Abstract
Circulatory Ferritin concentration varies with age, sex, and body composition. Studies that determine the relationship of different body weight measurements with plasma ferritin concentration in adolescents are lacking. A descriptive cross-sectional design was utilized. Data collection involved self-reporting demographics, blood samples, and body composition measures for a sample of 814 healthy Jordanian adolescents. Ferritin deficiency was observed in 55.8% of the study population. Simple linear regression showed that BMI, gender, location, and smoking status 2.5%, 3.9%, 0.4%, and 0.4%, respectively, associated positively with plasma ferritin level (p < 0.05). After controlling for gender, location, and smoking status, additional hierarchal multiple linear regression showed that BMI explained 2.2% of plasma ferritin (p < 0.000). However, the obesity-stratified hierarchal multiple linear regression, showed that BMI explained 2.1% of plasma ferritin in the overweight and obese (HI) adolescents (p = 0.02), but not in the under and normal weight (LO) adolescents (p = 0.91). After controlling for gender, location, and smoking status, the ANCOVA showed that plasma ferritin level was greater (p < 0.000) in the HI (19.00 ± 13.6) versus the LO (15.20 ± 10.4) obesity group. Our results indicated that normal ferritin level among obese people does not necessarily indicate normal iron storage.
Introduction
Ferritin is a protein pivotal for various vital body organs, processes, functions, and diseases. It has been implicated in coronary artery disease and malignancy and directly involved in sideroblastic anemias, neurodegenerative disorders, inflammation, and hemophagocytic syndrome1,2. However, it is particularly essential for iron storing3 and supply2. Ferritin level ranges are 18–115 μg/L for women and 30–300 μg/L for men while the range is 7–140 μg/L in children4.
Obesity is spreading across the globe, primarily in developing countries5, including Jordan6. A recent report shows that adolescent overweight and obesity prevalence in Jordan is reaching 30%7, which is greater than those in developed countries8 and around the world9. Additionally, it has been linked to coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, and musculoskeletal, immune, and nervous system disorders, with a profound impact on quality of life10. In fact, obesity has recently been classified as a disease5.
Several studies, with contradictory findings, have detected an association between ferritin and obesity with few reports concluding that high ferritin levels were associated with increased obesity11–13. However, others reported either similar14,15 or even lower16 ferritin concentration in obese versus normal weight adults.
In adolescents, the relationship of ferritin with obesity is still elusive with only few studies showing an inverse association between serum ferritin level and obesity2,16,17. Therefore, the present study aimed at assessing the association of body mass index (BMI) with plasma ferritin concentration in a sample of apparently healthy 7th–10th grade Jordanian students. Given previous results, we hypothesized that BMI is related to circulatory ferritin levels in adolescents. The results can help recognizing the importance of body composition for plasma ferritin levels.
Results
Participants
A total of 2691 adolescents gave parental consent and self-assent to participate in the study. Plasma ferritin level was obtained from 1046 while BMI was obtained from 2488 adolescents. As in Table 1, plasma ferritin and BMI measurements obtained concurrently from 873 adolescents. About half of the participants were females while the largest portions of the students were in the 9th grade.
Table 1.
The participant demographic (n = 873).
| Gender (% male) | 54.9 |
| Age (yrs, mean ± SD) | 14.6 ± 1.0 |
| Weight (kg, mean ± SD) | 56.3 ± 13.0 |
| Height (cm, mean ± SD) | 161.0 ± 9.0 |
| Smoking status (%Yes) | 65.3 |
| Grade | |
| 7 (%) | 21.4 |
| 8 (%) | 23.3 |
| 9 (%) | 32.6 |
| 10 (%) | 22.7 |
| Location | |
| Rural (%) | 52.6 |
| Urban (%) | 47.4 |
| Family income (%) | |
| Above poverty | 34.5 |
| Below poverty | 65.5 |
| Mother education level (%) | |
| Above bachelor degree | 37.9 |
| Below associate degree | 62.1 |
| Father education level (%) | |
| Above bachelor degree | 44.9 |
| Below associate degree | 55.1 |
| Ferritin status (μg/L, mean ± SD) | 16.1 ± 12.1 |
| Below normal (%) | 55.8 |
| Normal (%) | 44.2 |
| Obesity status (BMI, mean ± SD) | 21.6 ± 3.9 |
| Normal and under weight | 72.7 |
| Over weight and obese | 27.3 |
Normality test
The normality test revealed that ferritin was not normally distributed (W-S p-value < 0.000). The values were then log-transformed and used for analysis.
Relationship of Obesity Measures with Ferritin
The analysis revealed that 628 (72.7%) adolescents were in the LO while 236 (27.3%) adolescents were the HI obesity groups. Additionally, 382 (44.2%) adolescents were ferritin normal while 483 (55.8%) adolescents were ferritin deficient.
Individual simple linear regression for the entire sample showed that BMI, gender, location, and smoking status explained (p < 0.05) 2.5%, 3.9%, 0.4%, and 0.4%, respectively, of plasma ferritin level. However, father and mother education level, income, were not related (p < 0.05) to plasma ferritin level among the adolescent participants. Additional hierarchal multiple linear regression, after controlling for gender, location, and smoking status, showed that BMI explained 2.2% of plasma ferritin (p < 0.0001). However, as in Fig. 1, the obesity-stratified hierarchal multiple linear regression, showed that BMI explained 2.1% of plasma ferritin in the HI (p = 0.02), but not in the LO (p = 0.91), obesity groups.
Figure 1.
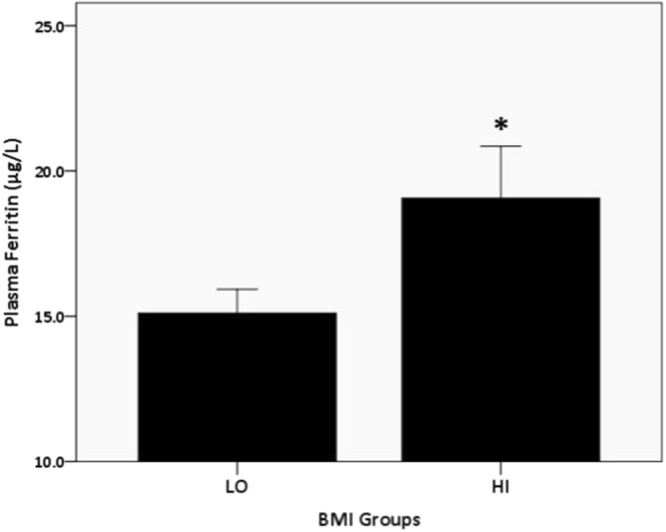
Relationship of BMI with plasma ferritin in the HI group. R2-change = 0.021; p < 0.02.
As in Fig. 2, the ANCOVA, after controlling for gender, location, and smoking status, showed that plasma ferritin level was greater (p < 0.000) in the HI (19.00 ± 13.6) versus the LO (15.20 ± 10.4) obesity group.
Figure 2.
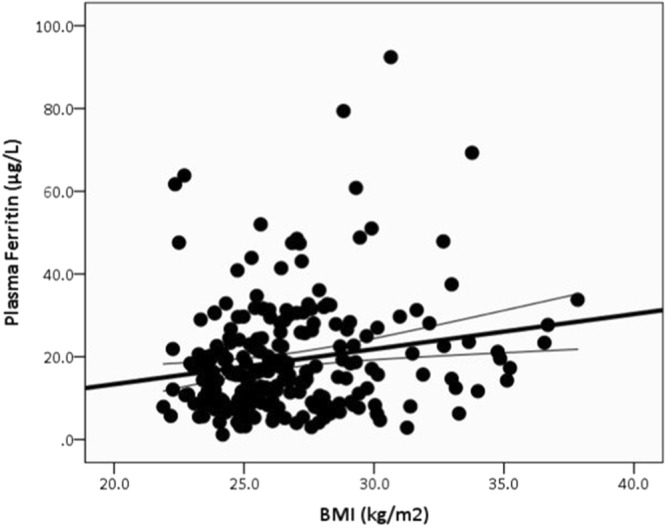
ANCOVA comparison in serum ferritin (μg/L) levels among high school students, after controlling for gender, smoking status, and location in the LO versus HI obesity groups. The data are presented in mean ± SE. *Indicate differences at p < 0.05.
Discussion
The study examined the relationship of plasma ferritin level with obesity among adolescents. Based on WHO classifications4, our results showed that more than half of the adolescents were ferritin deficient. Additionally, close to 1/3rd of the adolescents were classified as either obese or overweight. Interestingly, the comparisons showed that plasma ferritin was greater in the obese and overweight versus the normal weight adolescents. Most importantly, the results showed that plasma ferritin level was positively associated with BMI in the obese but not in the normal weight individuals.
Worldwide, iron depletion is the most common nutrient deficiency among adolescents18. The literature has identified athletic participation, menstruation, poor diet, and mismatch between ferritin demand and availability as possible risk factors for iron deficiency among adolescents19. Iron deficiency is also associated with decreased exercise capacity due to earlier onset of fatigue than healthy counterpart, which may result in stationary lifestyle, and subsequently possible weight gain11. Low iron level may also lead to anemia and its sequentially health problems including that might cause cognitive impairment, low immunity, and high risk pregnancies20.
Another cause of obesity among adolescents is the consumption of unbalanced and unhealthy meals, which consequently leads to further decline in iron stores21. Poor nutrition among children from lower socioeconomic status has also been implicated. These underprivileged children tend to consume low-cost meals, which is usually rich in fat and sugar and low in essential nutrients, such as iron22. These findings, along with our findings, indicate a need for developing strategies to improve eating habits to help restrain obesity in this population.
Importantly, BMI showed positive association with plasma ferritin concentration among obese adolescents, confirming previous studies13,14, showing greater circulatory ferritin level among obese adolescents13,14. Huang et al.12 reported that BMI is associated positively with plasma ferritin but negatively with serum iron in overweight/obese adolescents compared to the normal weight or underweight adolescents. Thus, according to Huang et al. results, circulatory ferritin does not reflect iron deficiency in overweight/obese adolescents. Furthermore, the current and previous findings suggest that different reference values of ferritin level should be considered when evaluating iron deficiency in overweight and obese adolescents. In addition, the need for different measures to assess iron level in obese adolescents is warranted. Conversely, other studies reported similar ferritin concentrations between obesity groups14. A negative relationship between ferritin level and BMI was also reported in Iranian girls17. These contradictory findings highlight the need for further studies to define the relationship between ferritin and obesity.
The mechanism for the positive relationship of obesity with ferritin is not fully understood. There is a growing evidence supports that obesity-related inflammatory process can increase ferritin level12,23 even with depleted iron stores24,25. Serum ferritin is an acute phase reactant protein, similar to CRP, which increases in response to inflammatory processes in obese people, including adipokines released by adipocytes2,16. Inflammatory cytokines are activated in response to obesity, and accordingly, hepcidin, an iron regulatory protein, is released as a defense mechanism resulting in decreased iron level and increased ferritin level17. This indicate that inflammation-induced obesity results in iron deficiency, though ferritin is elevated. Therefore, further research is needed to evaluate the inflammatory markers along with ferritin levels in adolescents, especially obese ones. Longitudinal and experimental studies that include monitoring ferritin level simultaneously with iron stores in obese versus normal weight adolescents are required to verify these relationships. A biomarker of inflammation should also be measured at the same time.
Our findings have important clinical implications. Given the rapidly increasing adolescence obesity (27.3% of our sample were obese), with the known link between obesity and iron deficiency (55.8% of our sample were ferritin deficient), guidelines for screening for obesity and iron deficiency should be promoted and implemented for this population. Additionally, routine monitoring of ferritin and iron levels are warranted among obese individuals. Special precautions are also advised when using ferritin level as an indicator of iron deficiency in obese adolescents, especially ferritin level in obese adolescents seems not reflect body iron storage26. Strategies to decrease iron deficiency and obesity in this high-risk population imperative to minimize the negative effects of iron deficiency and obesity. These strategies include promoting regular exercise and sufficient nourishment.
As with other studies, our study has some limitations. The first is the use of a cross-sectional design, which limits inferring causal relationships that may be obtained from a longitudinal design. A second limitation is with the relatively small sample size from a small country that may limit the generalizability of our findings. Another limitation is the use of BMI only as an indicator of obesity/overweight among adolescents. Including more detailed measures of body fat and fat distribution may strengthen our findings. In addition, this study did not measure biomarkers of inflammation, which if measured may verify any existing relationships. However, the current findings may serve as a platform for future longitudinal studies.
Conclusions
The results confirmed the positive association of obesity with plasma ferritin, especially among obese adolescents. Recognition of these results suggests taking this association into consideration when assessing iron deficiency for obese/overweight adolescents. Our results also imply that, although ferritin concentration in plasma/serum is the most pertinent indicator in monitoring iron stores in healthy persons27, it is of less value in assessing anemia in obese persons. However, more studies are needed to further establish ferritin relationship with various obesity measures using larger samples and longitudinal design.
Methods
Design, sampling and ethical approval
This is a descriptive cross-sectional study. The current study involved self-reporting and biometric data collection tools. The study was designed to assess the association of body mass index with circulatory plasma ferritin concentration in randomly selected adolescents in Jordan after being stratified for gender and school type. The inclusion criteria include students in grade 7–10, attends school regularly in any of the randomly selected schools, free of chronic diseases, and able to understand and write in Arabic fluently.
Written informed parental consents as well as informed child assents, approved by the required institutional review boards were obtained from all participants. Schools were contacted first and pre-arrangements with school principals were made in order to ensure that important teaching processes and school classes would not be interrupted. The study was approved and carried out in accordance with the guidelines and regulations of both the University of Science and Technology (JUST) Institutional Review Board (IRB) committee and the Jordanian Ministry of Education Ethics committee.
Study measurements were collected under university professor supervision and with the help of a trained research team and volunteers to ensure consistent and unified measurements. Self-reporting demographics were obtained in classes while blood samples and body composition measures were collected in the school nursing units. Blood withdrawals were performed by experienced nurses and medical laboratory technicians. Additionally, schoolteachers were available to facilitate data collection. All efforts were observed and precautions were taken to avoid possible risks that might be associated with data collection, especially before, during, and after blood withdrawal.
Blood samples
Venous blood samples were drawn from the arm and collected in an EDTA tube for ferritin analysis. Plasma ferritin concentrations were determined using Beckman Coulter Access Immunoassay Systems (Access Ferritin). The plasma ferritin cutoff value of 15 μg/L was used according to the WHO4 set for the study’s sample age range.
Physiological measures
A digital weighing scale was used to determine weight (Wt), while a standard tape measure at the umbilical level and widest portion of the buttocks was used to determine waist (W) and hip (H) circumferences, respectively, and height (HT). BMI was calculated using standard procedures28,29. BMI z-scores were calculated relative to Centers for Disease Control and Prevention gender-specific, BMI-for-age reference data30.
Statistical analyses
The SPSS package (version 21.0; Chicago, IL) was used to statistically analyze the data. Alpha was set a priori at p ≤ 0.05. Continuous tabulated data are presented in means ± SD while presented in means ± SE in figures. The adolescents were stratified according to obesity using BMI measure, to low (LO) and high (HI) obesity. The LO group included the adolescents who were classified as under and normal weight while the HI group included over weight and obese students, according to the CDC recommendations30.
Several individual linear regression models for the entire sample and sub-analysis (obesity-stratified) were used to establish the relationship of BMI, gender, age, smoking status (yes versus no), location (urban versus rural), family income (below versus above poverty line), and father and mother education level (below associate versus above bachelor degrees) with plasma ferritin. Additional obesity-stratified hierarchal multiple linear regression after controlling for gender, location, and smoking status related to plasma ferritin, was used to examine the relationship of obesity with plasma ferritin. One-way ANCOVA was used to compare plasma ferritin levels between the LO and HI obesity groups, after controlling for variables related to plasma ferritin.
Ferritin values were examined for normality using Welk-Shapiro (W-S) test. The values were then log-transformed and used for analysis.
Acknowledgements
This work was funded by Jordan University of Science and Technology/Deanship of Research [Research Grant No: KS/20160208]. The funding source had no role other than financial support.
Author Contributions
K.K.Sh., M.A.A., N.A. & A.B. contributed to the acquisition, analysis and interpretation of data. All authors drafted the article and approved the final version to be published.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Khulood K. Shattnawi, Mahmoud A. Alomari, Nihaya Al-Sheyab and Ayman Bani Salameh contributed equally.
Change history
1/24/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Garcia-casal, M., Pasricha, M., Martinez, R. & Peña-rosas, J. Serum or plasma ferritin concentration as an index of iron deficiency and overload (Protocol). 7 (2015). [DOI] [PMC free article] [PubMed]
- 2.Khan, A., Khan, W. M., Ayub, M., Humayun, M. & Haroon, M. Ferritin Is a Marker of Inflammation rather than Iron Deficiency in Overweight and Obese People. J. Obes. 2016 (2016). [DOI] [PMC free article] [PubMed]
- 3.Knovich, M. A., Storey, J. A., Coffman, L. G., Torti, S. V. & Torti, F. M. Ferritin for the clinician. Blood Rev [Internet]. 23(3), 95–104, 10.1016/j.blre.2008.08.001 Available from (2009). [DOI] [PMC free article] [PubMed]
- 4.WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations [Internet]. Vitamin and Mineral Nutrition Information System. [cited 2017 Jul 25]. p. 1–5. Available from, http://www.who.int/vmnis/indicators/serum_ferritin.pdf (2011).
- 5.Gupta N, Goel K, Shah P, Misra A. Childhood Obesity in Developing Countries: Epidemiology, Determinants, and Prevention. Endocr Rev. 2012;33(February):48–70. doi: 10.1210/er.2010-0028. [DOI] [PubMed] [Google Scholar]
- 6.Al Hourani, H. Food and Nutrition Profile: the Hashemite Kingdom of Jordan. 1–65 (2011).
- 7.Alomari, M. A., Al-Sheyab, N. A. & Ward K. D. Adolescent Waterpipe Use is Associated with Greater Body Weight: The Irbid-TRY. Subst Use Misuse [Internet]. Jun 7 [cited 2018 May 16] 53(7), 1194–202. Available from, http://www.ncbi.nlm.nih.gov/pubmed/29261407 (2018). [DOI] [PubMed]
- 8.OECD. OBESITY Update. (June) (2014).
- 9.MacPhee M. Global Childhood Obesity: How to Curb an Epidemic. 23(1):1–4 (2008). [DOI] [PubMed]
- 10.Serra-Majem, L. & Bautista-Castaño, I. Etiology of obesity: two “‘key issues’” and other emerging factors. Nutr Hosp [Internet]. [cited 2017 Jul 29] 28(5), 32–4332. Available from, http://www.redalyc.org/articulo.oa?id=309229028004 (2013). [DOI] [PubMed]
- 11.Aderibigbe OR, Pisa PT, Mamabolo RL, Kruger HS, Vorster HH. The relationship between indices of iron status and selected anthropometric cardiovascular disease risk markers in an African population: the THUSA study. Cardiovasc J Afr. 2011;22(5):249–56. doi: 10.5830/CVJA-2011-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, Y. et al. Relationship Between being Overweight and Iron Deficiency in Adolescents. Pediatr Neonatol [Internet]. 56(6), 386–92. Available from, 10.1016/j.pedneo.2015.02.003 (2015). [DOI] [PubMed]
- 13.Jeon YJ, Cho W, Park SH, Jung MH, Suh B. Serum ferritin level is higher in male adolescents with obesity: results from the Korean National Health and Nutrition Examination Survey 2010. Ann Pediatr Endocrinol Metab. 2013;18:141–7. doi: 10.6065/apem.2013.18.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif, M., Madani, M. & Tabatabaie, F. Comparative Evaluation of Iron Deficiency among Obese and Non-obese Children. Iran J Pediatr Hematol Oncol. 4(1) (2014). [PMC free article] [PubMed]
- 15.Ghadimi, R., Esmaili, H., Kheirkhah, D. & Tamaddoni, A. Is Childhood Obesity Associated with Iron Deficiency Anemia? Casp J Pediatr [Internet]. 1(2), 59–66. Available from, http://www.caspianjp.ir/article-1-40-en.pdf (2015).
- 16.Dandekar, U. S. Association between Serum Ferritin and Body Composition in Young Women [Internet]. University of Massachusetts - Amherst. Available from, http://scholarworks.umass.edu/theses/355 (2014).
- 17.Eftekhari, M. H., Mozaffari-Khosravi, H. & Shidfar, F. The relationship between BMI and iron status in iron-deficient adolescent Iranian girls. Public Health Nutr., 1–5 (2009). [DOI] [PubMed]
- 18. Gedefaw, L., Tesfaye, M., Yemane, T., Adisu, W. & Asres, Y. Anemia and iron deficiency among school adolescents: burden, severity, and determinant factors in southwest Ethiopia. Adolesc Health Med Ther [Internet]. 189. Available from, https://www.dovepress.com/anemia-and-iron-deficiency-among-school-adolescents-burden-severity-an-peer-reviewed-article-AHMT (2015). [DOI] [PMC free article] [PubMed]
- 19.Kohn, M. R., O’Dea, J. A. & Schlumbom, V. E. Iron deficiency in adolescent girls. Med Today [Internet]. 2000 [cited Jul 26] 1(6), 76–86. Available from, http://medicinetoday.com.au/2000/june/feature-article/iron-deficiency-adolescent-girls (2017).
- 20.Abbaspour, N., Hurrell, R. & Kelishadi, R. Review on iron and its importance for human health. J Res Med Sci Off J Isfahan Univ Med Sci [Internet]. 2014 Feb [cited May 13] 19(2), 164–74. Available from, http://www.ncbi.nlm.nih.gov/pubmed/24778671 (2018). [PMC free article] [PubMed]
- 21.Mesías M, Seiquer I, Navarro MP. Iron Nutrition in Adolescence. Crit Rev Food Sci Nutr. 2013;53(11):1226–37. doi: 10.1080/10408398.2011.564333. [DOI] [PubMed] [Google Scholar]
- 22.Pinhas-Hamiel O, et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes. 2003;27(3):416–8. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 23.Dignass, A., Farrag, K. & Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int J chronic Dis [Internet] 2018(Table 1), 9394060. Available from, https://www.hindawi.com/journals/ijcd/2018/9394060/%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/29744352%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5878890 (2018). [DOI] [PMC free article] [PubMed]
- 24.Turgeon O’brien, H., Blanchet, R., Gagné, D., Lauzière, J. & Vézina, C. Using Soluble Transferrin Receptor and Taking Inflammation into Account When Defining Serum Ferritin Cutoffs Improved the Diagnosis of Iron Deficiency in a Group of Canadian Preschool Inuit Children from Nunavik. Anemia [Internet]. [cited 2018 May 12]; Available from, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4921626/pdf/ANEMIA2016-6430214.pdf (2016). [DOI] [PMC free article] [PubMed]
- 25.Kaner G, Pekcan G, Pamuk G, Pamuk BO, Amoutzopoulos B. Is iron deficiency related with increased body weight? A cross-sectional study. Prog Nutr. 2016;18(2):102–10. [Google Scholar]
- 26.Alam F, Abdul Shakoor M, Syeda Sadia F. Increased Body Mass Index may lead to Hyperferritinemia Irrespective of Body Iron Stores. Pak J Med Sci. 2015;31(6):1521–6. doi: 10.12669/pjms.316.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beard, J. L. Iron deficiency: Assessment during pregnancy and its importance in pregnant adolescents. Am J Clin Nutr. 59(2 Suppl.) (1994). [DOI] [PubMed]
- 28.Adams, G. M & Beam, W. C. Exercise physiology: laboratory manual. McGraw-Hill, 308 (2008).
- 29.Alomari, M. A. et al. The clinical and nonclinical values of nonexercise estimation of cardiovascular endurance in young asymptomatic individuals. Scientific World Journal. 2012, 958752 (2012). [DOI] [PMC free article] [PubMed]
- 30.Krebs, N. F. et al. Assessment of Child and Adolescent Overweight and Obesity. Pediatrics [Internet]. Dec 1 [cited 2017 Aug 6] 120(Supplement), S193–228. Available from, http://www.ncbi.nlm.nih.gov/pubmed/18055652 (2007). [DOI] [PubMed]


