia J, Scott K. A subset of octopaminergic neurons that promotes feeding initiation in Drosophila melanogaster. PLOS ONE. 2018;13:e0198362. doi: 10.1371/journal.pone.0198362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizunami M, Matsumoto Y. Roles of Octopamine and Dopamine Neurons for Mediating Appetitive and Aversive Signals in Pavlovian Conditioning in Crickets. Front Physiol. 2017;8:1027. doi: 10.3389/fphys.2017.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheiner R, Steinbach A, Claßen G, Strudthoff N, Scholz H. Octopamine indirectly affects proboscis extension response habituation in Drosophila melanogaster by controlling sucrose responsiveness. J. Insect Physiol. 2014;69:107–117. doi: 10.1016/j.jinsphys.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 2005;25:1431–1441. doi: 10.1523/JNEUROSCI.4258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini, N., Giurfa, M., Sandoz, J.-C. & Avarguès-Weber, A. Aminergic neuromodulation of associative visual learning in harnessed honey bees. Neurobiol Learn Mem, 10.1016/j.nlm.2018.05.014 (2018). [DOI] [PubMed]
- 34.Watanabe K, et al. A Circuit Node that Integrates Convergent Input from Neuromodulatory and Social Behavior-Promoting Neurons to Control Aggression in Drosophila. Neuron. 2017;95:1112–1128.e7. doi: 10.1016/j.neuron.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claßen, G. & Scholz, H. Octopamine Shifts the Behavioral Response From Indecision to Approach or Aversion in Drosophila melanogaster. Front. Behav. Neurosci. 12 (2018). [DOI] [PMC free article] [PubMed]
- 36.Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nature Neuroscience. 2008;11:1059–1067. doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- 37.Pyakurel P, Privman Champaloux E, Venton BJ. Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord. ACS Chem Neurosci. 2016;7:1112–1119. doi: 10.1021/acschemneuro.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huetteroth W, et al. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr. Biol. 2015;25:751–758. doi: 10.1016/j.cub.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koon AC, Budnik V. Inhibitory control of synaptic and behavioral plasticity by octopaminergic signaling. J. Neurosci. 2012;32:6312–6322. doi: 10.1523/JNEUROSCI.6517-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliadi KG, Iliadi N, Boulianne GL. Drosophila mutants lacking octopamine exhibit impairment in aversive olfactory associative learning. European Journal of Neuroscience. 2017;46:2080–2087. doi: 10.1111/ejn.13654. [DOI] [PubMed] [Google Scholar]
- 41.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Sinakevitch I, Strausfeld NJ. Comparison of octopamine-like immunoreactivity in the brains of the fruit fly and blow fly. J. Comp. Neurol. 2006;494:460–475. doi: 10.1002/cne.20799. [DOI] [PubMed] [Google Scholar]
- 43.Busch S, Selcho M, Ito K, Tanimoto H. A map of octopaminergic neurons in the Drosophila brain. J. Comp. Neurol. 2009;513:643–667. doi: 10.1002/cne.21966. [DOI] [PubMed] [Google Scholar]
- 44.Schneider A, et al. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE. 2012;7:e52007. doi: 10.1371/journal.pone.0052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 46.Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avila FW, Bloch Qazi MC, Rubinstein CD, Wolfner MF. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc. Natl. Acad. Sci. USA. 2012;109:4562–4567. doi: 10.1073/pnas.1117689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Middleton CA, et al. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monastirioti M, et al. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J. Comp. Neurol. 1995;356:275–287. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stocker B, et al. Structural and Molecular Properties of Insect Type II Motor Axon Terminals. Front Syst Neurosci. 2018;12:5. doi: 10.3389/fnsys.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivlin PK, St. Clair RM, Vilinsky I, Deitcher DL. Morphology and molecular organization of the adult neuromuscular junction of Drosophila. J. Comp. Neurol. 2004;468:596–613. doi: 10.1002/cne.10977. [DOI] [PubMed] [Google Scholar]
- 52.O’Sullivan Angela, Lindsay Theodore, Prudnikova Anna, Erdi Balazs, Dickinson Michael, von Philipsborn Anne C. Multifunctional Wing Motor Control of Song and Flight. Current Biology. 2018;28(17):2705-2717.e4. doi: 10.1016/j.cub.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 53.Pech U, Pooryasin A, Birman S, Fiala A. Localization of the contacts between Kenyon cells and aminergic neurons in the Drosophila melanogaster brain using SplitGFP reconstitution. J. Comp. Neurol. 2013;521:3992–4026. doi: 10.1002/cne.23388. [DOI] [PubMed] [Google Scholar]
- 54.Miller, A. The internal anatomy and histology of the imago of Drosophila melanogaster. In Demerec (ed.) Biology of Drosophila 420–534 (1950).
- 55.Court, R. C. et al. A Systematic Nomenclature for the Drosophila Ventral Nervous System. bioRxiv122952, 10.1101/122952 (2017).
- 56.El-Kholy S, et al. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 2015;361:669–684. doi: 10.1007/s00441-015-2137-4. [DOI] [PubMed] [Google Scholar]
- 57.Ohhara Y, Kayashima Y, Hayashi Y, Kobayashi S, Yamakawa-Kobayashi K. Expression of β-adrenergic-like octopamine receptors during Drosophila development. Zool. Sci. 2012;29:83–89. doi: 10.2108/zsj.29.83. [DOI] [PubMed] [Google Scholar]
- 58.Papaefthimiou C, Theophilidis G. Octopamine–a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): Acceleration vs. inhibition. J. Insect Physiol. 2011;57:316–325. doi: 10.1016/j.jinsphys.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 59.Zornik E, Paisley K, Nichols R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides. 1999;20:45–51. doi: 10.1016/S0196-9781(98)00151-X. [DOI] [PubMed] [Google Scholar]
- 60.Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/S0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- 61.Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci. Lett. 2002;329:324–328. doi: 10.1016/S0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- 62.Solari P, et al. Opposite effects of 5-HT/AKH and octopamine on the crop contractions in adult Drosophila melanogaster: Evidence of a double brain-gut serotonergic circuitry. PLoS ONE. 2017;12:e0174172. doi: 10.1371/journal.pone.0174172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Fink C, El-Kholy S, Roeder T. The octopamine receptor octß2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2015;88:168–178. doi: 10.1002/arch.21211. [DOI] [PubMed] [Google Scholar]
- 64.Lim J, et al. The Octopamine Receptor Octβ2R Regulates Ovulation in Drosophila melanogaster. PLOS ONE. 2014;9:e104441. doi: 10.1371/journal.pone.0104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev. Biol. 2003;264:179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez-Valentín R, et al. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 2006;209:183–198. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- 67.Monastirioti M, Linn CE, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lange AB, Orchard I. Dorsal unpaired median neurons, and ventral bilaterally paired neurons, project to a visceral muscle in an insect. J. Neurobiol. 1984;15:441–453. doi: 10.1002/neu.480150605. [DOI] [PubMed] [Google Scholar]
- 69.Lee H-G, Rohila S, Han K-A. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS ONE. 2009;4:e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stengl M. Pheromone transduction in moths. Front Cell Neurosci. 2010;4:133. doi: 10.3389/fncel.2010.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhukovskaya MI, Polyanovsky AD. Biogenic Amines in Insect Antennae. Front Syst Neurosci. 2017;11:45. doi: 10.3389/fnsys.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Linn C. E., Roelofs W. L. Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth,Trichoplusia ni. Archives of Insect Biochemistry and Physiology. 1986;3(2):161–171. doi: 10.1002/arch.940030206. [DOI] [Google Scholar]
- 73.ZHUKOVSKAYA MARIANNA I. Selective regulation of sensitivity to odours of different behavioural significance in the American cockroach, Periplaneta americana. Physiological Entomology. 2008;33(2):162–166. doi: 10.1111/j.1365-3032.2008.00615.x. [DOI] [Google Scholar]
- 74.Vander Meer RK, Preston CA, Hefetz A. Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Naturwissenschaften. 2008;95:1155–1158. doi: 10.1007/s00114-008-0432-6. [DOI] [PubMed] [Google Scholar]
- 75.Andrés M, et al. Auditory Efferent System Modulates Mosquito Hearing. Curr. Biol. 2016;26:2028–2036. doi: 10.1016/j.cub.2016.05.077. [DOI] [PubMed] [Google Scholar]
- 76.Matheson T. Octopamine modulates the responses and presynaptic inhibition of proprioceptive sensory neurones in the locust Schistocerca gregaria. J. Exp. Biol. 1997;200:1317–1325. doi: 10.1242/jeb.200.9.1317. [DOI] [PubMed] [Google Scholar]
- 77.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selcho M, Wegener C. Immunofluorescence and Genetic Fluorescent Labeling Techniques in the Drosophila Nervous System. Immunocytochemistry and Related Techniques. 2015;101:39–62. [Google Scholar]
- 79.Klagges BR, et al. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J. Neurosci. 1996;16:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagh DA, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartenstein, V. Atlas of Drosophila development. (Cold Spring Harbor Laboratory Press, 1993).
- 83.Soler C, Daczewska M, Da Ponte JP, Dastugue B, Jagla K. Coordinated development of muscles and tendons of the Drosophila leg. Development. 2004;131:6041–6051. doi: 10.1242/dev.01527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author.