Abstract
Background:
Black carbon (BC) is a ubiquitous component of particulate matter (PM) emitted from combustion-related sources and is associated with a number of health outcomes.
Objectives:
We conducted a systematic review to evaluate the potential for cardiovascular morbidity and mortality following exposure to ambient BC, or the related component elemental carbon (EC), in the context of what is already known about the associations between exposure to fine particulate matter (PM2.5) and cardiovascular health outcomes.
Data Sources:
We conducted a stepwise systematic literature search of the PubMed database and employed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting our results.
Study Eligibility Criteria:
Studies meeting inclusion criteria (i.e., include a quantitative measurement of BC or EC used to characterize exposure and an effect estimate of the association of the exposure metric with ED visits, hospital admissions, or mortality due to cardiovascular disease) were evaluated for risk of bias in study design and results.
Study Appraisal and Synthesis Methods:
Risk of bias evaluations assess some aspects of internal validity of study findings based on study design, conduct, and reporting and identify potential issues related to confounding or other biases.
Results:
The results of our systematic review demonstrate similar results for BC or EC and PM2.5; that is, a generally modest, positive association of each pollutant measurement with cardiovascular emergency department visits, hospital admissions, and mortality. There is no clear evidence that health risks are greater for either BC or EC when compared to one another, or when either is compared to PM2.5.
Limitations:
We were unable to adequately evaluate the role of copollutant confounding or differential spatial heterogeneity for BC or EC compared to PM2.5.
Conclusions and Implications of Key Findings:
Overall, the evidence at present indicates that BC or EC is consistently associated with cardiovascular morbidity and mortality but is not sufficient to conclude that BC or EC is independently associated with these effects rather than being an indicator for PM2.5 mass.
Systematic Review Registration Number:
Not available.
Keywords: Air Pollution, Black Carbon, Particulate Matter, Cardiovascular, Mortality, Morbidity
1. Introduction
Black Carbon (BC) is a measured component of fine particulate matter (PM2.5) routinely measured in the U.S. and is generally present in submicron particles emitted from combustion-related sources including biomass burning, residential heating and cooking, industry, and transportation (U.S. EPA, 2009). There is strong evidence linking exposure to PM2.5 to an array of health effects, including premature mortality. There remain regional differences in PM2.5-related health effects reported in a number of epidemiologic studies that cannot be fully explained by geographical variations in ambient concentrations of PM2.5 (U.S. EPA, 2009). It has been hypothesized that a component or subset of more toxicologically active components of PM2.5 are influencing this variability, with a number of studies emphasizing a potential role of BC (Bell et al. 2007; Janssen et al. 2011).
There is a growing body of epidemiologic studies examining associations between BC and a number of adverse health outcomes, with early studies being summarized in reports by the U.S. EPA (2009, 2012) and the WHO (2012). In the last review of health evidence related to exposures to PM, completed in 2009, the U.S. EPA concluded that there was limited evidence that the chemical composition of PM would be a better surrogate to predict health effects related to PM than particle mass alone (U.S. EPA, 2009). The same conclusion was reached specifically for BC in a Report to Congress (U.S. EPA, 2012) that indicated the evidence for health effects associated with exposures to BC as a component of PM2.5 and PM2.5 as a whole were similar and that it would be difficult to identify effects solely attributable to BC. Other reviews using a source apportionment approach have found that exposures to source categories including BC are consistently associated with cardiovascular effects, but conclusions from these studies are limited to exposures to the source mixture and not BC alone (Stanek et al., 2011; Lippmann et al., 2013).
The WHO report (WHO, 2012) utilized an alternative approach, placing equal weight on risk estimates using both IQR and incremental increases in exposure. When incremental increases are used to estimate risk, and the same increment is used for both BC and PM2.5, pollutants with ambient concentrations that often differ by an order of magnitude or more, it becomes more difficult to compare the results for BC to the results for PM2.5 and to compare the results for BC across studies. This approach resulted in a different interpretation of the evidence and the conclusion that the associations between BC and health effects observed in epidemiologic studies were more robust than those observed for PM2.5. This conclusion is based mainly on the fact that in copollutant models including measures of both BC and PM2.5 mass, the effect estimates for BC were relatively unchanged, whereas the effect estimates for PM2.5 were attenuated. This led to WHO’s conclusion that “BC is a better indicator of harmful particulate substance from combustion sources (especially traffic) than undifferentiated PM mass” (WHO, 2012). Building on this conclusion that BC particles may pose a greater risk to health than other PM components, a recent study conducted a health impact assessment to estimate the public health burden of BC (Li et al., 2016).
Given the amount of evidence that has continued to accumulate since these reports and reviews were published, an updated evaluation is necessary. In particular, we have decided to focus on cardiovascular health effects as this is where the strongest evidence lies for the health effects of PM2.5 and BC. This includes evidence for a variety of endpoints that contribute to our knowledge on potential mechanisms and exposure pathways, such as associations with oxidative stress, inflammation and biomarkers of cardiac disease (e.g., fibrinogen, von Willebrand factor), as well as other sub-clinical markers of cardiac disease (e.g., heart rate variability, arrhythmia). In this systematic review, we evaluate studies of severe cardiac effects, emergency department (ED) visits and hospital admissions due to cardiovascular morbidity, as well as mortality attributed to cardiovascular disease among humans following short- or long-term exposure to ambient BC or EC, in order to assess associations in the context of what is already known about the relationship between PM2.5 and cardiovascular health outcomes. Specifically, we examine whether or not there is clear evidence for an independent effect of BC, separate from that attributed to PM2.5, on these health outcomes. We evaluate the differences between the risk for these outcomes following exposure to BC and PM mass, with an interest in the trends and strength of the relationships from studies we identified that were conducted in North America, Europe and Asia.
This systematic review uses the following Population, Exposure, Comparison, Outcome, Study Design (PECOS) statement: In any population of adults (ages 18+), including subgroups of susceptible individuals (P), what is the increase in risk of an emergency department visit, hospital admission or mortality related to a cardiovascular endpoint (O) per unit increase equal to the interquartile range (C) in μg/m3 of short-term or long-term ambient concentrations of BC or EC (E), observed in time-series and case-crossover studies (for short-term exposure) and cohort studies (for long-term exposure) (S)?
2. Methods
2.1. Definition of black carbon and elemental carbon
BC is carbonaceous material defined by light absorbing capacity. Elemental carbon (EC) is another measured component of particulate matter routinely measured in the U.S. and is strongly correlated with BC, although they are not identical and have fundamentally different operational definitions (Arnott et al. 2005). EC contains only carbon that is not bound to other elements, and is defined using thermo-optical techniques. There are several measurement techniques available to quantify concentrations of BC or BC analogs (e.g., EC). The most commonly used techniques can be classified into two groups (U.S. EPA, 2012). Filter-based optical methods measure light absorption which is proportional to the BC concentration and quantify it to a mass concentration. Thermal-optical methods measure the carbon fraction that resists removal through heating to a high temperature to quantify the EC concentration. BC and EC values from these measurement methods are highly correlated (U.S. EPA, 2012). Furthermore, published studies show that the BC:EC ratios derived by commercial instrumentation are generally within 30% (U.S. EPA, 2012). BC and EC are both indicators for carbon-rich combustion sources, and are often used interchangeably in the literature. Therefore, both were evaluated in this review. The terms “soot” and “black smoke” have also been used to describe BC, however, because the definitions of soot and black smoke can vary and are often imprecise, we did not include studies of soot or black smoke in this review.
2.2. Search strategy
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for our stepwise systematic literature search (Moher et al. 2009) (Figure 1). The PubMed database was used to search for relevant BC or EC references (see Figure 1 for search string) and the original search was conducted through September 30, 2015. An updated literature search using the same search strategy was conducted for the dates September 30, 2015 through June 15, 2017. Next, publications on cardiovascular health effects were identified in PubMed (search string also shown in Figure 1), and the overlapping records between the two searches were selected for consideration. Study inclusion criteria were then applied. Inclusion criteria were:
Figure 1.
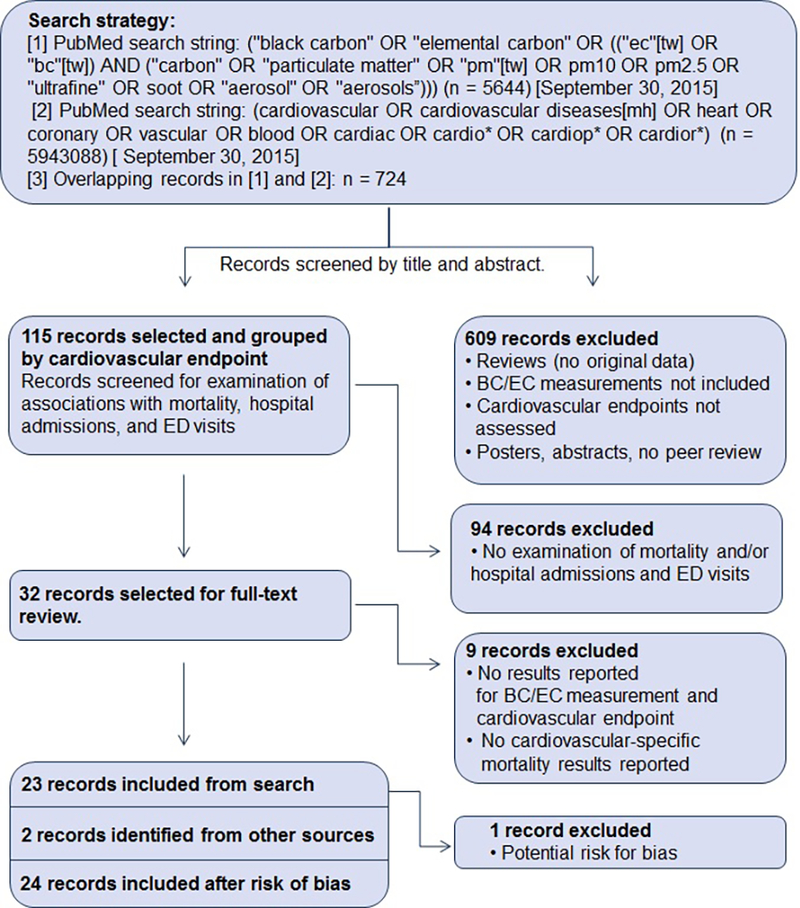
PRISMA flow diagram summarizing the systematic literature search and exclusion/inclusion criteria for epidemiologic studies of emergency department visits, hospital admissions and mortality (Black and White Figure)
Each study be an original, peer-reviewed research article
Each study be published in English
Each study include a quantitative measurement of BC or EC and PM2.5 used to characterize exposure
Each study include an effect estimate of the association of the exposure metric with ED visits, hospital admissions, or mortality due to cardiovascular disease.
The references were first screened by a single author (JLN) by title and abstract for potential relevance. The full text of each screened reference was reviewed by one author (either TJL or JDS) to identify characteristics of the study design and health effects reported to determine if the study would inform this review. Studies that did not report a main effect for BC or EC but did evaluate the ability of BC or EC to modify the effect of PM2.5 on a health effect were not included in the systematic review, but are characterized in the Discussion section.
2.3. Risk of bias evaluation
Studies meeting inclusion criteria were evaluated for risk of bias in results and study design. Risk of bias evaluations assess some aspects of internal validity of study findings based on study design, conduct, and reporting, and can identify potential issues associated with chance, bias or confounding. The risk of bias evaluation is a way to characterize potential strengths and weaknesses of individual studies more transparently. Published sources on systematic review were considered when developing a risk of bias framework for use in this review (Agency for Healthcare and Quality 2012; Higgins et al. 2011; Rooney et al. 2014). Epidemiologic studies were evaluated for evidence of by answering six questions related to confounding bias, exposure misclassification, selection bias, detection bias, disease misclassification, and selective reporting. Specifically, we examined the extent to which potential confounders were accounted for in the study population and exposure assessment, whether monitoring techniques were appropriate for the sample population, whether study sampling techniques were clearly described and included subject attrition, our confidence in the disease status of subjects reported in the studies, and whether all results were reported. In evaluating these six factors for risk of bias in each study, we assigned a “low”, “probably low”, “probably high”, or “high” risk of bias rating for each factor based on the answer to the question. Additional information is provided in Supplemental Material regarding these biases, criteria for assigning a risk of bias category (based on the OHAT risk-of-bias tool described in Rooney et al. 2014 and modified slightly to focus on aspects related to population-based epidemiologic studies).
2.4. Data extraction and synthesis
After studies meeting inclusion criteria were selected, study details and relevant results were extracted into tables (JDS) and reviewed for verification by an additional author (TJL). Results were preferentially extracted for fully-adjusted models, and in studies of acute exposure, for short-term cumulative lag periods (e.g., lag 0–1, lag 0–2) or short-term single day lag periods (e.g., 0, 1, 2). Continuous effect estimates were extracted to reflect associations with an increase in exposure concentration equal to the interquartile range (IQR) as reported in the study. Any inconsistencies between the two authors were discussed for clarification and agreement on final reporting. Results and trends were compared across studies to identify similarities and inconsistencies in cardiovascular effects associated with BC or EC. Studies that included results for both BC or EC and PM2.5 were deemed to be more informative and were highlighted in the text and included in figures.
3. Results
3.1. Search strategy
Our original PRISMA search for studies of BC or EC in the PubMed database yielded 5,644 records, while the search for cardiovascular health effects resulted in 5,943,088 records. Overlap between these two searches returned 724 epidemiologic records. No animal toxicological studies or studies from other scientific disciplines met our criteria for this review. Twenty-five studies were identified that examined ED visits, hospital admissions, or mortality: four for ED visits, ten for hospital admissions, and thirteen for mortality (three studies looked at both morbidity and mortality endpoints: Basagaña et al. 2015; Gan et al. 2011; Ito et al. 2011). Our updated literature search for the later time period yielded an additional four studies: one hospital admission study, one ED visit study, and two mortality studies (see Supplemental Material for details).
3.2. Risk of bias evaluation
The risk of bias framework developed for this review addressed relevant biases for this field of literature. In general, low risk was assigned for the majority of studies, especially for the selection bias, disease misclassification, and selective reporting categories of bias (Figure 2 and Supplementary Material). This was not surprising given that most of the studies included in this systematic review used similar study designs (i.e., time-series or case-crossover designs). The risk of bias for the confounding and exposure misclassification categories was “probably low” for most of the studies evaluated, largely due to study designs that focused on day-to-day changes in air pollution and the observed health endpoints. Two studies (de Kluizenaar et al. 2013; Zanobetti and Schwartz, 2006) received a “probably high” risk of bias for one category of bias (i.e., exposure measurement error) due to the imputation of missing data from a single BC monitor (Zanobetti and Schwartz, 2006) and the extrapolation of EC concentrations from measured concentrations of black smoke and emissions estimated from the EC component of PM10 (de Kluizenaar et al. 2013). One study (Crabbe, 2012) received a “high” risk of bias for one category of bias (i.e., selection bias). In this time-series analysis, on average, there was less than one hospital admission per day, which likely diminished the variance necessary to detect trends or patterns associated with air pollution. Based on concerns related to study quality, the study by Crabbe (2012) was not included in the review. Overall, due to the nature of the outcomes evaluated in this review (i.e., ED visits, hospital admissions and mortality) and the unlikeliness of detection bias associated with them, detection bias was not evaluated for these outcomes.
Figure 2.
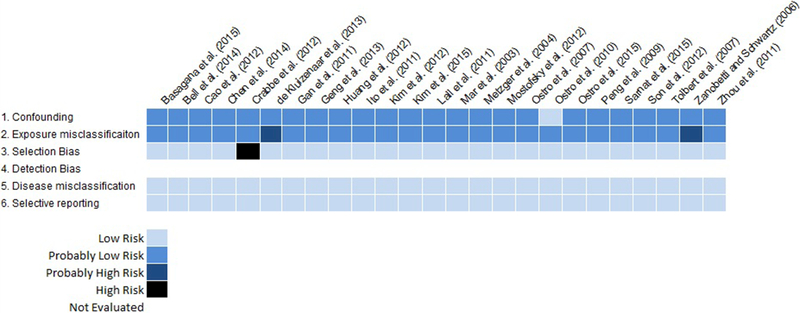
Risk of bias evaluation for epidemiologic studies of emergency department visits, hospital admissions and mortality. Due to the nature of these outcomes (i.e., emergency department visits, hospital admissions and mortality) and the unlikeliness of detection bias associated with them, detection bias was not evaluated for these outcomes. (Black and White Figure)
3.3. Emergency Department Visits
Four studies evaluated the association between short-term exposure to EC and ED visits for cardiovascular disease, including visits for any cardiovascular (Tolbert et al. 2007; Sarnat et al. 2015), ischemic heart disease (Sarnat et al. 2015), dysrhythmia (Sarnat et al. 2015), congestive heart failure (Metzger et al. 2004; Sarnat et al. 2015) or ischemic and hemorrhagic stroke (Chen et al. 2014). Each of these studies used measurements from outdoor fixed-site monitors to assign exposure to EC (and PM2.5). The impact of exposure measurement error from using a single outdoor fixed-site monitor to assign exposure to BC or EC may be greater than that for PM2.5 because of the more localized gradients around BC/EC sources (Clougherty et al. 2008). Sarnat et al. (2015) observed a modest, positive association between EC and all cardiovascular ED visits in the St. Louis metropolitan area, while the association with PM2.5 was null. When examining cause-specific ED visits, Sarnat observed the strongest associations with congestive heart failure for both EC and PM2.5, with smaller, generally positive associations for the other cardiovascular outcomes. In Atlanta, GA, Metzger et al. (2004) observed a modest increase in risk of congestive heart failure associated with an interquartile range (IQR) increase in the concentration of EC. Similar increases were observed for IQR increases in the concentration of PM2.5. Tolbert et al. (2007) extended the time-series in Atlanta, GA to include several additional years of ED visit and pollutant data. They observed smaller increases in risk for all CVD ED visits compared to associations reported by Metzger et al. (2004) for congestive heart failure ED visits. The associations of all CVD ED visits with EC and PM2.5 were similar when scaled to an increase equal to the IQR of the pollutant concentration. In a time-series study conducted in Taipei, Taiwan, Chen et al. (2014) observed generally null associations between ischemic stroke and IQR increases in both EC and PM2.5, but the associations with hemorrhagic stroke were similarly elevated for both EC and PM2.5. Quantitative results from these studies are presented in Figure 3 and study characteristics can be found in Table S1.
Figure 3.
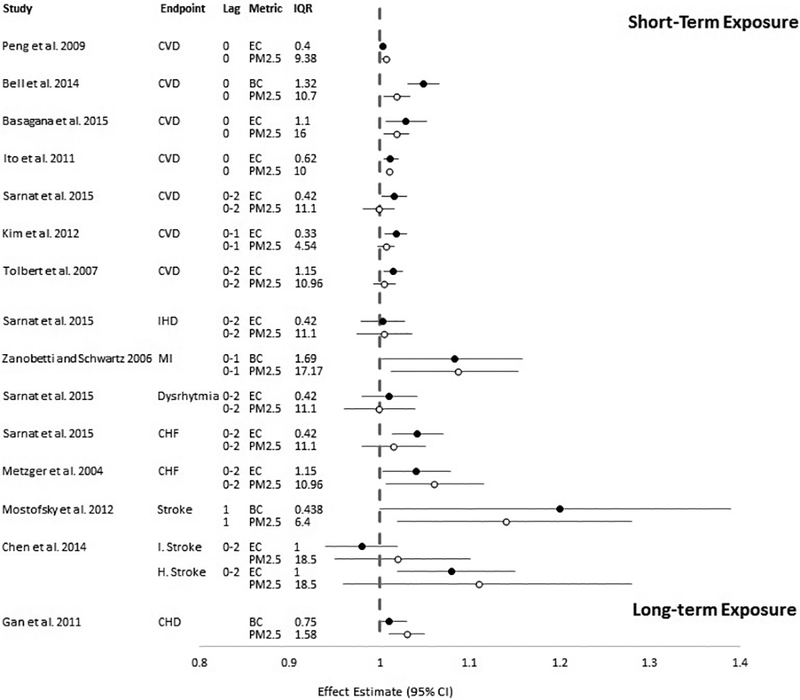
Association between exposure to BC or EC and PM2.5 and cardiovascular emergency department visits or hospital admissions per interquartile range (IQR) in mean (or median) pollutant concentration (in μg/m3). Circles represent point estimates, horizontal lines represent 95% confidence intervals for BC or EC (black circles) and PM2.5 (white circles) per increase in concentration equal to the IQR as reported in the study. CVD: emergency department visits or hospital admissions for any cardiovascular disease; IHD: ischemic heart disease; MI: myocardial infarction; CHF: congestive heart failure; I. Stroke: ischemic stroke; H. Stroke: hemorrhagic stroke; CHD: coronary heart disease. (Black and White Figure)
3.4. Hospital Admissions
Ten studies evaluated the association between BC or EC and cardiovascular hospital admissions. Three of these studies conducted multi-city analyses of short-term exposure and hospital admissions for any cardiovascular health endpoint. Five short-term exposure studies were conducted in a single city and included hospital admissions or emergency department visits for any cardiovascular endpoint, myocardial infarction (MI), or stroke. Each of these studies used measurements from outdoor fixed-site monitors to assign exposure to BC or EC and PM2.5, which could lead to greater exposure measurement error for BC or EC compared to PM2.5. An additional two studies evaluated long-term exposure to BC or EC and hospital admissions due to coronary heart disease. Quantitative results from these studies are presented in Figure 3 and study characteristics can be found in Table S1.
In order to evaluate the association between short-term exposure to PM2.5 and its components (including EC) with cardiovascular hospital admissions, Peng et al. (2009) utilized data from the Medicare cohort that includes adults 65 years and older across the United States (U.S.). The authors conducted a time-series analysis that included the years 2000–2006 and used county median concentrations of EC and PM2.5 from 119 counties to assign exposure. They observed very modest risks (i.e., <1% increase) of hospital admissions for any cardiovascular endpoint for an increase in exposure equal to the IQR for both EC and PM2.5. In another U.S.-based multi-city study, Bell et al. (2014) examined the association between short-term exposure to BC and PM2.5 and CVD hospital admissions in four counties across Connecticut and Massachusetts. It is noteworthy that, opposed to most of the other studies that relied on fixed-site monitors to assign exposure, Bell et al. (2014) used measurements from fixed-site monitors as inputs to a source apportionment analysis, which was then used to generate daily BC and PM2.5 estimates. Bell et al. (2014) observed positive associations between CVD hospital admissions and IQR increases in BC and PM2.5; the magnitude of the association was higher for BC than for PM2.5. Basagaña et al. (2015) conducted a multi-city study that included five European cities and examined the association between EC or PM2.5 and cardiovascular hospital admissions. They observed positive associations between cardiovascular hospital admissions and IQR increases in EC and PM2.5; the magnitudes of the associations were similar for EC and PM2.5.
Results from time-series analyses of short-term exposure to EC and hospital admissions conducted in single cities are generally similar to the results from multi-city studies. Ito et al. (2011) conducted a study in New York City, NY, and reported positive associations between cardiovascular hospital admissions and short-term exposure to both EC and PM2.5. In Denver, CO, Kim et al. (2012) observed modest positive associations between cardiovascular hospital admissions and short-term exposure to EC and PM2.5.
Several studies examined cause-specific CVD hospital admissions and observed associations with BC and PM2.5 that were generally higher in magnitude (though less precise, as evidenced by wider confidence intervals) than those observed when all CVD hospital admissions were included together. Zanobetti and Schwartz (2006) examined hospital admissions for myocardial infarction (MI) in Boston, MA, and observed positive associations with both BC and PM2.5 that were very similar in magnitude when evaluated for an increase in concentration equal to the IQR. Also in Boston, MA, Mostofsky et al. (2012) examined the association between hospital admissions for stroke onset and exposure to BC or PM2.5, and observed positive associations with both that were very similar in magnitude when evaluated for an increase in concentration equal to the IQR.
Gan et al. (2011) evaluated long-term exposure to BC and the association with hospital admissions for coronary heart disease, relying on spatio-temporal models that combine monitored and modeled concentrations to assign exposure. While the results of long-term exposure studies were similar in direction and magnitude to those that examined short-term exposure, the results (scaled to IQR) from short- and long-term exposure studies are not directly comparable due to the highly variable time scales used (i.e., daily exposures in short-term exposure studies and annual or multi-year exposures in long-term exposure studies). Gan et al. (2011) observed modest increases in coronary heart disease hospital admissions associated with IQR increases in 5-year average concentrations of BC and PM2.5 in Vancouver, British Columbia.
3.5. Mortality
Thirteen studies evaluated the association between BC or EC and cardiovascular mortality. Ten studies considered short-term exposure and mortality due to any cardiovascular health endpoint; of these studies, three conducted multi-city analyses while seven studies were conducted in a single city. An additional three studies evaluated long-term exposure to BC or EC and mortality due to cardiovascular health endpoints. Quantitative results from these studies are presented in Figure 4 and study characteristics can be found in Table S1.
Figure 4.
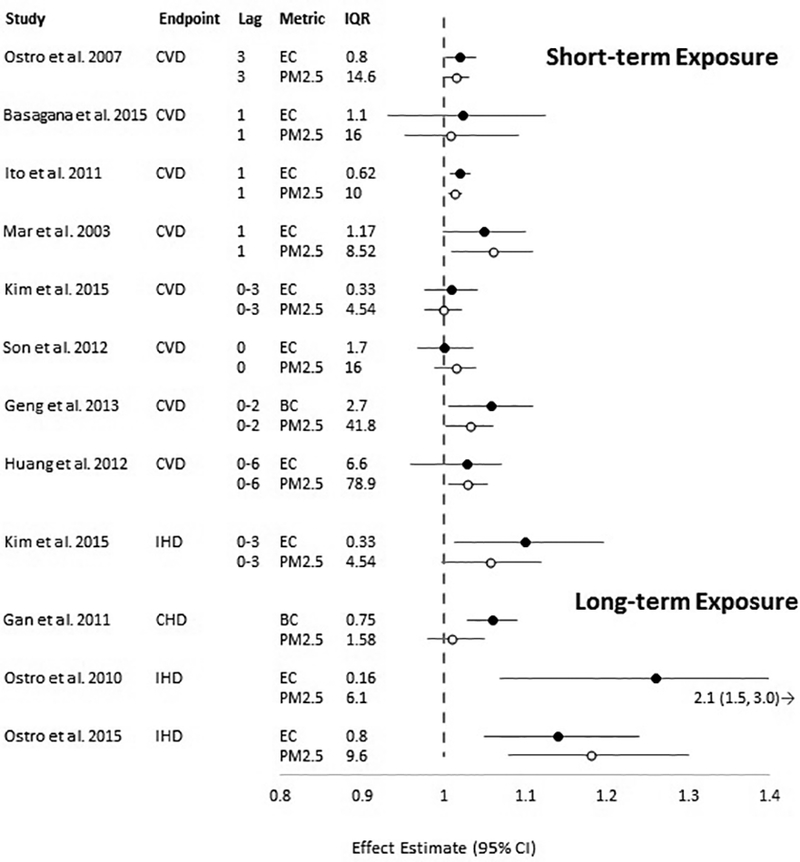
Association between exposure to BC or EC and PM2.5 and cardiovascular mortality per interquartile range (IQR) in mean (or median) pollutant concentration (in μg/m3). Circles represent point estimates, horizontal lines represent 95% confidence intervals for BC or EC (black circles) and PM2.5 (white circles) per increase in concentration equal to the IQR as reported in the study. CVD: mortality due to any cardiovascular disease; IHD: ischemic heart disease; CHD: coronary heart disease. (Black and White Figure)
Epidemiologic studies of short-term exposure to BC/EC and PM2.5 generally demonstrate modest, positive associations with mortality due to cardiovascular disease. In a time-series study conducted in six southern California counties, Ostro et al. (2007) observed modest, positive associations between increases in concentrations of EC and PM2.5 equal to the IQR and cardiovascular mortality. Basagaña et al. (2015) conducted a multicity study that included five European cities, and examined the association between EC or PM2.5 and CVD mortality. They observed positive associations between CVD mortality and IQR increases in EC and PM2.5; the magnitudes of the associations were similar for EC and PM2.5.
Results from time-series analyses of short-term exposure to EC and cardiovascular mortality conducted in single cities are generally similar to the results from multicity studies. Ito et al. (2011) reported similar positive associations between CVD mortality and short-term exposure to both EC and PM2.5. Mar et al. (2003) reported similar results from their time-series study conducted in Phoenix, AZ. In Denver, CO, Kim et al. (2015) observed modest positive associations between CVD mortality and short-term exposure to EC and PM2.5; they observed slightly larger associations when the analyses were restricted to mortality due to ischemic heart disease.
Several single-city time-series studies of cardiovascular mortality and short-term exposure to BC or EC were conducted in Asia, where concentrations of EC and BC were two to twelve times higher than those observed in the United States (Cao et al. 2012; Geng et al. 2013; Huang et al. 2012; Son et al. 2012; Table S1). Even in locations where concentrations of BC or EC were much higher, the associations with cardiovascular mortality were either near-null, or modestly positive, similar to those observed in the United States and Europe where concentrations are lower, providing evidence of a consistent, positive effect across the entire range of ambient concentrations reported in these studies.
Three studies evaluated long-term exposure to BC or EC and the association with mortality due to coronary heart disease (Gan et al. 2011) or ischemic heart disease (Ostro et al. 2010; 2015). Each study used a different method for assigning exposure (Table S1). Some of the results of long-term exposure studies were similar to those that examined short-term exposure (Gan et al. 2011), while others observed higher magnitude associations (Ostro et al. 2010, 2015). The results (scaled to IQR) from short- and long-term exposure studies are not directly comparable due to the highly variable time scales used (i.e., daily exposures in short-term exposure studies and annual or multi-year exposures in long-term exposure studies). Associations between long-term exposures to PM2.5 are often higher in magnitude compared to associations with short-term exposure (U.S. EPA, 2009). Gan et al. (2011) observed a modest increase in coronary heart disease mortality associated with an IQR increase in 5-year average concentrations of BC estimated using a land use regression model; the association with PM2.5 was closer to the null value. In an examination of the California Teacher’s Cohort, Ostro et al. (2010) observed positive associations between EC and PM2.5 concentrations measured at the nearest monitor to the participant’s residential address and mortality due to ischemic heart disease; the association was notably larger for PM2.5 than for EC. In a subsequent analysis that relied on a subset of the California Teacher’s Cohort and used a transport model to predict long-term exposure, Ostro et al. (2015) observed positive associations for both EC and PM2.5 that were similar in magnitude to each other, as well as to the association observed for EC in the Ostro et al. (2010) study.
4. Discussion
The results of our systematic review demonstrate generally similar risk for BC or EC and PM2.5; that is, generally modest, positive associations of each of these pollutant measurements with cardiovascular ED visits, hospital admissions and mortality. These results are consistent with our previous results that focused on populations with pre-existing disease (Nichols et al. 2013). Experimental evidence from studies of biomarkers of effect and subclinical CVD endpoints provide coherence and biological plausibility for the results observed in this review (Brook, 2010; Moller, 2011; U.S. EPA, 2009). There is no clear evidence that risk is greater for either BC or EC when compared to one another across studies, or when either is compared to PM2.5, when effect estimates are calculated for an increase in pollutant concentration equal to the IQR. Similarly, we observed no difference in the direction or magnitude of the association for cardiovascular ED visits, hospital admissions or mortality. The results of the few studies that looked at specific cardiovascular endpoints (e.g., MI, IHD, CHF) were consistent with the results for all cardiovascular endpoints grouped together, although the estimates for the cause-specific outcomes tended to be more imprecise (i.e., had wider confidence intervals), likely due to a reduction in sample size. There was limited evidence that the effect estimates from studies of long-term exposure might be higher than those of short-term exposure studies, although this is based on a small number of studies, and the higher effect estimates were observed by the same group of researchers and among the same cohort (Ostro et al. 2010, 2015). Additionally, the larger magnitude estimates were less precise than the other studies with smaller magnitude effect estimates.
Although our review is specific to BC or EC, our results are consistent with previous reviews that have concluded that there is no evidence that any PM2.5 sources or components are a better indicator for risk than particulate matter mass (Stanek et al. 2011; U.S. EPA, 2009). In order to facilitate the comparison of our results with these previous reviews, we placed more emphasis on studies that included results for both BC or EC and PM2.5. However, three studies (Lall et al. 2011; Zhou et al. 2011; de Kluizennar et al. 2013) examined only EC and did not include results for PM2.5. Lall et al. (2011) observed a positive association between CVD hospital admissions and short-term exposure to EC. In a time-series study conducted in two cities (Seattle, WA and Detroit, MI), Zhou et al. (2011) reported inconsistent associations between EC and cardiovascular mortality that varied depending on the city and season included in the analysis, though no quantitative results were presented. In a long-term exposure study conducted in the Netherlands, de Kluizennar et al. (2013) observed modest increases in hospital admissions for cerebrovascular disease associated with increases in the annual average concentration of EC. While these studies inform the relationship between these outcomes and EC exposures, the lack of results for PM2.5 limits interpretability of the EC results since a comparison cannot be made.
In an attempt to explain some of the regional heterogeneity in risk estimates observed for PM2.5 and CVD hospital admissions, Bell et al. (2009) examined the increase in CVD hospital admission per IQR increase in the fraction of PM2.5 total mass for a number of components. They observed risks for CVD hospitalizations that were higher in counties with higher EC, nickel or vanadium content. Consistent with our conclusions, Bell et al. (2009) concluded that “no single component is responsible for the harmful nature of PM”, but that the chemical composition of PM2.5 may help to explain observed geographic and seasonal heterogeneity in PM health effects. Similarly, Crouse et al. (2016) decomposed PM2.5 mass by component proportions and used joint models of PM2.5 mass and the component proportion to evaluate the association with cardio-metabolic mortality. They reported better model fit for the joint models than for models of PM2.5 mass or PM2.5 components alone. Their results provide evidence for the utility of modeling PM2.5 mass and components together. These studies underscore the value of examining PM2.5 components in the context of PM mass.
Because we wanted to evaluate the effect estimates for BC or EC in the context of the association observed for PM2.5, we evaluated effect estimates from each study that were scaled to an increase in pollutant concentration equal to the IQR for each pollutant. This allows for more direct comparison of the risk for BC or EC and PM2.5 than if each effect estimate were to be scaled to the same unit increase in pollutant concentration, which would be more useful in evaluating relative toxicity. This is especially important since BC and EC are components of PM2.5 mass, and we recognize that the contribution of individual components to PM2.5 mass can vary geographically. None of the studies included in this review included risk estimates based on categorical exposures, and thus assumed a linear concentration-response function. The assumption of a linear concentration-response function is well supported for the relationship between PM2.5 and cardiovascular outcomes (U.S. EPA, 2009), though there is relatively little evidence characterizing the shape of concentration-response function for BC or EC and cardiovascular outcomes. Generally, BC or EC concentrations were moderately correlated with PM2.5 (r = 0.4–0.75); a few studies reported weak correlations (i.e., r<0.4) (Gan et al. 2011; Sarnat et al. 2015) and a few reported strong correlations (i.e., r>0.75) (Mar et al. 2000; Ostro et al. 2010; Son et al. 2012).
We recognize that, on a mass basis, BC or EC has a greater relative toxicity compared to PM2.5 (Janssen et al. 2011). However, we were more interested in comparing the risk of BC or EC and PM2.5. BC or EC make up a small proportion of total PM2.5 mass and strategies aimed at lowering BC or EC may help to reduce the health effects attributed to PM2.5, but only to a small degree. Our focus on the health effects of BC or EC in the context of PM2.5, and comparing the risk per IQR increase in each of the pollutants underscores this point.
There are several limitations that we could not address in our review. We were unable to adequately evaluate the role of copollutant confounding in each of the studies included in this review. Several studies evaluated BC or EC in statistical models adjusted for PM2.5. Generally, the relationship between EC and cardiovascular morbidity or mortality was unchanged (Basagna et al. 2015; Sarnat et al. 2015, Cao et al. 2012; Kim et al. 2015) or slightly attenuated, though maintaining the same pattern or results and statistical significance (Gan et al. 2011; Mostofsky et al. 2012) when PM2.5 was included in the model. Still, it is possible that other traffic-related copollutants could explain (at least in part) the associations observed with BC or EC and PM2.5 in the studies included in this review. Secondly, sources of PM2.5 vary regionally, and thus the components of PM are likely to vary across study locations. Also, BC and EC concentrations may be more spatially variable than PM2.5 concentrations (Clougherty et al. 2008), which could lead to differential exposure measurement error, especially in the studies of short-term exposure which almost exclusively relied on fixed site monitors to assign exposure. BC and EC concentrations demonstrate much greater within-city gradients compared to within-city gradients of PM2.5, especially in areas with high volumes of on-road sources. If it is the case that spatial variability in BC or EC is not being adequately characterized, we would expect to see similar effect estimates for BC or EC and PM2.5, as we do here. Given the generally moderate correlations between BC or EC and PM2.5 in these studies, to the extent that pollutant concentrations vary together in short-term exposure studies (i.e., increase or decrease based on weather or other factors), using the IQRs to compute risk may help to reduce some aspects of this error (e.g., scaling error). The implication of differential measurement errors in long-term exposure estimates may be more complicated and depend on the scale of geographic coverage. Better spatial characterization of BC or EC concentrations in studies of short-term exposure in future studies would be useful in clarifying the role of differential exposure measurement error in our results. In addition, while meteorological variables such as temperature and humidity are often accounted for in epidemiologic studies of air pollution, the studies included in this review generally did not adjust for day-to-day variability in other meteorology parameters which may impact pollutant concentrations. In particular, wind speed and mixing height impact concentrations of all primary pollutants, including BC, by modifying atmospheric dilution conditions. In contrast, concentrations of PM2.5 would be expected to be somewhat less correlated with wind speed and mixing height due to the high contribution of secondary mass. Due to the consistency in the magnitude and direction of the effect estimates for BC or EC and PM2.5 observed in these studies, it does not appear that the failure to account for wind speed or mixing height contributed to a major source of bias. Consideration of the potential effects of atmospheric dilution on the association between BC and health outcomes in future studies would be informative.
A strength of our systematic review is the incorporation of an evaluation of risk of bias to our methods. This ensured critical evaluation of study design methods. Overall, we observed very similar results in our risk of bias evaluation across studies. Similarity in study design accounts for the majority of the consistency in the risk of bias ratings assigned across studies. Of the 25 studies identified by our literature search, one (Crabbe, 2012) was excluded due to concerns of potential bias and study quality. The remaining 24 studies were judged to be of sufficient quality to support the conclusions reached in this review. Another strength of our review was the evaluation of BC or EC in the context of PM2.5. Since BC and EC are components of PM2.5, their associations with health outcomes cannot be interpreted independently from the associations of particulate matter mass with the same health outcomes. While the inclusion of studies of long-term exposure is a strength of our review, the interpretation of the effects of long-term exposure on outcomes which are measured on a daily basis (e.g., ED visits, hospital admissions and mortality), can be difficult. In these studies it is often difficult to differentiate the effect of chronic exposure to moderate pollutant levels versus repeated acute exposure to higher concentrations.
Overall, we observed generally modest, positive associations between BC or EC and the cardiovascular endpoints. These associations were consistent with one another, and robust to different lag periods, exposure periods, and different cardiovascular disease endpoints. Importantly, these modest, positive associations were similar to those observed for PM2.5 in each study. The possibility that the similarities in results for BC or EC and PM2.5 could be due to greater exposure measurement error due to the greater spatial heterogeneity of BC and EC, especially in the presence of on-road sources, cannot be ruled out. Our evaluation of BC in the context of PM2.5 is consistent with previous reviews that have concluded that the evidence does not show that any single particulate matter source or component is a better indicator for risk than particulate matter mass (U.S. EPA, 2009, Stanek et al. 2011). That said, we recommend any future evaluations of particulate matter sources and components should be conducted in the context of PM2.5 mass in order to facilitate comparisons of the components to total particle mass and incorporate the appropriate spatial resolution for estimating exposure to PM components.
Supplementary Material
Acknowledgments:
The authors wish to thank Bryan Hubbell, Chad Bailey, Marion Hoyer, Molini Patel and Breanna Alman for comments on drafts of this work. This work is supported in part by an appointment to the Internship/Research Participation Program at Office of Research and Development (National Center for Environmental Assessment), U.S. Environmental Protection Agency (EPA), administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer: This manuscript has been reviewed by the U.S. Environmental Protection Agency and approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Financial Interests Declaration: The authors declare that they have no actual or potential competing financial interests.
References
- Agency for Healthcare Research and Quality (2012) Grading the strength of a body of evidence when assessing health care interventions –AHRQ and the effective health care program: An update: Draft report. Rockville, MD: Agency for Healthcare Research and Quality; http://effectivehealthcare.ahrq.gov/ehc/products/457/1163/gradingthestrengthofevidence_draftmethodschapter_20120625.pdf [PubMed] [Google Scholar]
- Arnott WP; Zielinska B; Rogers CF; Sagebiel J; Park K; Chow J; Moosmuller H; Watson JG; Kelly K; Wagner D; Sarofim A; Lighty J; Palmer G (2005) Evaluation of 1047-nm photoacoustic instruments and photoelectric aerosol sensors in source-sampling of black carbon aerosol and particle-bound PAHs from gasoline and diesel powered vehicles. Environ Sci Technol 39:5398–406. 10.1021/es049595e [DOI] [PubMed] [Google Scholar]
- Basagaña X; Jacquemin B; Karanasiou A; Ostro B; Querol X; Agis D; Alessandrini E; Alguacil J; Artiñano B; Catrambone M; de La Rosa JD; Díaz J; Faustini A; Ferrari S; Forastiere F; Katsouyanni K; Linares C; Perrino C; Ranzi A; Ricciardelli I; Samoli E; Zauli-Sajani S; Sunyer J; Stafoggia M; on behalf of the MED-PARTICLES Study group (2014) Short-term effects of particulate matter constituents on daily hospitalizations and mortality in five South-European cities: Results from the MED-PARTICLES project. Environ Int 75C:151–158. 10.1016/j.envint.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Bell ML; Dominici F; Ebisu K; Zeger SL; Samet JM (2007) Spatial and Temporal Variation in PM2. 5 Chemical Composition in the United States for Health Effects Studies. Environ Health Perspect 115:989–995. 10.1289/ehp.9621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML; Ebisu K, Peng RD, Samet JM, Dominici F. (2009) Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am J Crit Care Med 179: 1115–1120. 10.1164/rccm.200808-1240OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML; Ebisu K; Leaderer BP; Gent JF; Lee HJ; Koutrakis P; Wang Y; Dominici F; Peng RD (2014) Associations of PM2.5 constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥ 65 years of age. Environ Health Perspect 122:138–144. 10.1289/ehp.1306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD; Rajagopalan S; Pope CAIII; Brook JR; Bhatnagar A; Diez-Roux AV; Holguin F; Hong Y; Luepker RV; Mittleman MA; Peters A; Siscovick D; Smith SC Jr; Whitsel L; Kaufman JD (2010) Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121:2331–2378. 10.1161/cir.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- Cao J; Xu H; Xu Q; Chen B; Kan H (2012) Fine particulate matter constituents and cardiopulmonary mortality in a heavily polluted chinese city. Environ Health Perspect 120:373–378. 10.1289/ehp.1103671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S; Lin Y; Chang W; Lee CTe; Chan CC (2014) Increasing emergency room visits for stroke by elevated levels of fine particulate constituents. Sci Total Environ 473:446–450. 10.1016/j.scitotenv.2013.12.035 [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Wright RJ, Baxter LK, Levy JI (2008) Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants. Env Health 7:17 10.1186/1476-069X-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe H (2012) Risk of respiratory and cardiovascular hospitalisation with exposure to bushfire particulates: new evidence from Darwin, Australia. Environ Geochem Health 34:697–709. 10.1007/s10653-012-9489-4 [DOI] [PubMed] [Google Scholar]
- Crouse DL, Philip S, van Donkelaar A, Martin RV, Jessiman B, Peters PA, Weichenthal S, Brook JR, Hubbell B, Burnett RT. (2016) A new method to jointly estimate the mortality risk of long-term exposure to fine particulate matter and its components. Scientific Reports 6: 18916 10.1038/srep18916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kluizenaar Y; van Lenthe FJ; Visschedijk AJ; Zandveld PY; Miedema HM; Mackenbach JP (2013) Road traffic noise, air pollution components and cardiovascular events. Noise Health 15:388–397. 10.4103/1463-1741.121230 [DOI] [PubMed] [Google Scholar]
- Gan W; Koehoorn M; Davies H; Demers P; Tamburic L; Brauer M (2011) Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect 119:501–507. 10.1289/ehp.1002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F; Hua J; Mu Z; Peng L; Xu X; Chen R; Kan H (2013) Differentiating the associations of black carbon and fine particle with daily mortality in a Chinese city. Environ Res 120:27–32. 10.1016/j.envres.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- Huang W; Cao J; Tao Y; Dai L; Lu SE; Hou B; Wang Z; Zhu T (2012) Seasonal Variation of Chemical Species Associated With Short-Term Mortality Effects of PM2.5 in Xi’an, a Central City in China. Am J Epidemiol 175:556–566. 10.1093/aje/kwr342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K; Mathes R; Ross Z; Nádas A; Thurston G; Matte T (2011) Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect 119:467–473. 10.1289/ehp.1002667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen NA; Hoek G; Simic-Lawson M; Fischer P; van Bree L; Ten Brink H; Keuken M; Atkinson RW; Anderson HR; Brunekreef B; Cassee FR (2011) Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM10 and PM2.5. Environ Health Perspect 119:1691–1699. [Review] 10.1289/ehp.1003369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY; Dutton SJ; Sheppard L; Hannigan MP; Miller SL; Milford JB; Peel JL; Vedal S (2015) The short-term association of selected components of fine particulate matter and mortality in the Denver Aerosol Sources and Health (DASH) study. Environ Health 14:49 10.1186/s12940-015-0037-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY; Peel JL; Hannigan MP; Dutton SJ; Sheppard L; Clark ML; Vedal S (2012) The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect 120:1094–1099. 10.1289/ehp.1104721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lall R; Ito K; Thurston G (2011) Distributed lag analyses of daily hospital admissions and source-apportioned fine particle air pollution. Environ Health Perspect 119:455–460. 10.1289/ehp.1002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M; Chen LC; Gordon T; Ito K; Thurston GD (2013) National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Research report (Health Effects Institute) 5–13. http://www.ncbi.nlm.nih.gov/pubmed/24377209 [PubMed]
- Mar TF; Norris GA; Larson TV; Wilson WE; Koenig JQ (2003) Air pollution and cardiovascular mortality in Phoenix, 1995–1997 In Revised analyses of time-series studies of air pollution and health. (177–182). Cambridge, MA: Health Effects Institute; http://www.healtheffects.org/pubs/timeseries.pdf [Google Scholar]
- Metzger KB; Tolbert PE; Klein M; Peel JL; Flanders WD; Todd KH; Mulholland JA; Ryan PB; Frumkin H (2004) Ambient air pollution and cardiovascular emergency department visits. Epidemiology 15:46–56. 10.1097/01.ede.0000101748.28283.97 [DOI] [PubMed] [Google Scholar]
- Moher D; Liberati A; Tetzlaff J; Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269. Doi: 10.73226/0003-4819-151-4-20098180-00135 [DOI] [PubMed] [Google Scholar]
- Moller P; Mikkelsen L; Vesterdal LK; Folkmann JK; Forchhammer L; Roursgaard M; Danielsen PH; Loft S (2011) Hazard identification of particulate matter on vasomotor dysfunction and progression of atherosclerosis. Crit Rev Toxicol 41:339–368. [Review] 10.3109/10408444.2010.533152 [DOI] [PubMed] [Google Scholar]
- Mostofsky E; Schwartz J; Coull BA; Koutrakis P; Wellenius GA; Suh HH; Gold DR; Mittleman MA (2012) Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol 176:317–326. 10.1093/aje/kws018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JL; Owens EO; Dutton SJ; Luben TJ (2013) Systematic review of the effects of black carbon on cardiovascular disease among individuals with pre-existing disease Int J Public Health 58:707–724. [Review] 10.1007/s00038-013-0492-z [DOI] [PubMed] [Google Scholar]
- Ostro B; Feng WY; Broadwin R; Green S; Lipsett M (2007) The effects of components of fine particulate air pollution on mortality in California: Results from CALFINE. Environ Health Perspect 115:13–19. 10.1289/ehp.9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B; Hu J; Goldberg D; Reynolds P; Hertz A; Bernstein L; Kleeman MJ (2015) Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California teachers study cohort. Environ Health Perspect 123:549–556. 10.1289/ehp.1408565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B; Lipsett M; Reynolds P; Goldberg D; Hertz A; Garcia C; Henderson KD; Bernstein L (2010) Long-term exposure to constituents of fine particulate air pollution and mortality: Results from the California teachers study. Environ Health Perspect 118:363–369. 10.1289/ehp.0901181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R; Bell M; Geyh A; Mcdermott A; Zeger S; Samet J; Dominici F (2009) Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect 117:957–963. 10.1289/ehp.0800185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA; Boyles AL; Wolfe MS; Bucher JR; Thayer KA (2014) Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122:711–718. 10.1289/ehp.1307972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE; Winquist A; Schauer JJ; Turner JR; Sarnat JA (2015) Fine particulate matter components and emergency department visits for cardiovascular and respiratory diseases in the St. Louis, Missouri-Illinois, metropolitan area. Environ Health Perspect 123:437–444. 10.1289/ehp.1307776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JY; Lee JT; Kim KH; Jung K; Bell ML (2012) Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ Health Perspect 120:872–878. 10.1289/ehp.1104316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek LW; Sacks JD; Dutton SJ; Dubois JJB (2011) Attributing health effects to apportioned components and sources of particulate matter: An evaluation of collective results. Atmos Environ 45:5655–5663. 10.1016/j.atmosenv.2011.07.023 [DOI] [Google Scholar]
- Tolbert PE; Klein M; Peel JL; Sarnat SE; Sarnat JA (2007) Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol 17:S29–S35. 10.1038/sj.jes.7500625 [DOI] [PubMed] [Google Scholar]
- U.S. EPA (2009) Integrated science assessment for particulate matter (EPA/600/R-08/139F). Research Triangle Park, NC: U.S. Environmental Protection Agency, National Center for Environmental Assessment; http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=216546 [PubMed] [Google Scholar]
- U.S. EPA (2012) Report to Congress on black carbon (EPA-450/R-12–001). U.S. Environmental Protection Agency; http://www.epa.gov/blackcarbon/2012report/fullreport.pdf [Google Scholar]
- WHO (2012) Health Effects of Black Carbon. Janssen NAH, Gerlofs-Niljand ME, Lanki T, Salonen RO, Cassee F, Hoek G, Fischer P, Brunekreef B, Krzyzanowski M. ISBN 978 92 890 0265 3. http://www.euro.who.int/en/publications/abstracts/health-effects-of-black-carbon-2012
- Li Y, Henze DK, Jack D, Henderson BH, Kinney PL. (2016) Assessing public health burden associated with exposure to ambient black carbon in the United States. Science Total Environ, 539: 515–525. 10.1016/j.scitotenv.2015.08.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A; Schwartz J (2006) Air pollution and emergency admissions in Boston, MA. J Epidemiol and Community Health 60:890–895. 10.1136/jech.2005.039834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J; Ito K; Lall R; Lippmann M; Thurston G (2011) Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect 119:461–466. 10.1289/ehp.1002613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


