Abstract
The advent of new chemotherapeutic and immunotherapeutic treatments has markedly improved outcomes in patients with cancer. However, increasing numbers of elderly patients with cancer and prolonged periods of treatment have made the management of cardiovascular complications and treatment-induced cardiotoxicity an important concern, and onco-cardiology has received increasing attention. The number of patients with cardiotoxicity, particularly atherosclerotic lesions, and the usage of angiogenesis inhibitors have increased, making the involvement of onco-cardiologists essential for effective disease management. A paradigm shift in immunotherapy was caused by the development of immune checkpoint inhibitors. Because vascular endothelial growth factors (VEGF) in the cancer microenvironment and cancer immune function are interrelated angiogenesis inhibitors will most likely play an increasingly important role in combined immunotherapy. To ensure the optimal long-term diagnosis and long-term treatment of cancer and the effective management of treatment-related atherosclerotic diseases, the long-term continuous participation of onco-cardiologists is essential.
Keywords: Cancer, Cardiotoxicity, Vascular endothelial growth factors, Immune checkpoint inhibitors, Atherosclerosis
Introduction
In Japan, 1 in 2 individuals will have cancer during their lifetime. Progress in cancer therapy, particularly the development of chemotherapy and immunotherapy, has markedly improved outcomes in patients with cancer. On the other hand, Westernization of lifestyle and rapid aging of population have led to increasing numbers of patients with cancer and cardiovascular disease. The management of cardiotoxicity caused by new cancer treatments has become an important problem. Attention has focused on atherosclerotic disease occurring with the westernization of lifestyle, the aging of patients with cancer, and as cardiotoxicity associated with the prolonged use of anticancer therapy1, 2). Together with oncologists, onco-cardiologists, who actively participate in the diagnosis and treatment of both cancer and cardiovascular disease, aggressively contribute to the diagnosis and treatment of cardiotoxicity. Thereby, various cancer-specific and cancer-treatment-related problems are being solved3). In this paper, we outline the mechanism and management of atherosclerosis induced mainly by angiogenesis inhibitors, one of the most important factors, in patients with cancer treatment-related atherosclerosis.
1. Significance and Role of Angiogenesis Inhibitors in Patients with Cancer
Since Folkmann4) reported the relation between angiogenesis and cancer cell proliferation, attention has focused on intracellular signaling pathways involved in angiogenesis as an important target of molecular-targeted drugs in cancer therapy. The roles and clinical significance of angiogenic factors, consisting mainly of vascular endothelial growth factors (VEGF) and VEGF receptors, have been elucidated. The main site of action of angiogenic factors is the vascular endothelium. Angiogenic factors act to induce endothelium-dependent vascular relaxation to maintain blood flow and promote angiogenesis. Mobilization of circulating endothelial progenitor cells from bone marrow is induced to promote angiogenesis5). In healthy adults, VEGF plays an important role in wound healing and the repair of vascular endothelial injury by promoting the production of nitric oxide (NO) and prostacyclin (PGI2) in vascular endothelial cells to maintain normal blood flow. In pathological environments such as ischemic heart disease, VEGF secretion is stimulated by hypoxia-inducible factors (HIFs) associated with tissue ischemia, promoting compensatory angiogenesis. In the microenvironment of cancer, VEGF produced by cancer cells induces angiogenesis and proliferation required for the extension of cancer. In addition, VEGF plays important roles in the proliferation and metastasis of tumor tissue6, 7). In fact, VEGF is expressed in many types of cancers, including colorectal cancer, liver cancer, lung cancer, thyroid cancer, breast cancer, gastrointestinal cancer, renal cancer, bladder cancer, ovarian cancer, cervical cancer, angiosarcoma, germ cell tumors, and intracranial tumors. Angiogenesis inhibitors that target angiogenic factors were therefore developed for cancer therapy8). In 2004, bevacizumab, a representative anti-angiogenic agent, was developed as an anti-VEGF human monoclonal antibody. Its indications include colorectal cancer and have been expanded to include non–small-cell lung cancer, breast cancer, malignant glioma, ovarian cancer, and uterine cancer. Bevacizumab has been given to many patients with cancer and been reported to be effective9).
2. Anti-Angiogenetic Agents and Drug-Induced Hypertension
Because angiogenesis inhibitors do not directly target cancer cells, these agents were initially suspected to be highly effective with few adverse reactions at the time of initial development. However, cardiotoxicity such as hypertension, thromboembolism, heart failure, and ischemic heart disease was reported in patients who received angiogenesis inhibitors. The incidence of hypertension was particularly high, and such cardiotoxicity required appropriate management. The incidences of hypertension caused by representative angiogenesis inhibitors are shown in Table 11, 10–13). The times and the incidences of elevated blood pressure differed considerably according to the angiogenesis inhibitor being received.
Table 1. Incidence of hypertension after treatment with representative anti-angiogenetic agents.
| Agents | Hypertension (%) |
|---|---|
| Monoclonal antibody-based tyrosine kinase inhibitors | |
| bevacizumab | 23.6 |
| ado-trastuzumab emtansine | 5.1 |
| Small molecular tyrosine kinase inhibitors | |
| sorafenib | 15.3 |
| sunitinib | 21.6 |
| axitinib | 40.1 |
| regorafenib | 44.4 |
| lenvatinib | 67.8 |
| pasopanib | 42.0 |
| vandetanib | 24.2 |
| mTOR (mammalian target of rapamycin) inhibitors | |
| everolimus | 4–13 |
| temsirolimus | 7 |
Quoted from the following articles:
(01) Yeh ET, et al, JACC 2009; 53: 2231–2247.
(10) Zamorano Jl, et al, Eur Heart J.2016; 37 (36): 2768–2801.
(11) Moudgil R, et al. Can J Cardiol 2016; 32: 863–870.
(12) Wang Z, et al. Eur J Clin Pharmacol 2014; 70: 225–231
(13) Schlumberger M, et al. N Engl J Med 2015; 372: 621–630.
A total of 197 patients with cancer who received bevacizumab in our hospital were studied retrospectively. The time of initiating treatment with the antihypertensive drugs and the time of onset of proteinuria were investigated. Grade 2 or higher hypertension developed in 38.6% of the patients who received bevacizumab, and antihypertensive drugs were required to control blood pressure. Proteinuria was positive in 41.6% of the patients. The times of elevated blood pressure varied from the day after starting treatment to within 1 week after starting treatment in some patients. In other patients, blood pressure rose after 4 or more weeks, and treatment was required. These various periods suggested that the mechanisms of blood pressure elevation varied considerably. As the dose of bevacizumab increased, increasing numbers of patients had elevated blood pressure and proteinuria, indicating that cardiotoxicity was dose-dependent14).
The main site of action of angiogenesis inhibitors is the vascular endothelium. Angiogenesis inhibitors are thought to act primarily on microvessels 150–200 µm in diameter. The mechanism by which angiogenesis inhibitors are thought to act on elevated blood pressure is shown in Fig. 1. Treatment with angiogenesis inhibitors causes vasoconstriction associated with decreases in vasodilators such as NO and PGI2, resulting in vasoconstriction (vasoconstriction). In addition, vascular smooth muscle cell proliferation, platelet aggregation, thrombosis, and leukocyte adsorption to the vascular endothelium occur, promoting vascular endothelial dysfunction and the formation of plaque. On the other hand, vascular endothelial dysfunction and hypoxia promote the production of endothelin-1 (ET-1), causing vasoconstriction. Continuous treatment with angiogenesis inhibitors promotes a reduction in the peripheral arteriolar bed and capillary rarefaction associated with microthrombus formation, leading to microangiopathy (anatomical rarefaction). Consequently, hypertension and thromboembolism associated with atherosclerosis are induced, leading to drug-induced atherosclerosis6, 7, 15–19).
Fig. 1.
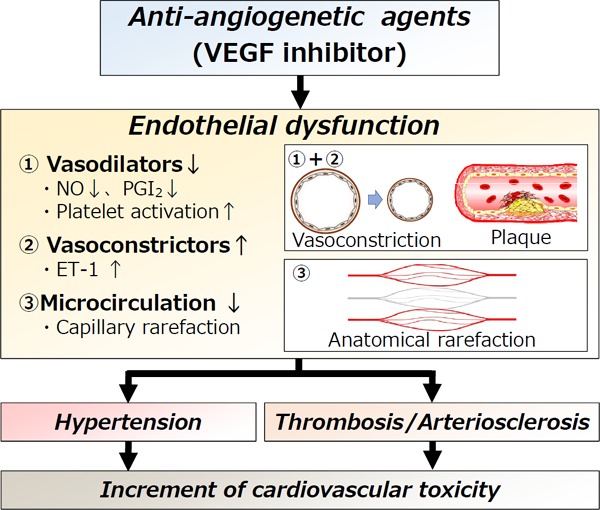
Mechanism of cardiotoxicity associated with anti-angiogenetic agents (VEGF inhibitors).
① Decrease in vasodilator factors: NO and PGI2, which are maintained by VEGF, are disturbed, resulting in vasospasm (vasoconstriction). In addition, platelet activity increases, leading to the formation of plaque (plaque). ② Increase in vasoconstrictors: ET-1 is increased by vascular endothelial dysfunction. ③ Microangiopathy: Microangiopathy occurs in association with a decrease in the peripheral vascular bed and microthrombus formation, resulting in capillary rarefaction and increased peripheral vascular resistance. This change becomes irreversible (anatomical rarefaction).
Quoted from the following articles:
(06) Chen, HX. and Cleck, JN. Nat Rev Clin Oncol 2009; 6: 465–477.
(07) Cameron AC et al., Can J Cardiol 2016; 32: 852–862.
(15) Zhu X et al. Am J Kidney Dis. 2007; 49: 186–193.
(16) Kappers MH et al., J Hypertens. 2009; 27: 2297–2309.
(17) Izzedine H et al. Ann Oncol. 2009; 20: 807–815.
(18) Vaklavas C et al. The Oncologist. 2010; 15: 1230–141.
(19) Kappers MH et al., Hypertension. 2010 Oct; 56(4): 675–681.
(20) de Jesus-Gonzalez N et al. Hypertension. 2012; 60: 607–615
The time of elevated blood pressure differs according to the action of VEGF inhibitors, as shown in Fig. 2. Immediately after the start of treatment, the functions of NO and PGI2 are disturbed, and functional rarefaction due to vasoconstriction mainly occurs, resulting in reversible elevation of blood pressure. Continuous treatment with VEGF inhibitors results in a reduction in capillary rarefaction, and elevation of blood pressure in the chronic phase is attributed to anatomical rarefaction. Long-term treatment with angiogenesis inhibitors for more than several years has been reported to cause aortic dissection. Adequate caution should thus be exercised with respect to cardiotoxicity affecting medium and large blood vessels20–22).
Fig. 2.
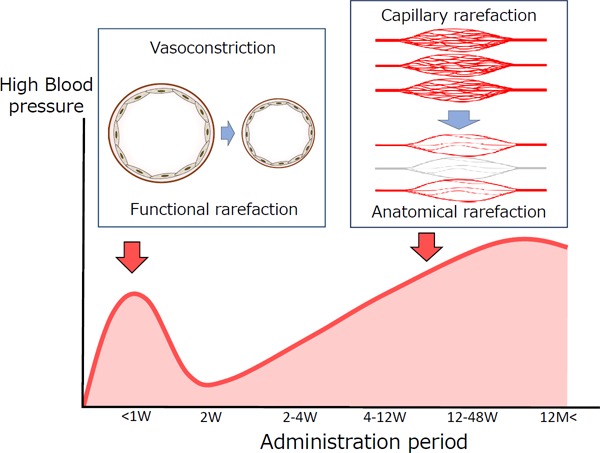
Timing and mechanism of hypertension caused by anti-VEGF treatment
During the early phase of treatment with anti-VEGF drugs, blood pressure is increased by vasoconstriction caused by disturbance of NO and PGI2 (functional rarefaction). This change occurs immediately after starting treatment in some patients and is irreversible. However, plaque is formed during long-term treatment, resulting in microangiopathy (capillary rarefaction). Peripheral vascular resistance increases, resulting in irreversible changes (anatomical rarefaction).
Quoted from the following articles:
(20) de Jesus-Gonzalez N et al. Hypertension. 2012; 60: 607–615.
(21) Mourad JJ et al. Annals of Oncology 2008; 19: 927–934.
(22) Takada M et al. International Heart Journal 2018 (in press).
3. Multi-Targeted Tyrosine Kinase Inhibitors (TKIs) and Atherosclerotic Disease
Angiogenesis-related factors are known to include various factors besides VEGF, such as platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and angiopoietin-123). The receptors of these factors are important targets for molecular-targeted drugs. Multi-targeted tyrosine kinase inhibitors (TKIs) are anticancer drugs that were developed to target VEGF and other angiogenesis receptors. Chronic myeloid leukemia (CML) is a representative disease for which TKIs are used. Because more than 90% of CML cases are caused by a Philadelphia chromosome abnormality, the Bcr-Abl tyrosine kinase inhibitor imatinib was developed as first-line treatment and has markedly improved outcomes in patients with CML24). Second-generation TKIs such as dasatinib, nilotinib and bosutinib were developed to treat patients with imatinibrefractory disease. Ponatinib was developed to manage the most intractable Bcr-AblT315 mutations, enabling remission to be induced in most patients with CML25).
On the other hand, the occurrence of arterial thromboembolism in patients with CML who receive TKIs has received considerable attention. In the PACE trial, assessing the clinical outcomes of treatment with ponatinib in patients with Bcr-Abl T315I mutations, the occurrence of arterial thromboembolism received considerable attention. The incidence of serious arterial occlusion was high (20%). More specifically, the incidence of cardiovascular thromboembolism was 16%, cerebrovascular occlusion 13%, peripheral vascular occlusion 14%, and venous thromboembolism 6%26, 27).
Apart from ponatinib, dasatinib causes hypertension, pulmonary arterial hypertension (PAH), and platelet dysfunction. Nilotinib causes hypertension, peripheral arterial disease (PAD), ischemic heart disease (IHD), cerebrovascular accidents (CVA), hyperglycemia, and dyslipidemia. Bosutinib causes hypertension. These findings indicate that each drug has different cardiotoxicity profiles. Many aspects of mechanism of cardiotoxicity caused by TKIs several years after starting treatment remain unclear. However, cardiotoxicity caused by treatment for CML was characterized by a high remission rate as well as by long-term treatment with TKIs for several years or longer. This is also related to the fact that TKIs have multiple targets as shown in Table 2. Besides VEGFR, TKIs target PDGFR, discoidin domain receptor 1 (DDR1), SRC, and cKIT. Besides these targets, various factors, including FGF, HGF, and Tie-2 are known to be extensively disturbed as targets of angiogenesis28, 29). It is extremely interesting that imatinib is virtually free of cardiovascular adverse reactions, despite having nearly the same targets30). Ponatinib is a potent inhibitor of VEGFR1-3, but has a different cardiotoxicity profile from that of sunitinib and sorafenib, which can strongly inhibit VEGFR 1-3. Cardiotoxicity is thus considered to involve not only on-target factors, but also off-target factors. New findings obtained from the cardiotoxicity caused by cancer treatment are expected to suggest that cardiotoxicity involves not only on-target factors, but also off-target factors. Such new findings obtained from the cardiotoxicity of these anticancer treatments suggest that studying differences in and the target sites of these drugs might provide new important clues to the mechanism of atherosclerosis31–33).
Table 2. The target sites of Bcr-Abl tyrosine kinase inhibitors and cardiotoxicity in patients with chronic myelogenous leukemia.
| Kinase/TKI | Imatinib | Nilotinib | Dasatinib | Bostinib | Ponatinb |
|---|---|---|---|---|---|
| Bcr-Abl | + | ++ | ++ | ++ | |
| Bcr-Abl (T315I) | ++ | ||||
| VEGFR | ++ | ++ | |||
| FGFR | ++ | ++ | |||
| PDGFR | + | + | ++ | ++ | |
| SRC | ++ | ++ | + | ||
| DDR1 | + | + | ++ | ||
| Tie2 | ++ | ||||
| cKIT | + | + | ++ | ++ |
| Cardiotoxicity/TKI | Imatinib | Nilotinib | Dasatinib | Bostinib | Ponatinb |
|---|---|---|---|---|---|
| PAOD | ++ | +/− | ++ | ||
| IHD/CVA | + | + | |||
| VTE | + | ||||
| Pulmonary hypertension | + | ||||
| Platelet dysfunction | + | + | |||
| Hypertension | + | ++ | |||
| Hyperglycemia | a | + | |||
| Dyslipidemia | a | + |
Bcr-Abl tyrosine kinase inhibitors used to treat chronic myelogenous leukemia have multiple target sites, and each drug is associated with different cardiovascular adverse reactions.
a: Imatinib has been shown to have positive effects on glucose blood levels, as well as lipid profile.
Abbreviations: CVA: cerebrovascular accident, DDR1: discoidin domain receptor 1, HT: hypertension, IHD: ischemic heart disease, PAOD peripheral arterial occlusive disease, PDGFR: platelet-derived growth factor receptor, TKI: Tyrosine kinase inhibitors, EGFR: vascular endothelial growth factor receptor, VTE: venous thromboembolism.
Quoted from the following articles:
(28) Moslehi JJ, Deininger M. J Clin Oncol 2015; 33: 4210–4218.
(29) Pasvolsky O et al., Cardio-Oncology 2015; 1: 5–15.
4. Immunotherapy and Cardiovascular Disorders
The development of immune checkpoint inhibitors has completely changed the conventional concept of anticancer therapy. Immune checkpoint inhibitors targeting CTLA4 or PD-1/PD-L1 have improved outcomes in many types of cancer. On the other hand, immune-related adverse events (irAE), associated with different characteristics and mechanisms from those of conventional anticancer treatment, have been reported34–36). Important cardiovascular irAE include myocarditis, pericarditis, vasculitis, and venous thrombosis37–39).
In the cancer microenvironment, sites of cancer cell proliferation are associated with neovascularization, and VEGF is associated with anticancer immune response in contrast to conventional angiogenesis. As shown in Fig. 3, cancer cells create a microenvironment that allows them to avoid cancer immune surveillance along with tumor-reactive T cells, thereby allowing cancer-cell proliferation and metastasis. At that time, VEGF has accumulated in many cancers, and VEGF produced by these tumors can directly disturb the maturation of dendritic cells (DC) and activate antigen-specific regulatory T cells (T-reg)40–44).
Fig. 3.
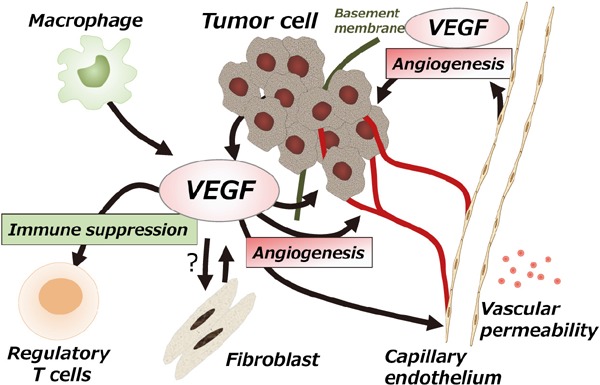
Multiple mechanisms of VEGF in the tumor microenvironment
Vascular endothelial growth factors (VEGF) have multiple functions in the tumor microenvironment. VEGF secreted by vascular endothelial cells modulates factors such as endothelial cell proliferation during angiogenesis, reconstruction of the extracellular matrix, and vascular permeability, thereby maintaining the function of vascular endothelial cells. In contrast, in the tumor microenvironment, VEGF secreted by tumor cells acts to promote tumor cell infiltration and survival. VEGF also modulates tumor immune response by inhibiting dendritic cell function and regulating the function of suppressor T cells. The functions of VEGF are also related to tumor fibroblasts and macrophages. Quoted from the following articles:
(40) Goal HL, Mercurio AM. Nat Rev Cancer 2013; 13: 871–882.
(41) Terme M et al. Caner Res 2013; 73: 539–549.
In combination immunotherapy using these characteristics, combining angiogenesis inhibitors with immune checkpoint inhibitors has been reported to have good outcomes45, 46). In the future, angiogenesis inhibitors might play a different role from the conventional role in immunotherapy-based anticancer treatment. On the other hand, measures are needed against irAE caused by immune checkpoint inhibitors and cardiotoxicity such as vascular disorders and thromboembolism caused by concurrent treatment with angiogenesis inhibitors.
5. Management of Drug-Induced Atherosclerosis
The diagnosis and treatment of drug-induced atherosclerosis in patients with cancer is challenging because the treatment of cancer has priority. Although ponatinib is associated with a high rate of serious cardiovascular complications exceeding 10%, patients who have CML associated with T315I mutations have refractory and fatal disease requiring treatment with ponatinib. Early treatment, including measures to prevent serious atherosclerosis, is therefore essential to appropriately treat cancer in patients with CML. Angiogenesis inhibitors are known to dose-dependently cause cardiotoxicity. Moreover, among patients who received ponatinib, the presence of 2 or more risk factors, such as advanced age, hypertension, diabetes mellitus, and dyslipidemia, was associated with high incidences of vascular disorders. The risk of cardiotoxicity should thus be evaluated by assessing cardiovascular risk factors and stratifying the cardiovascular risk47).
Aspirin and statins are currently considered prophylactic treatment for cardiotoxicity caused by cancer therapy-related atherosclerosis. Patients with many risk factors for atherosclerosis who are at a high risk for thrombosis should be considered candidates for treatment with aspirin and statins. Although treatment with antiplatelet drugs plus oral anticoagulants does not reduce the risk of cardiovascular events in patients with PAD48), treatment with aspirin, which has antiplatelet activity, plus statins to prevent vascular endothelial dysfunction is considered relatively safe in patients with cancer. However, the management of cancer-associated thrombosis remains poorly understood. Long-term antithrombotic therapy including new anticoagulants to reduce the risks of thrombosis and bleeding should be considered49). In the near future, the advent of angiogenesis inhibitors and immune checkpoint inhibitors and the effectiveness of these treatments will increase the need for treating patients with chronic cancer and cancer survivors. The long-term outcomes of survivors of breast cancer after treatment are known to depend largely on atherosclerotic changes50), and the surveillance and treatment of atherosclerotic lesions after the treatment of cancer have received considerable attention. Further studies including the close monitoring of further patients and intervention by cardiologists are needed51, 52).
Conclusions
The development of new cancer treatments has caused a paradigm shift in the diagnosis and treatment of cancer, which may have an impact during the next 20 to 30 years. Conventional short-term anticancer and palliative therapy should be reconsidered. In patients with cancer therapy-induced atherosclerosis, continuous surveillance should be performed for longer periods than those initially planned in clinical trials currently in progress. It is expected that new knowledge not only about the effectiveness for cancer, but also about long-term adverse effects will be obtained. Longterm toxic effects should be minimized, thereby allowing cancer therapy to be appropriately maintained.
Acknowledgments
The author wishes to express his gratitude to Wataru Shioyama (Osaka International Cancer Institute, Japan) for their inspiring lectures.
Conflict of Interest
The author (M.M.) has received lecture and manuscript fees from Daiichi Sankyo Company, Ltd, Bayer Yakuhin, Ltd, Bristol-Myers Squibb, Pfizer Japan Inc. and research funds from Bayer Yakuhin, Ltd, Bristol-Myers Squibb, Novartis pharma KK.
References
- 1). Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complication of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004; 109: 3122-3131 [DOI] [PubMed] [Google Scholar]
- 2). Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016; 375: 1457-1467 [DOI] [PubMed] [Google Scholar]
- 3). Albini A, Pennesi G, Donatelli F, Cammarota R, DeFlora S, Noonan DM. Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention. J Natl Cancer Inst 2010; 102: 14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285: 1182-1186 [DOI] [PubMed] [Google Scholar]
- 5). Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001; 7: 1194-1201 [DOI] [PubMed] [Google Scholar]
- 6). Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 2009; 6: 465-477 [DOI] [PubMed] [Google Scholar]
- 7). Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol 2016; 32: 852-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004; 9 Suppl 1: 2-10 [DOI] [PubMed] [Google Scholar]
- 9). Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N Engl J Med 2004; 350: 2335-2342 [DOI] [PubMed] [Google Scholar]
- 10). Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, ESC Scientific Document Group 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37: 2768-2801 [DOI] [PubMed] [Google Scholar]
- 11). Moudgil R, Yeh ET. Mechanisms of Cardiotoxicity of Cancer Chemotherapeutic Agents: Cardiomyopathy and Beyond. Can J Cardiol. 2016; 32: 863-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Wang Z, Xu J, Nie W. Risk of hypertension with regorafenib in cancer patients: a systematic review and metaanalysis. Eur J Clin Pharmacol. 2014; 70: 225-231 [DOI] [PubMed] [Google Scholar]
- 13). Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372: 621-630 [DOI] [PubMed] [Google Scholar]
- 14). Komori K, Mukai M, Ishitoko C, Sugitani K, Nakata Y, Tei G, Masu Y, Shioyama W, Awata N, Hori M. Relationship between hypertension and proteinuria associated with Bevacizumab: Retrospective study on cardiac toxicity. Annals of Oncology 2013; 24 (Suppl 9): ix57 [Google Scholar]
- 15). Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007; 49: 186-193 [DOI] [PubMed] [Google Scholar]
- 16). Kappers MH, van Esch JH, Sleijfer S, Danser AH, van den Meiracker AH. Cardiovascular and renal toxicity during angiogenesis inhibition: clinical and mechanistic aspects. J Hypertens. 2009; 27: 2297-2309 [DOI] [PubMed] [Google Scholar]
- 17). Izzedine H, Ederhy S, Goldwasser F, Soria JC, Milano G, Cohen A, Khayat D, Spano JP. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009; 20: 807-815 [DOI] [PubMed] [Google Scholar]
- 18). Vaklavas C, Lenihan D, Kurzrock R, Tsimberidou AM. Antivascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target?. The Oncologist. 2010; 15: 1230-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010; 56: 675-681 [DOI] [PubMed] [Google Scholar]
- 20). de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertension. 2012; 60: 607-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Annals of Oncology 2008; 19: 927-934 [DOI] [PubMed] [Google Scholar]
- 22). Takada M, Yasui T, Oka T, Shioyama W, Kuroda T, Nakai Y, Nishimura K, Mukai M, Fujita M. Aortic dissection and cardiac dysfunction emerged coincidentally during the longterm treatment with angiogenesis inhibitors for metastatic renal cell carcinoma: A case report of onco-cardiology. International Heart Journal 2018. (in press) [DOI] [PubMed] [Google Scholar]
- 23). Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438: 967-974 [DOI] [PubMed] [Google Scholar]
- 24). Chen MH1, Kerkelä R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008; 118: 84-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah NP, Kantarjian H, PACE Investigators A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuahc C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Lustgarten S, Rivera VM, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes TP, Shah NP, Kantarjian HM. Ponatinib efficacy and safety in Philadelphia chromosome – positive leukemia: final 5-year results of the phase 2 PACE trial. Blood 2018; 132: 393-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Nicolini FE, Basak GW, Kim DW, Olavarria E, Pinilla-Ibarz J, Apperley JF, Hughes T, Niederwieser D, Mauro MJ, Chuah C, Hochhaus A, Martinelli G, DerSarkissian M, Duh MS, McGarry LJ, Kantarjian HM, Cortes JE. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer 2017; 123: 2875-2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Moslehi JJ, Deininger M. Tyroshine kinae inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015; 33: 4210-4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Pasvolsky O, Leader A, Iakobishvili Z, Wasserstrum Y, Kornowski R, Raanani P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardio-Oncology 2015; 1: 5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol. 2016; 2: 625-632 [DOI] [PubMed] [Google Scholar]
- 31). Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med 2013; 369: 1779-1781 [DOI] [PubMed] [Google Scholar]
- 32). Loren CP, Aslan JE, Rigg RA, Nowak MS, Healy LD, Gruber A, Druker BJ, McCarty OJ. The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb Res 2014; 135: 155-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Moslehi J: The cardiovascular perils of cancer survivorship. N Engl J Med 2013; 368: 1055-1056 [DOI] [PubMed] [Google Scholar]
- 34). Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J1, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016; 375: 1749-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Hauhan A, Burkeen G, Houranieh J, Arnold S, Anthony L. Immune checkpoint-associated cardiotoxicity: case report with systematic review of literature. Ann Oncol. 2017; 28: 2034-2038 [DOI] [PubMed] [Google Scholar]
- 36). Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017; 28: 368-376 [DOI] [PubMed] [Google Scholar]
- 37). Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001; 291: 319-322 [DOI] [PubMed] [Google Scholar]
- 38). Varricchi G, Galdiero MR, Tocchetti CG. Cardiac Toxicity of Immune Checkpoint Inhibitors. Circulation. 2017; 136: 1989-1992 [DOI] [PubMed] [Google Scholar]
- 39). Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA. National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018; 36: 1714-1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Goal HL, Mercurio AM: VEGF targets the tuore cell. Nat Rev Cancer 2013; 13: 871-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Caner Res 2013. 73:539-549 [DOI] [PubMed] [Google Scholar]
- 42). Kawakami Y, Yaguchi T, Sumimoto H, Kudo-Saito C, Iwata-Kajihara T, Nakamura S, Tsujikawa T, Park JH, Popivanova BK, Miyazaki J, Kawamura N. Improvement of cancer immunotherapy by combining molecular targeted therapy. Front Oncol. 2013; 3: 136. 10.3389/fonc.2013.00136 eCollection 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, Feyen K, Tawney J, Hanahan D, Michael IP, Bergers G. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017; 9: eaak9679. 10.1126/scitranslmed.aak9679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Campesato LF, Merghoub T. Antiangiogenic therapy and immune checkpoint blockade go hand in hand. Ann Transl Med. 2017; 5: 497. 10.21037/atm.2017.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1-10 [DOI] [PubMed] [Google Scholar]
- 46). Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity 2013; 39: 61-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017; 35: 893-911 [DOI] [PubMed] [Google Scholar]
- 48). Anand S, Yusuf S, Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, Sussex B, Liu L, Guzman R, Cina C, Crowell R, Keltai M, Gosselin G. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. Engl J Med 2007; 357: 217-227 [DOI] [PubMed] [Google Scholar]
- 49). Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018; 72: 89-93 [DOI] [PubMed] [Google Scholar]
- 50). Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. M. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017; 2: 88-93 [DOI] [PubMed] [Google Scholar]
- 51). Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ. ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007; 25: 3991-4008 [DOI] [PubMed] [Google Scholar]
- 52). Montazeri K, Unitt C, Foody JM, Harris JR, Partridge AH, Moslehi J. ABCDE steps to prevent heart disease in breast cancer survivors. Circulation 2014; 130: e157-e159 [DOI] [PubMed] [Google Scholar]


