Abstract
Aims: Coronary artery atherosclerosis in patients needing carotid revascularization has not been fully clarified. The aim of this study was to evaluate the stenotic severity and plaque characteristics of coronary arteries by coronary computed tomography angiography (CTA) in patients scheduled for carotid-artery stenting (CAS) or carotid endarterectomy (CEA).
Methods: We performed coronary CTA after carotid ultrasound (US) in 164 patients (81.7% male, aged 68.1 ± 12.2 years) from 2014 to 2016. Of all, 70 were scheduled for CAS or CEA (CAS/CEA group) and 94 were not (non-CAS/CEA group). Carotid US and coronary CTA were compared for the evaluation of stenotic severity and plaque characteristics of each vessel between CAS/CEA and non-CAS/CEA groups.
Results: Between the two groups, there were significant differences in the presence of significant stenosis (SS: ≥ 70% stenosis of coronary artery) (55.7% vs. 39.4%, P = 0.038), triple-vessel disease (TVD)/left main trunk (LMT) (SS in each of three epicardial vessels and/or LMT) (24.3% vs. 7.5%, P = 0.0025), and high-risk plaque (HRP: positive remodeling and/or low attenuation) (55.7% vs. 24.5%, P < 0.0001). CAS/CEA was independently associated with TVD/LMT (OR = 2.30, 95%CI: 1.14–8.59, P = 0.026) and HRP (OR = 3.17, 95%CI: 1.57–6.54, P = 0.0012) in multivariable logistic regression analysis. Similarly, vulnerable plaque (78.6% vs. 2.1%, P < 0.0001) as well as severe stenosis of carotid artery (98.6% vs. 0%, P < 0.0001) was seen more often in CAS/CEA than in non-CAS/CEA group.
Conclusions: The prevalence of TVD/LMT and HRP determined by coronary CTA is higher in patients needing CAS/CEA than in those without. Management of systemic atherosclerosis is required in the perioperative period of CAS/CEA.
Keywords: Coronary computed tomography angiography, Carotid artery revascularization, Plaque characteristics, Systemic atherosclerosis
See editorial vol. 25: 1005–1006
Introduction
Ischemic stroke and coronary artery disease (CAD) share common risk factors and similar pathological mechanisms. Several studies have shown that patients with a history of ischemic stroke are frequently complicated by asymptomatic CAD1). Furthermore, the previous study about patients with ischemic stroke has shown that the risk of CAD is particularly high in patients with carotid artery diseases2, 3). Carotid endarterectomy (CEA) has been established as a standard treatment, and additionally carotid-artery stenting (CAS) has become another option for carotid artery stenosis4, 5). Careful evaluation and management before carotid artery revascularization are recommended for the prevention of perioperative cardiovascular events1). Moreover, CAD is the major cause of death in patients who survive an ischemic stroke6, 7). Nevertheless, the extent of CAD, especially plaque characteristics, in patients scheduled for carotid revascularization has not been fully clarified.
Coronary computed tomographic angiography (CTA) provides valuable information not only about luminal stenosis but also about plaque characteristics. High-risk plaque (HRP: positive remodeling and/or low attenuation) determined by CTA was associated with a high risk of acute coronary syndrome (ACS)8–10). Modern CT equipment provides excellent images with less radiation exposure and lower contrast medium dose requirement, extending the potential use of CTA in preoperative assessment.
Aim
The purposes of the present study were to evaluate the stenotic severity and plaque characteristics of coronary arteries in patients scheduled for carotid artery revascularization and to determine the factors associated with the presence of significant stenosis (SS) and HRP.
Patients and Methods
Patients
We prospectively performed coronary CTA for 70 consecutive patients who were performed carotid ultrasonography (US) and scheduled for CAS or CEA (CAS/CEA group) from January 2014 to December 2016, after excluding 13 after percutaneous coronary intervention (PCI) and/or coronary artery bypass grafting, 5 patients with advanced chronic kidney disease (CKD; estimated glomerular filtration rate [eGFR] < 30 ml/min/1.73 m2), and 6 who did not provide agreement to participate in our research. Of 24 cases who were excluded, 6 (25.0%) had a history of ACS. Clinical features, coronary CTA and carotid US findings were compared with those of the patients in non-CAS/CEA group (n = 94) who underwent coronary CTA within 3 months after carotid US during the same period. In non-CAS/CEA group, the purposes of coronary CTA were investigation of chest symptoms in 16 patients, electrocardiographic or echocardiographic abnormalities in 5, preoperative screening of surgery in 59, and any complaint other than chest symptoms in 14. Meanwhile, carotid US was for preoperative screening of surgery in 58, carotid artery screening after cerebral infarction in 8, and screening for systemic atherosclerosis in 28.
Both coronary CTA and carotid US were performed at Fujita Health University Hospital. Similarly, both CAS and CEA were all performed at Fujita Health University Hospital, Department of Comprehensive Strokology or Neurosurgery. The study was approved by the Institutional Review Board and the ethics committee at Fujita Health University.
Carotid US
Carotid US was performed for the evaluation of stenotic severity and plaque characteristics of carotid arteries in the examined patients. Two-dimensional B-mode and color Doppler images of carotid artery were obtained using the Vivid E9 system (GE Healthcare, Tokyo, Japan) with 2.4–10.0 MHz broadband linear array transducer or Aplio 500 system (Toshiba Medical Systems, Tokyo, Japan) with 11 MHz broadband linear array transducer. All measurements were performed according to a standard protocol by an experienced vascular ultrasonographer who was unaware of both the coronary CTA findings and clinical data. Data were collected on intima-media thickness (IMT), the presence of plaque, plaque score (PS), locations of plaque, surface characteristics, echogenicity, and degree of stenosis. IMT was calculated as the averaged IMT of both distal common carotid arteries. Plaque was designated as focal intima-media thickening greater than or equal to 1.1 mm. PS were measured by summing all plaque thicknesses11). According to the measurement technique of the North American Symptomatic Carotid Endarterectomy Trial, luminal stenosis were quantified as mild (1% to 49%), moderate (50% to 69%), or severe (70% to 100%)12). Plaques were categorized as vulnerable when they had at least one of the following features: irregular or ulcerated appearance on the surface or intraplaque echolucency according to the Gray–Weale classification13, 14).
Coronary CTA
We used 320-slice CT (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan) in all patients. To obtain the coronary artery calcium score (CACS), a non-enhanced scan was performed, using a prospectively electrocardiography (ECG)-triggered scan protocol: detector configuration 320 × 0.5 mm, rotation time 275 ms. Then we have reconfigured the 0.5–3 mm. The total CACS was computed from all calcified lesions by means of Agatston score. A 320-slice CT was performed with 0.5 mm detector elements, rotation speed of 275 or 350 ms, and scanner settings of 300–580 mA and 100–135 kV. For the contrast-enhanced scan, 20.4 mgI · kg–1· s–1 of contrast medium was injected for 12 s followed by 20 ml saline at 3.0 ml/s. Axial scan was performed with prospective gated scan for one heart beat at a heart rate of < 65 beats per min for half reconstruction and in two or three heart beats for heart rate ≥ 65 beats per min for segment reconstruction. The raw CT data were reconstructed using algorithms optimized for electrocardiogram-gated segment reconstruction. The reconstructed CT image data were transferred to a computer workstation for post-processing (ZIOSTATION System 1000 or Amin/ZIO, Tokyo, Japan).
CTA images were evaluated on axial, coronal, sagittal, cross-sectional, and curved multiplanar reformation images. CTA interpretation was performed by consensus between two cardiologists who were unaware of both the CTA findings and outcome data; M. M. reviewed every study, in addition to a review by one more cardiologist (H. K. or S. M.). Coronary artery segments > 2 mm were evaluated for the presence of plaques and atherosclerotic stenosis. Stenotic lesions were quantified for lumen diameter stenosis by visual estimation and graded as none (no luminal stenosis), mild (1% to 39%), moderate (40% to 69%), severe or occluded (70% to 99%), or occluded, as per the guidelines of the Society of Cardiovascular Computed Tomography15). Luminal stenosis ≥ 70% was defined as SS, like in our previous article9). The patients with SS in only one or two coronary arteries, and those with SS in each of the three epicardial vessels and/or left main trunk (LMT), were named as single-vessel disease/double-vessel disease and triple-vessel disease (TVD)/LMT, respectively. HRP was defined as plaque manifesting positive remodeling and/or low attenuation. Low attenuation was defined as plaque with minimum CT density, namely, < 30 Hounsfield units (HU). Coronary arterial remodeling was defined as a change in the vessel area at the plaque site in comparison with the reference segment set proximal to the legion in a normal-appearing vessel segment (reference area). The remodeling index was reported as positive when the area at the plaque site was at least 20% larger than the reference segment16–18).
Clinical Profiling
Information of medical histories and categorical risk factor status were collected. Medication treated for at least 2 months was considered to be on medication. Laboratory data including lipid profile, HbA1c, high-sensitive C-reactive protein, and Troponin I (TnI) were obtained within 2 weeks before or after coronary CTA. Current or previous daily cigarette use was defined as smoking. Hypertension was defined by blood pressures ≥ 140/90 mmHg or current antihypertensive medication. Dyslipidemia was defined by a low-density lipoprotein cholesterol level ≥ 140 mg/dl or a high-density lipoprotein cholesterol level < 40 mg/dl or a triglyceride level ≥ 150 mg/dl, or treatment with lipid-lowering medication. Diabetes was defined as a fasting blood glucose ≥ 126 mg/dl or HbAlc (NSGP) ≥ 6.5% or treatment with hypoglycemic medication.
Statistical Analysis
Shapiro–Wilk test was used to assess the normality of continuous data. Variables with a normal distribution are expressed as mean values ± standard deviation, and asymmetrically distributed data are given as median and interquartile range. Categorical variables were presented as frequency (percentage). Differences between two groups were evaluated by Mann–Whitney test or Student's t-test for continuous variables and by chi-square test for categorical variables. Odds ratio (OR) and 95% confidence intervals (CIs) were applied by univariate and multivariate logistic regression analyses to determine any factors associated with SS, TVD/LMT, or HRP. Variables with P < 0.05 by univariate analysis were included in the multivariate logistic analysis. In addition, Kappa statistics was used to determine intra-observer and inter-observer reproducibility for measurements of carotid and coronary imaging from 20 randomly selected patients. All statistical analyses were carried out using SPSS 21 software program (SPSS Inc., Chicago, IL, USA).
Results
We examined 164 patients (81.7% male, aged 68.1 ± 12.2 years), including 70 undergoing CAS or CEA (CAS/CEA group). Of 70 patients, 8 (11.4%) had chest symptoms. Significant differences were noted in age, dyslipidemia, diabetes, and a history of ischemic stroke between CAS/CEA and non-CAS/CEA groups. As proportion of drug treatment, there were significant differences in antiplatelet, beta-blocker, statin, and antidiabetic agents between the two groups (Table 1). Carotid US demonstrated that PS was significantly higher in the CAS/CEA group than in the non-CAS/CEA group. Furthermore, severe stenosis unilaterally were in 59 patients, bilaterally in 10, and moderate unilateral stenosis in the remaining one in CAS/CEA group, whereas 4 patients had moderate stenosis and 5 mild stenosis in non-CAS/CEA group. Vulnerable plaque was detected in 55 patients (78.6%) of CAS/CEA group and 2 (2.1%) of non-CAS/CEA group. On the other hand, coronary CTA revealed significant differences in CACS, SS, TVD/LMT, and the presence of HRP between CAS/CEA and non-CAS/CEA group (Table 1, Fig. 1).
Table 1. Baseline clinical characteristics and imaging analysis.
| CAS/CEA | non-CAS/CEA | P | |
|---|---|---|---|
| (n = 70) | (n = 94) | ||
| Clinical Characteristics | |||
| Age, years | 71.9 ± 7.1 | 65.3 ± 14.3 | 0.0044 |
| Male, n (%) | 59 (84.3) | 75 (79.8) | 0.46 |
| BMI, kg/m2 | 22.9 ± 3.3 | 23.1 ± 3.8 | 0.96 |
| Hypertension, n (%) | 48 (68.6) | 64 (68.1) | 0.95 |
| Dyslipidemia, n (%) | 49 (70.0) | 39 (41.5) | 0.0003 |
| Diabetes, n (%) | 20 (28.6) | 12 (12.8) | 0.012 |
| Ischemic Stroke, n (%) | 55 (78.6) | 8 (8.5) | < 0.0001 |
| Smoking, n (%) | 51 (72.9) | 62 (66.0) | 0.34 |
| eGFR, ml/min/1.73 m2 | 65.3 ± 17.8 | 64.8 ± 18.9 | 0.70 |
| Medications | |||
| Antiplatelet, n (%) | 70 (100) | 28 (29.8) | < 0.0001 |
| ACEi or ARB, n (%) | 31 (44.3) | 39 (41.5) | 0.72 |
| Ca blocker, n (%) | 26 (37.1) | 41 (43.6) | 0.40 |
| Beta-blocker, n (%) | 11 (15.7) | 30 (31.9) | 0.016 |
| Statin, n (%) | 45 (66.2) | 37 (39.4) | 0.0007 |
| Fibrate, n (%) | 0 (0.0) | 1 (1.1) | 0.29 |
| DM oral drug or Insulin, n (%) | 17 (24.3) | 10 (10.6) | 0.020 |
| Carotid US | |||
| IMT, mm | 0.93 ± 0.36 | 0.94 ± 0.36 | 0.93 |
| PS | 14.4 | 6.7 | < 0.0001 |
| [11.3–16.8] | [1.7–10.9] | ||
| Mild stenosis, n (%) | 0 (0) | 5 (5.3) | 0.017 |
| Moderate stenosis, n (%) | 1 (1.4) | 4 (4.3) | 0.28 |
| Severe stenosis, n (%) | 69 (98.6) | 0 (0) | < 0.0001 |
| Unilateral, n (%) | 59 (84.3) | 0 (0) | < 0.0001 |
| Bilateral, n (%) | 10 (14.3) | 0 (0) | < 0.0001 |
| Vulnerable, n (%) | 55 (78.6) | 2 (2.1) | < 0.0001 |
| Coronary CTA | |||
| CACS | 292.4 | 118.0 | 0.0096 |
| [56.0–637.0] | [0–512.5] | ||
| SS, n (%) | 39 (55.7) | 37 (39.4) | 0.038 |
| SVD/DVD, n (%) | 22 (31.4) | 30 (31.9) | 0.95 |
| TVD/LMT, n (%) | 17 (24.3) | 7 (7.5) | 0.0025 |
| HRP, n (%) | 39 (55.7) | 23 (24.5) | < 0.0001 |
CAS, carotid-artery stenting; CEA, carotid endarterectomy; BMI, body mass index; eGFR, estimated glomerular filtration rate; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin-receptor blockers; Ca blocker, Calcium channel blockers; DM, diabetes mellitus; IMT, intima-media thickness; PS, plaque score; CACS, coronary artery calcium score; SS, significant stenosis; SVD, single vessel disease; DVD, double vessel disease; TVD, triple vessel disease; LMT, left main trunk; HRP, high risk plaque
Fig. 1.
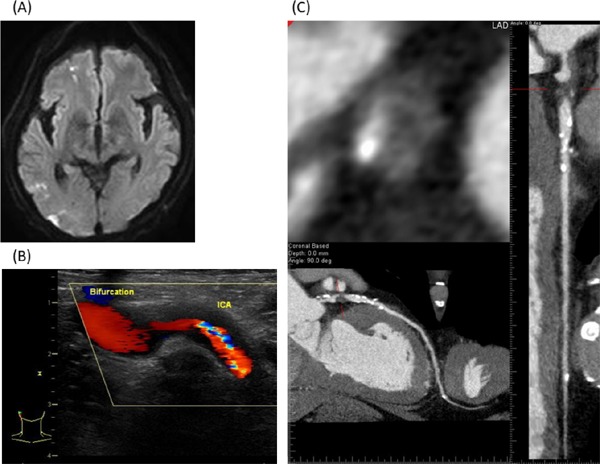
Representative case. A 72-year-old man suffered multiple cerebral infarctions (A). Before CEA for severe stenosis of right internal carotid artery (B), SS with HRP was detected in the proximal LAD (C), though he had never experienced chest symptom.
Blinded assessment for the variability of both stenotic severity and plaque vulnerability on each vessel was performed in a randomly selected, representative subgroup of 20 studies: The kappa statistics for intraobserver and inter-observer variabilities were 0.90 and 0.80 for carotid vulnerable plaque, 0.96 and 0.84 for carotid stenotic severity, 0.69 and 0.69 for coronary HRP, and 0.89 and 0.80 for coronary stenotic severity, respectively.
On the basis of coronary CTA, there were significant differences in age, diabetes, eGFR, and CAS/CEA between patients with and without SS. Age, diabetes, and eGFR were independent factors associated with SS in multivariate analysis (Table 2A). Similarly, there were significant differences in diabetes and CAS/CEA between patients with and without TVD/LMT. Diabetes (OR = 3.98, 95%CI: 1.50–10.55, P = 0.0060) and CAS/CEA (OR = 2.30, 95%CI: 1.14–8.59, P = 0.026) were independent factors associated with TVD/LMT after adjustment for age and sex (Table 2B). Furthermore, there were significant differences in age, sex, diabetes, smoking, and CAS/CEA between patients with and without HRP. After adjustment for sex, diabetes, and smoking, CAS/CEA (OR = 3.17, 95%CI: 1.57–6.54, P = 0.0012) was an independent factor associated with HRP, as well as age (Table 2C).
Table 2. Clinical profiles in patients with and without SS, TVD/LMT, or HRP.
| (A) SS | ||||||
|---|---|---|---|---|---|---|
| SS ( + ) | SS (−) | Univariate |
Multivariate |
|||
| (n = 76) | (n = 88) | OR (95%CI) | P | OR (95%CI) | P | |
| Age, years | 71.4 ± 8.6 | 65.3 ± 14.1 | 1.05 (1.02–1.09) | 0.0009 | 0.96 (0.93–0.99) | 0.019 |
| Male, n (%) | 64 (84.2) | 70 (79.6) | 1.37 (0.61–3.07) | 0.44 | 1.10 (0.46–2.65) | 0.83 |
| BMI, kg/m2 | 23.1 ± 3.6 | 23.0 ± 3.7 | 0.99 (0.91–1.08) | 0.88 | ||
| Hypertension, n (%) | 55 (72.4) | 57 (64.8) | 1.42 (0.73–2.77) | 0.30 | ||
| Dyslipidemia, n (%) | 47 (61.8) | 41 (46.6) | 1.86 (0.99–3.49) | 0.051 | ||
| Diabetes, n (%) | 21 (27.6) | 11 (12.5) | 2.67 (1.19–5.99) | 0.014 | 2.48 (1.07–6.04) | 0.035 |
| Smoking, n (%) | 56 (73.7) | 57 (64.8) | 1.52 (0.78–2.98) | 0.22 | ||
| eGFR, ml/min/1.73 m2 | 60.7 ± 18.2 | 68.7 ± 17.8 | 1.03 (1.01–1.05) | 0.0045 | 1.02 (1.00–1.04) | 0.034 |
| CAS/CEA, n (%) | 39 (51.3) | 31 (35.2) | 1.93 (1.03–3.63) | 0.038 | 1.48 (0.74–2.97) | 0.26 |
| (B) TVD/LMT | ||||||
|---|---|---|---|---|---|---|
| TVD/LMT (+) | TVD/LMT (−) | Univariate |
Multivariate |
|||
| (n = 24) | (n = 140) | OR (95%CI) | P | OR (95%CI) | P | |
| Age, years | 71.0 ± 9.0 | 67.6 ± 12.6 | 1.03 (0.99–1.08) | 0.19 | 0.98 (0.93–1.03) | 0.50 |
| Male, n (%) | 20 (83.3) | 114 (81.4) | 1.14 (0.36–3.61) | 0.82 | 1.08 (0.27–3.54) | 0.90 |
| BMI, kg/m2 | 22.8 ± 2.9 | 23.1 ± 3.7 | 1.02 (0.91–1.17) | 0.69 | ||
| Hypertension, n (%) | 18 (75.0) | 94 (67.1) | 1.46 (0.55–3.95) | 0.44 | ||
| Dyslipidemia, n (%) | 16 (66.7) | 72 (51.4) | 1.89 (0.76–4.70) | 0.16 | ||
| Diabetes, n (%) | 11 (45.8) | 21 (15.0) | 4.79 (1.90–12.12) | 0.0012 | 3.98 (1.50–10.55) | 0.0060 |
| Smoking, n (%) | 16 (66.7) | 97 (69.3) | 0.89 (0.35–2.23) | 0.80 | ||
| eGFR, ml/min/1.73 m2 | 65.6 ± 18.0 | 64.9 ± 18.5 | 1.00 (0.98–1.02) | 0.86 | ||
| CAS/CEA, n (%) | 17 (70.8) | 53 (37.9) | 3.99 (1.55–10.25) | 0.0025 | 2.30 (1.14–8.59) | 0.026 |
| (C) HRP | ||||||
|---|---|---|---|---|---|---|
| HRP (+) | HRP (−) | Univariate |
Multivariate |
|||
| (n = 62) | (n = 102) | OR (95%CI) | P | OR (95%CI) | P | |
| Age, years | 71.7 ± 7.2 | 66.0 ± 14.0 | 1.05 (1.02–1.09) | 0.0020 | 0.96 (0.92–1.00) | 0.029 |
| Male, n (%) | 57 (91.9) | 77 (75.5) | 3.70 (1.34–10.26) | 0.0055 | 3.30 (1.00–12.40) | 0.051 |
| BMI, kg/m2 | 23.4 ± 4.2 | 22.8 ± 3.2 | 0.96 (0.87–1.05) | 0.33 | ||
| Hypertension, n (%) | 45 (72.6) | 67 (65.7) | 1.38 (0.69–2.76) | 0.35 | ||
| Dyslipidemia, n (%) | 39 (62.9) | 49 (48.0) | 1.83 (0.96–3.50) | 0.063 | ||
| Diabetes, n (%) | 16 (25.8) | 16 (15.7) | 1.87 (0.86–4.08) | 0.012 | 1.33 (0.57–3.10) | 0.51 |
| Smoking, n (%) | 50 (80.7) | 63 (61.8) | 2.58 (1.22–5.44) | 0.0097 | 1.41 (0.55–3.69) | 0.47 |
| eGFR, ml/min/1.73 m2 | 62.0 ± 17.0 | 66.9 ± 18.9 | 1.02 (1.00–1.04) | 0.090 | ||
| CAS/CEA, n (%) | 39 (62.9) | 31 (30.4) | 3.88 (2.00–7.56) | < 0.0001 | 3.17 (1.57–6.54) | 0.0012 |
OR, odds ratio; CI, confidence interval; other abbreviations like in Table 1
Moreover, we examined the factors associated with TVD/LMT and HRP in patients scheduled for CAS/CEA. There were significant differences in chest symptom, carotid vulnerable plaque, CACS, and TnI elevation between patients with and without TVD/LMT. After adjustment for age, sex, carotid vulnerable plaque, and TnI elevation, the patients with chest symptom and high CACS score had higher prevalence of TVD/LMT in CAS/CEA group. In contrast, there were no factors associated with HRP (Table 3).
Table 3. Factors associated with the presence of TVD/LMT and HRP in patients scheduled for CEA/CAS.
| TVD/LMT |
HRP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | ||||||||
| TVD/LMT | TVD/LMT | OR | p | OR | p | HRP | HRP | OR | p | |
| (+) (n = 17) | (−) (n = 53) | (95%CI) | (95%CI) | (+) (n = 39) | (−) (n = 31) | (95%CI) | ||||
| Age, years | 71.4 ± 9.8 | 72.0 ± 6.0 | 1.00 | 0.90 | 1.06 | 0.32 | 71.4 ± 7.1 | 72.5 ± 7.1 | 1.02 | 0.57 |
| (0.94–1.09) | (0.94–1.19) | (0.96–1.10) | ||||||||
| Male, n (%) | 13 (76.5) | 46 (86.8) | 2.02 | 0.33 | 2.65 | 0.30 | 34 (87.2) | 25 (80.7) | 1.63 | 0.46 |
| (0.47–7.84) | (0.41–17.07) | (0.44–6.24) | ||||||||
| BMI, kg/m2 | 22.8 ± 2.7 | 23.0 ± 3.5 | 1.01 | 0.93 | 23.5 ± 3.4 | 22.2 ± 3.2 | 0.88 | 0.073 | ||
| (0.86–1.20) | (0.75–1.02) | |||||||||
| Hypertension, n (%) | 12 (70.6) | 36 (67.9) | 1.13 | 0.84 | 28 (71.8) | 20 (64.5) | 1.40 | 0.52 | ||
| (0.36–4.03) | (0.51–3.90) | |||||||||
| Dyslipidemia, n (%) | 12 (70.6) | 37 (69.8) | 1.04 | 0.95 | 28 (71.8) | 21 (67.7) | 1.21 | 0.71 | ||
| (0.32–3.70) | (0.43–3.41) | |||||||||
| Diabetes, n (%) | 8 (47.1) | 12 (22.6) | 3.04 | 0.060 | 13 (33.3) | 7 (22.6) | 1.71 | 0.32 | ||
| (0.90–9.75) | (0.60–5.24) | |||||||||
| Ischemic Stroke, n (%) | 12 (70.6) | 43 (81.1) | 1.79 | 0.37 | 31 (79.5) | 24 (77.4) | 1.13 | 0.83 | ||
| (0.48–6.14) | (0.35–3.58) | |||||||||
| Smoking, n (%) | 12 (70.6) | 40 (75.5) | 1.28 | 0.69 | 29 (74.4) | 23 (74.2) | 1.01 | 0.99 | ||
| (0.35–4.21) | (0.34–2.97) | |||||||||
| eGFR, ml/min/1.73 m2 | 66.8 ± 19.1 | 64.8 ± 17.5 | 0.99 | 0.81 | 63.1 ± 16.9 | 68.0 ± 18.8 | 1.02 | 0.15 | ||
| (0.03–13.00) | (0.99–1.05) | |||||||||
| Chest symptom, n (%) | 5 (29.4) | 3 (5.7) | 6.94 | 0.014 | 15.25 | 0.018 | 4 (10.3) | 4 (12.9) | 1.30 | 0.73 |
| (1.50–37.88) | (1.61–183.47) | (0.28–5.94) | ||||||||
| Bilateral Carotid Stenosis, n (%) | 2 (11.8) | 8 (15.1) | 1.33 | 0.73 | 7 (18.0) | 3 (9.7) | 2.04 | 0.32 | ||
| (0.29–9.48) | (0.51–10.17) | |||||||||
| Carotid Vulnerable plaque, n (%) | 16 (94.1) | 39 (73.6) | 5.74 | 0.047 | 1.72 | 0.64 | 32 (82.1) | 23 (74.2) | 1.59 | 0.43 |
| (1.02–108.38) | (0.20–37.40) | (0.50–5.14) | ||||||||
| CACS | 860.0 | 149.1 | 1.00 | < 0.0001 | 1.00 | < 0.0001 | 421.5 | 98.8 | 1.00 | 0.063 |
| [412.4–1931.2] | [47.0–444.6] | (1.00–1.00) | (1.00–1.00) | [149.1–637.0] | [28.0–596.9] | (1.00–1.00) | ||||
| TnI > 0.04 ng/mL, n (%) | 4 (23.5) | 1 (1.9) | 16.00 | 0.0060 | 21.46 | 0.051 | 3 (7.7) | 2 (6.5) | 1.21 | 0.84 |
| (2.15–327.44) | (0.98–786.50) | (0.19–9.64) | ||||||||
Discussion
In the current study, more than 50% of the patients scheduled for carotid revascularization without a history of CAD had significant stenoses on coronary CTA, though only 11% of them had chest symptoms. Furthermore, the prevalence of TVD/LMT in CAS/CEA group was 24%, and the independent predictors of TVD/LMT were chest symptom and CACS. On the other hand, more than 50% of the patients in CAS/CEA group had HRPs in the coronary tree. CAS/CEA was independently associated with TVD/LMT and the presence of HRP in multivariable logistic regression analysis. Although several studies have documented the prevalence of CAD in patients scheduled for carotid revascularization19, 20), few studies have assessed it by coronary CTA21). Moreover, to our knowledge, this is the first study to evaluate not only stenotic severity but also plaque characteristics of the patients scheduled for carotid revascularization using coronary CTA.
Presence of Coronary Artery Disease
In the CREST trial, a mega-trial comparing CAS and CEA, the prevalence of previous cardiovascular disease was 44% and that of previous coronary-artery bypass surgery was 20%4). In the EVA-3S trial, a comparison between CAS and CEA in symptomatic patients, 12% of the enrolled patients had a history of prior myocardial infarction and 13% had that of prior surgery or angioplasty for coronary artery22). In several studies, preoperative coronary angiography (CAG) was performed at a single session after cerebral angiography in patients with severe carotid artery stenoses. Significant coronary artery stenoses were present in 60–77% of all examined patients including a history of CAD19, 23). On the other hand, stress myocardial scintigraphy was performed in the other studies. Myocardial ischemia was detected in approximately 25% to 60% of the patients before carotid artery revascularization24–26). In the only study assessing coronary artery atherosclerosis by CTA in patients scheduled for CEA, severe stenosis (≥ 70%) of coronary arteries was identified in approximately 50% of the patients undergoing CEA21).
Coronary CTA, a less invasive modality than conventional CAG, provides valuable information in advance regarding atherosclerosis in the whole coronary tree, whereas invasive CAG has been performed in a single session just before or after carotid stenting in several studies19). It is very helpful in preventing cardiac events after CAS/CEA to know the state of coronary atherosclerosis in advance, because patients at risk can be stratified and the treatment of complicating CAD in them decided. In our study, diabetes mellitus (DM) as well as CAS/CEA was also an independent predictor for both the presence of SS and TVD/LMT. DM has been reported as an important predictor for TVD/LMT, and recently, coronary CTA may be considered as a screening for even asymptomatic patients in a specific condition27).
MPI with exercise or adenosine stress has also been well used as a low invasive examination for assessing CAD24–26). For patients with severe carotid stenosis, we should be cautious about using adenosine, because systemic vasodilation from adenosine may worsen cerebral ischemia. On the other hand, coronary CTA, revealing the severity of stenotic atherosclerotic lesions, has been used effectively as a gatekeeper to CAG28). The introduction of modern scanners including 320-slice CT has enabled imaging with less motion artifact and a lower dose of contrast medium.
Interestingly, in non-CAS/CEA group, mean IMT was less than 0.8 mm in about 20% of the patients (n = 7) with SS on coronary CTA. This result was consistent with current consensus by ACC/AHA guidelines: routine measurement of IMT is not recommended in clinical practice for risk assessment29).
Characteristics of Coronary Plaques
Previously, we revealed that the CT characteristics of culprit lesions in ACS include positive remodeling and low-attenuation plaque16). On the basis of this result, we reported that HRP showing positive remodeling and/or low attenuation was associated with the development of ACS in the future8, 9). Furthermore, the CTA detection of HRP enhanced the predictive accuracy of the clinical and angiographic characteristics for future coronary events18). Finally, statin treatment for HRP results in decreases in the plaque and necrotic core volume30). In this way, non-invasive assessment of plaque characteristics using coronary CTA may be useful for both prognostic stratification and primary prevention of coronary events.
In our previous study about patients undergoing CTA for suspected CAD, the prevalence of HRP was 12% in all segments and 7% in segments after excluding target lesions for scheduled PCI8, 9, 31). On the other hand, in the present study, it reached 56% in the patients scheduled for carotid revascularization, and CAS/CEA indication was an independent factor associated with the presence of HRP. Interestingly, the prevalence of carotid vulnerable plaque was also high in CAS/CEA group (79%). Comparisons between carotid and coronary artery indicated that stenotic severity of carotid arteries was directly related to the stroke risk in patients with symptomatic carotid artery disease, whereas the extent of luminal stenosis in coronary arteries was not always associated with ACS occurrence32, 33). This means that carotid lesions for the indication of revascularization consist of not only severe stenosis but also vulnerable plaque simultaneously. Consequently, many of the CAS/CEA candidates with carotid vulnerable plaques have vulnerable plaques also in coronary arteries34).
Finally, we made an attempt to identify the patients with TVD/LMT and HRP in the coronary tree using conventional risk factors and laboratory findings in CAS/CEA candidates. The presence of chest symptom and CACS were independent predictors for TVD/LMT. CACS is a surrogate marker of the total burden of coronary atherosclerosis, and is useful for predicting the presence of CAD and cardiac events in the future35). On the other hand, no factors were associated with the presence of HRP. The only strategy to identify the patients at high risk for developing ACS may be to perform coronary CTA.
Study Limitation
The present study has several limitations. First, this was a single-center study consisting of a limited number of patients scheduled for CAS/CEA. CAS/CEA candidates were prospectively enrolled, but as a control group, non-CAS/CEA group consisted of all patients undergoing coronary CTA after carotid US. That may have introduced selection bias. Second, advanced CKD patients were excluded though consecutive patients undergoing CAS/CEA were studied. Finally, stenotic severity on CTA can be overestimated because of coronary calcification and motion artifact.
Conclusions
The prevalence of significant stenoses in TVD/LMT and HRP determined by coronary CTA is higher in patients scheduled for carotid revascularization than in those without. Cautious management of systemic atherosclerosis is required in the perioperative period of CAS/CEA.
Acknowledgments
None.
Author Contributions
M. Hoshino reviewed CTA images and wrote the initial draft of the manuscript.
H. Kawai designed the study, reviewed CTA images, and assisted M. Hoshino in writing the manuscript.
M. Sarai and S. Motoyama advised on this project, reviewed CTA images, and assisted in the preparation of the manuscript.
A. Sadato, M. Hayakawa, and I. Nakahara contributed to patient recruitment.
Y. Nagahara and K. Miyajima contributed to data collection.
H. Takahashi contributed to analysis and interpretation of data.
Y. Hirose and Y. Ozaki supervised this project.
Financial Support
None.
Conflict of Interest
None.
References
- 1). Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, Taubert KA. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation. 2003; 108: 1278-1290 [DOI] [PubMed] [Google Scholar]
- 2). Yoo J, Yang JH, Choi BW, Kim YD, Nam HS, Choi HY, Lee HS, Cha MJ, Choi D, Nam CM, Jang Y, Lee DH, Kim J, Heo JH. The frequency and risk of preclinical coronary artery disease detected using multichannel cardiac computed tomography in patients with ischemic stroke. CerebroVasc Dis. 2012; 33: 286-294 [DOI] [PubMed] [Google Scholar]
- 3). Morris DR, Ayabe K, Inoue T, Sakai N, Bulbulia R, Halliday A, Goto S. Evidence-Based Carotid Interventions for Stroke Prevention: State-of-the-art Review. J Atheroscler Thromb. 2017; 24: 373-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, CREST Investigators Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010; 363: 11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004; 351: 1493-1501 [DOI] [PubMed] [Google Scholar]
- 6). Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z, Oxford Vascular Study Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005; 366: 1773-1783 [DOI] [PubMed] [Google Scholar]
- 7). Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005; 36: 2748-2755 [DOI] [PubMed] [Google Scholar]
- 8). Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009; 54: 49-57 [DOI] [PubMed] [Google Scholar]
- 9). Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015; 66: 337-346 [DOI] [PubMed] [Google Scholar]
- 10). Takamura K, Fujimoto S, Kondo T, Hiki M, Kawaguchi Y, Kato E, Daida H. Incremental Prognostic Value of Coronary Computed Tomography Angiography: High-Risk Plaque Characteristics in Asymptomatic Patients. J Atheroscler Thromb. 2017; 24:1174-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, Yoneda S, Kimura K, Kamada T. Ultrasonic evaluation of early carotid atherosclerosis. Stroke. 1990; 21: 1567-1572 [DOI] [PubMed] [Google Scholar]
- 12). Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999; 30: 1751-1758 [DOI] [PubMed] [Google Scholar]
- 13). Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino). 1988; 29: 676-681 [PubMed] [Google Scholar]
- 14). Picano E, Paterni M. Ultrasound tissue characterization of vulnerable atherosclerotic plaque. Int J Mol Sci. 2015; 16: 10121-10133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP, Society of Cardiovascular Computed Tomography SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009; 3: 122-136 [DOI] [PubMed] [Google Scholar]
- 16). Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007; 50: 319-326 [DOI] [PubMed] [Google Scholar]
- 17). Motoyama S, Kondo T, Anno H, Sugiura A, Ito Y, Mori K, Ishii J, Sato T, Inoue K, Sarai M, Hishida H, Narula J. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J. 2007; 71: 363-366 [DOI] [PubMed] [Google Scholar]
- 18). Kawai H, Motoyama S, Sarai M, Ito H, Takahashi H, Harigaya H, Kan S, Ishii J, Anno H, Murohara T, Ozaki Y. Adding coronary computed tomography angiography to invasive coronary angiography improves prediction of cardiac events. Circ J. 2014; 78: 2735-2740 [DOI] [PubMed] [Google Scholar]
- 19). Hofmann R, Kypta A, Steinwender C, Kerschner K, Grund M, Leisch F. Coronary angiography in patients undergoing carotid artery stenting shows a high incidence of significant coronary artery disease. Heart. 2005; 91: 1438-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Huang KL, Chang YJ, Chang CH, Chang TY, Liu CH, Hsieh IC, Wong HF, Wai YY, Chen YW, Yip BS, Lee TH. Impact of coexisting coronary artery disease on the occurrence of cerebral ischemic lesions after carotid stenting. PLoS One. 2014; 9: e94280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Kim JH, Heo SH, Nam HJ, Youn HC, Kim EJ, Lee JS, Kim YS, Kim HY, Koh SH, Chang DI. Preoperative Coronary Stenosis Is a Determinant of Early Vascular Outcome after Carotid Endarterectomy. J Clin Neurol. 2015; 11: 364-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lièvre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touzé E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X, EVA-3S Investigators Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis. N Engl J Med. 2006; 355: 1660-1671 [DOI] [PubMed] [Google Scholar]
- 23). Wu YW, Lin MS, Lin YH, Chao CL, Kao HL. Prevalence of concomitant atherosclerotic arterial diseases in patients with significant cervical carotid artery stenosis in Taiwan. Int J Cardiovasc Imaging. 2007; 23: 433-439 [DOI] [PubMed] [Google Scholar]
- 24). Chimowitz MI, Poole RM, Starling MR, Schwaiger M, Gross MD. Frequency and severity of asymptomatic coronary disease in patients with different causes of stroke. Stroke. 1997; 28: 941-945 [DOI] [PubMed] [Google Scholar]
- 25). Urbinati S, Di Pasquale G, Andreoli A, Lusa AM, Ruffini M, Lanzino G, Pinelli G. Frequency and prognostic significance of silent coronary artery disease in patients with cerebral ischemia undergoing carotid endarterectomy. Am J Cardiol. 1992; 69: 1166-1170 [DOI] [PubMed] [Google Scholar]
- 26). Sconocchini C, Racco F, Pratillo G, Alesi C, Zappelli L. Patients with carotid stenosis and clinical history negative for coronary disease: usefulness of the ergometric test for the identification of ischemic myocardial disease. MinerVa Med. 1997; 88: 173-181 [PubMed] [Google Scholar]
- 27). Kang SH, Park GM, Lee SW, Yun SC, Kim YH, Cho YR, Park HW, Suh J, Yang DH, Kang JW, Lim TH, Jung CH, Koh EH, Lee WJ, Kim MS, Lee KU, Park JY. Long-Term Prognostic Value of Coronary CT Angiography in Asymptomatic Type 2 Diabetes Mellitus. JACC Cardiovasc Imaging. 2016; 9: 1292-1300 [DOI] [PubMed] [Google Scholar]
- 28). Shaw LJ, Hausleiter J, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Kim YJ, Cheng VY, Chow BJ, Cury RC, Delago AJ, Dunning AL, Feuchtner GM, Hadamitzky M, Karlsberg RP, Kaufmann PA, Leipsic J, Lin FY, Chinnaiyan KM, Maffei E, Raff GL, Villines TC, Labounty T, Gomez MJ, Min JK, CONFIRM Registry Investigators Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol. 2012; 60: 2103-2114 [DOI] [PubMed] [Google Scholar]
- 29). Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63: 2935-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, Sanda Y, Anno H, Kondo T, Wong ND, Narula J, Ozaki Y. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010; 7: 691-698 [DOI] [PubMed] [Google Scholar]
- 31). Kawai H, Sarai M, Motoyama S, Harigaya H, Ito H, Sanda Y, Biswas S, Anno H, Ishii J, Murohara T, Ozaki Y. Coronary plaque characteristics in patients with mild chronic kidney disease. Analysis by 320-row area detector computed tomography. Circ J. 2012; 76: 1436-1441 [DOI] [PubMed] [Google Scholar]
- 32). Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis. 2013; 227: 193-200 [DOI] [PubMed] [Google Scholar]
- 33). The European Carotid Surgery Trialists Collaborative Group Risk of stroke in the distribution of an asymptomatic carotid artery. Lancet. 1995; 345: 209-212 [PubMed] [Google Scholar]
- 34). Zhao Q, Zhao X, Cai Z, Li F, Yuan C, Cai J. Correlation of coronary plaque phenotype and carotid atherosclerotic plaque composition. Am J Med Sci. 2011; 342: 480-485 [DOI] [PubMed] [Google Scholar]
- 35). Yamamoto H, Kitagawa T, Kihara Y. Clinical implications of the coronary artery calcium score in Japanese patients. J Atheroscler Thromb. 2014; 21: 1101-1108 [DOI] [PubMed] [Google Scholar]


