Abstract
Introduction:
Hyperkalemia (potassium >5.0 mEq/L) affects heart failure patients with renal disease regardless of the use of renin–angiotensin–aldosterone system inhibitors (RAASi). The open-label TOURMALINE study showed that patiromer, a sodium-free, nonabsorbed potassium binder, lowers serum potassium of hyperkalemic patients similarly when given with or without food; unlike prior studies, patients were not required to be taking RAASi. We conducted post hoc analyses to provide the first report of patiromer in patients not taking RAASi.
Methods:
Hyperkalemic patients received patiromer, 8.4 g/d to start, adjusted to achieve and maintain serum potassium of 3.8 to 5.0 mEq/L. If taking RAASi, stable doses were required. The primary end point was the proportion of patients with serum potassium 3.8 to 5.0 mEq/L at week 3 or 4. This analysis presents data by patients taking or not taking RAASi.
Results:
Demographics and baseline characteristics were similar in patients taking (n = 67) and not taking RAASi (n = 45). Baseline mean (SD) serum potassium was 5.37 (0.37) mEq/L and 5.42 (0.43) mEq/L in patients taking and not taking RAASi, respectively. Mean (SD) daily patiromer doses were similar (10.7 [3.2] and 11.5 [4.0] g, respectively). The primary end point was achieved in 85% (95% confidence interval [CI]: 74-93) of patients taking RAASi and in 84% (95% CI: 71-94) of patients not taking RAASi. From baseline to week 4, the mean (SE) change in serum potassium was −0.67 (0.08) mEq/L in patients taking RAASi and −0.56 (0.10) mEq/L in patients not taking RAASi (both P < .0001 vs baseline, P = nonsignificant between groups). Adverse events were reported in 26 (39%) patients taking RAASi and 25 (54%) not taking RAASi; the most common adverse event was diarrhea (2% and 11%, respectively; no cases were severe). Five patients (2 taking RAASi) reported 6 serious adverse events; none considered related to patiromer.
Conclusions:
Patiromer was effective and generally well-tolerated for hyperkalemia treatment, whether or not patients were taking RAAS inhibitors.
Keywords: heart failure, hyperkalemia, chronic kidney disease, patiromer, RAAS inhibitor
Introduction
Management of heart failure in patients with chronic kidney disease (CKD) is often complicated by hyperkalemia (serum potassium >5.0 mEq/L), even for patients not taking renin–angiotensin–aldosterone system inhibitors (RAASi).1–3 RAASi are used to manage these conditions, decrease proteinuria, slow CKD progression, and improve survival in patients with heart failure. RAASi are also associated with a higher incidence of hyperkalemia that may result in their discontinuation or administration at suboptimal doses.4–11 Indeed, in an analysis of records of 205,108 patients with hyperkalemia, Epstein et al found that relatively few patients were prescribed maximum doses of RAASi.12 Furthermore, ∼20% of hyperkalemia events led to RAASi dose downtitration and ∼25% of hyperkalemia events led to RAASi discontinuation.
Patiromer is a nonabsorbed, potassium-binding polymer that uses calcium as a counterexchange ion.13 It is approved for the treatment of hyperkalemia in the United States and European Union.14,15 TOURMALINE was a phase 4, prospective, open-label, 4-week randomized trial that compared the efficacy and safety of patiromer administered without food versus with food. Unlike prior studies of patiromer,16,17 the TOURMALINE trial did not require patients to be taking RAASi.18 The study showed that patiromer lowers serum potassium similarly when given with or without food.18 The prescribing information now recommends that patiromer can be given with or without food. Patiromer binds potassium in the gastrointestinal tract, and based on this mechanism of action, we hypothesized that the effect of patiromer on serum potassium in hyperkalemic patients would be similar regardless of RAASi use. Therefore, we conducted a post hoc analysis using data from TOURMALINE to provide the first report of patiromer efficacy in patients not taking RAASi.
Methods and Materials
Study Design and Oversight
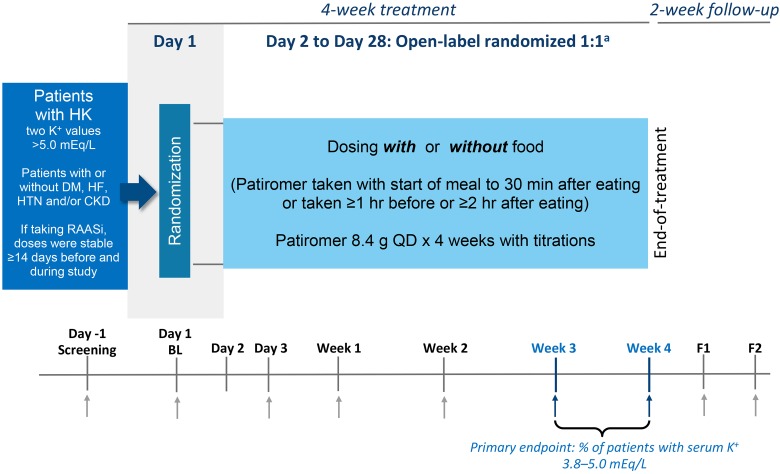
TOURMALINE was an open-label study of patients with hyperkalemia (Figure 1; clinicaltrials.gov identifier, NCT02694744). Patients and site study staff were not blinded to treatment assignment (ie, with or without food groups); sponsor, contractors, and personnel supporting the study who did not require access to patient source documents or to the electronic data capture system were blinded to treatment assignment. This post hoc analysis is exploratory in nature and should be interpreted appropriately. Detailed methodology of the study has been previously reported18 and is described briefly here. The protocol was approved by a central institutional review board, all patients provided written informed consent, and the study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines.
Figure 1.
TOURMALINE study design. aRandomization was stratified by screening K+, race, and history of diabetes mellitus; although patients were randomized on the morning of the baseline visit (day 1), patients did not initiate with or without food dosing until day 2. Abbreviations and symbols: ↑, scheduled blood draw; BL, baseline; F1, follow-up visit 1; F2, follow-up visit 2; HK, hyperkalemia; K+, potassium; QD, once daily.
Study Population (Key Criteria)
Adult patients (≥18 years old) with 2 potassium values >5.0 mEq/L at the screening visit, each obtained from separate venipunctures, in different arms when possible, were enrolled. If taking RAASi, β-blockers, or diuretics at baseline, patients were required to have been on a stable dose for ≥14 days prior to screening. While every effort was made to keep these medications stable during the study, doses could be adjusted at the discretion of the investigator. Patients could have clinically stable diabetes mellitus (type 1 or type 2), heart failure, hypertension, and/or CKD, but none of these disorders was a requirement for study entry. Patients with heart, liver, or kidney transplant; dialysis or probable need for dialysis during study period; or unstable medical conditions were excluded.
Procedure
Patients were randomized 1:1 to a group that received patiromer with food or a group that received patiromer without food, both at a starting dose of 8.4 g once daily. Patients were instructed to take their patiromer dose around a specific meal, which was chosen for each patient so that the patiromer dose was maximally separated from their other concomitant oral medications. Patiromer daily doses could be increased or decreased during the study by 8.4 g/d to a maximum of 25.2 g/d to achieve and maintain potassium levels within the target range of 3.8 to 5.0 mEq/L, as described by Pergola et al.18
Following day 1, randomized patients attended scheduled study visits on day 3 and then on week 1, 2, 3, and 4. Potassium levels were measured at each scheduled visit. For safety, patients were followed weekly for 2 weeks after the last dose of patiromer. Potassium was measured in whole blood locally by point-of-care device (iSTAT; Abbott Point-of-Care, Princeton, New Jersey) for eligibility and dose titration decisions by the investigator and in serum at a central clinical laboratory for efficacy and safety evaluations.
End Points
The prespecified primary and secondary efficacy end points in TOURMALINE were the proportion (95% confidence interval [CI]) of patients with serum potassium in the target range of 3.8 to 5.0 mEq/L at week 3 or 4 and the least squares (LS) mean (standard error [SE]) change in serum potassium from baseline to week 4, respectively. Safety variables included adverse events (AEs), clinical laboratory test results, vital signs, and early withdrawal data. For this post hoc analysis, results were analyzed for patients taking versus not taking RAASi.
Statistical Analysis
The efficacy and safety populations included all patients who were randomized and had taken at least one dose of patiromer. Because taking patiromer with and without food did not impact serum potassium reduction in the main analysis, the with and without food treatment groups were merged to increase the sample size in each of the analysis groups here which are presented by RAASi use at baseline.
The exact (Clopper-Pearson) method was used to obtain proportion of responders (patients with serum potassium in the target range [3.8-5.0 mEq/L] at either week 3 or week 4) and its 95% CI. The proportion of responders in prespecified subgroups by baseline serum potassium (<5.5 or ≥5.5 mEq/L), race (white vs all other), estimated glomerular filtration rate (eGFR; ≥30 or <30 mL/min/1.73 m2), and diabetes mellitus (yes vs no) was analyzed similarly.
Mean change in serum potassium from baseline to week 4 was analyzed by analysis of covariance (ANCOVA) model to estimate the difference between groups taking and not taking RAASi. This model included baseline serum potassium as a continuous covariate and baseline RAASi use, race (white vs all others), and history of diabetes mellitus as categorical covariates. This ANCOVA model was also used to evaluate mean change in serum potassium from baseline to each visit during treatment and from end of treatment to last available follow-up visit. The Kaplan-Meier survival function was used to estimate the median time and 95% CI in days to achieve serum potassium levels within the target range. Log-rank testing was used to compare groups.
For all analyses, descriptive statistics were summarized as mean and SE for continuous variables or proportions for categorical variables. Continuous variables for baseline demographic and clinical characteristics were summarized as mean and standard deviation (SD). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina), with statistical significance set at P < .05.
Results
Disposition and Baseline Characteristics
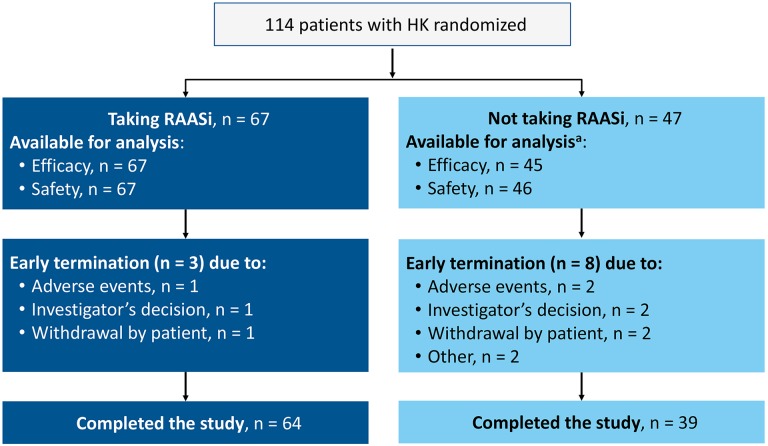
Of 114 patients randomized, 67 (59%) were taking RAASi at baseline. Figure 2 shows disposition for patients taking and not taking RAASi. Specific RAASi taken by >1 patient included the angiotensin-converting enzyme inhibitors lisinopril (n = 30, including 1 who received lisinopril in combination with hydrochlorothiazide), enalapril (n = 10), ramipril (n = 2), and benazepril (n = 2, including 1 who received benazepril with amlodipine) and the angiotensin II receptor blockers losartan (n = 13) and valsartan (n = 5, including 1 who received valsartan with hydrochlorothiazide). One patient each received candesartan, fosinopril, irbesartan, and olmesartan (olmesartan in combination with amlodipine and hydrochlorothiazide). Four patients were receiving spironolactone (3 in combination with one of the above RAASi).
Figure 2.
Disposition of patients taking and not taking RAAS inhibitors. aExcluded from the efficacy analysis: 1 patient who did not receive patiromer and 1 patient with a protocol violation and no postbaseline serum potassium observations. Excluded from the safety analysis: 1 patient who did not receive patiromer. HK indicates hyperkalemia; RAASi, renin–angiotensin–aldosterone system inhibitor.
Overall, baseline characteristics were similar between groups (Table 1), except for an imbalance in mean (SD) eGFR: 45.8 (26.4) mL/min/1.73 m2 in those taking RAASi versus 34.7 (23.1) in those not taking RAASi (P = .0238). Patients’ prior medications were generally similar between groups (Table 2), except there were numerically fewer patients taking β-blockers (P = .0842) among patients taking RAASi. There was no difference in the proportion of patients taking non-RAASi diuretics.
Table 1.
Baseline Demographic and Clinical Characteristics.
| Taking RAAS Inhibitors (n = 67) | Not Taking RAAS Inhibitors (n = 45) | Total (N = 112) | |
|---|---|---|---|
| Men, n (%) | 42 (63) | 31 (69) | 73 (65) |
| Age, years, mean (SD) | 66 (13) | 67 (10) | 67 (12) |
| Race, n (%) | |||
| White | 51 (76) | 41 (91) | 92 (82) |
| Black | 13 (19) | 1 (2) | 14 (13) |
| Hispanic Latino ethnicity | 38 (57) | 25 (56) | 63 (56) |
| Serum K+,a mEq/L, mean (SD) levels | 5.37 (0.37) | 5.42 (0.43) | 5.39 (0.40) |
| <5.5 mEq/L, n (%) | 40 (60) | 26 (58) | 66 (59) |
| ≥5.5 mEq/L, n (%) | 27 (40) | 19 (42) | 46 (41) |
| Diabetes mellitus, n (%) | 55 (82) | 37 (82) | 92 (82) |
| eGFR, mL/min/1.73 m2 | |||
| Mean (SD) | 45.8 (26.4) | 34.7 (23.1) | 41.3 (25.6) |
| Median (IQR) | 35.0 (24.0, 70.0) | 25.0 (20.0, 41.0) | 31.5 (22.0, 59.0) |
| CKD, n (%) | 48 (72) | 37 (82) | 85 (76) |
| CKD stage 3a | 5 (8) | 4 (9) | 9 (8) |
| CKD stage 3b | 12 (18) | 8 (18) | 20 (18) |
| CKD stage 4 | 24 (36) | 19 (42) | 43 (38) |
| CKD stage 5 | 1 (2) | 5 (11) | 6 (5) |
| Heart failure, n (%) | 6 (9) | 4 (9) | 10 (9) |
| NYHA class I/II | 4 (6) | 4 (9) | 8 (7) |
| NYHA class III | 2 (3) | 0 (0) | 2 (2) |
| Myocardial infarction, n (%) | 3 (5) | 4 (9) | 7 (6) |
| Hypertension, n (%) | 67 (100) | 38 (84) | 105 (94) |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; K+, potassium; NYHA, New York Heart Association; RAAS, renin–angiotensin–aldosterone system; SD, standard deviation.
a Baseline serum K+ is defined as the mean of serum K+ from the central laboratory on 2 consecutive days (day −1 and day 1) immediately prior to the first dose of patiromer.
Table 2.
Medications of Interest Used Within 30 Days Prior to Screening Visit.
| n (%) | Taking RAAS Inhibitors (n = 67) | Not Taking RAAS Inhibitors (n = 45) | Total (N = 112) |
|---|---|---|---|
| Non-RAAS inhibitor diuretica | 24 (36) | 16 (36) | 40 (36) |
| Insulin | 26 (39) | 15 (33) | 41 (37) |
| β-blocker | 30 (45) | 28 (62) | 58 (52) |
| β-agonistb | 4 (6) | 4 (9) | 8 (7) |
| NSAID (systemic) | |||
| Ibuprofen | 2 (3) | 1 (2) | 3 (3) |
| Aspirin | 23 (34) | 21 (47) | 44 (39) |
| Coxib | 1 (2) | 0 | 1 (<1) |
| Lactulose | 0 | 1 (2) | 1 (<1) |
| Sodium polystyrene sulfonatec | 1 (2) | 2 (4) | 3 (3) |
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; RAAS, renin–angiotensin–aldosterone system.
a All non-RAAS inhibitor diuretics were potassium-wasting.
b β-agonists given for obstructive airway.
c Sodium polystyrene sulfonate not allowed within 7 days prior to screening.
Efficacy
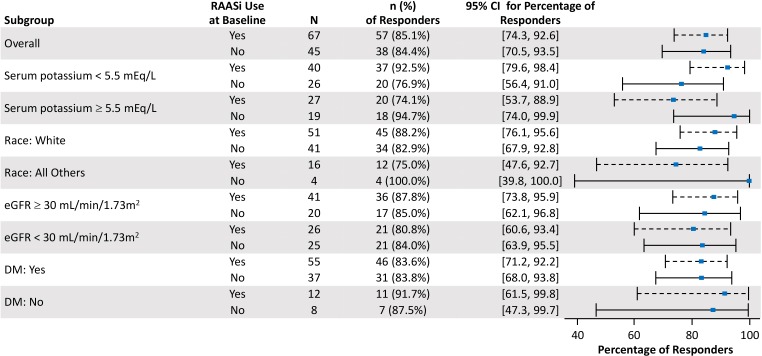
Similar proportions of patients taking and not taking RAASi achieved target levels of serum potassium (3.8-5.0 mEq/L) at week 3 or 4 (85%; 95% CI: 74-93 and 84%; 95% CI: 71-94, respectively; Figure 3) and at any study visit (89% and 96%, respectively). An analysis of prespecified subpopulations showed no apparent differences in the proportion of responders among those taking and not taking RAASi (Figure 3).
Figure 3.
Forest plot of responders at either week 3 or 4 by subgroup. Responders were defined as patients achieving target serum potassium of 3.8 to 5.0 mEq/L. DM indicates diabetes mellitus; eGFR, estimated glomerular filtration rate; RAASi, renin–angiotensin–aldosterone system inhibitor.
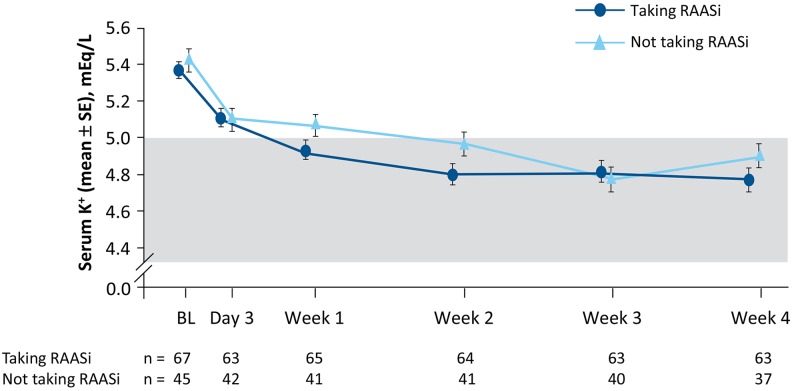
Mean serum potassium decreased from baseline similarly in both groups (Figure 4). The LS mean (SE) change in serum potassium from baseline to week 4 was statistically significant (P < .0001) for patients taking (−0.67 [0.08]) or not taking RAASi (−0.56 [0.10]) and was not different between groups (P = .27). Using unadjusted means and a paired t test, the mean change from baseline (SE) was also statistically significant (P < .0001) for patients taking (−0.60 [0.06]) or not taking RAASi (−0.52 [0.09]) and was not different between groups (P = .52). The median time to achieving serum potassium in the target range was 8 days in both groups (P = .0832 for taking RAASi vs not).
Figure 4.
Mean (SE) serum potassium over time by baseline RAAS inhibitor use. The shaded area represents the target range for serum potassium (3.8-5.0 mEq/L). BL indicates baseline; K+, potassium; RAAS, renin–angiotensin–aldosterone system; SE, standard error.
In the follow-up period (after stopping patiromer treatment), LS mean (SE) serum potassium levels increased from end of treatment by 0.32 (0.09) mEq/L and 0.33 (0.11) mEq/L in the RAASi and not taking RAASi groups, respectively (P < .005 for each vs end of treatment; P = .924 for taking RAASi vs not). The mean (SE) follow-up times were 14 (0.2) and 15 (0.7) days, for patients taking or not taking RAASi, respectively. In the RAASi group, the proportion of patients with potassium ≥5.5 mEq/L at the end of treatment was 13.4%, and after patiromer discontinuation increased to 17.5% at the first week and to 20.6% at the second week of follow-up; the proportions for those not taking RAASi were 8.9%, 35% and 34.1%, respectively. These differences were not statistically significant. Similar to baseline, there were differences in eGFR (mean [SD]) at the first and second week of follow-up; the eGFR in patients taking RAASi was greater than in those not taking RAASi (first week, 49.5 [28.8] mL/min/1.73 m2, 39.8 [28.6] mL/min/1.73 m2; second week, 50.5 [29.6] mL/min/1.73 m2, 38.7 [26.9] mL/min/1.73 m2, respectively).
Patiromer Dosing and Titration
The mean (SD) daily patiromer dose was 10.7 (3.2) g and 11.5 (4.0) g, respectively, in patients taking and not taking RAASi (median doses 8.4 g and 10.2 g, respectively). The mean (SD) daily dose prescribed at week 3 (the last titrated dose) was 12.5 (6.0) g and 13.9 (6.1) g, respectively. This dose was reached following a similar mean (SD) number of titrations per patient in each group: 0.6 (0.7) titrations in the RAASi group and 0.7 (0.7) among those not taking RAASi; the mean (SD) time to first titration was also similar (11.8 [7.3] days and 11.0 [5.7] days, respectively). Uptitrations occurred in 13% to 20% of patients at each of the weekly visits, and 2 patients in each group were downtitrated (on RAASi, both at week 3; and not on RAASi, 1 each at week 1 and week 2).
No RAASi medication (including spironolactone) was changed (new start, dose modification, or discontinuation) during the treatment or follow-up periods of the study. One patient in the group not taking RAASI discontinued a potassium-wasting diuretic (hydrochlorothiazide) during the follow-up period.
Safety/Tolerability
Adverse events (AEs) were reported in 26 (39%) and 25 (54%) patients taking and not taking RAASi, respectively (Table 3). AEs were mild to moderate in most patients. The most common AE was diarrhea (5% overall); all cases were mild. Additional AEs (none severe) occurring in ≥3% of patients overall included increase in blood creatine phosphokinase (all mild), constipation, anemia, headache, and urinary tract infection, all without obvious patterns in relation to taking or not taking RAASi. Overall, gastrointestinal disorders occurred in 14 (12%) patients, 4 (6%) who were taking RAASi and 10 (22%) who were not; all gastrointestinal AEs were mild to moderate in severity.
Table 3.
Safety Summary.
| n (%) | Taking RAAS Inhibitors (n = 67) | Not taking RAAS Inhibitors (n = 46) | Total (N = 113) |
|---|---|---|---|
| With ≥1 AE | 26 (39) | 25 (54) | 51 (45) |
| Severity | |||
| Mild | 13 (19) | 13 (28) | 26 (23) |
| Moderate | 12 (18) | 10 (22) | 22 (19) |
| Severe | 1 (2) | 2 (4) | 3 (3) |
| Most common AEsa | |||
| Diarrhea | 1 (2) | 5 (11) | 6 (5) |
| Increased blood creatine phosphokinase | 2 (3) | 2 (4) | 4 (4) |
| Constipation | 1 (2) | 3 (7) | 4 (4) |
| Anemia | 0 | 3 (7) | 3 (3) |
| Headache | 0 | 3 (7) | 3 (3) |
| Urinary tract infection | 2 (3) | 1 (2) | 3 (3) |
| With ≥1 treatment-related AE | 7 (10) | 6 (13) | 13 (12) |
| Most common treatment-related AEsa | |||
| Diarrhea | 0 | 3 (7) | 3 (3) |
| Constipation | 1 (2) | 2 (4) | 3 (3) |
| With ≥1 AE leading to study discontinuation | 1 (2) | 2 (4) | 3 (3) |
| With ≥1 serious AEb | 2 (3) | 3 (7) | 5 (4) |
| Deaths | 0 | 1 (2) | 1 (<1) |
| Prespecified laboratory values of interest | |||
| Serum K+ <3.5 mEq/L | 0 | 0 | 0 |
| Serum Mg <1.2 mg/dLc | 0 | 0 | 0 |
| Serum Mg <1.4 mg/dLc | 2 (3) | 3 (7) | 5 (5) |
Abbreviations: AE, adverse event; K+, potassium; Mg, magnesium; RAAS, renin–angiotensin–aldosterone system.
a Occurring in ≥3 patients in the total group.
b None were considered related to patiromer in the judgment of the investigators.
c One patient had no postbaseline serum Mg value.
AEs considered to be related to patiromer occurred in 13 (12%) patients overall; the most common treatment-related AEs were constipation and diarrhea (Table 3). Treatment-related hypomagnesemia was reported in 1 patient in each group and headache in 2 patients not taking RAASi. Three patients discontinued the study due to AEs, 1 who was taking RAAS inhibitor and 2 who were not (Table 3). Six serious AEs were reported for 5 patients (2 taking RAASi); none were considered related to patiromer. Among patients taking RAASi, 1 experienced angina and chest pain on 2 occasions and 1 experienced claudication. Among patients not taking RAASi, 1 experienced acute kidney injury and 1 experienced anemia. In 1 patient not taking RAASi, a serious AE (cardiopulmonary arrest, described by Pergola et al18) was fatal and was considered not related to patiromer; the safety review board assessed the death as related to cardiovascular causes (sudden cardiac death) and unlikely to be related to hypokalemia or hyperkalemia.
Serum potassium remained ≥3.5 mEq/L in all patients during the treatment phase and during follow-up. Single episodes of low normal levels (3.7-4.0 mEq/L) were reported for 1 (2%) patient not taking RAASi and 3 (4%) patients receiving RAASi. Mean (SD) serum magnesium at baseline was 2.1 (0.3) mg/dL among those taking RAASi and 2.2 (0.3) mg/dL among those not taking RAASi and remained in the normal range (1.8-2.4 mg/dL) throughout the study. There was a small mean (SD) reduction in serum magnesium from baseline to week 4 of −0.1 (0.2) in those taking RAASi and −0.2 (0.2) among those who were not; values returned to baseline by the end of the 2-week follow-up period. Five patients experienced serum magnesium <1.4 mg/dL during the study period (Table 3); in 4 of these patients, serum magnesium was <1.8 mg/dL at baseline. No patient experienced serum magnesium <1.2 mg/dL. There were no clinically relevant changes in eGFR, creatinine, or vital signs during the study.
Discussion
The main finding of TOURMALINE was that patiromer is similarly effective when administered with or without food,18 which allowed us to combine data from both treatment arms for this subgroup analysis. Furthermore, the design of the study allowed us to examine whether the potassium-lowering effect of patiromer was modified by concomitant RAASi use. We show that once-daily patiromer was similarly effective in treating hyperkalemia whether or not patients were taking RAASi, with 84% to 85% of patients achieving serum potassium between 3.8 and 5.0 mEq/L. This was achieved with similar number and extent of dose titrations of patiromer in both patient groups. These findings show that patients on RAASi, which increases the risk of hyperkalemia, respond to patiromer treatment the same as those not taking RAASi.
In our post hoc analysis, the 2 groups were relatively well balanced with respect to baseline characteristics. The one exception being a lower baseline eGFR in patients not taking RAASi, which could be explained by these patients having more advanced CKD as a group and being less likely to have tolerated or received background RAASi therapy due to physicians’ concerns regarding hyperkalemia. Despite the imbalance in baseline eGFR between the 2 groups, the subpopulation responder analysis showed no differences in the efficacy of patiromer when stratified for patients with eGFR above (or equal to) and less than 30 mL/min/1.73 m2.
In the follow-up period after patiromer treatment was stopped, serum potassium levels increased similar to that seen in the placebo arm of the Two-Part, Single-Blind, Phase 3 Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia (OPAL-HK) trial during the 8-week randomized withdrawal phase.17 Over the 8 weeks of the withdrawal phase of OPAL-HK, 56% of the placebo group required discontinuation of RAASi therapy to adequately maintain serum potassium levels <5.5 mEq/L. Likely due to the shorter duration of follow-up in our study (2 weeks), no patients required changes to or discontinuation of their RAASi dosing. At the same time, we observed a greater proportion of patients with serum potassium levels ≥5.5 mEq/L in the group not taking RAASi in the follow-up period. As noted earlier, compared to patients taking RAASi, eGFR was lower in patients not taking RAASi at baseline as well as during the follow-up period which likely contributed to the increased occurrence of serum potassium ≥5.5 mEq/L after patiromer was discontinued.
Overall, the AE profile was acceptable and similar in both groups. The AEs of diarrhea (all mild) and constipation (none severe) were numerically higher in patients not taking RAASi. However, given the small patient numbers, the post hoc nature of this analysis, and the fact that gastrointestinal AEs occur (though uncommonly) in patients taking RAASi, it is unclear whether this difference could simply reflect what is observed in clinical trials of patiromer and the observation is a chance occurrence. Safety findings were consistent with previous patiromer clinical studies.
Study Limitations
There were several limitations to the current analyses, including that TOURMALINE was designed to detect food effects on patiromer efficacy and safety and was not designed a priori to test any potential impact of RAASi on the potassium-lowering effect of patiromer. Importantly, this was an open-label study with no placebo group. The study did include a follow-up phase, which was mainly for safety monitoring purposes, and showed an increase in serum potassium upon drug withdrawal. In this post hoc analysis, patient numbers were relatively small and variability was wide; thus, power to show differences between subgroups was limited. Therefore, these findings are considered exploratory and the data should be interpreted with caution. The group differences in eGFR (lower mean values in the non-RAASi group) and use of β-blockers (greater in the non-RAASi group) reveal some important distinctions among hyperkalemic patients treated with or without RAASi. β-blockers inhibit renin production and potassium uptake, predisposing patients for hyperkalemia,19 and impaired kidney function limits the patients’ ability to excrete potassium. Thus, these 2 baseline characteristics likely explain why patients not on RAASi had hyperkalemia requiring treatment with patiromer and perhaps their intolerance of RAASi therapy. This also might explain the greater proportion of hyperkalemia upon discontinuation of patiromer in those not taking RAASi. Nonetheless, regardless of the driver for hyperkalemia (worse kidney function and/or β-blockade) or RAASi use, patiromer was effective at treating hyperkalemia and maintaining target serum potassium levels during the study treatment period.
Conclusion
We show for the first time that patiromer appears to be equally effective and well tolerated when used without or with RAASi. Further research (eg, AMBER trial clinicaltrials.gov identifier: NCT03071263) will evaluate whether patiromer enables optimal use and produces beneficial outcomes associated with RAASi therapy in patients with or at risk for hyperkalemia.
Acknowledgments
Writing and editorial support services were provided by Impact Communication Partners, Inc., and funded by Relypsa, Inc., a Vifor Pharma Group Company, which were acknowledged by the authors.
Authors’ Note: The work was conducted at multiple research sites, including those of the authors.
Author Contributions: The authors made substantial contributions to the conception or design of the work (C.G., J.Y., P.E.P.) or to the acquisition, analysis, or interpretation of data for the work (R.A.K., C.G., J.Y., A.C., P.E.P.); participated in critically revising the manuscript (R.A.K., C.G., J.Y., A.C., P.E.P.); approved the final version to be published (R.A.K., C.G., J.Y., A.C., P.E.P.); and agreed to be accountable for all aspects of the work (R.A.K., C.G., J.Y., A.C., P.E.P.).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Charles Hennekens served as guest editor for this paper. R.A.K. has no conflicts to declare. C.G., A.C., and J.Y. report employment by Relypsa, Inc., a Vifor Pharma Group Company. P.E.P. reports receiving honoraria from Akebia, Astra-Zeneca, Keryx, Reata, and ExThera; and reports serving as a consultant or participating in advisory boards for Akebia, Vifor, and Keryx. As the principal investigator for many pharmaceutical companies, his institution has received research support.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored and funded by Relypsa, Inc., a Vifor Pharma Group Company.
ORCID iD: Robert A. Kloner  http://orcid.org/0000-0002-6258-0544
http://orcid.org/0000-0002-6258-0544
References
- 1. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nilsson E, Gasparini A, Arnlov J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–284. [DOI] [PubMed] [Google Scholar]
- 3. Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5(3):531–548. [DOI] [PubMed] [Google Scholar]
- 4. Allen LA, Fonarow GC, Liang L, et al. American Heart Association’s Get With the Guidelines Heart Failure I. Medication initiation burden required to comply with heart failure guideline recommendations and hospital quality measures. Circulation. 2015;132(14):1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balamuthusamy S, Srinivasan L, Verma M, et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am Heart J. 2008;155(5):791–805. [DOI] [PubMed] [Google Scholar]
- 6. Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 8. Curtis LH, Mi X, Qualls LG, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165(6):979–986.e1. [DOI] [PubMed] [Google Scholar]
- 9. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273(18):1450–1456. [PubMed] [Google Scholar]
- 10. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. [DOI] [PubMed] [Google Scholar]
- 11. Yildirim T, Arici M, Piskinpasa S, et al. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3-5 in clinical practice: a safety concern? Ren Fail. 2012;34(9):1095–1099. [DOI] [PubMed] [Google Scholar]
- 12. Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(suppl 11):S212–S220. [PubMed] [Google Scholar]
- 13. Li L, Harrison SD, Cope MJ, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21(5):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veltassa® (patiromer) [package insert]. Prescribing Information. Redwood City, CA: Relypsa, Inc, a Vifor Pharma Group Company; 2018. https://www.veltassa.com/pi.pdf. Accessed July 2018. [Google Scholar]
- 15. Veltassa® (patiromer) [package insert]. SmPC. European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004180/human_med_002141.jsp&mid=WC0b0. Accessed July 2018.
- 16. Bakris GL, Pitt B, Weir MR, et al. AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–161. [DOI] [PubMed] [Google Scholar]
- 17. Weir MR, Bakris GL, Bushinsky DA, et al. OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. [DOI] [PubMed] [Google Scholar]
- 18. Pergola PE, Spiegel DM, Warren S, Yuan J, Weir MR. Patiromer lowers serum potassium when taken without food: comparison to dosing with food from an open-label, randomized, parallel group hyperkalemia study. Am J Nephrol. 2017;46(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben Salem C, Badreddine A, Fathallah N, Slim R, Hmouda H. Drug-induced hyperkalemia. Drug Saf. 2014;37(9):677–692. [DOI] [PubMed] [Google Scholar]