Abstract
This is the largest study to-date to report on outcomes of care for a national sample of doula-supported adolescent births (n = 1,892, birth years 2000 to 2013). Descriptive statistics were calculated for maternal demographics, risk profiles, labor/birth interventions and occurrences, and birth outcomes. In this national sample, childbearing adolescents and their neonates experienced improved health outcomes and lower rates of intervention relative to national statistics for adolescent deliveries in the United States. Key findings are consistent with previous studies on the effects of doula care for marginalized and medically underserved communities. Results strengthen the case for doulas as a perinatal care strategy for improving maternal and infant health outcomes and decreasing inequities among childbearing adolescents.
Keywords: adolescence, pregnancy outcomes, childbirth, doulas, cesarean
Introduction
The United States has the highest teenage birth rate among all industrialized countries at 22.3 per 1,000 live births (Kearney & Levine, 2012; Martin, Hamilton, Osterman, Driscoll, & Mathews, 2017), and young women in the United States experience very poor maternal and infant health outcomes. Adolescents between the ages of 15 and 19 years have some of the highest preterm birth rates (9.91%), low birth weight (LBW) rates (9.48%), and fetal demise rates (6.66/1,000) of all age groups in the United States (MacDorman & Gregory, 2015; Martin et al., 2017). Women under the age of 20 years also experience high rates of intervention during birth, including a 20.4% cesarean surgery rate, and a 63.5% epidural rate (Martin et al., 2017; Osterman & Martin, 2011). Initial breastfeeding rates are low at 50.7% (National Center for Health Statistics, 2015). Collectively, these outcomes highlight the health inequities young childbearing women face and are cause for great concern, given the lifelong health implications for neonates who receive a less than optimal start at birth (Kramer et al., 2008; Mueller, Bakacs, Combellick, Grigoryan, & Dominguez-Bello, 2015; Sakala, Romano, & Buckley, 2016). Doula care as a strategy for improving health outcomes is explored in this study through a retrospective analysis of a national dataset of doula-supported adolescent births occurring between 2000 and 2013 in the United States (n = 1,892).
The United States has the highest teenage birth rate among all industrialized countries at 22.3 per 1,000 live births and young women in the United States experience very poor maternal and infant health outcomes.
Literature Review
Doulas are non-medical, childbirth support professionals who provide emotional, physical, educational, and advocacy support to pregnant persons and their families during the childbearing year (DONA International, 2012). Birth doulas commonly work within a continuity of care model, where care begins in the prenatal period and continues through to support in labor and in the first few weeks of the postpartum period.1 This continuity model is particularly well documented in marginalized communities and at-risk populations, including childbearing adolescents, where doulas work extensively in the prenatal period on education, emotional support, wellness strategies, and engagement with social services in order to help the client meet basic needs, address underlying social determinants of health, and begin preparation for childbirth and parenting (Everson, 2015; Gentry, Nolte, Gonzalez, Pearson, & Ivey, 2010; Gruber, Cupito, & Dobson, 2013; HealthConnect One, 2014; Kozhimannil et al., 2016). Previous research suggests that continuous support during the perinatal period by someone who is neither part of the clinical care team nor part of the immediate childbearing family (i.e., a doula) may lead to (a) improved clinical outcomes for both the pregnant woman and newborn; (b) cost savings through decreased interventions and improved health outcomes; and (c) improved maternal–infant bonding and parenting experiences (HealthConnect One, 2014; Hodnett, Gates, Hofmeyr, & Sakala, 2013; Kozhimannil, Hardeman, Attanasio, Blauer-Peterson, & O’Brien, 2013; Kozhimannil et al., 2016; Steel, Frawley, Adams, & Diezel, 2015).
Doula care and continuous labor support have been found effective, to varying degrees, for the following birth outcomes: decreased average lengths of labor (Campbell, Lake, Falk, & Backstrand, 2006; Hodnett et al., 2013; Nommsen-Rivers, Mastergeorge, Hansen, Cullum, & Dewey, 2009); reduced rates of instrumental vaginal birth (forceps and vacuum extraction), cesarean surgery, and pharmacologic pain management (Campbell et al., 2006; Gruber et al., 2013; Hodnett et al., 2013; Kozhimannil, Attanasio, Hardeman, & O’Brien, 2013; Kozhimannil et al., 2014; Nommsen-Rivers et al., 2009); increased breastfeeding rates, maternal-infant bonding/positive interactions, and positive childbearing experiences (Edwards et al., 2013; Gruber et al., 2013; Hodnett et al., 2013; Kozhimannil et al., 2013; Nommsen-Rivers et al., 2009; Vonderheid, Kishi, Norr, & Klima, 2011); and deceased rates of LBW, preterm birth, and low 5-minute Apgar scores (Campbell et al., 2006; Gruber et al., 2013; Hodnett et al., 2013; Kozhimannil et al., 2013; Kozhimannil et al., 2016). Thus, in their 2013 Cochrane systematic review on continuous labor support, Hodnett et al. (2013, p. 16) conclude by asserting that, “continuous support during labour should be the norm, rather than the exception…Given the clear benefits and absence of adverse effects of continuous labour support, policymakers should consider including it as a covered service for all women.”
Furthermore, doula support may be especially beneficial for medically underserved and marginalized communities that experience significant health inequities, including racial and ethnic minority women (Edwards et al., 2013; Hardeman & Kozhimannil, 2016; HealthConnect One, 2014; Kozhimannil et al., 2014; Kozhimannil et al., 2016), low-income women (Campbell et al., 2006; Hardeman & Kozhimannil, 2016; Kozhimannil et al., 2016; Kozhimannil et al., 2013; Nommsen-Rivers et al., 2009), and childbearing adolescents (Arat, 2013; Coley & Nichols, 2016; Edwards et al., 2013; Gentry et al., 2010; Gruber et al., 2013; Hans et al., 2013; Humphries & Korfmacher, 2012; Vonderheid et al., 2011; Wen, Korfmacher, Hans, & Henson, 2010). These studies have universally demonstrated benefits associated with doula support for marginalized communities, but are subject to methodological shortcomings, including small sample sizes and location- or program-specific results. Furthermore, studies focusing specifically on outcomes of doula care for childbearing adolescents remain limited. This study describes maternal and neonatal health outcomes from a national sample of doula-supported adolescent births using data collected between 2000 and 2013 by the DONA International birth doula data project. This is the largest study to-date examining the effects of doula care for adolescent childbearing women and is one of the few to report on outcomes from a national sample.
Methods
Data Collection
Data were collected between 2000 and 2013 using the DONA International birth doula data collection form, developed in 1995 to gather data on doula-supported birth outcomes. The data collection form includes 35 demographic and perinatal health variables. Participation in the data collection process is voluntary for both the doula and the pregnant woman,2 and data may be submitted by all DONA International doulas, including certified doulas, certification candidates, or non-certified doulas. The data collection form is completed by the doula following a client’s birth and then is sent to the DONA International headquarters where DONA volunteers enter data into a Master (electronic) Data File. The DONA Master Data File is jointly managed by DONA International and researchers at University of North Carolina at Chapel Hill; this study focuses on all adolescent entries in the dataset between 2000 and 2013, where “adolescent” is defined as all clients in the DONA Master Data File who were between the ages of 15 and 19 years at the time of birth. All analyses using the adolescent dataset were approved by the Institutional Review Board at Oregon State University. All pregnant women whose data are included in the DONA dataset signed a “Client Confidentiality Release” form that gave their doula permission to send deidentified data to DONA International for use in research and evaluation; continued care with the doula was not contingent on whether or not the childbearing woman gave this permission.
Inclusion Criteria
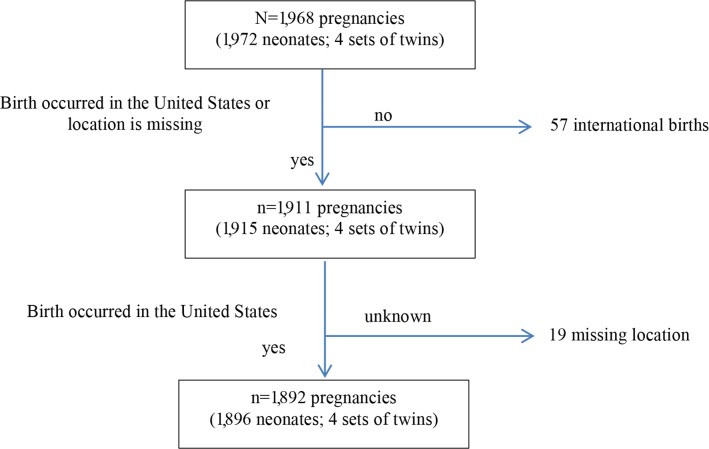
The 2000 to 2013 DONA Master Data File contains 35,645 records of which 2,046 were for adolescents between the ages of 15 and 19 years. After 74 duplicate entries were removed, the complete adolescent dataset contained 1,968 births (n = 1,972 neonates; four sets of twins). Births with a known location outside the United States were then excluded (n = 57), as well as births with location unspecified (n = 19). Thus, the final sample for this study consisted of n = 1,892 adolescents (1,896 neonates; see Figure 1).
Figure 1. Sample size delimitation. Delimitation begins with all unduplicated entries entered into the DONA International Birth Doula dataset for adolescents who gave birth between ages 15 to 19 (birth years 2000 to 2013). Final analyses are limited to adolescent women who gave birth with a DONA International birth doula in the United States.
Data Analysis
The objective of this study was to describe outcomes of care for a national sample of childbearing adolescents who received doula care. In keeping with this objective, our main analyses consisted of calculating basic frequencies, measures of central tendency, measures of variability, and confidence intervals (CIs), as applicable. For all analyses, denominators are limited to those women and neonates who were at risk for the given outcome. For example, the denominator for birth weight is all liveborn neonates with stillbirths excluded. Actual denominators (i.e., the denominator of women or liveborn neonates less missing data for a given variable) are included throughout. All analyses were conducted using IBM SPSS Version 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Participant Characteristics
Data on 1,892 adolescents (1,896 neonates) were contributed by 574 different DONA International doulas. Adolescent client characteristics are reported in Table 1. Women of color comprised over half the sample (54.2%). The mean maternal age was 17.8 years (standard deviation, 1.20). Just over half (52.7%) of the women were referred to their doula by the hospital, while “other” comprised 45.2% of referrals; the specifics of these referral sources are unknown, but may include childbirth educators, clinicians, case managers, or family/friends (Coley & Nichols, 2016). Payment for doula services varied with the majority (64.8%) of doulas indicating the hospital as their payment source, followed by volunteerism (22.8%), private pay (6.1%), third-party reimbursement (3.3%), and “other” sources (2.9%).
TABLE 1. Demographic Characteristics and Pregnancy Occurrences for 1,892 Adolescents in the DONA International Dataset.
| Characteristics | n (%) | |
|---|---|---|
| Race/ethnicityab | ||
| White | 860 (45.9) | |
| Black | 387 (20.7) | |
| Hispanic or Latino | 437 (23.3) | |
| Asian | 99 (5.3) | |
| Native American | 28 (1.5) | |
| Other | 63 (3.4) | |
| Maternal age at birth (years) | ||
| 15 | 102 (5.4) | |
| 16 | 211 (11.2) | |
| 17 | 362 (19.1) | |
| 18 | 543 (28.7) | |
| 19 | 674 (35.6) | |
| Referral sourcec | ||
| DONA International | 38 (2.0) | |
| Hospital | 984 (52.7) | |
| Other | 844 (45.2) | |
| Place of birthd | ||
| Home | 13 (0.7) | |
| Hospital | 1,655 (87.8) | |
| Birth center | 217 (11.5) | |
| Other | 1 (0.1) | |
| Clinical care providere | ||
| Midwife | 313 (16.8) | |
| Obstetrician/gynecologist | 1,206 (64.7) | |
| Combination | 103 (5.5) | |
| Family practice doctor | 243 (13.0) | |
| Method of payment for doulaf | ||
| Private pay | 112 (6.1) | |
| Third-party reimbursement | 61 (3.3) | |
| Volunteer | 419 (22.8) | |
| Hospital | 1,191 (64.8) | |
| Other | 54 (2.9) | |
| Childbirth education classes attendedg | ||
| Yes | 693 (38.0) | |
| No | 1,131 (62.0) | |
| Parityh | ||
| Nulliparous | 1,690 (90.3) | |
| Multiparous | 182 (9.7) | |
| Pregnancy occurrencesi | ||
| Pregnancy-induced hypertension | 67 (3.7) | |
| Gestational diabetes mellitus | 22 (1.2) | |
| High riskj | 56 (3.1) | |
| Other (unspecified) | 114 (6.3) | |
| Multiple gestationj | ||
| Twins | 4 (0.2) | |
As identified by the doula contributor.
Missing data for 18 women.
Missing data for 26 women.
Missing data for 6 women.
Missing data for 27 women.
Missing data for 55 women.
Missing data for 68 women.
Missing data for 20 women.
Missing data for 83 women.
No definition is given for what constitutes a “high-risk” pregnancy on the data form.
Prenatal Risk Profile
Ninety percent of clients were nulliparous. Fourteen percent of the sample were identified by the doula as having a “higher-risk” pregnancy, a category that included risk factors such as pregnancy-induced hypertension (3.7%) and gestational diabetes mellitus (1.2%). An additional 3.1% were marked as “high-risk” (this option was not defined on the data collection form), and 6.3% had “other” pregnancy-related risk factors, without specification. These categories were not mutually exclusive, meaning doulas could check more than one option to characterize the risk profile for a particular client’s pregnancy. Four sets of twins (n = 8 neonates) are included in this sample (Table 1).
Birthing Care
The vast majority (64.7%) of women in this sample received care from an obstetrician, 16.8% from midwives, 13.0% from family practice doctors, and the remaining 5.5% received co-care from more than one type of provider during the prenatal period. Births occurred in hospitals for 87.8% of the sample, while a smaller portion of adolescents birthed in a hospital-affiliated or freestanding birth center (11.5%) or at home (0.7%). Given that 90% of the women in the sample were nulliparous, the rate of attendance at childbirth education classes was lower than expected at only 38.0% (Table 1).
Induction and Augmentation
Thirty percent of women in this sample began labor via pharmacological induction (i.e., prostaglandins, synthetic oxytocin, misoprostol), and 35.4% had their labors augmented via synthetic oxytocin (these categories were not mutually exclusive). Forty-three percent of women birthed without either pharmacological induction or augmentation. Just under half of the sample (47.5%) had their membranes artificially ruptured (AROM). Continuous, external electronic fetal monitoring was used in 64.1% of labors, while 17.2% of women had intermittent fetal monitoring (either by Doppler or electronic fetal monitoring), and the remaining 18.6% had internal electronic fetal monitoring (Table 2).
TABLE 2. Labor Interventions and Occurrences for 1,892 Adolescents in the DONA International Dataset.
| Outcome | Median (IQR) |
|---|---|
| Labor length in hoursa | |
| Maternal self-report of length of laborb | 12.0 (8.0–18.0) |
| Length of labor from admission to birthc | 10.0 (7.0–15.1) |
| Labor length in hours for births without pharmacological induction | |
| Maternal self-report of length of labord | 13.0 (9.0–18.69) |
| Length of labor from admission to birthe | 9.5 (6.17–14.0) |
| Labor length in hours for births without pharmacological induction or augmentation | |
| Maternal self-report of length of laborf | 12.0 (8.0–17.0) |
| Length of labor from admission to birthg | 8.0 (5.0–12.0) |
| Doula support | |
| Length in hoursh | 8.0 (5.0–12.0) |
| n (%) | |
| Pharmacologic pain reliefij | |
| Epidural before 5 cm | 507 (27.5) |
| Epidural after 5 cm | 509 (27.6) |
| IV pain medications | 988 (53.6) |
| Other (unspecified) | 159 (8.6) |
| Interventionsij | |
| Pharmacological induction | 548 (29.7) |
| Artificial rupture of membranes | 875 (47.5) |
| Synthetic oxytocin augmentation | 653 (35.4) |
| Monitoringk | |
| Intermittent fetal monitoring | 295 (17.2) |
| Continuous fetal monitoring | 1,096 (64.1) |
| Internal fetal monitoring | 318 (18.6) |
Note. IQR = interquartile range; IV = intravenous.
Specific stages of labor are not delineated on the data form.
Missing data for 395 women.
Missing data for 292 women.
Missing data for 287 women.
Missing data for 215 women.
f Missing data for 190 women.
Missing data for 140 women.
Missing data for 84 women.
These categories are not mutually exclusive.
These questions were not asked as discrete variables (yes/no). Rather, only the presence of the intervention was noted. As such, blank cells in the dataset could indicate either “no” or “unknown.” In order to estimate the number of unknown cases, we averaged the number of missing data points from six variables with high degrees of apparent reliability in the dataset: ethnicity, place of birth, method of birth, labor doula hours, NICU admission, and breastfeeding. Using this approach, we estimated the unknown rate for this variable to be n = 30. Because main interventions and pharmacologic pain relief choices are generally well known by doulas given their focus on supporting physiologic birth, we believe this rate to reasonable.
Missing data for 164 women.
Length of Labor and Doula Support
The median length of doula support for the sample was 8.0 hours (interquartile range, IQR, 5.0–12.0 hours). Length of labor is reported in this dataset via two variables: maternal self-report of labor duration and a separate field calculated from time of admission until time of birth (Table 2). The median length of labor for the sample according to maternal self-report was 12.0 hours (IQR, 8.0–18.0 hours). The median length of labor from admission to birth was 10.0 hours (IQR, 7.0–15.1 hours). If the sample is limited to spontaneous labors only (i.e., no pharmacological induction), the median length of labor according to maternal self-report was 13.0 hours (IQR, 9.0–18.69 hours), and the median length from time of admission to birth was 9.50 hours (IQR, 6.17–14.0 hours). If the sample is further limited to spontaneous labors that also did not have pharmacological augmentation, the median length of labor according to maternal self-report was 12.0 hours (IQR, 8.0–17.0 hours), and the median length from time of admission to birth was 8.0 hours (IQR, 5.0–12.0 hours).
Pharmacologic Pain Relief
Just over half (55.1%) of the women in this sample had epidural anesthesia during labor, split roughly evenly between administration before 5 cm dilated (27.5%) and after 5 cm dilated (27.6%). Additionally, 53.6% of the women in this sample received intravenous pain medications, and 8.6% received another, unspecified form of pharmacologic pain relief. These categories were not mutually exclusive. Seventeen percent of women birthed with no pharmacologic pain medication or anesthesia (Table 2).
Mode of Birth
The spontaneous vaginal birth (SVB) rate for the entire sample was 79.3%. An additional 8.1% of women had an assisted vaginal birth (forceps or vacuum extraction), and 12.6% gave birth via cesarean surgery. Almost all (97.5%) of the cesareans were unplanned, and nine of the multiparas had “vaginal birth after cesarean” (VBAC) listed as their mode of birth. However, previous cesarean surgery was not asked on the data collection form under obstetric history and, thus, a VBAC success rate cannot be calculated. When the sample was limited to term, singleton, liveborn neonates only, the rates of SVB, assisted vaginal, and cesarean birth were not significantly different from the overall sample (see Table 3). Of the four sets of twins, three sets were born vaginally, and one set was born via unplanned cesarean.
TABLE 3. Birth Outcomes for 1,896 Neonates Born to Adolescents in the DONA International Dataset.
| Outcome | n (%) |
|---|---|
| Mode of birtha | |
| Spontaneous vaginalb | 1,480 (79.3) |
| Assisted vaginal (forceps or vacuum) | 151 (8.1) |
| Cesarean | 236 (12.6) |
| If cesarean, was this cesarean planned? | |
| Yes | 6 (2.5) |
| No | 230 (97.5) |
| Mode of birth for term, singleton, liveborn neonatesc | |
| Spontaneous vaginald | 1,390 (79.3) |
| Assisted vaginal (forceps or vacuum) | 142 (8.1) |
| Cesarean | 220 (12.6) |
| Gestational age at birthe | |
| Prematuref | 92 (4.9) |
| Posttermg | 56 (3.1) |
| Gestational age at birth for singletons only | |
| Prematureh | 88 (4.7) |
| Posttermi | 52 (2.9) |
| Birth weight in grams, median (IQR)j | 3,193 (2,762–3,243) |
| Low birth weight (<2,500 g) Macrosomic (>4,000 g) | 169 (10.2)111 (6.7) |
| Birth weight in grams for term, singleton neonates, median (IQR)k | 3,197 (2,771–3,628) |
| Low birth weight (<2,500 g) Macrosomic (>4,000 g) | 110 (7.0)110 (7.0) |
| Neonates with birthing woman <30 minutes after birthl | 1,148 (61.3) |
| Neonates with immediate health concernsm | 172 (9.2) |
| Neonate was admitted to the NICU | 106 (5.6) |
| Initial breastfeedingn | 1,119 (59.7) |
| Initial breastfeeding for term, singleton neonateso | 1,070 (60.3) |
Note. IQR = interquartile range; NICU = neonatal intensive care unit.
Missing data for 29 women.
Nine of these births were VBACs (vaginal births after cesarean).
Missing data for 25 women.
Nine of these births were VBACs (vaginal births after cesarean).
These data come from two questions on the data collection form. The preterm question is asked as “Baby outcome: premature.” The postterm question is asked as “Pregnancy: Gestation >42 weeks.”
Missing data for four neonates.
Missing data for 79 neonates.
Missing data for four neonates.
Missing data for 79 neonates.
Missing data for 224 neonates.
Missing data for 214 neonates.
Missing data for four neonates.
Missing data for eight neonates.
Missing data for four neonates.
Missing data for three neonates.
Gestational Age and Birth Weight
Ninety-two percent of neonates were considered full term at birth, while 4.9% were premature and 3.1% were postterm (>42 weeks). The median birth weight was 3,193 g (IQR, 2,762–3,243 g). Ten percent of neonates were born LBW (<2,500 g), and 6.7% were macrosomic (>4,000 g). When the sample was limited to term, singleton neonates, the median birth weight was 3,197 g (IQR, 2,771–3,628 g); 7.0% of term, singleton neonates were LBW, and 7.0% were macrosomic (Table 3).
Fetal and Neonatal Mortality and Morbidity
Nine percent of neonates in the sample experienced immediate health concerns following birth, and 5.6% were admitted to the neonatal intensive care unit (NICU). This dataset contains a total of 10 stillbirths, for a fetal demise rate of 5.27/1,000 (95% CI, 2.53–9.69). Additionally, there were nine missing entries for the stillbirth variable; none of these cases contained any postbirth data (e.g., breastfeeding or NICU admission), suggesting that the adolescent left the doula’s care before, during, or following birth, and postbirth outcomes are, thus, unknown. The data collection form does not specify the timing of demise (e.g., antenatal or intrapartum) nor does it provide cause of death. However, from free-text “notes” fields and other variables in the dataset, it can be determined that all known fetal demises were born vaginally. Birth weights for 7 of the 10 demises ranged from 1 to 6 lbs at birth. Of these, one fetus was noted as premature, and one woman had preeclampsia listed as a pregnancy complication. The final three fetuses were delivered vaginally with no known birth weights; one pregnancy was indicated as “high risk” by the doula, but no further information was given. Maternal ages for women who experienced losses were as follows: two women were 15 years; three women were 17 years; three women were 18 years; and two women were 19 years.
Breastfeeding and Immediate Contact
The initial breastfeeding rate for the entire sample was 59.7%. For term, singleton neonates only, the initial breastfeeding rate was 60.3%. Over half (61.3%) of neonates were united with the childbearing woman within 30 minutes of birth.
Discussion
In this national sample of doula-supported adolescent births, childbearing adolescents and their neonates experienced improved health outcomes and lower rates of intervention relative to national statistics for adolescent deliveries in the United States (MacDorman & Gregory, 2015; Martin et al., 2017; National Center for Health Statistics, 2015; Osterman & Martin, 2011). Key findings are also consistent with previously reported data on outcomes of doula support for socially marginalized and underserved communities (Campbell et al., 2006; Kozhimannil et al., 2013; Kozhimannil et al., 2016; Nommsen-Rivers et al., 2009). Rates of cesarean surgery (12.6%) and prematurity (4.9%) are substantially lower than rates reported nationally for adolescent childbearing women (20.4% for cesarean nationally, 9.91% for prematurity nationally), as is the epidural anesthesia rate for vaginal births (45.8% in this sample vs. 63.5% nationally; Martin et al., 2017; Osterman & Martin, 2011). Doula-supported women in this sample also experienced a 60% initial breastfeeding rate, which is improved relative to rates reported nationally for adolescents (50.7%; National Center for Health Statistics, 2015). Sixty-one percent of neonates were united with the birthing woman within 30 minutes of the birth; such immediate contact is a known correlate of breastfeeding success and maternal-infant bonding (Moore, Anderson, Bergman, & Dowswell, 2012). Table 4 compares select outcomes from this study, national statistics, and other select doula studies.
TABLE 4. Review of Select Outcomes Comparing 1,892 Adolescent Births (1,896 Neonates) in the DONA International Dataset With National Adolescent Datasets and Existing Doula Care Studies.
| National Data for Adolescents in the United Statesa | Gruber et al., 2013c—Adult Women With Doulas | Gruber et al., 2013c—Adolescent Women With Doulas | Kozhimannil, Vogelsang, Hardeman, & Prasad, 2016d—Doula Cohort | Nommsen-Rivers et al., 2009e—Doula Cohort | Campbell et al., 2006f—Doula Cohort | This Study—Doula-Supported Adolescent Births | |
|---|---|---|---|---|---|---|---|
| Sample size (n) | Varies | n = 51 | n = 46 | n = 1,935 | n = 44 | n = 298 | n = 1,892 |
| Sample composition | Adolescents, national statistics for the United States from NCHS | Adults enrolled in a childbirth program in North Carolina receiving doula care | Adolescents enrolled in a childbirth program in North Carolina receiving doula care | Medicaid recipients enrolled in a non-profit doula program in one metropolitan city in the Midwest | Low-income women in a regional hospital of northern California receiving doula support | Low-income women in an ambulatory care center in New Jersey receiving doula support | Doula-supported adolescents, national sample |
| Cesarean | 20.4% | 21.6% | 17.4% | 20.4% | 27.3% | 18.9% | 12.6% |
| Preterm birth | 9.91% | ND | ND | 4.7% | ND | ND | 4.9% (all)4.7% (singleton) |
| Low birth weight | 9.48% | 3.9% | 0.0% | 7.1% | ND | ND | 10.2% (7.0% term, singleton). |
| Pregnancy-induced hypertension | ND | ND | ND | 3.2% | 4.6% | ND | 3.7% |
| Gestational diabetes | ND | ND | ND | 5.4% | 4.6% (gestational or chronic) | ND | 1.2% |
| Fetal demise | 6.66/1,000b | ND | ND | ND | ND | ND | 5.27/1,000 |
| Epidural use for singleton deliveries | 63.5% (only vaginal births) | 49.0% (only vaginal births) | 58.7% (only vaginal births) | 25.8% | ND | 85.0% | 55.1% (all);45.8% (only vaginal births) |
| Initial breastfeeding | 50.7% | 90.2% | 67.4% | ND | 63.6% | ND | 59.7% |
Note. NCHS = National Center for Health Statistics; ND = no data available.
Data sources: Osterman and Martin (2011); National Center for Health Statistics (2015); MacDorman and Gregory (2015); Martin et al. (2017).
Fetal death refers to the intrauterine demise of a fetus between 20 weeks gestation through birth. Data source: MacDorman and Gregory (2015).
Data were collected between January 2008 and December 2010. The program used DONA-trained doulas, not necessarily certified.
Data were collected between January 1, 2010 and January 31, 2014; only singleton births were included. The program used DONA-trained doulas, not necessarily certified.
Data were collected between January 1, 2010 and January 31, 2014; only singleton births were included. The program used DONA-trained doulas, not necessarily certified.
Data were collected between 1998 and 2002; only nulliparous, singleton, term, “low-risk” pregnancies were included. Trained lay doula support was utilized by this hospital-based program.
Additionally, when benchmarked against Healthy People 2020, outcomes for doula-supported adolescent births in this sample are exceeding key maternal and infant health population-level targets, including cesarean (12.6% vs. 23.9% target) and preterm birth (4.9% vs. 11.4% target) (Office of Disease Prevention and Health Promotion, 2016). The fetal demise rate of this sample (5.27/1,000) is on par with the target objective of 5.6/1,000 and is lower than the fetal demise rate for adolescents nationally (6.6/1,000) (MacDorman & Gregory, 2015; Office of Disease Prevention and Health Promotion, 2016). However, the overall rate of LBW in this sample (10.2%) remains higher than both the 7.8% Healthy People 2020 target, and the national rate of 9.48% for childbearing adolescents (Martin et al., 2017). When delimited to term, singleton neonates, the LBW rate of 7.0% meets the national target. The initial breastfeeding rate in this sample (60%), while improved relative to adolescents nationally, is still below the Healthy People 2020 ever breastfed goal of 81.9%.
When compared to previously published data on outcomes associated with doula support for adult women (Gruber et al., 2013), adolescent women (Gruber et al., 2013), and low-income women (Campbell et al., 2006; Kozhimannil et al., 2016; Nommsen-Rivers et al., 2009), key outcomes from this sample are generally consistent with or better for cesarean, preterm, and epidural use, yet are on par or worse for breastfeeding and LBW rates. These findings require further investigation utilizing a matched cohort study design for the DONA sample and a comparison group drawn from national vital records data. Given that the DONA sample contains a high proportion of intersectional marginalization and higher risk young women (i.e., women of color, young ages of 15 to 17 years, and nulliparity) (Coley et al., 2015; Martin et al., 2017), it is possible that the positive outcomes identified here are functionally underestimated. Were this sample to be compared to a group matched for risk, it is possible that outcomes would indicate an even more pronounced improvement with doula care. A matched cohort study that controls for the risk level of doula- and non-doula-supported adolescents will be imperative for testing this hypothesis.
One of the most significant findings from this study is the notably low rate of cesarean surgery (12.6%). Given their young age—combined with their largely nulliparous status—women in this sample are at the beginning of the reproductive phase of the life course and are thus likely to have additional children. Ensuring full reproductive options for subsequent births is imperative as the United States aspires to reduce the national cesarean rate through the prevention of primary cesareans (American College of Obstetricians and Gynecologists & Society for Maternal-Fetal Medicine, 2014; Spong, Berghella, Wenstrom, Mercer, & Saade, 2012). Indeed, in a joint statement released by the American College of Obstetricians & Gynecologists and the Society for Maternal-Fetal Medicine on safe prevention of the primary cesarean birth, the authors state that: “one of the most effective tools to improve labor and delivery outcomes is the continuous presence of support personnel, such as a doula” (American College of Obstetricians and Gynecologists & Society for Maternal-Fetal Medicine, 2014, p. 13).
Additionally, the prematurity rate is also markedly lower than national rates for adolescents. Continuity of care from the prenatal through to the immediate postpartum period by birth doulas is most thoroughly documented for communities considered at-risk. For adolescents, doula care models targeting young women, explicitly, often design programming and services around a life course and social determinants of health framework (Kim & Saada, 2013; Kozhimannil et al., 2016; Pies & Kotelchuck, 2014). In this model, services both begin early on in the prenatal period and are more extensive in nature, and commonly include childbirth and parenting preparation, nutrition, housing security, physical activity, substance abuse, trauma-informed care, safe sleeping options, stress reduction strategies, employment and educational options, transportation, healthy relationships, and intimate partner violence, for example (Everson, 2015; Gentry et al., 2010; Gruber et al., 2013; HealthConnect One, 2014). Previous research (Commonsense Childbirth, n.d.; Kozhimannil et al., 2016; Kozhimannil et al., 2013; Sandall, Soltani, Gates, Shennan, & Devane, 2016) has demonstrated a correlation between targeted perinatal support models (e.g., doula care, midwifery care) and lowered prematurity rates. Current research (Bussières et al., 2015; Entringer, Buss, Andersen, Chicz-DeMet, & Wadhwa, 2011; Entringer, Buss, & Wadhwa, 2015; Hobel, Goldstein, & Barrett, 2008; Lu et al., 2010; Spicer et al., 2013) suggests that one biological pathway by which such models can impact prematurity rates is through mitigation of the cortisol response that results from the chronic stresses young childbearing adolescents embody as members of a marginalized (and often stigmatized) community.
In this national sample of doula-supported adolescent births, childbearing adolescents and their neonates experienced improved health outcomes and lower rates of intervention relative to national statistics for adolescent deliveries in the United States.
As such, with expanded continuity models, the potential for doula care to decrease preterm birth is significant in terms of both cost savings and lifelong well-being. Babies born prematurely are at risk for severe health problems and lifetime disabilities (Bastek et al., 2008; March of Dimes, 2013). Prematurity is also now the leading cause of death in children under the age of 5 years globally (Liu et al., 2015), and the leading contributor to infant death in the United States, accounting for more than one-third of all infant demises (Matthews, MacDorman, & Thoma, 2015). Furthermore, premature birth costs the United States more than $26.2 billion per year, including $16.9 billion in medical and health-care costs for the newborn; $1.9 billion in labor and birth costs associated with care of the childbearing woman; $611 million for early intervention services delivered between the ages of birth and 3 years; $1.1 billion for special education services delivered between the ages of 3 and 21 years; and $5.7 billion in lost wages for individuals born prematurely and who suffer lifelong disability and health issues (March of Dimes, 2013). The balance of evidence (Kozhimannil et al., 2013; Kozhimannil et al., 2014; Kozhimannil et al., 2016) to-date suggests that modest investment in doula care has the potential to decrease the substantial costs associated with adverse clinical health outcomes (e.g., prematurity) while also providing cost savings by reducing interventions at birth (e.g., cesarean).
Furthermore, this sample of doula-supported adolescent births included a small percentage of home (0.7%), birth center (11.5%), and midwife-attended (16.8%) deliveries, which are also associated with reductions in preterm birth as well as cesarean surgery (Cheyney et al., 2014; Sandall et al., 2016). Thus, the notable decreases in cesarean surgery and prematurity may reflect the shared impact of both doula and midwifery care as complementary patient-centered models.
Overall, findings from this study indicate the potential for doula care as a best practice to improve health outcomes and promote healthy, safe, and physiologic birth among adolescent childbearing women. With greater than 50% adolescents of color in the sample, these findings are particularly interesting given the well-documented negative health consequences of multiple intersecting forms of race-, class-, and age-based oppression (Coley et al., 2015; Everson & Ostrach, 2017). In addition, it is noteworthy that generally positive outcomes were achieved despite the low rate of childbirth education attendance among women in this sample (Hollowell, Oakley, Kurinczuk, Brocklehurst, & Gray, 2011). This is a key practice implication for childbirth educators and other professionals involved in perinatal education and care. Doulas and childbirth educators must work together and refer in reciprocity to ensure childbearing adolescents have access to culturally appropriate childbirth education and doula care and to clarify any misperceptions about the role of doulas or perinatal educators that serve as barriers to use (Coley & Nichols, 2016; Hardeman & Kozhimannil, 2016).
Additionally, despite improved outcomes relative to national datasets of adolescents and a tendency for doula-supported adolescent births to meet or exceed Healthy People 2020 objectives, rates of LBW, prematurity, fetal demise, and breastfeeding are still less than optimal. Larger social determinants of health prior to and during the childbearing year must be addressed if we are to achieve excellent outcomes for all young childbearing families (Viner et al., 2012; Kearney & Levine, 2012; Entringer et al., 2015; Office of Disease Prevention and Health Promotion, 2016). Ensuring interprofessional collaboration between childbirth educators, clinicians, and doulas may also help to further achieve optimal health outcomes and promote physiologic birth through the powerful combination of coordinated, patient-centered care (Zielinski, Brody, & Low, 2016).
Limitations
There are several limitations of this study. First, the DONA birth doula data collection form was not designed by researchers; the data project was initially conceived of as an internal evaluation mechanism, not a formal research database. As such, there are limitations related to the data collection tool itself, particularly in the wording of questions, and key co-variables on which data are not collected. In addition, the data form does not indicate the number of prenatal visits that occurred nor the specific services provided prior to the birth; however, the literature indicates that adolescents are likely to receive expanded services in the prenatal period because of their known risk for poor birth outcomes and status as a marginalized community. Furthermore, data were entered first by contributing doulas (who do not have clinical training and must rely on information provided by the primary clinician or the client for documentation), and then, secondarily, entered into a master data file by DONA International volunteers. Thus, data entry errors at either point in the process may occur, though it is likely that simple transcription errors are random and not systematic (Aday & Cornelius, 2006; Arts, De Keizer, & Scheffer, 2002). Demise data are also problematic. Since precise gestational age information is not available, the timing of any demises cannot be ascertained. It is also difficult to determine the confounding effects that midwifery-led care may have had on the lower rates of interventions and the improved health outcomes. Finally, additional, firm conclusions regarding the effects of doula care in childbearing adolescents cannot be made without the benefit of an adequately powered study with an appropriately matched comparison group. Future research should investigate the effectiveness of doulas and continuous labor support, controlling for clinical model of care (midwifery vs. obstetric) and focusing on the ways in which duration, timing, and quality of doula support may differentially impact maternal and infant health outcomes among childbearing adolescents.
Implications for Practice
In 2008, Berghella et al. published guidelines for the evidence-based management of labor and birth. The authors reviewed 41 birth practices, and of these, only three received a Grade “A” recommendation, meaning that the U.S. Preventative Services Task Force “strongly recommends that clinicians provide [the service] to eligible patients” (Berghella, Baxter, & Chauhan, 2008, p. 447). Doula care was one of the three to receive this high recommendation. Following, in a report on evidence-based birthing care released by leading advocacy organization, Childbirth Connection (a program of the National Partnership for Women and Families), doulas were cited as an effective strategy for optimizing outcomes within a best value framework (Sakala & Corry, 2008).
The growing body of research on the efficacy of doula care, especially for at-risk populations—combined with positive results reported here—makes a strong case for the implementation of doulas as a cost-effective strategy for improving maternal and infant health outcomes and decreasing inequities among childbearing adolescents. Childbirth educators and doulas must collaborate to optimize healthy and safe birth outcomes for young families. Firstly, childbirth educators can promote doula care as a best practice strategy through accurate descriptions of doula care and referrals of young clients to doulas. Secondly, doulas should help young clients to access childbirth education classes. The combined effect of doula care with perinatal education is likely to show an even greater improvement in birth outcomes for childbearing adolescents and other marginalized communities. We recommend that insurers, perinatal educators, clinicians, and public health policies support the utilization of doulas to improve care experiences and advance reproductive justice for young families in the United States.
Biographies
COURTNEY L. EVERSON is a Medical Anthropologist, Academic Faculty, and Dean of Graduate Studies in the Midwifery Sciences Program at the Midwives College of Utah in Salt Lake City, Utah. Dr. Everson is also a birth and postpartum doula, a perinatal health educator, the Director of Research Education for the Midwives Alliance of North America Division of Research, co-Vice President for the Association of Midwifery Educators, and serves on the Boards of Directors for the Academic Collaborative for Integrative Health and the Midwifery Education Accreditation Council.
MELISSA CHEYNEY is an Associate Professor of medical anthropology and reproductive biology in the Anthropology Program at Oregon State University in Corvallis, Oregon. Dr. Cheyney is also a Certified Professional Midwife, licensed in the State of Oregon, and Chair of the Division of Research for the Midwives Alliance of North America.
MARIT L. BOVBJERG is a Clinical Assistant Professor in the Epidemiology Program, College of Public Health and Human Sciences, at Oregon State University in Corvallis, Oregon. Dr. Bovbjerg is also the Director of Data Quality for the Midwives Alliance of North America Division of Research.
Footnotes
In this article, we focus on birth doulas specifically. Birth doulas should be distinguished from postpartum doulas, who work strictly in the extended postpartum period.
The DONA International birth doula data collection form does not explicitly ask about gender identification of the client. Given national statistics that show an increasing number of adolescents who identify as gender non-binary and gender non-conforming, it is likely that not all participants in the dataset identify as women and we acknowledge here the gender continuum that may be represented in this adolescent dataset.
References
- Aday, L. A., & Cornelius, L. J. (2006). Designing and conducting health surveys: A comprehensive guide (3rd ed.). San Francisco, CA: Jossey-Bass. [Google Scholar]
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. (2014). Obstetric care consensus no. 1: Safe prevention of the primary cesarean delivery. Obstetrics and Gynecology, 123(3), 693–711. 10.1097/01.AOG.0000444441.04111.1d [DOI] [PubMed] [Google Scholar]
- Arat, G. (2013). Doulas’ perceptions of single mothers’ risk and protective factors, and aspirations relative to child-birth. Qualitative Report, 18(2), 1–11. [Google Scholar]
- Arts, D. G., De Keizer, N. F., & Scheffer, G. J. (2002). Defining and improving data quality in medical registries: A literature review, case study, and generic framework. Journal of the American Medical Informatics Association, 9(6), 600–611. 10.1197/jamia.M1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastek, J. A., Sammel, M. D., Paré, E., Srinivas, S. K., Posencheg, M. A., & Elovitz, M. A. (2008). Adverse neonatal outcomes: Examining the risks between preterm, late preterm, and term infants. American Journal of Obstetrics and Gynecology, 199(4), 367.e1–36367. 10.1016/j.ajog.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Berghella, V., Baxter, J. K., & Chauhan, S. P. (2008). Evidence-based labor and delivery management. American Journal of Obstetrics and Gynecology, 199(5), 445–454. 10.1016/j.ajog.2008.06.093 [DOI] [PubMed] [Google Scholar]
- Bussières, E. -L., Tarabulsy, G. M., Pearson, J., Tessier, R., Forest, J. -C., & Giguère, Y. (2015). Maternal prenatal stress and infant birth weight and gestational age: A meta-analysis of prospective studies. Developmental Review, 36, 179–199. [Google Scholar]
- Campbell, D. A., Lake, M. F., Falk, M., & Backstrand, J. R. (2006). A randomized control trial of continuous support in labor by a lay doula. Journal of Obstetric, Gynecologic & Neonatal Nursing, 35(4), 456–464. 10.1111/j.1552-6909.2006.00067.x [DOI] [PubMed] [Google Scholar]
- Cheyney, M., Bovbjerg, M., Everson, C., Gordon, W., Hannibal, D., & Vedam, S. (2014). Outcomes of care for 16,924 planned home births in the United States: The Midwives Alliance of North America Statistics Project, 2004 to 2009. Journal of Midwifery & Women’s Health, 59(1), 17–27. 10.1111/jmwh.12172 [DOI] [PubMed] [Google Scholar]
- Commonsense Childbirth. (n.d.). The JJ Way® easy access prenatal clinics. Retrieved from http://www.commonsensechildbirth.org/the-birth-place-birthing-center/easy-accessprenatal-clinic/
- DONA International. (2012). Position paper: The doula’s contribution to modern maternity care. Chicago, IL: Author. [Google Scholar]
- Coley, S. L., & Nichols, T. R. (2016). Understanding factors that influence adolescent mothers’ doula use: A qualitative study. The Journal of Perinatal Education, 25(1), 46–55. 10.1891/1058-1243.25.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley, S. L., Nichols, T. R., Rulison, K. L., Aronson, R. E., Brown-Jeffy, S. L., & Morrison, S. D. (2015). Race, socioeconomic status, and age: Exploring intersections in preterm birth disparities among teen mothers. International Journal of Population Research, 2015, e617907–10. 10.1155/2015/617907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, R. C., Thullen, M. J., Korfmacher, J., Lantos, J. D., Henson, L. G., & Hans, S. L. (2013). Breastfeeding and complementary food: Randomized trial of community doula home visiting. Pediatrics, 132(Suppl 2), S160–S166. 10.1542/peds.2013-1021P [DOI] [PubMed] [Google Scholar]
- Entringer, S., Buss, C., Andersen, J., Chicz-DeMet, A., & Wadhwa, P. D. (2011). Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosomatic Medicine, 73(6), 469–474. 10.1097/PSY.0b013e31821fbf9a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer, S., Buss, C., & Wadhwa, P. D. (2015). Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. 10.1016/j.psyneuen.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson, C. (2015). “I’m a mom too!” – Stigma, support & contested identities among adolescent mothers in the United States. Doctoral dissertation, Oregon State University. Retrieved from http://hdl.handle.net/1957/56267
- Everson, C., & Ostrach, B. (2017). Pathologized bodies and deleterious birth outcomes: Iatrogenic effects of teen pregnancy stigma Ostrach, B, Lerman, S, & Singer, M (Eds.), Stigma syndemics: New directions in biosocial health. Lanham, MD: Rowman & Littlefield, Lexington Books. [Google Scholar]
- Gentry, Q. M., Nolte, K. M., Gonzalez, A., Pearson, M., & Ivey, S. (2010). “Going beyond the call of doula”: A grounded theory analysis of the diverse roles community-based doulas play in the lives of pregnant and parenting adolescent mothers. Journal of Perinatal Education, 19(4), 24–40. 10.1624/105812410X530910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, K. J., Cupito, S. H., & Dobson, C. F. (2013). Impact of doulas on healthy birth outcomes. The Journal of Perinatal Education, 22(1), 49–58. 10.1891/1058-1243.22.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans, S. L., Thullen, M., Henson, L. G., Lee, H., Edwards, R. C., & Bernstein, V. J. (2013). Promoting positive mother–infant relationships: A randomized trial of community doula support for young mothers. Infant Mental Health Journal, 34(5), 446–457. 10.1002/imhj.21400 [DOI] [Google Scholar]
- Hardeman, R. R., & Kozhimannil, K. B. (2016). Motivations for entering the doula profession: Perspectives from women of color. Journal of Midwifery & Women’s Health, 61(6), 773–780. 10.1111/jmwh.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HealthConnect One. (2014). The perinatal revolution. Chicago, IL: Author. [Google Scholar]
- Hobel, C. J., Goldstein, A., & Barrett, E. S. (2008). Psychosocial stress and pregnancy outcome. Clinical Obstetrics and Gynecology, 51(2), 333–348. 10.1097/GRF.0b013e31816f2709 [DOI] [PubMed] [Google Scholar]
- Hodnett, E. D., Gates, S., Hofmeyr, G. J., & Sakala, C. (2013). Continuous support for women during childbirth. The Cochrane Database of Systematic Reviews, 7, CD003766 10.1002/14651858.CD003766.pub5 [DOI] [PubMed] [Google Scholar]
- Hollowell, J., Oakley, L., Kurinczuk, J. J., Brocklehurst, P., & Gray, R. (2011). The effectiveness of antenatal care programmes to reduce infant mortality and preterm birth in socially disadvantaged and vulnerable women in high-income countries: A systematic review. BMC Pregnancy and Childbirth, 11(1), 13 10.1186/1471-2393-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, M. L., & Korfmacher, J. (2012). The good, the bad, and the ambivalent: Quality of alliance in a support program for young mothers. Infant Mental Health Journal, 33(1), 22–33. 10.1002/imhj.20334 [DOI] [PubMed] [Google Scholar]
- Kearney, M. S., & Levine, P. B. (2012). Why is the teen birth rate in the United States so high and why does it matter? Journal of Economic Perspectives, 26(2), 141–166. 10.1257/jep.26.2.141 [DOI] [PubMed] [Google Scholar]
- Kim, D., & Saada, A. (2013). The social determinants of infant mortality and birth outcomes in Western developed nations: A cross-country systematic review. International Journal of Environmental Research and Public Health, 10(6), 2296–2335. 10.3390/ijerph10062296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil, K. B., Attanasio, L. B., Hardeman, R. R., & O’Brien, M,. (2013). Doula care supports near-universal breastfeeding initiation among diverse, low-income women. Journal of Midwifery & Women’s Health, 58(4), 378–382. 10.1111/jmwh.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil, K. B., Attanasio, L. B., Jou, J., Joarnt, L. K., Johnson, P. J., & Gjerdingen, D. K. (2014). Potential benefits of increased access to doula support during childbirth. The American Journal of Managed Cared, 20(8), 1–7. [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil, K. B., Hardeman, R. R., Alarid-Escudero, F., Vogelsang, C. A., Blauer-Peterson, C., & Howell, E. A. (2016). Modeling the cost-effectiveness of doula care associated with reductions in preterm birth and cesarean delivery. Birth, 43(1), 20–27. 10.1111/birt.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil, K. B., Hardeman, R. R., Attanasio, L. B., Blauer-Peterson, C., & O’Brien, M. (2013). Doula care, birth outcomes, and costs among Medicaid beneficiaries. American Journal of Public Health, 103(4), e113–e121. 10.2105/AJPH.2012.301201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil, K. B., Vogelsang, C. A., Hardeman, R. R., & Prasad, S. (2016). Disrupting the pathways of social determinants of health: Doula support during pregnancy and childbirth. The Journal of the American Board of Family Medicine, 29(3), 308–317. 10.3122/jabfm.2016.03.150300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M. S., Aboud, F., Mironova, E., Vanilovich, I., Platt, R. W., Matush, L., … Shapiro, S. (2008). Breastfeeding and child cognitive development: New evidence from a large randomized trial. Archives of General Psychiatry, 65(5), 578–584. 10.1001/archpsyc.65.5.578 [DOI] [PubMed] [Google Scholar]
- Liu, L., Oza, S., Hogan, D., Perin, J., Rudan, I., Lawn, J. E., … Black, R. E. (2015). Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. The Lancet, 385(9966), 430–440. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- Lu, M. C., Kotelchuck, M., Hogan, V., Jones, L., Wright, K., & Halfon, N. (2010). Closing the Black-White gap in birth outcomes: A life-course approach. Ethnicity & Disease, 20(1 Suppl 2), S2–62–2–76. [PMC free article] [PubMed] [Google Scholar]
- MacDorman, M. F., & Gregory, E. (2015). Fetal and perinatal mortality, United States, 2013. National Vital Statistics Reports, 64(8), 1–24. [PubMed] [Google Scholar]
- March of Dimes. (2013). The impact of premature birth on society. Retrieved from http://www.marchofdimes.org/mission/the-economic-and-societal-costs.aspx
- Martin, J. A., Hamilton, B. E., Osterman, M. J., Driscoll, A. K., & Mathews, T. J. (2017). Births: Final data for 2015. National Vital Statistics System, 66(1), 1–70. [PubMed] [Google Scholar]
- Matthews, T. J., MacDorman, M. F., & Thoma, M. E. (2015). Infant mortality statistics from the 2013 period linked birth/infant death data set. National Vital Statistics System, 64(9), 1–30. [PubMed] [Google Scholar]
- Moore, E. R., Anderson, G. C., Bergman, N., & Dowswell, T. (2012). Early skin-to-skin contact for mothers and their healthy newborn infants. The Cochrane Database of Systematic Reviews, 5(5), CD003519 10.1002/14651858.CD003519.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, N. T., Bakacs, E., Combellick, J., Grigoryan, Z., & Dominguez-Bello, M. G. (2015). The infant microbiome development: Mom matters. Trends in Molecular Medicine, 21(2), 109–117. 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2015). Health, United States, 2014. Hyattsville, MD: U.S. Government Printing Office. [Google Scholar]
- Nommsen-Rivers, L. A., Mastergeorge, A. M., Hansen, R. L., Cullum, A. S., & Dewey, K. G. (2009). Doula care, early breastfeeding outcomes, and breastfeeding status at 6 weeks postpartum among low-income primiparae. Journal of Obstetric, Gynecologic and Neonatal Nursing, 38(2), 157–173. 10.1111/j.1552-6909.2009.01005.x [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. (2016). Maternal, infant, and child health. Retrieved from http://www.healthypeople.gov/2020/topicsobjectives/topic/maternal-infant-and-child-health
- Osterman, M. J., & Martin, J. A. (2011). Epidural and spinal anesthesia use during labor: 27-state reporting area, 2008. National Vital Statistics Reports, 59(5), 1–14. [PubMed] [Google Scholar]
- Pies, C., & Kotelchuck, M. (2014). Bringing the MCH life course perspective to life. Maternal and Child Health Journal, 18(2), 335–338. 10.1007/s10995-013-1408-5 [DOI] [PubMed] [Google Scholar]
- Sakala, C., & Corry, M. (2008). Evidence-based maternity care: What it is and what it can achieve. New York, NY: Milbank Memorial Fund. [Google Scholar]
- Sakala, C., Romano, A. M., & Buckley, S. J. (2016). Hormonal physiology of childbearing, an essential framework for maternal-newborn nursing. Journal of Obstetric, Gynecologic and Neonatal Nursing, 45(2), 264–275. 10.1016/j.jogn.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Sandall, J., Soltani, H., Gates, S., Shennan, A., & Devane, D. (2016). Midwife-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews, 4, CD004667 10.1002/14651858.CD004667.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer, J., Werner, E., Zhao, Y., Choi, C. W., Lopez-Pintado, S., Feng, T., … Monk, C. (2013). Ambulatory assessments of psychological and peripheral stress-markers predict birth outcomes in teen pregnancy. Journal of Psychosomatic Research, 75(4), 305–313. 10.1016/j.jpsychores.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong, C. Y., Berghella, V., Wenstrom, K. D., Mercer, B. M., & Saade, G. R. (2012). Preventing the first cesarean delivery: Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstetrics and Gynecology, 120(5), 1181–1193. http://10.1097/AOG.0b013e3182704880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel, A., Frawley, J., Adams, J., & Diezel, H. (2015). Trained or professional doulas in the support and care of pregnant and birthing women: A critical integrative review. Health & Social Care in the Community, 23(3), 225–241. 10.1111/hsc.12112 [DOI] [PubMed] [Google Scholar]
- Viner, R. M., Ozer, E. M., Denny, S., Marmot, M., Resnick, M., Fatusi, A., & Currie, C. (2012). Adolescence and the social determinants of health. The Lancet, 379(9826), 1641–1652. 10.1016/S0140-6736(12)60149-4 [DOI] [PubMed] [Google Scholar]
- Vonderheid, S. C., Kishi, R., Norr, K. F., & Klima, C. (2011). Group prenatal care and doula care for pregnant women Handler, A, Kennelly, J, & Peacock, N (Eds.), Reducing racial/ethnic disparities in reproductive and perinatal outcomes ( 369–399). New York, NY: Springer. [Google Scholar]
- Wen, X., Korfmacher, J., Hans, S. L., & Henson, L. G. (2010). Young mothers’ involvement in a prenatal and postpartum support program. Journal of Community Psychology, 38(2), 172–190. 10.1002/jcop.20358 [DOI] [Google Scholar]
- Zielinski, R. E., Brody, M. G., & Low, L. K. (2016). The value of the maternity care team in the promotion of physiologic birth. Journal of Obstetric, Gynecologic and Neonatal Nursing, 45(2), 276–284. 10.1016/j.jogn.2015.12.009 [DOI] [PubMed] [Google Scholar]