Abstract
We report a case of Tangier disease with Leriche syndrome and bleeding tendency. In this male patient, nasal hemorrhage had been observed frequently throughout childhood. At 46 years old, he experienced effort angina, and coronary angiography demonstrated 75% stenosis in the right coronary artery. Orange-colored tonsils, mild hepatosplenomegaly and very low levels of serum high-density lipoprotein cholesterol (HDL-C) were observed, and the patient was diagnosed with Tangier disease. At 52 years old, effort angina recurred. Coronary angiography revealed 75% stenosis of the left main trunk, left anterior descending, and right coronary arteries. Stenosis of the brachiocephalic and right common iliac arteries was also recorded. Stents were implanted, and coronary artery bypass surgery was performed. At 53 years old, 15 months after surgery, the patient reported intermittent claudication, coldness of feet, and impotence. Aortic angiography showed progression of the stenosis at the bifurcation of the common iliac artery. The patient was diagnosed with Leriche syndrome, and aorta–left external iliac artery graft bypass surgery was performed. After surgery, oozing from subcutaneous tissue and leaking from the anastomotic region were observed. Additional analysis revealed two single-nucleotide polymorphisms (V825I and N935T) in the ATP-binding cassette transporter A1 (ABCA1) gene, and accumulation of small dense low-density lipoprotein together with low levels of HDL-C. In Tangier disease, HDL-C is markedly decreased because of ABCA1 deficiency. However, this is the first reported case to exhibit extensive atherosclerosis and bleeding tendency. This patient had atypical extensive and multiple atherosclerotic lesions, accompanied by Leriche syndrome and uncontrollable bleeding.
Keywords: Tangier disease, Leriche syndrome, Atherosclerosis, HDL, ABCA1
Introduction
Tangier disease is characterized by orange-colored tonsils, mild hepatosplenomegaly, and a decline in high-density lipoprotein cholesterol (HDL-C) concentrations in the blood. A mutation of the ATP-binding cassette transporter A1 (ABCA1) gene has been indicated as the gene responsible for Tangier disease1–4). ABCA1 transports cholesterol at the plasma membrane and is expressed throughout the body5). In Tangier disease, it is believed that cholesterol is deposited in various tissues (vessel wall, β cells etc.), and we previously reported severely calcified coronary artery images by intravascular ultrasonography and impaired insulin secretion in ABCA1 deficiency6, 7).
Here, we describe a case of Tangier disease with extensive atherosclerotic lesions, accompanied by Leriche syndrome and bleeding tendency. Although atherosclerosis is a characteristic of Tangier disease, its frequency and severity are unclear. Therefore, we analyzed 56 papers (78 cases) and a review paper8) (54 cases) and investigated the frequency and severity of atherosclerosis and bleeding tendency in Tangier disease.
Case Presentation
A 53-year-old man was admitted to our hospital complaining of impotence, intermittent claudication, and a feeling of coldness in his lower extremities. He had a history of smoking from 17 to 52 years of age, 15 cigarettes per day (Brinkman Index: 525). The patient's father had type 2 diabetes. His mother had angina pectoris, type 2 diabetes, and renal insufficiency of unknown origin. His elder brother suffered a cerebral infarction at the age of 53. His younger sister died from sudden renal failure at 48 years of age.
From childhood, he often experienced nosebleeds. When he was 46 years old, he was diagnosed with effort angina. Coronary angiography revealed 75% stenosis in the right coronary artery #2 and a stent was implanted. At 52 years old, he again had chest pain on effort. Left coronary angiography revealed 75% stenosis in the left main trunk ostium (Fig. 1A) and moderate to severe diffuse stenosis from the ostium to the distal portion of the right coronary artery (Fig. 1B). Because of the severe coronary artery stenosis, including the left main trunk and right coronary artery, he was scheduled for coronary artery bypass graft (CABG) surgery in our hospital. He was administrated aspirin, ethyl icosapentate, and pitavastatin. At that time, because he had profound systemic atherosclerosis, we re-evaluated the atherosclerosis risk factors. His body mass index was 23.4 kg/m2. Orange-colored swollen tonsils (Fig. 2A, B), and mild hepatosplenomegaly were observed. HDL-C was 2 mg/dL and apolipoprotein A-1 (ApoA1) was undetectable. In addition, low hemoglobin and platelet counts were observed, consistent with a previous report7). Mean platelet volume was indicative of larger platelets (Fig. 3A). In addition, activated partial thromboplastin time was prolonged. Bleeding time was over 10 minutes (Supplemental Table 1). Among the risk factors for atherosclerosis, HDL-C was 2 mg/dL. The patient was diagnosed with Tangier disease. Fasting blood sugar level was 121 mg/dL, fasting insulin 5.9 µU/mL, and hemoglobin A1c (NGSP) was 4.9%. A 75 g oral glucose tolerance test was not performed.
Fig. 1.
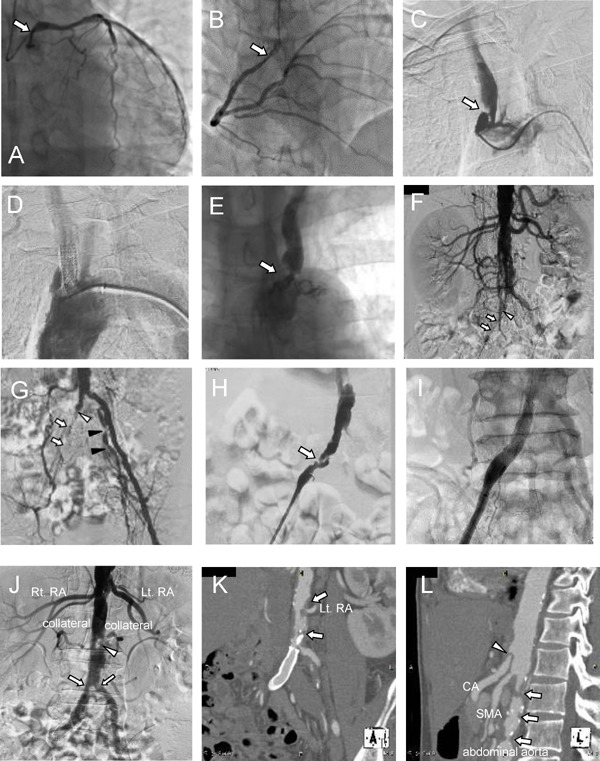
Coronary and aortic angiography before and after coronary artery bypass graft surgery at the age of 52
In the left coronary artery (LCA), 75% stenosis was observed in the left main trunk ostium (A). In the right coronary artery ostium (RCA), moderate to severe diffuse stenosis was observed (B). In addition, multiple severe calcified lesion were observed in both the LCA and RCA on coronary angiography.
Angiography of the aortic arch revealed irregular and eccentric stenosis of the brachiocephalic artery (C). This lesion was treated with stent implantation (8 × 27 mm) (D). A huge lesion was observed in the left subclavian artery (E).
Angiography of the abdominal aorta and lower limbs showed severe stenosis in the right common iliac artery (open arrow) (F, G), whereas the right internal iliac artery was not contrasted (open arrowhead). In addition, severe stenosis was found in the left internal iliac artery (closed arrowhead). Angiography of the right lower limb revealed stenosis with dissection in the right common iliac artery (H). This lesion was treated with stent implantation (10×60 mm) (I).
Abdominal aortic angiography, 2 years after coronary artery bypass graft surgery, when the patient was 53 years old. During angiography of the aortic arch, newly developed multiple severe stenoses were observed at the bifurcation of the common iliac artery. The arrowhead indicates the dissection of the abdominal aorta, which had not been observed 2 years previously (Fig. 1-F)) (J). Three-dimensional CT angiography of the coronal section showed severe stenosis with calcification at the ostium of the left renal artery and near the bifurcation of the common iliac artery (K). Three-dimensional CT angiography in the sagittal view showed multiple severe irregular stenoses of the abdominal aorta (open arrow) and dissection of the celiac artery (open arrowhead) (L).
Fig. 2.
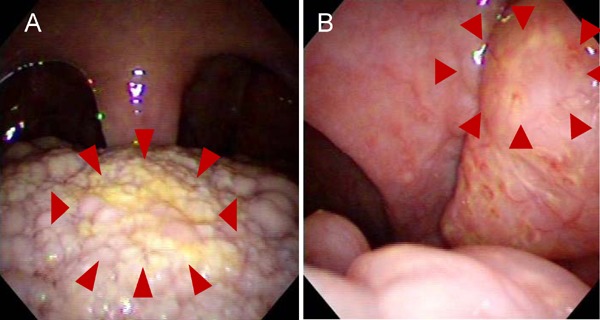
Photos of tonsils of the proband. The lingual tonsil (A) and pharyngeal tonsil (B) were orange-colored and swelling, which is a typical characteristic in Tangier disease
Fig. 3.
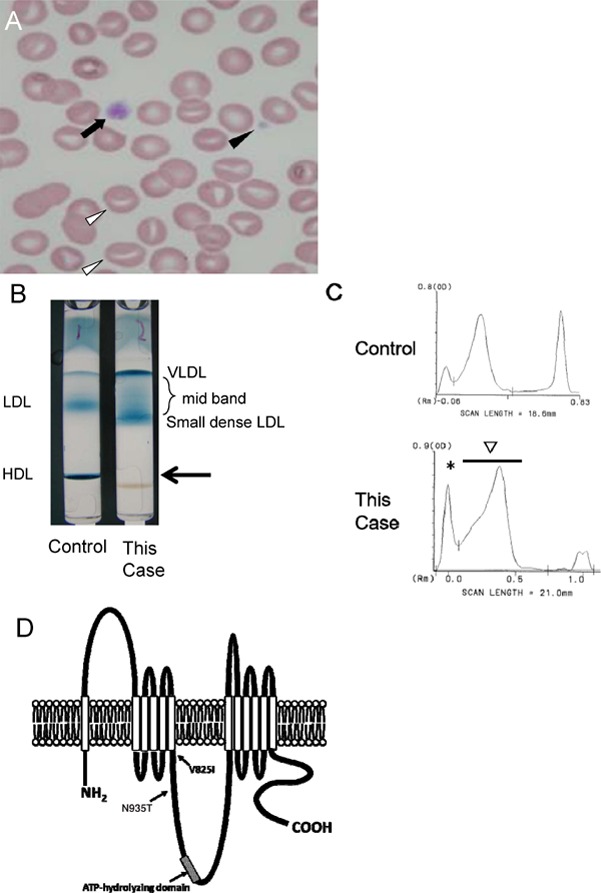
Peripheral blood was stained by Giemsa (×1000). Both normal platelets (closed arrow) as well as abnormal giant ones (closed arrowhead) were observed. In addition, erythrocytes with numerous stomatocytes (arrowhead) were observed (A).
Lipoprotein agarose gel electrophoresis was carried out. In this case, HDL was not observed at the arrow (B). The peak of VLDL (*) was high, and a mid-band was observed between VLDL (*) and LDL, suggesting accumulation of remnant. In this case, the second peak (arrow) was moved to the right, indicating accumulation of small-sized LDL (C). A putative model of ABCA1 mutation, V825I and N935T (D).
Supplemental Table 1. Clinical characteristics and Hemostasis Coagulation Tests of this case.
| Proband | Normal range | ||
|---|---|---|---|
| Height | 160 cm | ||
| Weight | 60.0 kg | ||
| BMI | 23.4 kg/m2 | ||
| white blood cell | 5,400 /µL | 3,800∼8,500 /µL | |
| red blood cell | 3.67 × 106 /µL | 4.00∼5.00 × 106 µL | |
| Hemoglobin | 12.3 g/dL | ↓ | 13.0∼16.80 g/dL |
| Hematocrit | 35.6% | ↓ | 38.0∼52.0% |
| total cholesterol | 98 mg/dL | 130∼219 mg/dL | |
| HDL-C | 2 mg/dL | ↓↓↓ | 40∼70 mg/dL |
| LDL-C | 89 mg/dL | 61∼139 mg/dL | |
| triglyceride | 67 mg/dL | 35∼149 mg/dL | |
| lipoprotein (a) | 2 mg/dL | ∼40 mg/dL | |
| apoprotein A-1 | < 5 mg/dL | ↓↓↓ | 119∼155 mg/dL |
| Platelet counts | 6.3 × 104 /µL | ↓↓ | 10.0∼40.0 × 4 /µL |
| MPV | 12.9 fL | ↑ | 7.5∼11.0 fL |
| PDW | 17.8% | ↑ | 15.2∼17.2% |
| PCT | 0.081% | ↓ | 0.1∼0.3% |
| PT | 90% | 80∼120% | |
| PT-INR | 1.07 | 0.87∼1.11 | |
| APTT | 40.8 sec | ↑ | 24.1∼35.3 sec |
| Bleeding time | > 10 minute | ↑↑ | 1.0∼5.0 minutes |
Abbreviations: HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; MPV, mean platelet volume; PDW, platelet distribution width; PCT, plateletcrit.
We evaluated the patient's systemic condition, especially the peripheral arteries. Irregular and eccentric stenosis of the brachiocephalic artery was observed (Fig. 1C). The lesion was treated with stent implantation (8 × 27 mm) (Fig. 1D). In addition, severe stenosis was found in the left subclavian artery (Fig. 1E) and was also treated with stent implantation. Angiography of the abdominal aorta and lower limbs revealed severe stenosis in the right common iliac artery (Fig. 1F), whereas the right internal iliac artery was not contrasted (Fig. 1G). In addition, severe stenosis was found in the left internal iliac artery (Fig. 1G). In the right common iliac artery, stenosis with dissection was observed (Fig. 1H) and treated with stent implantation (10 × 60 mm) (Fig. 1I). After treating the peripheral arteries, we performed CABG (right internal thoracic artery-posterior descending branch, AV node branch, and left internal thoracic artery-left anterior descending branch #8). At 5 days after surgery, cardiac tamponade occurred and was successfully controlled by platelet transfusion and pericardial drainage.
Six months later, the patient again suffered from effort angina. On coronary angiography, the radial artery graft between posterior descending branch #4 and AV node branch #4 was completely obstructed. We implanted a drug-eluting stent in the proximal right coronary artery to relieve the unprotected ischemic area.
Thirteen months later, at 53 years old, 1 year after the previous CABG, the patient was admitted to hospital complaining of impotence, intermittent claudication and a feeling of coldness in his lower extremities—a symptom of Leriche syndrome. The ankle– brachial pressure index was 0.79 on the right and 0.66 on the left, respectively. Angiography revealed severe stenosis at the bifurcation of the common iliac artery and dissection of the abdominal aorta (Fig. 1J). Three-dimensional computed tomography (CT) angiography showed incremental detritus stenosis with calcification at the ostium of the left renal artery and near the bifurcation of the common iliac artery (Fig. 1K, L). The patient was diagnosed with Leriche syndrome and underwent aorta–external iliac artery bypass surgery and replacement of the abdominal aorta with a blood vessel prosthesis. Histology of tissue obtained from the abdominal aorta indicated an aggregation of foam cells (Fig. 5A, B). On the following day, difficulty in hemostasis was again observed after surgery. As in the previous bypass surgery, we transfused platelet and managed to achieve hemostasis. However, internal bleeding could not be controlled; he suffered from compartment syndrome and died from rhabdomyolysis of the lower extremities.
To evaluate the etiology of the progressive atherosclerosis, we performed additional analyzes. Polyacrylamide gel electrophoresis (Fig. 3B, C) revealed a mid-band and increased peak of small dense low density lipoprotein (LDL). A mid-band suggested an increase of remnants, and small dense LDL were known as pro-atherogenic lipoproteins. However, an α-band (HDL) was not detected.
In terms of ABCA1 gene mutation, this patient had compound heterozygosity for V825I and N935T. V825I has been previously reported and is located in the 6th transmembrane domains. N935T is a novel mutation, located between 6th transmembrane domain and ATP-hydrolyzing domain (Fig. 3D).
Discussion
Unfortunately, our case died from uncontrollable bleeding. On admission, we observed a prolonged bleeding time, which indicated platelet dysfunction, thrombocytopenia, and giant platelets in peripheral blood (Fig. 1L). There is one previous study that investigated impaired platelet activation in ABCA1 deficiency9); the authors concluded that impaired release of the content of dense bodies may explain the defective activation of ABCA1-deficient platelets by collagen and low concentrations of thrombin. In other hypoalphalipoproteinemic diseases such as apolipoprotein A1 deficiency and lecithin-cholesterol acyltransferase deficiency, there have been no case reports about bleeding tendency. To examine whether this conclusion might be universal among Tangier patients, we reviewed all previously published case reports. However, there was no clear report of bleeding tendency. On the other hand, we previously reported decreased expression of the Rho GTPase family, cdc42, in Tangier disease10). We assumed that ABCA1 and cdc42 have intracellular colocalization11). Interestingly, in a recent report of a patient with de novo cdc42 mutation12), the patient had macrothrombocytopenia, which is completely consistent with our case (Fig. 3A). Although the precise molecular mechanism has not been completely elucidated, we are assuming that our patient might have had impaired or dysfunctional interaction between ABCA1 and cdc42, inducing macrothrombocytopenia and bleeding tendency in addition to defective HDL-C.
Atherosclerosis is a characteristic of Tangier disease; however, its frequency and severity are unclear. Therefore, we analyzed 56 papers (78 cases) and a review paper8) (54 cases) and investigated the frequency and severity of atherosclerosis and bleeding tendency in Tangier disease. From our analysis, we were not able to find any patient with such extensive atherosclerotic lesions as in our case. The literature search also revealed that angina was observed in 33 cases (24.8%) and other vascular diseases in 29 cases (21.8%) of total 133 cases of Tangier disease (Table 1, 2). It has been considered that Tangier patients might have a pro-atherogenic profile, due to very low levels of HDL-C, which is actually not common. In contrast, our Tangier disease case had extensive severe atherosclerosis. Furthermore, this is the first case of Tangier disease accompanied by Leriche syndrome. Schaefer EJ et al. indicated that LDL-C levels were typically lower than normal in Tangier disease13). This was explained by a twofold increase in LDL-C catabolism. However, it was also reported that the low levels of LDL-C in Tangier patients were rich in small dense LDL13). In our case, small dense LDL was markedly elevated, and this could have been involved in the extensive atherosclerosis.
Table 1. Clinical and laboratory features in Tangier disease.
| age | gender | angina | other vascular disease | TCho | LDL-C | HDL-C | TG | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 24 | M | - | - | 53 | 16 | 0 | 284 | 1961 | Fredrickson DS et al. |
| 2 | 25 | F | - | - | 63 | 30 | 0 | 351 | 1961 | Fredrickson DS et al. |
| 3 | 25 | F | - | - | 46 | 38 | 4 | 72 | 1964 | Fredrickson DS et al. |
| 4 | 29 | F | - | - | 89 | 80 | 6 | 131 | 1964 | Fredrickson DS et al. |
| 5 | 72 | M | + | + | 74 | 47 | 2 | 207 | 1965 | Hoffman HN et al. |
| 6 | 48 | M | + | + | 69 | 13 | 0 | 213 | 1965 | Hoffman HN et al. |
| 7 | 37 | M | - | - | 47 | 8 | 332 | 1967 | Kocen RS et al. | |
| 8 | 25 | F | - | - | 84 | 70 | 2 | 163 | 1967 | Engel WK et al. |
| 9 | 33 | F | - | - | 84 | 49 | 5 | 154 | 1967 | Engel WK et al. |
| 10 | 40 | M | - | - | 68 | 4 | 122 | 1968 | Kummer H et al. | |
| 11 | 3 | F | - | - | 70 | 7 | 155 | 1970 | Kracht J et al. | |
| 12 | 5 | M | - | - | 1971 | Bale PM et al. | ||||
| 13 | 15 | F | - | - | 59 | 47 | 7 | 136 | 1971 | Bale PM et al. |
| 14 | 53 | F | + | + | 95 | 9 | 180 | 1972 | Lindeskog GR et al. | |
| 15 | 62 | M | + | + | 60 | 0 | 230 | 1974 | Haas LF et al. | |
| 16 | 8 | F | - | - | 83 | 5 | 105 | 1974 | Greten H et al. | |
| 17 | 6 | F | - | - | 1974 | Stanios W et al. | ||||
| 18 | 10 | M | - | - | 57 | 35 | 2 | 110 | 1975 | Fetrans VJ et al. |
| 19 | 7 | M | - | - | 72 | 37 | 2 | 180 | 1975 | Fetrans VJ et al. |
| 20 | 56 | M | - | + | 114 | 6 | 269 | 1975 | Utermann G et al. | |
| 21 | 2 | F | - | - | 64 | 181 | 1976 | Assman G et al. | ||
| 22 | 56 | M | - | - | 60 | 6 | 100 | 1976 | Assman G et al. | |
| 23 | 53 | M | - | - | 51 | 0 | 170 | 1976 | Assman G et al. | |
| 24 | 56 | F | - | - | 90 | 5 | 348 | 1976 | Assman G et al. | |
| 25 | 56 | M | - | + | 42 | 22 | 0 | 297 | 1977 | Brook JG et al. |
| 26 | 14 | F | - | - | 59 | 49 | 0 | 102 | 1978 | Herbert PN et al. |
| 27 | 69 | F | - | + | 116 | 101 | 6 | 114 | 1978 | Dyck PJ et al |
| 28 | 19 | F | - | - | 80 | 39 | 4 | 214 | 1982 | Suarez BK et al. |
| 29 | 20 | F | - | - | 177 | 158 | 8 | 240 | 1982 | Frith RW et al. |
| 30 | 26 | M | - | - | 73 | 73 | 8 | 124 | 1982 | Frith RW et al. |
| 31 | 19 | M | - | - | 138 | 134 | 8 | 178 | 1982 | Frith RW et al. |
| 32 | 29 | F | - | - | 69 | 0 | 145 | 1983 | Ohtaki et al | |
| 33 | 31 | F | - | - | 60 | 2 | 88 | 1983 | Ohtaki et al | |
| 34 | 15 | M | - | - | 1984 | Dechelotte P et al | ||||
| 35 | 62 | M | + | + | 79 | 1 | 146 | 1984 | Vergani CG et al | |
| 36 | 28 | F | - | - | 50 | 15 | 8 | 175 | 1984 | Tarao K et al |
| 37 | 26 | F | - | - | 39 | 8 | 132 | 1984 | Tarao K et al | |
| 38 | 38 | M | - | - | 55 | 2 | 190 | 1985 | Gibbels E et al | |
| 39 | 53 | M | - | - | 98 | 2 | 355 | 1985 | Pietrini V et al | |
| 40 | 36 | M | - | - | 52 | 233 | 1986 | Clerc M et al | ||
| 41 | 65 | M | - | - | 28 | 1 | 202 | 1987 | Pressly TA et al | |
| 42 | 61 | F | - | - | 106 | 7 | increased | 1987 | Schmalbruch H et al | |
| 43 | 62 | M | - | - | 72 | 6 | 297 | 1987 | Frohlich J et al | |
| 44 | 27 | M | - | - | 46 | 0 | 244 | 1988 | Bracco G et al | |
| 45 | 55 | F | - | + | 73 | 1.5 | 658 | 1989 | Reinhard W.H. et al | |
| 46 | 50 | M | - | - | 103 | 545 | 1989 | Leal Luna A et al | ||
| 47 | 14 | M | - | - | 25 | 5 | 98 | 1990 | Lo W.D. et al | |
| 48 | M | - | - | 23 | 1 | 40 | 1990 | Kunitake S.T. et al | ||
| 49 | M | - | - | 30 | 1 | 78 | 1990 | Kunitake S.T. et al | ||
| 50 | 36 | M | 127 | 124 | 1990 | Schmitz G et al | ||||
| 51 | 28 | M | 35 | 89 | 1990 | Schmitz G et al | ||||
| 52 | 43 | M | - | 10 | 1991 | Dumon MF et al | ||||
| 53 | 47 | M | + | 28 | 6 | 232 | 1991 | Matsuzawa Y et al | ||
| 54 | 46 | 123 | 0 | 1991 | Antoine JC et al | |||||
| 55 | 61 | F | 109 | 1 | 249 | 1993 | Fazio R et al | |||
| 56 | 48 | F | + | 2 | 1993 | Cheung MC et al | ||||
| 57 | 52 | F | + | + | 115 | 8.6 | 3 | 185 | 1994 | C. Serfaty-Lacrosniere et al |
| 58 | 37 | M | + | + | 58 | 21 | 1 | 365 | 1994 | C. Serfaty-Lacrosniere et al |
| 59 | 40 | M | - | - | 40 | 23 | 0 | 242 | 1994 | C. Serfaty-Lacrosniere et al |
| 60 | 56 | F | 130 | 2 | 1994 | Frosini G et al | ||||
| 61 | 29 | M | + | - | 143 | 3.87 | 164 | 1994 | Burnett JR et al | |
| 62 | 40 | M | - | - | 46 | 19 | 0 | 242 | 1994 | Barnard GF et al |
| 63 | 36 | F | 104 | 123 | 1996 | No authors listed | ||||
| 64 | 39 | F | - | 89 | < 10 | 487 | 1996 | Mentis SW et al | ||
| 65 | 57 | F | + | 1998 | Neuman M et al | |||||
| 66 | 8 | F | 88.2 | 6.58 | 194 | 1998 | Lachaux A et al | |||
| 67 | 1 | M | 84.4 | 3.87 | 265 | 1998 | Lachaux A et al | |||
| 68 | 55 | M | + | 36 | 2 | 143 | 2000 | Ohnishi M et al | ||
| 69 | 48 | M | + | 28 | 6 | 232 | 2000 | Komuro R et al | ||
| 70 | 50 | F | + | + | 92.9 | 63.9 | 3.87 | 124 | 2001 | Bertolini S et al |
| 71 | 48 | M | + | 96.3 | 56.8 | 5 | 75 | 2002 | Ishii J et al | |
| 72 | 20 | M | - | 61 | 0 | 114 | 2002 | Guo Z et al | ||
| 73 | 69 | M | - | 34 | 0.8 | 187 | 2002 | Guo Z et al | ||
| 74 | 57 | M | + | 22 | 4 | 88 | 2002 | Guo Z et al | ||
| 75 | 56 | M | + | 25 | 1 | 112 | 2002 | Takami H et al | ||
| 76 | 54 | F | - | - | 108 | absent | absent | 2003 | Zuchner S et al | |
| 77 | 32 | F | - | - | 75.9 | 1.94 | 162 | 2003 | Kolovou GD et al | |
| 78 | 29 | M | - | - | 27 | 3 | 231 | 2003 | Grobusch MP et al | |
| 79 | 36 | M | - | - | 63 | not detectable | not detectable | 2004 | Sinha S et al | |
| 80 | 52 | M | - | - | 159 | 105 | 3.87 | 204 | 2004 | Hovingh GK et al |
| 81 | 38 | M | + | + | 89 | 50.3 | 3.87 | 177 | 2004 | Hovingh GK et al |
| 82 | 42 | F | + | 147 | 108 | 3.87 | 228 | 2004 | Albrecht C et al | |
| 83 | 42 | F | - | - | 66 | 52 | 4 | 37 | 2004 | Guan JZ et al |
| 84 | 53 | M | 41 | 4 | 2004 | Morchen M et al | ||||
| 85 | 72 | F | 2004 | Herrmann WA et al | ||||||
| 86 | 42 | F | + | 136 | 108 | 1.55 | 133 | 2006 | Slatter TL et al | |
| 87 | 17 | M | 2006 | Cai Z et al | ||||||
| 88 | 24 | M | - | 33 | 10 | 1 | 100 | 2006 | Espinel J et al | |
| 89 | 65 | M | + | + | 70 | 29 | 5.5 | 299 | 2007 | Imai R et al |
| 90 | 15 | F | - | - | 127 | 5.79 | 166 | 2008 | Theaudin M et al | |
| 91 | 55 | F | 81 | 4 | 384 | 2008 | Sperti C et al | |||
| 92 | 49 | M | + | + | 60 | 0 | 2008 | Schippling S et al | ||
| 93 | 57 | M | + | 78 | 37 | 5 | 178 | 2008 | Bektas M et al | |
| 94 | 35 | F | - | - | 2009 | Miyachi K et al | ||||
| 95 | 31 | F | 98 | 87 | 1 | 66 | 2009 | Maekawa M et al | ||
| 96 | 74 | M | - | 69 | 3.55 | 42 | 2009 | Koseki M et al | ||
| 97 | 44 | M | + | 64 | 2.5 | 272 | 2009 | Koseki M et al | ||
| 98 | 71 | F | + | 59 | 6 | 162 | 2009 | Koseki M et al | ||
| 99 | 54 | M | + | 35 | 0 | 395 | 2009 | Koseki M et al | ||
| 100 | 62 | M | + | 65.8 | 19.4 | 1.93 | 274.6 | 2009 | Hooper AJ et al | |
| 101 | 37 | M | + | + | 58 | 4 | 184 | 2009 | Sampietro T et al | |
| 102 | 40 | M | - | - | 67 | 2.32 | 114.3 | 2010 | Cameron J et al | |
| 103 | 55 | F | 105 | 3 | 384 | 2010 | Pichit P et al | |||
| 104 | 53 | F | + | 141 | 5 | 138 | 2010 | Pichit P et al | ||
| 105 | 43 | - | - | 1.93 | 2012 | Zyss J et al | ||||
| 106 | 52 | - | - | 3.09 | 2012 | Zyss J et al | ||||
| 107 | 39 | + | + | 1.16 | 2012 | Zyss J et al | ||||
| 108 | 50 | M | - | + | 5.02 | 2012 | Zyss J et al | |||
| 109 | 22 | M | - | - | 92 | 49 | 6 | 184 | 2012 | Rader DJ et al |
| 110 | 76 | F | - | - | 34.8 | 19.3 | 0.38 | 283 | 2012 | Fasano T et al |
| 111 | 33 | M | + | + | 108 | 46.4 | 5.41 | 283 | 2012 | Fasano T et al |
| 112 | 6 | F | 61.8 | 34.8 | 2.32 | 133 | 2012 | Fasano T et al | ||
| 113 | 32 | M | - | - | 50.3 | not available | 1.16 | 186 | 2012 | Fasano T et al |
| 114 | 0 | M | 96.7 | 22 | 5.03 | 133 | 2012 | Fasano T et al | ||
| 115 | 69 | F | - | + | 143 | 104 | 11.6 | 133 | 2012 | Fasano T et al |
| 116 | 37 | M | - | 166 | not available | 5.41 | 1187 | 2012 | Fasano T et al | |
| 117 | 60 | F | - | + | 217 | 139 | 27.8 | 310 | 2012 | Fasano T et al |
| 118 | 54 | M | - | + | 224 | 128 | 22 | 390 | 2012 | Fasano T et al |
| 119 | 52 | M | - | + | 228 | 155 | 18.9 | 328 | 2012 | Fasano T et al |
| 120 | 45 | F | + | + | 60 | 34 | unmeasurable | 103 | 2012 | Pervaiz MA et al |
| 121 | 59 | F | + | + | 57 | 31 | 2 | 2012 | Feng W et al | |
| 122 | 38 | F | - | + | 124 | 106 | < 5 | 138 | 2013 | Negi SI et al |
| 123 | 51 | M | - | - | 48 | 8 | 1 | not detectable | 2014 | Lucchi T et al |
| 124 | 58 | F | + | 60 | 2 | 448 | 2014 | Sechi A et al | ||
| 125 | 12 | M | - | - | 48 | 0 | 0.6 | 319 | 2014 | Sahiner N et al |
| 126 | 3 | M | 60 | 41.4 | < 3.1 | 2014 | Ravesloot et al | |||
| 127 | 22 | F | 50 | 27 | 3.1 | 108 | 2015 | Brunham LR et al | ||
| 128 | 26 | M | - | 65 | 34 | 7.7 | 114 | 2015 | Brunham LR et al | |
| 129 | 4 | F | 49.9 | 14.7 | 5.41 | 151 | 2015 | Brunham LR et al | ||
| 130 | 16 | M | - | - | 86 | 49.8 | < 5 | 86 | 2015 | Per H et al |
| 131 | 17 | M | - | - | 59 | 2 | 107 | 2016 | Murano T et al | |
| 132 | 43 | M | - | 149 | 110 | 5 | 2016 | Nagappa M et al |
Abbreviations: The same cases were described with preference to the latest report. TCho, total cholesterol (mg/dL); LDL-C, low density lipoprotein-cholesterol (mg/dL); HDL-C, high density lipoprotein-cholesterol (mg/dL); TG, triglycerides (mg/dL).
Table 2. Clinical characteristics and lipid profiles of Tangier patients divided by presence or absence of atherosclerosis.
| male | CVD(+) | CVD(−) | p-value |
|---|---|---|---|
| (n = 26) | (n = 41) | ||
| age | 51.1 ± 10.3 | 34.9 ± 17.7 | < 0.001 |
| TCho (mg/dL) | 65.8 (42.0, 89.0) | 58.0 (46.3, 72.0) | 0.347 |
| LDL-C (mg/dL) | 52.1 ± 44.4 | 46.9 ± 40.9 | 0.377 |
| HDL-C (mg/dL) | 3.87 (1.00, 5.31) | 2.00 (1.00, 5.00) | 0.555 |
| TG (mg/dL) | 231 (173, 286) | 184 (112, 242) | 0.110 |
| female | CVD(+) | CVD(−) | p-value |
|---|---|---|---|
| (n = 17) | (n = 26) | ||
| age | 54.2 ± 9.71 | 28.8 ± 18.1 | < 0.001 |
| TCho (mg/dL) | 105 (63.3, 130) | 73.0 (59.8, 89.0) | 0.049 |
| LDL-C (mg/dL) | 85.9 ± 44.7 | 53.9 ± 37.7 | 0.038 |
| HDL-C (mg/dL) | 3.87 (2.00, 6.00) | 5.00 (1.97, 7.00) | 0.932 |
| TG (mg/dL) | 150 (133, 217) | 159 (131, 206) | 0.709 |
These statistical analyses were performed using the STATA version 11.0 (Stata, College Station, TX, USA) statistics software package. Data are expressed as mean ± s.d. or median (interquartile range; 25–75%) because of histogram. All participants were using t-tests or Wilcoxon's signed rank tests appropriately.
TCho, total cholesterol; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol; TG, triglycerides.
Regarding the mutational analysis of ABCA1 gene, there is a report that V825I was associated with coronary artery disease while having no effect on HDL-C or ApoA1 levels14). In addition, the V825I mutation is located in the transmembrane domain15). Frikke-Schmidt et al. genotyped single-nucleotide polymorphisms of 69,259 individuals and found that V825I affected the risk of coronary artery disease16). On the other hand, Yin et al. suggested that there was no significant association between the V825I polymorphism and the risk of atherosclerosis17). Thus, the association between V825I and cardiovascular disease is controversial. In our case, the other mutation, N935T is a novel mutation, located between 6th transmembrane domain and ATP-hydrolyzing domain. We consider that the feature of this novel mutation might be associated with transportation of cholesterol.
There still remain many unknown points regarding the pathophysiology of Tangier disease. Further investigation is required to assess the incidence and the mechanism of atherosclerosis and bleeding tendency in Tangier patients.
COI Statement
The authors have no conflicts of interest to declare in association with this study.
References
- 1). Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denèfle P, Assmann G: Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet, 1999; 22: 352-355 [DOI] [PubMed] [Google Scholar]
- 2). Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G: The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet, 1999; 22: 347-351 [DOI] [PubMed] [Google Scholar]
- 3). Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR: Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet, 1999; 22: 336-345 [DOI] [PubMed] [Google Scholar]
- 4). Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S: Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res, 2007; 48: 299-306 [DOI] [PubMed] [Google Scholar]
- 5). Wang N1, Silver DL, Thiele C, Tall AR: ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem, 2001; 276: 23742-23747 [DOI] [PubMed] [Google Scholar]
- 6). Komuro R, Yamashita S, Sumitsuji S, Hirano K, Maruyama T, Nishida M, Matsuura F, Matsuyama A, Sugimoto T, Ouchi N, Sakai N, Nakamura T, Funahashi T, Matsuzawa Y: Tangier disease with continuous massive and longitudinal diffuse calcification in the coronary arteries : demonstration by the sagittal images of intravascular ultrasonography. Circulation, 2000; 101: 2446-2448 [DOI] [PubMed] [Google Scholar]
- 7). Koseki M, Matsuyama A, Nakatani K, Inagaki M, Nakaoka H, Kawase R, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Sandoval JC, Ohama T, Nakagawa-Toyama Y, Matsuura F, Nishida M, Ishigami M, Hirano K, Sakane N, Kumon Y, Suehiro T, Nakamura T, Shimomura I, Yamashita S: Impaired insulin secretion in four Tangier disease patients with ABCA1 mutations. J Atheroscler Thromb, 2009; 16: 292-296 [DOI] [PubMed] [Google Scholar]
- 8). Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED, Smith MP, Jr, Pritchard PH, Frohlich J, Lees RS, et al. : Homozygous Tangier disease and cardiovascular disease. Atherosclerosis, 1994; 107: 85-98 [DOI] [PubMed] [Google Scholar]
- 9). Nofer JR, Herminghaus G, Brodde M, Morgenstern E, Rust S, Engel T, Seedorf U, Assmann G, Bluethmann H, Kehrel BE: Impaired platelet activation in familial high density lipoprotein deficiency (Tangier disease). J Biol Chem, 2004; 279: 34032-34037 [DOI] [PubMed] [Google Scholar]
- 10). Hirano K, Matsuura F, Tsukamoto K, Zhang Z, Matsuyama A, Takaishi K, Komuro R, Suehiro T, Yamashita S, Takai Y, Matsuzawa Y: Decreased expression of a member of the Rho GTPase family, Cdc42Hs, in cells from Tangier disease - the small G protein may play a role in cholesterol efflux. FEBS Lett, 2000; 484: 275-279 [DOI] [PubMed] [Google Scholar]
- 11). Tsukamoto K, Hirano K, Tsujii K, Ikegami C, Zhongyan Z, Nishida Y, Ohama T, Matsuura F, Yamashita S, Matsuzawa Y: ATP-binding cassette transporter-1 induces rearrangement of actin cytoskeletons possibly through Cdc42/N-WASP. Biochem Biophys Res Commun, 2001; 287: 757-765 [DOI] [PubMed] [Google Scholar]
- 12). Takenouchi T, Kosaki R, Niizuma T, Hata K, Kosaki K: Macrothrombocytopenia and developmental delay with a de novo CDC42 mutation: Yet another locus for thrombocytopenia and developmental delay. Am J Med Genet A, 2015; 167A: 2822-2825 [DOI] [PubMed] [Google Scholar]
- 13). Schaefer EJ, Brousseau ME, Diffenderfer MR, Cohn JS, Welty FK, O'Connor J, Jr, Dolnikowski GG, Wang J, Hegele RA, Jones PJ: Cholesterol and apolipoprotein B metabolism in Tangier disease. Atherosclerosis, 2001; 159: 231-236 [DOI] [PubMed] [Google Scholar]
- 14). Tan JH, Low PS, Tan YS, Tong MC, Saha N, Yang H, Heng CK: ABCA1 gene polymorphisms and their associations with coronary artery disease and plasma lipids in males from three ethnic populations in Singapore. Hum Genet, 2003; 113: 106-117 [DOI] [PubMed] [Google Scholar]
- 15). Singaraja RR, Brunham LR, Visscher H, Kastelein JJ, Hayden MR: Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol, 2003; 23: 1322-1332 [DOI] [PubMed] [Google Scholar]
- 16). Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Steffensen R, Tybjaerg-Hansen A: Genetic variation in ABCA1 predicts ischemic heart disease in the general population. Arterioscler Thromb Vasc Biol, 2008; 28: 180-186 [DOI] [PubMed] [Google Scholar]
- 17). Yin YW, Wang Q, Sun QQ, Hu AM, Liu HL: ATP-binding cassette transporter 1 C69T and V825I polymorphisms in the development of atherosclerosis: a metaanalysis of 18,320 subjects. Thromb Res, 2015; 135: 130-136 [DOI] [PubMed] [Google Scholar]


