Abstract
A well-established development of increasing disease severity leads from sepsis through systemic inflammatory response syndrome, septic shock, multiple organ dysfunction syndrome, and cellular and organismal death. Less commonly discussed are the equally well-established coagulopathies that accompany this. We argue that a lipopolysaccharide-initiated (often disseminated intravascular) coagulation is accompanied by a proteolysis of fibrinogen such that formed fibrin is both inflammatory and resistant to fibrinolysis. In particular, we argue that the form of fibrin generated is amyloid in nature because much of its normal α-helical content is transformed to β-sheets, as occurs with other proteins in established amyloidogenic and prion diseases. We hypothesize that these processes of amyloidogenic clotting and the attendant coagulopathies play a role in the passage along the aforementioned pathways to organismal death, and that their inhibition would be of significant therapeutic value, a claim for which there is considerable emerging evidence.
Keywords: sepsis, SIRS, dormant bacteria, septic shock, MODS
Sepsis is a disease with high mortality. 1 2 3 4 5 6 7 However, the original notion of sepsis as the invasion of blood and tissues by pathogenic microorganisms has long come to be replaced, in the antibiotic era, by the recognition that in many cases, the main causes of death arise not so much from the replication of the pathogen per se but from the host's “innate immune” response to the pathogen. 8 9 10 11 In particular, microbial replication is not even necessary (and most bacteria in nature are dormant 12 13 14 15 16 ), as this response is driven by very potent 17 inflammation-inducing agents such as the lipopolysaccharides (LPSs) of gram-negative bacteria 18 and equivalent cell wall materials such as lipoteichoic acids from gram-positive bacteria. 19 20 21 22 To this end, such release may even be worsened (i.e., the Jarisch–Herxheimer reaction 23 24 25 26 ) by antibiotic therapy. 27 28 29 30 In unfavorable cases, this leads to an established cascade ( Fig. 1 ) 31 in which the innate immune response, involving proinflammatory cytokines such as interleukins 6, 8, and 1β, monocyte chemoattractant protein-1, and tissue necrosis factor α, 32 becomes a “cytokine storm” 33 34 35 36 37 leading to a “systemic inflammatory response syndrome” (SIRS), 38 39 40 41 42 43 septic shock, 4 multiple organ failure 44 (MOF, also known as multiple organ dysfunction syndrome, MODS 45 46 ), and finally organismal death. All of the above is well known and may be taken as a noncontroversial background. Nevertheless, it is still unclear whether apoptotic 47 and necrotic 48 cell death is minimal 49 or significant. Despite this knowledge, “the recent inability of activated protein C to show an outcome benefit in a randomized controlled multicenter trial 2 and the subsequent withdrawal of the product from commercial use add to the growing stockpile of failed therapeutics for sepsis.” 50 (This last failure was probably due to an excessively anticoagulant activity.)
Fig. 1.
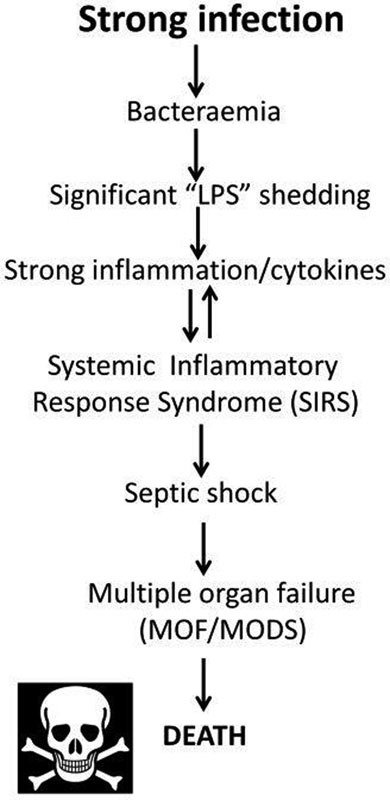
A standard cascade illustrating the progression of infection through sepsis, systemic inflammatory response syndrome, and death.
Most recently 51 (but see also Churpek et al 52 ), definitions of sepsis have come to be based on organ function and the Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) Scores. 53 These latter take into account the multisystem nature of sepsis and include respiratory, hemostatic (but only based on platelet counts), hepatic, cardiovascular, renal, and central nervous system measurements. A SOFA score of 2 or greater typically means at least a 10% mortality rate. Specifically, sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is defined as a subset of sepsis in which underlying circulatory and cellular metabolism abnormalities are profound enough to increase mortality substantially.
Table 1 (based on Vincent et al 53 ) shows the potential values that contribute to the SOFA score.
Table 1. Potential values that contribute to the SOFA score a .
| SOFA score | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
|
Respiration
PaO 2 /FiO 2 (mm Hg) |
<400 | <300 | <200 (with respiratory support) | <100 (with respiratory support) |
|
Coagulation
10 −3 /platelets/mm |
<150 | <100 | <50 | <50 |
|
Liver
Bilirubin mg/dL (μM) |
1.2–1.9 (20–32) |
2–5.9 (33–101) |
6–11.9 (102–204) |
>12 (>204) |
|
Cardiovascular
Hypotension |
MA
p
< 70
mm Hg |
Dopamine ≤ 5
b
or
dobutamine (any dose) |
Dopamine > 5 or epinephrine ≤0.1 or norepinephrine ≤ 0.1 |
Dopamine > 15 or epinephrine > 0.1 or norepinephrine > 0.1 |
|
CNS
Glasgow Coma Score |
13–14 | 10–12 | 6–9 | <6 |
|
Renal
Creatinine, mg/dL (μM) or urine output |
1.2–1.9 (110–170) |
2–3.4 (171–299) |
3.5–4.9 (300–440) Or <500 mL/d |
>5 (>440) or <200 mL/d |
Abbreviations: CNS, central nervous system; SOFA, Sequential (Sepsis-Related) Organ Failure Assessment.
Based on Vincent et al 53 and shows the potential values that contribute to the SOFA score.
Catecholamine and adrenergic agents administered for at least 1 hour; doses in μg/kg/min.
Absent from Fig. 1 , and from the usual commentaries of this type, is any significant role of coagulopathies, although these too are a well-established accompaniment of SIRS/sepsis, 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 and they will be our focus here. They form part of an emerging systems biology analysis, 16 18 70 71 72 73 74 75 76 77 78 79 80 in which iron dysregulation and an initially minor infection (e.g., in rheumatoid arthritis 81 82 ) are seen to underpin the etiology of many chronic inflammatory diseases normally considered (as once were gastric ulcers 83 ) to lack a microbial component.
Here we develop these ideas for those conditions that are recognized as involving a genuine initial microbial invasion, together with sepsis and inflammation driven (in particular) by the cell wall components of bacteria (although we note that the same kinds of arguments apply to viruses 84 and to other infections).
Normal Blood Coagulation and Coagulopathies
Historically, there are two main pathways of activation described that lead “normal” blood coagulation to form a clot, as occurs, for example, in response to vessel wall damage or exposure of blood to negatively charged surfaces. They have been expertly reviewed many times 85 86 87 88 89 90 (e.g., are known in the older literature, respectively, as “extrinsic” and “intrinsic” pathways). Fig. 2 shows a basic model of coagulation (redrawn from Kell and Pretorius 74 under a CC-BY license); typically, assembly of fibrin fibers proceeds in a stepwise fashion. In short, after damage to a blood vessel, collagen is exposed and factor (F) VII interacts with tissue factor (TF), forming a complex called TF-FVIIa. FXa and its cofactor Va form the prothrombinase complex and activate thrombin through prothrombin. Finally, the terminal stages of the coagulation pathway happens, where a cross-linked fibrin polymer is formed as a result of fibrinogen (typically present in plasma at 2–4 g/L) conversion to fibrin and cross-linking due to the activation of FXIII, a transglutaminase. Thrombin activates FXIII into FXIIIa, which, in turn, acts on soluble and insoluble fibrin to polymerize it into insoluble cross-linked fibrin clot. This fibrin clot, when viewed under a scanning electron microscope, consists of individually visible fibrin fibers, discussed in the next paragraphs (see Fig. 3A for a representative healthy clot structure created when thrombin is added to plasma. 74 91 92 93
Fig. 2.
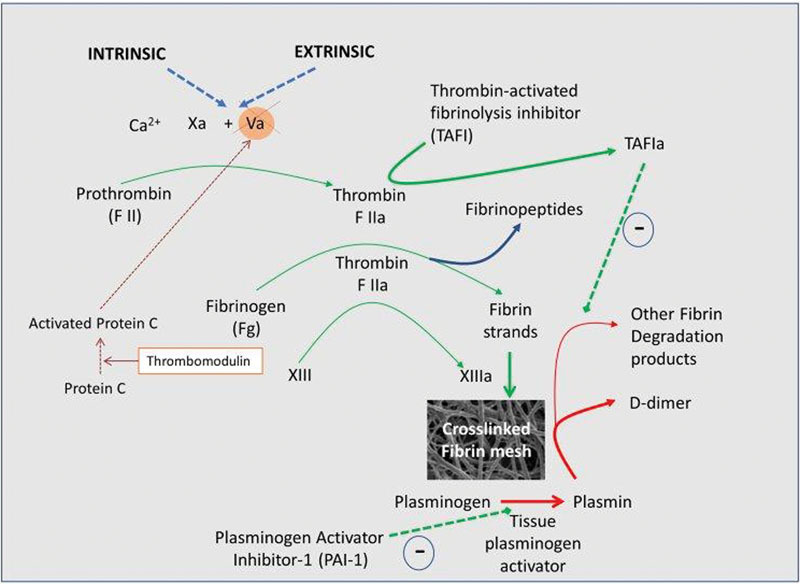
The classical coagulation pathways, where assembly of fibrin fibers proceeds in a stepwise fashion (redrawn from Kell and Pretorius 74 under an open access CC-BY license).
Fig. 3.
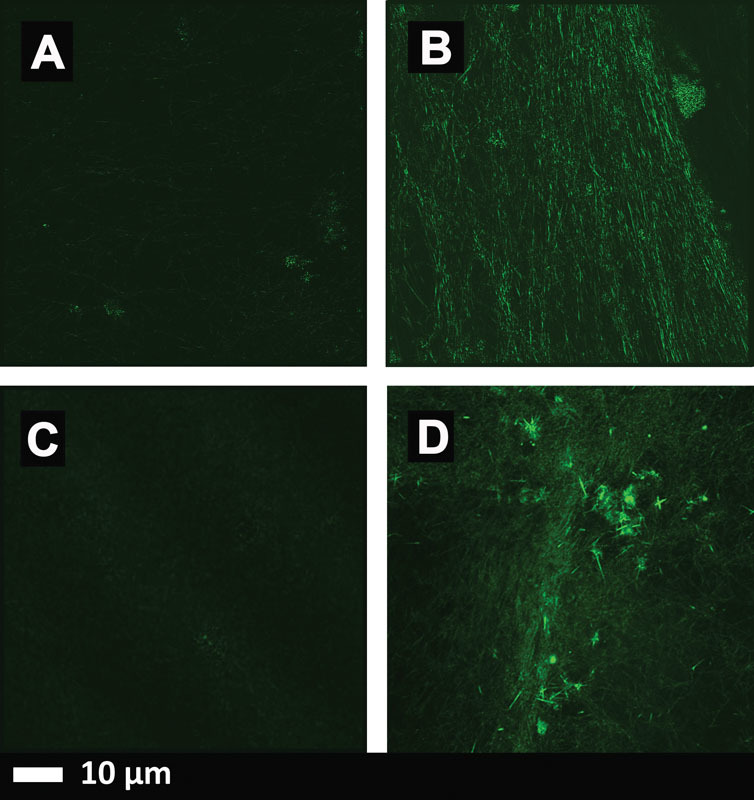
The results of thrombin-mediated blood clotting. (A–C) Micrographs taken with a Zeiss LSM 800 superresolution Airyscan confocal microscopy using the α Plan-Apochromat 63x/1.46 Oil DIC M27 Elyra objective. (D) Micrograph taken with a Zeiss LSM 510 META confocal microscope with a Plan-Apochromat 63x/1.4 Oil DIC objective. ( A ) Healthy platelet poor plasma (PPP) with added thioflavin T (ThT) (5-μM exposure concentration) and thrombin. ( B ) The same PPP, with added lipopolysaccharide (LPS) (0.2 ng/L exposure concentration), followed by addition ThT and thrombin. ( C ) The same PPP, with added LPS followed by LPS-binding protein (2 ng/L final exposure concentration) followed by addition ThT and thrombin. ( D ) PPP, with added LPS (0.2 ng/L exposure concentration), followed by addition ThT and thrombin.
The normal picture of fibrinogen polymerization involves the removal of two fibrinopeptides (i.e., fibrinopeptides A and B) from fibrinogen, which is normally rich in α-helices, leading to its self-association through “knobs and holes,” but with otherwise no major changes in secondary structure. 77 80
Coagulopathies occur when the rate of clot formation or dissolution is unusually fast or slow, and in the case of chronic inflammatory diseases, these seem largely to coexist as hypercoagulation and hypofibrinolysis, arguably implying a common cause. 74 In a series of papers, we have shown in several diseases, such as stroke, 94 95 96 type 2 diabetes, 93 97 Alzheimer's disease, 79 98 99 and hereditary hemochromatosis, 92 that the fibrin clots induced by added thrombin adopted the form of “dense matted deposits” instead of their usual “spaghetti-like” appearance. The same kinds of effect could also be induced by unliganded (i.e., free) iron, 92 100 101 102 although no molecular explanation was (or could be) given. We pick this up in the Amyloid-Like Conformational Transitions in Fibrin(ogen) section. First however, we need to deal with two other topics.
Endotoxin-Induced “Disseminated Intravascular Coagulation”
Endotoxin (LPS) may also induce a runaway form of hypercoagulation 57 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 known as disseminated intravascular coagulation (DIC). There is significant evidence now that DIC is reasonably well defined 46 118 119 120 and that it can directly lead to MOF and death (Cunningham and Nelson, 121 and see the following). We hypothesize here that the form of clotting in DIC in fact involves autocatalytic fibrin(ogen) self-organization leading to amyloid formation, which is consistent with the faster clot formation in the presence of endotoxin 98 and which we have recently shown can occur in vitro with miniscule amounts of LPS. 80 In particular, this may be a major contributor to the various stages of sepsis, SIRS, MODS, and ultimately of organismal death.
Prions, Protein Free Energies, and Amyloid Proteins
Although it was originally shown that at least some proteins, when denatured and renatured, could revert to their original conformation, 122 123 implying that this was (isoenergetic with) the one of lowest free energy, this is now known not to be universal. Leaving aside chaperones and the like, one field in which proteins of the same sequence are well known to adopt radically different conformations, with a much more extensive β-sheet component (that is indeed thermodynamically more stable), is that of prion biology. 124 125 Thus, the prion protein is normally in an α-helix-rich conformation known as PrP c . However, it can also adopt a proteinase K-resistant form of the same sequence, known as PrP Sc . 126 127 128 129 130 131 The PrP c and PrP sc conformations and the catalysis of the conversion to itself by the latter of the former are very well known. The key point for us here, however, is indeed that this definitely implies 77 124 125 128 132 133 134 135 136 that proteins that may initially fold into a certain, ostensibly “native,” conformation can in fact adopt stable and more β-sheet-rich conformations of a lower free energy, separated from that of the original conformation by a potentially significant energy barrier.
Amyloid-Like Conformational Transitions in Fibrin(ogen)
As mentioned earlier, the general view (also see the following) is that no major secondary structural changes occur during normal fibrin formation. 77 85 87 However, we know of at least three circumstances in which fibrin can (i.e., is known to) adopt a β-sheet-rich conformation: (1) in the case of specific mutant sequences of the fibrinogen a chain, 137 138 139 140 141 142 143 (2) when fibrin is stretched mechanically beyond a certain limit, 144 145 146 147 148 149 150 and (3) when formed in the presence of certain small molecules, including bacterial LPS. 76 80 151 Thus, it is well established that fibrin can form β-sheet-rich amyloids, although it is assumed that conventional blood clotting involves only a “knobs-and-holes” mechanism, without any major changes in secondary structure. 85 86 87 88 89 90 152 153 We hypothesize here that the “dense matted deposits” seen earlier are in fact β-sheet-rich amyloids, and that it is this coagulopathy in particular that contributes significantly to the procession of sepsis along or through the cascade of toxicity outlined in Fig. 1 . To be specific, we consider that the binding of the LPS must be to fibrinogen itself since only this is preexisting and we have demonstrated it directly using isothermal calorimetry. 80 We note too that there is almost no “free” LPS except immediately after its addition/liberation from a bacterium, and that the kinetics of fibrinogen polymerization during thrombin-induced clotting are so fast that it is not necessary to invoke subsequent binding of LPS to protofibrils and so on as part of the mechanism of amyloidogenesis and toxicity.
In particular, thioflavin T (ThT) is a stain whose fluorescence (when excited at 440–450 nm or so) is massively enhanced upon binding to β-sheet-rich amyloids 154 155 156 157 158 159 160 161 162 163 (whose conformation differs markedly from that of “normal” β-sheets in proteins, else it would stain most such proteins). Fig. 3A to C show micrographs taken of clots with a Zeiss superresolution microscope using Airyscan technology (Carl Zeiss), and Fig. 3D shows a micrograph taken using a Zeiss confocal microscope (see legend for specific detail). Fig. 3A is a micrograph of healthy platelet poor plasma (PPP) with added ThT and thrombin. This is a representative micrograph to show “normal” clot structure, whereas Fig. 3B and D shows healthy PPP with added LPS and ThT. High-resolution Airyscan technology ( Fig. 3B ) shows ThT binding to areas where β-sheet-rich amyloids were induced by LPS. Fig. 3C shows PPP preexposed to LPS, followed by exposure to LPS-binding protein, ThT, and thrombin. LPS-binding protein was able to reverse the formation of the β-sheet-rich amyloids areas created by preexposure to LPS. Confocal microscopy ( Fig. 3D ) also shows this ThT binding to β-sheet-rich amyloid areas. However, individual binding areas are not as clearly visible as with the Airyscan technology. Nonetheless, the extent of β-amyloid formation in the LPS-treated over the two controls is very striking.
We also note the important analyses of Strickland et al to the effect that β-amyloid can interact with fibrin(ogen) 164 165 166 167 168 169 170 and cause it to become refractory to fibrinolysis. 170 171 172 173
Inflammatory Nature of Fibrin
The fact that fibrin itself is, or can be, inflammatory is well established 108 174 175 176 177 178 179 and does not need further elaboration. Our main point here is that in none of these studies has it been established whether (or to what extent) the fibrin is in an amyloid form or not so far. Certainly, it is very well established that amyloids can be inflammatory. 180 181 182 183
Further Evidence for the “Trigger” Role of LPS in Large-Scale Amyloid Formation
In our previous studies, 80 we found that LPS (endotoxin) at a concentration of just 0.2 ng/L could trigger the conversion of some 10 8 times more fibrinogen molecules, 80 and that the fibrin fibers so formed were amyloid in nature. (A very large amplification of structural molecular transitions could also be induced by LPS in a nematic liquid crystal. 184 185 186 ) Only some kind of autocatalytic processes can easily explain this kind of polymerization, just as occurs in prions, 77 129 131 where iron may also be involved. 70 71 187 188 189 To be explicit, the only feasible explanation is one in which an initial fibrinogen molecule with bound LPS adopts, at least on the loss of its fibrinopeptides, conformations in which the subsequent fibrinopeptide-less fibrinogens must also change their conformations to bind to it and so on as fibrinogens become protofibrils, protofibrils become fibrils, and so on. Put another way, if LPS is the only (and highly substoichiometric) addition to thrombin-induced fibrin formation, there must be an “autocatalytic process,” somewhat analogous to a prion, that must be taking place since rather than having conventional strands of fibrin, we have amorphous, denatured β sheets.
Cytotoxicity of Amyloids
Cytotoxicity of amyloids is so well known 98 182 190 191 192 193 194 195 196 197 that it barely needs rehearsing. However, the relative toxicities of soluble material, protofibrils, fibrils, and so on are less well understood, 198 in part because they can equilibrate with each other even if added as a “pure” component (of a given narrow NB equilibrate range). Although the larger fibrils are much more easily observable microscopically, there is a great deal of evidence that it is the smaller ones that are the more cytotoxic. 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 So far as is known, almost all (cf. Holm et al 217 ) the established forms of amyloid are cytotoxic. However, the tests have not yet been performed for the fibrin version since it has only very recently been discovered. 77 80 This is an urgent task for the future.
Sequelae Consistent with the Role of Amyloids in the “Sepsis Cascade” to Organ Failure and Death
If vascular or systemic amyloidogenesis really is a significant contributor to the worsening patient conditions as septic shock moves toward MOF/MODS and death, with the cytotoxic amyloids (whether from fibrin and/or otherwise) in effect being largely responsible for the MOF, then one might expect it to be visible as amyloid deposits in organs such as the kidney (whether as biopsies or postmortem). It is certainly possible to find evidence for this, 218 219 220 221 222 223 and our proposal is that such amyloid should be sought using ThT or other suitable staining in autopsy tissue.
Hypo- or Hypertensive States
A hallmark of most of the chronic, inflammatory diseases that we have considered here and elsewhere (as cited) is that they are either normotensive or (to varying degrees) hypertensive. By contrast, sepsis and septic shock are strongly hypotensive (accompanied by hypoperfusion), 224 225 226 227 228 and their normalization is considered a crucial factor for lowering mortality. Consequently, this bears a brief discussion. Of course, at one level, it is common in biology that something can be a stimulus (e.g., of blood pressure) at a low concentration and can be an inhibitor at a high concentration (this is known as “hormesis” 229 230 231 ). At a descriptive level, this is clearly happening here. As it stands, however, we can find no literature that has compared changes in tension as the dose of endotoxin is varied, with the doses given in such studies of endotoxin-induced shock normally being sufficient to induce significant hypotension. 232 233 234 It is, however, of considerable interest that this endotoxin-induced hypotension (and other sequelae) could be relieved by antithrombin (e.g., 233 235 236 237 238 239 240 241 242 243 244 245 246 247 ), implying a contributing role for coagulopathies in the hypotension otherwise observed, albeit other mechanisms are possible. 238
How Might This Understanding Lead to Improved Treatment Options?
Over the years, there have been many high-profile failures of therapies for various aspects of severe inflammation, sepsis, septic shock, and SIRS. These include therapies aimed at endotoxin itself (Centoxin), 248 249 250 and the use of recombinant activated protein C 251 or Drotrecogin alfa. 2 250 252 253 Anticytokine and anti-inflammatory treatments have also had, at best, mixed results. 254 255
However, the overall picture that we have come to is given in Fig. 4 . This implies that we might hope to stop the progress of the sepsis/SIRS/MODS cascade at any (preferably several 256 ) of several other places, including through iron chelation, 70 71 257 258 the use of anti-inflammatory agents, the use of anticoagulants such as heparin 178 or antithrombin, 233 238 243 and the use of stimulants of fibrinolysis. 259 The success of heparin 260 261 (see also Zarychanski et al, van Roessel et al, and Okamoto et al 262 263 264 ) is especially noteworthy in the context of the present hypothesis, though it may have multiple (not simply directly anticoagulant) actions. 265 266 The same is true of antithrombin. 233 235 236 237 238 239 240 241 242 243 244 245 246 247 267 268 However, antithrombin has also not been efficacious, especially in combination with heparin 269 and is globally not recommended in sepsis therapy guidelines. 4 270 Indeed, suppressing coagulation in sepsis globally may be inimical, as it is thought to serve a protective role in the initial stages of the disease. 271 It is also noteworthy that high-density lipoprotein (HDL) cholesterol is a protective against sepsis 18 272 273 274 (HDL are antioxidant 275 and anti-inflammatory 276 and can also bind and neutralize endotoxin 277 278 279 ). Therefore, the beneficial role of certain statins in sepsis 280 281 should be seen in the context of their much more potent anti-inflammatory role 70 rather than in any (modest) role involved in lowering overall serum cholesterol. Phospholipid emulsions may also serve. 282
Fig. 4.
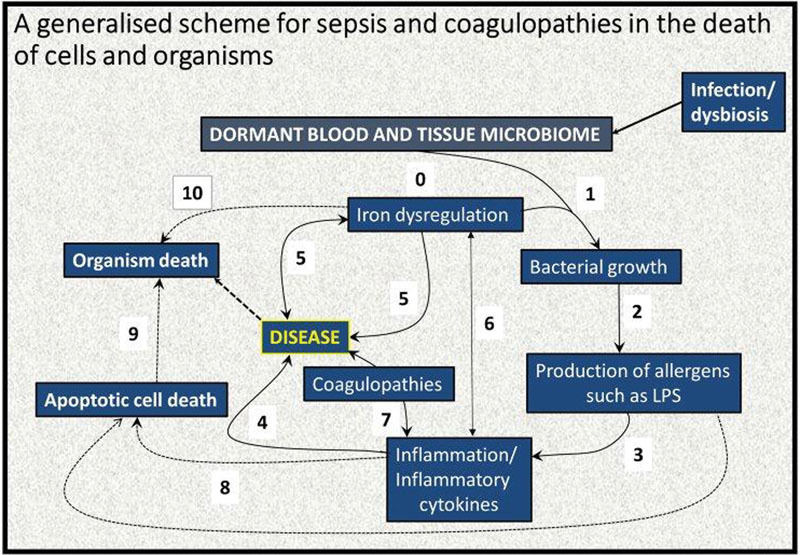
A systems biology model of the development of coagulopathies during sepsis, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome. An elementary systems biology model of how iron dysregulation can stimulate dormant bacterial growth that can, in turn, lead to antigen production (e.g., of lipopolysaccharide [LPS]) that can then trigger inflammation leading to cell death 70 71 and a variety of diseases. While it is recognized that this simple diagram is very far from capturing the richness of these phenomena, there is abundant evidence for each of these steps, starting with ( 0 ) an infection/gut dysbiosis and the creation of a (dormant) blood and tissue microbiome. This is typically accompanied by ( 1 ) iron dysregulation, which is known to be present in many diseases, as both cause and result ( 5 ) and as an important cause of inflammation ( 6 ) and even organism death ( 10 ). Iron, in turn, feeds bacterial growth ( 2 ), leading to production of, for example, LPS with an accompanying upregulated inflammatory cytokine profile ( 3 ), leading to disease ( 4 ). In inflammation both apoptotic death ( 8 ) and coagulopathies ( 7 ) are well-known phenomena. In turn, apoptotic death can lead to organism death ( 9 ).
We have noted previously (reviewed in Kell and Pretorius 74 ) that such “dense matter deposits” (now recognized as amyloid forms) are much more resistant to fibrinolysis than is “normal” fibrinogen. The working hypothesis here is that the β-sheet-rich forms are more resistant to proteolysis because (as in prions, where the structures are known) the residues normally targeted by the relevant proteases are no longer exposed. Clearly, the removal of such structures would benefit from the development of novel proteases to which they are susceptible.
Recombinant soluble human thrombomodulin (TM-α) is a novel anticoagulant drug and has been found to have significant efficacy in the treatment of sepsis-based DIC, 247 283 284 285 286 287 288 289 290 291 292 293 albeit fully powered randomized trials are awaited, 294 295 again adding further weight to our hypothesis. As Okamoto et al 264 point out, “In the European Union and the USA, the 2012 guidelines of the Surviving Sepsis Campaign do not recommend treatment for septic DIC. 4 296 In contrast, in Japan, aggressive treatment of septic DIC is encouraged,” 297 298 299 300 and that “that Japan is one of the countries that most effectively treats patients with septic DIC.” 264 A recent meta-analysis of randomized controlled trials for the efficacy and safety of anticoagulant therapy demonstrated that such therapy has a survival benefit in those with sepsis-induced DIC, but not in the overall population with sepsis or even in populations with sepsis-induced coagulopathy. 271 We note that the influence of soluble TM may be mediated by its indirect thrombin inhibition by binding and not localizing it to a site where protein C is activated.
Thus, if it is accepted that the type of fibrin that is formed is substantially of the amyloid variety, then anticoagulant and other drugs that inhibit or reverse such amyloid processes should also be of value, 301 as they seem to be in Alzheimer-type dementia. 165 302 303
Concluding Remarks
We conclude by showing our line of thought in Fig. 5 . There is by now abundant evidence that coagulopathies involving fibrin clots are a major part of sepsis, SIRS, septic shock, MODS, DIC, and organismal death. We have invoked further evidence that the type of fibrin involved is an amyloid form and have suggested that it is this that is especially damaging. This definitely needs to be tested further, for instance, using appropriate stains 155 304 and/or X-ray measurements 305 306 307 in concert with cellular toxicity assays. The former could easily be performed in or near the intensive therapy unit. LPS and other substances have now been shown to cause anomalous forms of fibrin, which opens up many novels lines of work, such that reducing or eliminating them might be worthwhile, for example, with LPS-binding protein. Finally, as a corollary of the above, we suggest that anticoagulant therapies that inhibit or reverse those β-amyloid forms of fibrin production will be especially valuable. To this end, lowering the levels of fibrinogen itself would seem to be a desirable aim. 308
Fig. 5.
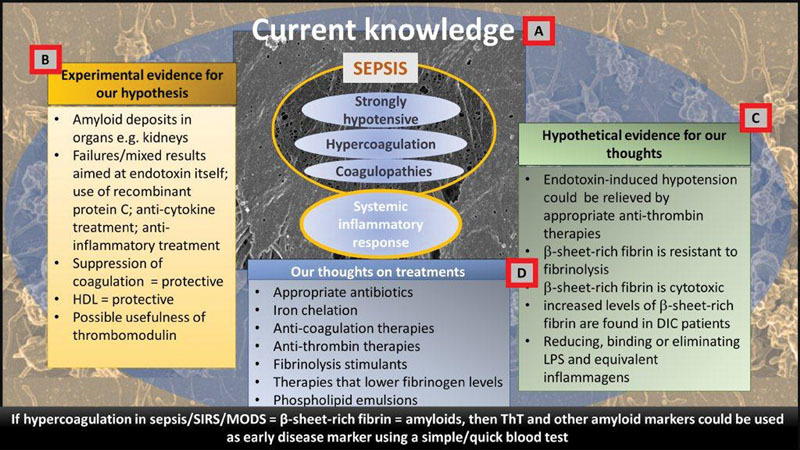
A schematic representation outlining our hypothesis based on current knowledge (A) , experimental evidence for our hypothesis (B) , hypothetical evidence ( C ), and finally our thoughts on (new) treatment regime approaches and early disease diagnosis ( D ).
Acknowledgments
D.B.K. thanks Prof. Nigel Harper for a very useful discussion. We also thank the referees and the journal editors for exceptionally careful and thoughtful reviews that helped improve the manuscript considerably.
Funding Statement
Funding We thank the Biotechnology and Biological Sciences Research Council (grant BB/L025752/1) as well as the National Research Foundation (NRF) and Medical Research Council (MRC) of South Africa for supporting this collaboration.
Conflict of Interest The authors have nothing to disclose.
Ethical Approval Disclosure
Ethical approval was granted by the University of Pretoria for all human studies (Human Ethics Committee: Faculty of Health Sciences) to Etheresia Pretorius.
References
- 1.Martin G S, Mannino D M, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri V M, Thompson B T, Barie P S et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 3.Angus D C, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(09):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 4.Dellinger R P, Levy M M, Rhodes A et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(02):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson E K, Rubenstein A R, Radin G T, Wiener R S, Walkey A J. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(03):625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann C, Scherag A, Adhikari N K et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(03):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 7.Beck M K, Jensen A B, Nielsen A B, Perner A, Moseley P L, Brunak S. Diagnosis trajectories of prior multi-morbidity predict sepsis mortality. Sci Rep. 2016;6:36624. doi: 10.1038/srep36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J.The immunopathogenesis of sepsis Nature 2002420(6917):885–891. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(02):145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 10.Russell J A. Management of sepsis. N Engl J Med. 2006;355(16):1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 11.Wiersinga W J, Leopold S J, Cranendonk D R, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5(01):36–44. doi: 10.4161/viru.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaprelyants A S, Gottschal J C, Kell D B.Dormancy in non-sporulating bacteria FEMS Microbiol Rev 199310(3-4):271–285. [DOI] [PubMed] [Google Scholar]
- 13.Kell D B, Kaprelyants A S, Weichart D H, Harwood C R, Barer M R. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie van Leeuwenhoek. 1998;73(02):169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 14.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(01):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 15.Buerger S, Spoering A, Gavrish E, Leslin C, Ling L, Epstein S S. Microbial scout hypothesis, stochastic exit from dormancy, and the nature of slow growers. Appl Environ Microbiol. 2012;78(09):3221–3228. doi: 10.1128/AEM.07307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kell D, Potgieter M, Pretorius E. Individuality, phenotypic differentiation, dormancy and ‘persistence’ in culturable bacterial systems: commonalities shared by environmental, laboratory, and clinical microbiology. F1000 Res. 2015;4:179. doi: 10.12688/f1000research.6709.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew W Y, Bayna E, Molle E D et al. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One. 2013;8(04):e61057. doi: 10.1371/journal.pone.0061057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kell D B, Pretorius E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr Biol. 2015;7(11):1339–1377. doi: 10.1039/c5ib00158g. [DOI] [PubMed] [Google Scholar]
- 19.Morath S, Geyer A, Hartung T. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J Exp Med. 2001;193(03):393–397. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schröder N W, Morath S, Alexander C et al. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278(18):15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 21.Baik J E, Ryu Y H, Han J Y et al. Lipoteichoic acid partially contributes to the inflammatory responses to Enterococcus faecalis. J Endod. 2008;34(08):975–982. doi: 10.1016/j.joen.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Jeon J H, Kim S K, Baik J E et al. Lipoteichoic acid of Staphylococcus aureus enhances IL-6 expression in activated human basophils. Comp Immunol Microbiol Infect Dis. 2012;35(04):363–374. doi: 10.1016/j.cimid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Belum G R, Belum V R, Chaitanya Arudra S K, Reddy B S. The Jarisch-Herxheimer reaction: revisited. Travel Med Infect Dis. 2013;11(04):231–237. doi: 10.1016/j.tmaid.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Cheung C M, Chee S P. Jarisch-Herxheimer reaction: paradoxical worsening of tuberculosis chorioretinitis following initiation of antituberculous therapy. Eye (Lond) 2009;23(06):1472–1473. doi: 10.1038/eye.2008.204. [DOI] [PubMed] [Google Scholar]
- 25.Fekade D, Knox K, Hussein K et al. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med. 1996;335(05):311–315. doi: 10.1056/NEJM199608013350503. [DOI] [PubMed] [Google Scholar]
- 26.Guerrier G, D'Ortenzio E. The Jarisch-Herxheimer reaction in leptospirosis: a systematic review. PLoS One. 2013;8(03):e59266. doi: 10.1371/journal.pone.0059266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prins J M, van Deventer S J, Kuijper E J, Speelman P. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob Agents Chemother. 1994;38(06):1211–1218. doi: 10.1128/aac.38.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirikae T, Nakano M, Morrison D C. Antibiotic-induced endotoxin release from bacteria and its clinical significance. Microbiol Immunol. 1997;41(04):285–294. doi: 10.1111/j.1348-0421.1997.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 29.Holzheimer R G.Antibiotic induced endotoxin release and clinical sepsis: a review J Chemother 200113(Spec No 1):159–172. [DOI] [PubMed] [Google Scholar]
- 30.Lepper P M, Held T K, Schneider E M, Bölke E, Gerlach H, Trautmann M. Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 2002;28(07):824–833. doi: 10.1007/s00134-002-1330-6. [DOI] [PubMed] [Google Scholar]
- 31.Levy M M, Fink M P, Marshall J C et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(04):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 32.Bozza F A, Salluh J I, Japiassu A M et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(02):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Elia R V, Harrison K, Oyston P C, Lukaszewski R A, Clark G C. Targeting the “cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20(03):319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison C. Sepsis: calming the cytokine storm. Nat Rev Drug Discov. 2010;9(05):360–361. doi: 10.1038/nrd3162. [DOI] [PubMed] [Google Scholar]
- 35.Oldstone M B, Rosen H. Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. Curr Top Microbiol Immunol. 2014;378:129–147. doi: 10.1007/978-3-319-05879-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tisoncik J R, Korth M J, Simmons C P, Farrar J, Martin T R, Katze M G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(01):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26(06):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Weigand M A, Hörner C, Bardenheuer H J, Bouchon A. The systemic inflammatory response syndrome. Best Pract Res Clin Anaesthesiol. 2004;18(03):455–475. doi: 10.1016/j.bpa.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101(03):189–198. doi: 10.1254/jphs.crj06010x. [DOI] [PubMed] [Google Scholar]
- 40.Ratzinger F, Schuardt M, Eichbichler K et al. Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS One. 2013;8(12):e82946. doi: 10.1371/journal.pone.0082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichsoellner M, Raggam R B, Wagner J, Krause R, Hoenigl M. Clinical evaluation of multiple inflammation biomarkers for diagnosis and prognosis for patients with systemic inflammatory response syndrome. J Clin Microbiol. 2014;52(11):4063–4066. doi: 10.1128/JCM.01954-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunne W M., Jr Laboratory diagnosis of sepsis? No SIRS, not just yet. J Clin Microbiol. 2015;53(08):2404–2409. doi: 10.1128/JCM.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stubljar D, Skvarc M. Effective strategies for diagnosis of systemic inflammatory response syndrome (SIRS) due to bacterial infection in surgical patients. Infect Disord Drug Targets. 2015;15(01):53–56. doi: 10.2174/1871526515666150320161804. [DOI] [PubMed] [Google Scholar]
- 44.Brown K A, Brain S D, Pearson J D, Edgeworth J D, Lewis S M, Treacher D F.Neutrophils in development of multiple organ failure in sepsis Lancet 2006368(9530):157–169. [DOI] [PubMed] [Google Scholar]
- 45.Johnson D, Mayers I. Multiple organ dysfunction syndrome: a narrative review. Can J Anaesth. 2001;48(05):502–509. doi: 10.1007/BF03028318. [DOI] [PubMed] [Google Scholar]
- 46.Gando S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38(02):S35–S42. doi: 10.1097/CCM.0b013e3181c9e31d. [DOI] [PubMed] [Google Scholar]
- 47.Laster S M, Wood J G, Gooding L R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141(08):2629–2634. [PubMed] [Google Scholar]
- 48.Sridharan H, Upton J W. Programmed necrosis in microbial pathogenesis. Trends Microbiol. 2014;22(04):199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5(01):66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer M. Biomarkers in sepsis. Curr Opin Pulm Med. 2013;19(03):305–309. doi: 10.1097/MCP.0b013e32835f1b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singer M, Deutschman C S, Seymour C W et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(08):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Churpek M M, Snyder A, Han X et al. qSOFA, SIRS, and early warning scores for detecting clinical deterioration in infected patients outside the ICU. Am J Respir Crit Care Med. 2017;195(07):906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent J L, Moreno R, Takala J et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(07):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 54.Eisele B, Lamy M. Clinical experience with antithrombin III concentrates in critically ill patients with sepsis and multiple organ failure. Semin Thromb Hemost. 1998;24(01):71–80. doi: 10.1055/s-2007-995825. [DOI] [PubMed] [Google Scholar]
- 55.Satran R, Almog Y. The coagulopathy of sepsis: pathophysiology and management. Isr Med Assoc J. 2003;5(07):516–520. [PubMed] [Google Scholar]
- 56.Dempfle C E. Coagulopathy of sepsis. Thromb Haemost. 2004;91(02):213–224. doi: 10.1160/TH03-03-0182. [DOI] [PubMed] [Google Scholar]
- 57.Kinasewitz G T, Yan S B, Basson B et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit Care. 2004;8(02):R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iba T, Gando S, Murata A et al. Predicting the severity of systemic inflammatory response syndrome (SIRS)-associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J Trauma. 2007;63(05):1093–1098. doi: 10.1097/01.ta.0000251420.41427.d3. [DOI] [PubMed] [Google Scholar]
- 59.Ogura H, Gando S, Iba T et al. SIRS-associated coagulopathy and organ dysfunction in critically ill patients with thrombocytopenia. Shock. 2007;28(04):411–417. doi: 10.1097/shk.0b013e31804f7844. [DOI] [PubMed] [Google Scholar]
- 60.Gando S. Role of fibrinolysis in sepsis. Semin Thromb Hemost. 2013;39(04):392–399. doi: 10.1055/s-0033-1334140. [DOI] [PubMed] [Google Scholar]
- 61.Hoppensteadt D, Tsuruta K, Cunanan J et al. Thrombin generation mediators and markers in sepsis-associated coagulopathy and their modulation by recombinant thrombomodulin. Clin Appl Thromb Hemost. 2014;20(02):129–135. doi: 10.1177/1076029613492875. [DOI] [PubMed] [Google Scholar]
- 62.Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39(05):559–566. doi: 10.1055/s-0033-1343894. [DOI] [PubMed] [Google Scholar]
- 63.Ostrowski S R, Berg R M, Windeløv N A et al. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J Crit Care. 2013;28(05):586–596. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Saracco P, Vitale P, Scolfaro C, Pollio B, Pagliarino M, Timeus F. The coagulopathy in sepsis: significance and implications for treatment. Pediatr Rep. 2011;3(04):e30. doi: 10.4081/pr.2011.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Semeraro N, Ammollo C T, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Hemost. 2015;41(06):650–658. doi: 10.1055/s-0035-1556730. [DOI] [PubMed] [Google Scholar]
- 66.Simmons J, Pittet J F. The coagulopathy of acute sepsis. Curr Opin Anaesthesiol. 2015;28(02):227–236. doi: 10.1097/ACO.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muronoi T, Koyama K, Nunomiya S et al. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb Res. 2016;144:169–175. doi: 10.1016/j.thromres.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Tan L, Huang Y, Pan X et al. Administration of bone marrow stromal cells in sepsis attenuates sepsis-related coagulopathy. Ann Med. 2016;48(04):235–245. doi: 10.3109/07853890.2016.1157725. [DOI] [PubMed] [Google Scholar]
- 69.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Kell D B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kell D B. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 2010;84(11):825–889. doi: 10.1007/s00204-010-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kell D B, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(04):748–773. doi: 10.1039/c3mt00347g. [DOI] [PubMed] [Google Scholar]
- 73.Pretorius E, Kell D B. Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integr Biol. 2014;6(05):486–510. doi: 10.1039/c4ib00025k. [DOI] [PubMed] [Google Scholar]
- 74.Kell D B, Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen) Integr Biol. 2015;7(01):24–52. doi: 10.1039/c4ib00173g. [DOI] [PubMed] [Google Scholar]
- 75.Potgieter M, Bester J, Kell D B, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. 2015;39(04):567–591. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kell D B, Kenny L C. A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol. 2016;3:60. doi: 10.3389/fmed.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kell D B, Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Prog Biophys Mol Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Pretorius E, Akeredolu O O, Soma P, Kell D B. Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med (Maywood) 2017;242(04):355–373. doi: 10.1177/1535370216681549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pretorius E, Bester J, Kell D B. A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease. J Alzheimers Dis. 2016;53(04):1237–1256. doi: 10.3233/JAD-160318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pretorius E, Mbotwe S, Bester J, Robinson C J, Kell D B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface. 2016;13(122):2.0160539E7. doi: 10.1098/rsif.2016.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ebringer A, Rashid T, Wilson C. Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper. Autoimmun Rev. 2010;9(04):216–223. doi: 10.1016/j.autrev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Ebringer A. London: Springer; 2012. Rheumatoid Arthritis and Proteus. [Google Scholar]
- 83.Marshall B J, Warren J R.Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration Lancet 19841(8390):1311–1315. [DOI] [PubMed] [Google Scholar]
- 84.Itzhaki R F, Lathe R, Balin B J et al. Microbes and Alzheimer's disease. J Alzheimers Dis. 2016;51(04):979–984. doi: 10.3233/JAD-160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weisel J W. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–299. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 86.Cilia La Corte A L, Philippou H, Ariëns R A. Role of fibrin structure in thrombosis and vascular disease. Adv Protein Chem Struct Biol. 2011;83:75–127. doi: 10.1016/B978-0-12-381262-9.00003-3. [DOI] [PubMed] [Google Scholar]
- 87.Undas A, Ariëns R A. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31(12):e88–e99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 88.Undas A, Nowakowski T, Cieśla-Dul M, Sadowski J. Abnormal plasma fibrin clot characteristics are associated with worse clinical outcome in patients with peripheral arterial disease and thromboangiitis obliterans. Atherosclerosis. 2011;215(02):481–486. doi: 10.1016/j.atherosclerosis.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 89.Wolberg A S. Determinants of fibrin formation, structure, and function. Curr Opin Hematol. 2012;19(05):349–356. doi: 10.1097/MOH.0b013e32835673c2. [DOI] [PubMed] [Google Scholar]
- 90.Undas A. Fibrin clot properties and their modulation in thrombotic disorders. Thromb Haemost. 2014;112(01):32–42. doi: 10.1160/TH14-01-0032. [DOI] [PubMed] [Google Scholar]
- 91.Pretorius E, Vermeulen N, Bester J, Lipinski B, Kell D B. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol Mech Methods. 2013;23(05):352–359. doi: 10.3109/15376516.2012.762082. [DOI] [PubMed] [Google Scholar]
- 92.Pretorius E, Bester J, Vermeulen N, Lipinski B, Gericke G S, Kell D B. Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One. 2014;9(01):e85271. doi: 10.1371/journal.pone.0085271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pretorius E, Bester J, Vermeulen N et al. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc Diabetol. 2015;14:30. doi: 10.1186/s12933-015-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pretorius E, Windberger U B, Oberholzer H M, Auer R E. Comparative ultrastructure of fibrin networks of a dog after thrombotic ischaemic stroke. Onderstepoort J Vet Res. 2010;77(01):E1–E4. doi: 10.4102/ojvr.v77i1.4. [DOI] [PubMed] [Google Scholar]
- 95.Pretorius E, Swanepoel A C, Oberholzer H M, van der Spuy W J, Duim W, Wessels P F. A descriptive investigation of the ultrastructure of fibrin networks in thrombo-embolic ischemic stroke. J Thromb Thrombolysis. 2011;31(04):507–513. doi: 10.1007/s11239-010-0538-5. [DOI] [PubMed] [Google Scholar]
- 96.Pretorius E, Steyn H, Engelbrecht M, Swanepoel A C, Oberholzer H M. Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul Fibrinolysis. 2011;22(08):696–700. doi: 10.1097/MBC.0b013e32834bdb32. [DOI] [PubMed] [Google Scholar]
- 97.Pretorius E, Oberholzer H M, van der Spuy W J, Swanepoel A C, Soma P. Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinolysis. 2011;22(06):463–467. doi: 10.1097/MBC.0b013e3283468a0d. [DOI] [PubMed] [Google Scholar]
- 98.Bester J, Soma P, Kell D B, Pretorius E. Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS) Oncotarget. 2015;6(34):35284–35303. doi: 10.18632/oncotarget.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lipinski B, Pretorius E. Iron-induced fibrin formation may explain vascular pathology in Alzheimer's disease. Folia Neuropathol. 2014;52(02):205. [PubMed] [Google Scholar]
- 100.Lipinski B, Pretorius E. Novel pathway of iron‑induced blood coagulation: implications for diabetes mellitus and its complications. Pol Arch Med Wewn. 2012;122(03):115–122. [PubMed] [Google Scholar]
- 101.Lipinski B, Pretorius E. Iron-induced fibrin in cardiovascular disease. Curr Neurovasc Res. 2013;10(03):269–274. doi: 10.2174/15672026113109990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pretorius E, Vermeulen N, Bester J, Lipinski B. Novel use of scanning electron microscopy for detection of iron-induced morphological changes in human blood. Microsc Res Tech. 2013;76(03):268–271. doi: 10.1002/jemt.22163. [DOI] [PubMed] [Google Scholar]
- 103.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(01):20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duburcq T, Tournoys A, Gnemmi V et al. Impact of obesity on endotoxin-induced disseminated intravascular coagulation. Shock. 2015;44(04):341–347. doi: 10.1097/SHK.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 105.Bick R L. Disseminated intravascular coagulation: a review of etiology, pathophysiology, diagnosis, and management: guidelines for care. Clin Appl Thromb Hemost. 2002;8(01):1–31. doi: 10.1177/107602960200800103. [DOI] [PubMed] [Google Scholar]
- 106.Kaneko T, Wada H. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. J Clin Exp Hematop. 2011;51(02):67–76. doi: 10.3960/jslrt.51.67. [DOI] [PubMed] [Google Scholar]
- 107.Levi M.The coagulant response in sepsis and inflammation Hamostaseologie 2010300110–12., 14–16 [PubMed] [Google Scholar]
- 108.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16 01:S11–S21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 109.Khemani R G, Bart R D, Alonzo T A, Hatzakis G, Hallam D, Newth C J. Disseminated intravascular coagulation score is associated with mortality for children with shock. Intensive Care Med. 2009;35(02):327–333. doi: 10.1007/s00134-008-1280-8. [DOI] [PubMed] [Google Scholar]
- 110.Levi M, van der Poll T. Disseminated intravascular coagulation: a review for the internist. Intern Emerg Med. 2013;8(01):23–32. doi: 10.1007/s11739-012-0859-9. [DOI] [PubMed] [Google Scholar]
- 111.Thachil J, Toh C H. Current concepts in the management of disseminated intravascular coagulation. Thromb Res. 2012;129 01:S54–S59. doi: 10.1016/S0049-3848(12)70017-8. [DOI] [PubMed] [Google Scholar]
- 112.Wada H, Matsumoto T, Yamashita Y, Hatada T. Disseminated intravascular coagulation: testing and diagnosis. Clin Chim Acta. 2014;436:130–134. doi: 10.1016/j.cca.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 113.Wu L C, Lin X, Sun H. Tanshinone IIA protects rabbits against LPS-induced disseminated intravascular coagulation (DIC) Acta Pharmacol Sin. 2012;33(10):1254–1259. doi: 10.1038/aps.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Z, Li J N, Bai Z Q, Lin X. Antagonism by salvianolic acid B of lipopolysaccharide-induced disseminated intravascular coagulation in rabbits. Clin Exp Pharmacol Physiol. 2014;41(07):502–508. doi: 10.1111/1440-1681.12242. [DOI] [PubMed] [Google Scholar]
- 115.Yu P X, Zhou Q J, Zhu W W et al. Effects of quercetin on LPS-induced disseminated intravascular coagulation (DIC) in rabbits. Thromb Res. 2013;131(06):e270–e273. doi: 10.1016/j.thromres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Nguyen T C, Gushiken F, Correa J I et al. A recombinant fragment of von Willebrand factor reduces fibrin-rich microthrombi formation in mice with endotoxemia. Thromb Res. 2015;135(05):1025–1030. doi: 10.1016/j.thromres.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeerleder S, Hack C E, Wuillemin W A. Disseminated intravascular coagulation in sepsis. Chest. 2005;128(04):2864–2875. doi: 10.1378/chest.128.4.2864. [DOI] [PubMed] [Google Scholar]
- 118.Gando S, Saitoh D, Ogura H et al. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(01):145–150. doi: 10.1097/01.CCM.0000295317.97245.2D. [DOI] [PubMed] [Google Scholar]
- 119.Semeraro N, Ammollo C T, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2(03):e2010024. doi: 10.4084/MJHID.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gando S, Meziani F, Levi M. What's new in the diagnostic criteria of disseminated intravascular coagulation? Intensive Care Med. 2016;42(06):1062–1064. doi: 10.1007/s00134-016-4257-z. [DOI] [PubMed] [Google Scholar]
- 121.Cunningham F G, Nelson D B. Disseminated intravascular coagulation syndromes in obstetrics. Obstet Gynecol. 2015;126(05):999–1011. doi: 10.1097/AOG.0000000000001110. [DOI] [PubMed] [Google Scholar]
- 122.Anfinsen C B, Haber E, Sela M, White F H., Jr The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc Natl Acad Sci U S A. 1961;47:1309–1314. doi: 10.1073/pnas.47.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anfinsen C B.Principles that govern the folding of protein chains Science 1973181(4096):223–230. [DOI] [PubMed] [Google Scholar]
- 124.Cohen F E, Prusiner S B. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 125.Harrison P M, Chan H S, Prusiner S B, Cohen F E. Thermodynamics of model prions and its implications for the problem of prion protein folding. J Mol Biol. 1999;286(02):593–606. doi: 10.1006/jmbi.1998.2497. [DOI] [PubMed] [Google Scholar]
- 126.Prusiner S B. Prions. Proc Natl Acad Sci U S A. 1998;95(23):13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aguzzi A. Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J Neurochem. 2006;97(06):1726–1739. doi: 10.1111/j.1471-4159.2006.03909.x. [DOI] [PubMed] [Google Scholar]
- 128.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 129.Aguzzi A, Lakkaraju A K. Cell biology of prions and prionoids: a status report. Trends Cell Biol. 2016;26(01):40–51. doi: 10.1016/j.tcb.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 130.Colby D W, Prusiner S B. Prions. Cold Spring Harb Perspect Biol. 2011;3(01):a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prusiner S B. Biology and genetics of prions causing neurodegeneration. Annu Rev Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collinge J, Clarke A R.A general model of prion strains and their pathogenicity Science 2007318(5852):930–936. [DOI] [PubMed] [Google Scholar]
- 133.Aguzzi A, Calella A M. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89(04):1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- 134.Ashe K H, Aguzzi A. Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion. 2013;7(01):55–59. doi: 10.4161/pri.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watts J C, Condello C, Stöhr J et al. Serial propagation of distinct strains of Aβ prions from Alzheimer's disease patients. Proc Natl Acad Sci U S A. 2014;111(28):10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woerman A L, Stöhr J, Aoyagi A et al. Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci U S A. 2015;112(35):E4949–E4958. doi: 10.1073/pnas.1513426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benson M D, Liepnieks J, Uemichi T, Wheeler G, Correa R. Hereditary renal amyloidosis associated with a mutant fibrinogen alpha-chain. Nat Genet. 1993;3(03):252–255. doi: 10.1038/ng0393-252. [DOI] [PubMed] [Google Scholar]
- 138.Hamidi Asl L, Liepnieks J J, Uemichi T et al. Renal amyloidosis with a frame shift mutation in fibrinogen aalpha-chain gene producing a novel amyloid protein. Blood. 1997;90(12):4799–4805. [PubMed] [Google Scholar]
- 139.Serpell L C, Benson M, Liepnieks J J, Fraser P E. Structural analyses of fibrinogen amyloid fibrils. Amyloid. 2007;14(03):199–203. doi: 10.1080/13506120701461111. [DOI] [PubMed] [Google Scholar]
- 140.Gillmore J D, Lachmann H J, Rowczenio D et al. Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A alpha-chain amyloidosis. J Am Soc Nephrol. 2009;20(02):444–451. doi: 10.1681/ASN.2008060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Picken M M. Fibrinogen amyloidosis: the clot thickens! Blood. 2010;115(15):2985–2986. doi: 10.1182/blood-2009-12-236810. [DOI] [PubMed] [Google Scholar]
- 142.Stangou A J, Banner N R, Hendry B M et al. Hereditary fibrinogen A alpha-chain amyloidosis: phenotypic characterization of a systemic disease and the role of liver transplantation. Blood. 2010;115(15):2998–3007. doi: 10.1182/blood-2009-06-223792. [DOI] [PubMed] [Google Scholar]
- 143.Haidinger M, Werzowa J, Kain R et al. Hereditary amyloidosis caused by R554L fibrinogen Aα-chain mutation in a Spanish family and review of the literature. Amyloid. 2013;20(02):72–79. doi: 10.3109/13506129.2013.781998. [DOI] [PubMed] [Google Scholar]
- 144.Zhmurov A, Brown A E, Litvinov R I, Dima R I, Weisel J W, Barsegov V. Mechanism of fibrin(ogen) forced unfolding. Structure. 2011;19(11):1615–1624. doi: 10.1016/j.str.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Litvinov R I, Faizullin D A, Zuev Y F, Weisel J W. The α-helix to β-sheet transition in stretched and compressed hydrated fibrin clots. Biophys J. 2012;103(05):1020–1027. doi: 10.1016/j.bpj.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhmurov A, Kononova O, Litvinov R I, Dima R I, Barsegov V, Weisel J W. Mechanical transition from α-helical coiled coils to β-sheets in fibrin(ogen) J Am Chem Soc. 2012;134(50):20396–20402. doi: 10.1021/ja3076428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kreplak L, Doucet J, Dumas P, Briki F. New aspects of the alpha-helix to beta-sheet transition in stretched hard alpha-keratin fibers. Biophys J. 2004;87(01):640–647. doi: 10.1529/biophysj.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guthold M, Liu W, Sparks E A et al. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys. 2007;49(03):165–181. doi: 10.1007/s12013-007-9001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu W, Carlisle C R, Sparks E A, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8(05):1030–1036. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miserez A, Guerette P A. Phase transition-induced elasticity of α-helical bioelastomeric fibres and networks. Chem Soc Rev. 2013;42(05):1973–1995. doi: 10.1039/c2cs35294j. [DOI] [PubMed] [Google Scholar]
- 151.Kell D B, Pretorius E. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. bioRxiv preprint. bioRxiv. 2016:54734. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 152.Weisel J W. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5 01:116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 153.Wolberg A S. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21(03):131–142. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 154.Biancalana M, Makabe K, Koide A, Koide S. Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol. 2009;385(04):1052–1063. doi: 10.1016/j.jmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Biancalana M, Koide S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 2010;1804(07):1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol. 2010;3(01):1–18. doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kuznetsova I M, Sulatskaya A I, Uversky V N, Turoverov K K. Analyzing thioflavin T binding to amyloid fibrils by an equilibrium microdialysis-based technique. PLoS One. 2012;7(02):e30724. doi: 10.1371/journal.pone.0030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kuznetsova I M, Sulatskaya A I, Uversky V N, Turoverov K K. A new trend in the experimental methodology for the analysis of the thioflavin T binding to amyloid fibrils. Mol Neurobiol. 2012;45(03):488–498. doi: 10.1007/s12035-012-8272-y. [DOI] [PubMed] [Google Scholar]
- 159.Kuznetsova I M, Sulatskaya A I, Maskevich A A, Uversky V N, Turoverov K K. High fluorescence anisotropy of thioflavin T in aqueous solution resulting from its molecular rotor nature. Anal Chem. 2016;88(01):718–724. doi: 10.1021/acs.analchem.5b02747. [DOI] [PubMed] [Google Scholar]
- 160.Lindberg D J, Wranne M S, Gilbert Gatty M, Westerlund F, Esbjörner E K. Steady-state and time-resolved Thioflavin-T fluorescence can report on morphological differences in amyloid fibrils formed by Aβ(1-40) and Aβ(1-42) Biochem Biophys Res Commun. 2015;458(02):418–423. doi: 10.1016/j.bbrc.2015.01.132. [DOI] [PubMed] [Google Scholar]
- 161.Sulatskaya A I, Kuznetsova I M, Turoverov K K. Interaction of thioflavin T with amyloid fibrils: fluorescence quantum yield of bound dye. J Phys Chem B. 2012;116(08):2538–2544. doi: 10.1021/jp2083055. [DOI] [PubMed] [Google Scholar]
- 162.Younan N D, Viles J H. A comparison of three fluorophores for the detection of amyloid fibers and prefibrillar oligomeric assemblies. ThT (thioflavin T); ANS (1-anilinonaphthalene-8-sulfonic acid); and bisANS (4,4′-dianilino-1,1′-binaphthyl-5,5′-disulfonic acid) Biochemistry. 2015;54(28):4297–4306. doi: 10.1021/acs.biochem.5b00309. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X, Ran C. Dual functional small molecule probes as fluorophore and ligand for misfolding proteins. Curr Org Chem. 2013;17(06):6. doi: 10.2174/1385272811317060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ahn H J, Zamolodchikov D, Cortes-Canteli M, Norris E H, Glickman J F, Strickland S. Alzheimer's disease peptide beta-amyloid interacts with fibrinogen and induces its oligomerization. Proc Natl Acad Sci U S A. 2010;107(50):21812–21817. doi: 10.1073/pnas.1010373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahn H J, Glickman J F, Poon K L et al. A novel Aβ-fibrinogen interaction inhibitor rescues altered thrombosis and cognitive decline in Alzheimer's disease mice. J Exp Med. 2014;211(06):1049–1062. doi: 10.1084/jem.20131751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cortes-Canteli M, Strickland S. Fibrinogen, a possible key player in Alzheimer's disease. J Thromb Haemost. 2009;7 01:146–150. doi: 10.1111/j.1538-7836.2009.03376.x. [DOI] [PubMed] [Google Scholar]
- 167.Cortes-Canteli M, Zamolodchikov D, Ahn H J, Strickland S, Norris E H. Fibrinogen and altered hemostasis in Alzheimer's disease. J Alzheimers Dis. 2012;32(03):599–608. doi: 10.3233/JAD-2012-120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cortes-Canteli M, Mattei L, Richards A T, Norris E H, Strickland S. Fibrin deposited in the Alzheimer's disease brain promotes neuronal degeneration. Neurobiol Aging. 2015;36(02):608–617. doi: 10.1016/j.neurobiolaging.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Paul J, Strickland S, Melchor J P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204(08):1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zamolodchikov D, Strickland S. A possible new role for Aβ in vascular and inflammatory dysfunction in Alzheimer's disease. Thromb Res. 2016;141 02:S59–S61. doi: 10.1016/S0049-3848(16)30367-X. [DOI] [PubMed] [Google Scholar]
- 171.Cortes-Canteli M, Paul J, Norris E H et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66(05):695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zamolodchikov D, Strickland S. Aβ delays fibrin clot lysis by altering fibrin structure and attenuating plasminogen binding to fibrin. Blood. 2012;119(14):3342–3351. doi: 10.1182/blood-2011-11-389668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zamolodchikov D, Berk-Rauch H E, Oren D A et al. Biochemical and structural analysis of the interaction between β-amyloid and fibrinogen. Blood. 2016;128(08):1144–1151. doi: 10.1182/blood-2016-03-705228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Akassoglou K, Adams R A, Bauer J et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101(17):6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Levi M, van der Poll T, Büller H R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 176.Flick M J, LaJeunesse C M, Talmage K E et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble J A, Zacharowski K.Novel aspects of fibrin(ogen) fragments during inflammation Mol Med 201117(5-6):568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jennewein C, Paulus P, Zacharowski K. Linking inflammation and coagulation: novel drug targets to treat organ ischemia. Curr Opin Anaesthesiol. 2011;24(04):375–380. doi: 10.1097/ACO.0b013e3283489ac0. [DOI] [PubMed] [Google Scholar]
- 179.Schuliga M. The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediators Inflamm. 2015;2015:437695. doi: 10.1155/2015/437695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cahill C M, Lahiri D K, Huang X, Rogers J T. Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim Biophys Acta. 2009;1790(07):615–628. doi: 10.1016/j.bbagen.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hirohata M, Ono K, Yamada M. Non-steroidal anti-inflammatory drugs as anti-amyloidogenic compounds. Curr Pharm Des. 2008;14(30):3280–3294. doi: 10.2174/138161208786404173. [DOI] [PubMed] [Google Scholar]
- 182.Minter M R, Taylor J M, Crack P J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer's disease. J Neurochem. 2016;136(03):457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 183.Spaulding C N, Dodson K W, Chapman M R, Hultgren S J. Fueling the fire with fibers: bacterial amyloids promote inflammatory disorders. Cell Host Microbe. 2015;18(01):1–2. doi: 10.1016/j.chom.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin I H, Miller D S, Bertics P J, Murphy C J, de Pablo J J, Abbott N L.Endotoxin-induced structural transformations in liquid crystalline droplets Science 2011332(6035):1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miller D S, Abbott N L. Influence of droplet size, pH and ionic strength on endotoxin-triggered ordering transitions in liquid crystalline droplets. Soft Matter. 2013;9(02):374–382. doi: 10.1039/C2SM26811F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carter M C, Miller D S, Jennings J et al. Synthetic mimics of bacterial lipid A trigger optical transitions in liquid crystal microdroplets at ultralow picogram-per-milliliter concentrations. Langmuir. 2015;31(47):12850–12855. doi: 10.1021/acs.langmuir.5b03557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Singh N, Haldar S, Tripathi A K, McElwee M K, Horback K, Beserra A. Iron in neurodegenerative disorders of protein misfolding: a case of prion disorders and Parkinson's disease. Antioxid Redox Signal. 2014;21(03):471–484. doi: 10.1089/ars.2014.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Singh N. The role of iron in prion disease and other neurodegenerative diseases. PLoS Pathog. 2014;10(09):e1004335. doi: 10.1371/journal.ppat.1004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Singh N, Asthana A, Baksi S et al. The prion-ZIP connection: from cousins to partners in iron uptake. Prion. 2015;9(06):420–428. doi: 10.1080/19336896.2015.1118602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ahmed M, Davis J, Aucoin D et al. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat Struct Mol Biol. 2010;17(05):561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hefti F, Goure W F, Jerecic J, Iverson K S, Walicke P A, Krafft G A. The case for soluble Aβ oligomers as a drug target in Alzheimer's disease. Trends Pharmacol Sci. 2013;34(05):261–266. doi: 10.1016/j.tips.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 192.Kayed R, Lasagna-Reeves C A. Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis. 2013;33 01:S67–S78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- 193.Liu B, Moloney A, Meehan S et al. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J Biol Chem. 2011;286(06):4248–4256. doi: 10.1074/jbc.M110.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meyer-Luehmann M, Spires-Jones T L, Prada Cet al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease Nature 2008451(7179):720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Miranda S, Opazo C, Larrondo L F et al. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer's disease. Prog Neurobiol. 2000;62(06):633–648. doi: 10.1016/s0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 196.Rival T, Page R M, Chandraratna D S et al. Fenton chemistry and oxidative stress mediate the toxicity of the beta-amyloid peptide in a Drosophila model of Alzheimer's disease. Eur J Neurosci. 2009;29(07):1335–1347. doi: 10.1111/j.1460-9568.2009.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sengupta U, Nilson A N, Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Uversky V N. Mysterious oligomerization of the amyloidogenic proteins. FEBS J. 2010;277(14):2940–2953. doi: 10.1111/j.1742-4658.2010.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Janson J, Ashley R H, Harrison D, McIntyre S, Butler P C. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48(03):491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 200.Bucciantini M, Giannoni E, Chiti Fet al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases Nature 2002416(6880):507–511. [DOI] [PubMed] [Google Scholar]
- 201.Kayed R, Head E, Thompson J Let al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis Science 2003300(5618):486–489. [DOI] [PubMed] [Google Scholar]
- 202.Baglioni S, Casamenti F, Bucciantini M et al. Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci. 2006;26(31):8160–8167. doi: 10.1523/JNEUROSCI.4809-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Glabe C G. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27(04):570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 204.Konarkowska B, Aitken J F, Kistler J, Zhang S, Cooper G J. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 2006;273(15):3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 205.Meier J J, Kayed R, Lin C Y et al. Inhibition of human IAPP fibril formation does not prevent beta-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am J Physiol Endocrinol Metab. 2006;291(06):E1317–E1324. doi: 10.1152/ajpendo.00082.2006. [DOI] [PubMed] [Google Scholar]
- 206.Haass C, Selkoe D J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(02):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 207.Xue W F, Hellewell A L, Gosal W S, Homans S W, Hewitt E W, Radford S E. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem. 2009;284(49):34272–34282. doi: 10.1074/jbc.M109.049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aitken J F, Loomes K M, Scott D W et al. Tetracycline treatment retards the onset and slows the progression of diabetes in human amylin/islet amyloid polypeptide transgenic mice. Diabetes. 2010;59(01):161–171. doi: 10.2337/db09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xue W F, Hellewell A L, Hewitt E W, Radford S E. Fibril fragmentation in amyloid assembly and cytotoxicity: when size matters. Prion. 2010;4(01):20–25. doi: 10.4161/pri.4.1.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fändrich M.Oligomeric intermediates in amyloid formation: structure determination and mechanisms of toxicity J Mol Biol 2012421(4-5):427–440. [DOI] [PubMed] [Google Scholar]
- 211.Göransson A L, Nilsson K PR, Kågedal K, Brorsson A C. Identification of distinct physiochemical properties of toxic prefibrillar species formed by Aβ peptide variants. Biochem Biophys Res Commun. 2012;420(04):895–900. doi: 10.1016/j.bbrc.2012.03.097. [DOI] [PubMed] [Google Scholar]
- 212.Stefani M. Structural features and cytotoxicity of amyloid oligomers: implications in Alzheimer's disease and other diseases with amyloid deposits. Prog Neurobiol. 2012;99(03):226–245. doi: 10.1016/j.pneurobio.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 213.Dobson C M. Weinheim, Germany: Wiley-VCH; 2013. The amyloid phenomenon and its significance; pp. 1–19. [Google Scholar]
- 214.Pillay K, Govender P. Amylin uncovered: a review on the polypeptide responsible for type II diabetes. BioMed Res Int. 2013;2013:826706. doi: 10.1155/2013/826706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Trikha S, Jeremic A M. Distinct internalization pathways of human amylin monomers and its cytotoxic oligomers in pancreatic cells. PLoS One. 2013;8(09):e73080. doi: 10.1371/journal.pone.0073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang S, Liu H, Chuang C L et al. The pathogenic mechanism of diabetes varies with the degree of overexpression and oligomerization of human amylin in the pancreatic islet β cells. FASEB J. 2014;28(12):5083–5096. doi: 10.1096/fj.14-251744. [DOI] [PubMed] [Google Scholar]
- 217.Holm N K, Jespersen S K, Thomassen L V et al. Aggregation and fibrillation of bovine serum albumin. Biochim Biophys Acta. 2007;1774(09):1128–1138. doi: 10.1016/j.bbapap.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 218.Barton C H, Vaziri N D, Gordon S, Tilles S. Renal pathology in end-stage renal disease associated with paraplegia. Paraplegia. 1984;22(01):31–41. doi: 10.1038/sc.1984.9. [DOI] [PubMed] [Google Scholar]
- 219.Jadoul M, Garbar C, Noël H et al. Histological prevalence of beta 2-microglobulin amyloidosis in hemodialysis: a prospective post-mortem study. Kidney Int. 1997;51(06):1928–1932. doi: 10.1038/ki.1997.262. [DOI] [PubMed] [Google Scholar]
- 220.Ben-Dov I Z, Pizov G, Ben-Chetrit E, Rubinger D, Or R. Fatal nephrotic syndrome complicating allogeneic stem cell transplantation: a case report. Nephrol Dial Transplant. 2009;24(09):2946–2949. doi: 10.1093/ndt/gfp261. [DOI] [PubMed] [Google Scholar]
- 221.Lachmann H J. Secondary AA amyloidosis. Contemp Hematol. 2010:179–189. [Google Scholar]
- 222.Lucas S, Fernandez R.The Autopsy Pathology of Sepsis-Related Death, Severe Sepsis and Septic Shock - Understanding a Serious KillerIn:, ed.Rijeka: InTech Open; 2012. Available at: http://www.intechopen.com/books/severe-sepsis-and-septic-shock-understanding-a-serious-killer/theautopsy-pathology-of-sepsis-and-septic-shock. Accessed July 27, 2017 [Google Scholar]
- 223.Díez R, Madero M, Gamba G, Soriano J, Soto V. Renal AA amyloidosis in patients with type 2 diabetes mellitus. Nephron Extra. 2014;4(02):119–126. doi: 10.1159/000363625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gross P A. Hypotension and mortality in septic shock: the “golden hour”. Crit Care Med. 2006;34(06):1819–1820. doi: 10.1097/01.CCM.0000220054.95214.7D. [DOI] [PubMed] [Google Scholar]
- 225.Kumar A, Roberts D, Wood K E et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(06):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 226.Bagshaw S M, Lapinsky S, Dial S et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35(05):871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 227.Holler J G, Bech C N, Henriksen D P, Mikkelsen S, Pedersen C, Lassen A T. Nontraumatic hypotension and shock in the emergency department and the prehospital setting, prevalence, etiology, and mortality: a systematic review. PLoS One. 2015;10(03):e0119331. doi: 10.1371/journal.pone.0119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gamper G, Havel C, Arrich J et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016;2:CD003709. doi: 10.1002/14651858.CD003709.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Calabrese E J. Hormesis and medicine. Br J Clin Pharmacol. 2008;66(05):594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cox L A., Jr Hormesis without cell killing. Risk Anal. 2009;29(03):393–400. doi: 10.1111/j.1539-6924.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 231.Mattson M P. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008;27(02):155–162. doi: 10.1177/0960327107083417. [DOI] [PubMed] [Google Scholar]
- 232.Wang D, Wei J, Hsu K et al. Effects of nitric oxide synthase inhibitors on systemic hypotension, cytokines and inducible nitric oxide synthase expression and lung injury following endotoxin administration in rats. J Biomed Sci. 1999;6(01):28–35. doi: 10.1007/BF02256421. [DOI] [PubMed] [Google Scholar]
- 233.Harada N, Okajima K, Isobe H, Uchiba M. Antithrombin reduces endotoxin-induced hypotension by enhancing pulmonary sensory neuron activation in rats. Thromb Haemost. 2006;95(06):1011–1018. doi: 10.1160/TH05-09-0637. [DOI] [PubMed] [Google Scholar]
- 234.Cayla C, Todiras M, Iliescu R et al. Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J. 2007;21(08):1689–1698. doi: 10.1096/fj.06-7175com. [DOI] [PubMed] [Google Scholar]
- 235.Uchiba M, Okajima K, Murakami K, Okabe H, Takatsuki K.Attenuation of endotoxin-induced pulmonary vascular injury by antithrombin III Am J Physiol 1996270(6 Pt 1):L921–L930. [DOI] [PubMed] [Google Scholar]
- 236.Fourrier F, Jourdain M, Tournoys A. Clinical trial results with antithrombin III in sepsis. Crit Care Med. 2000;28(09):S38–S43. doi: 10.1097/00003246-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 237.Hoffmann J N, Vollmar B, Römisch J, Inthorn D, Schildberg F W, Menger M D. Antithrombin effects on endotoxin-induced microcirculatory disorders are mediated mainly by its interaction with microvascular endothelium. Crit Care Med. 2002;30(01):218–225. doi: 10.1097/00003246-200201000-00031. [DOI] [PubMed] [Google Scholar]
- 238.Isobe H, Okajima K, Uchiba M, Harada N, Okabe H. Antithrombin prevents endotoxin-induced hypotension by inhibiting the induction of nitric oxide synthase in rats. Blood. 2002;99(05):1638–1645. doi: 10.1182/blood.v99.5.1638. [DOI] [PubMed] [Google Scholar]
- 239.Iba T, Kidokoro A. High-dose antithrombin therapy for sepsis: mechanisms of action. Shock. 2002;18(05):389–394. doi: 10.1097/00024382-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 240.Ostermann H. Antithrombin III in Sepsis. New evidences and open questions. Minerva Anestesiol. 2002;68(05):445–448. [PubMed] [Google Scholar]
- 241.Iba T, Kidokoro A, Fukunaga M et al. Antithrombin ameliorates endotoxin-induced organ dysfunction more efficiently when combined with danaparoid sodium than with unfractionated heparin. Intensive Care Med. 2005;31(08):1101–1108. doi: 10.1007/s00134-005-2707-0. [DOI] [PubMed] [Google Scholar]
- 242.Wiedermann C J. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006;10(01):209. doi: 10.1186/cc4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Komura H, Uchiba M, Mizuochi Y et al. Antithrombin inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by monocytes in vitro through inhibition of Egr-1 expression. J Thromb Haemost. 2008;6(03):499–507. doi: 10.1111/j.1538-7836.2007.02869.x. [DOI] [PubMed] [Google Scholar]
- 244.Levi M, Schouten M, van der Poll T. Sepsis, coagulation, and antithrombin: old lessons and new insights. Semin Thromb Hemost. 2008;34(08):742–746. doi: 10.1055/s-0029-1145256. [DOI] [PubMed] [Google Scholar]
- 245.Iba T, Saitoh D. Efficacy of antithrombin in preclinical and clinical applications for sepsis-associated disseminated intravascular coagulation. J Intensive Care. 2014;2(01):66. doi: 10.1186/s40560-014-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(09):1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 247.Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103(03):253–261. doi: 10.1007/s12185-015-1904-z. [DOI] [PubMed] [Google Scholar]
- 248.Helmerhorst E J, Maaskant J J, Appelmelk B J. Anti-lipid A monoclonal antibody centoxin (HA-1A) binds to a wide variety of hydrophobic ligands. Infect Immun. 1998;66(02):870–873. doi: 10.1128/iai.66.2.870-873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Marks L. The birth pangs of monoclonal antibody therapeutics: the failure and legacy of Centoxin. MAbs. 2012;4(03):403–412. doi: 10.4161/mabs.19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Murphy S T, Bellamy M C. The quest for the magic bullet: Centoxin, Drotrecogin Alfa and lessons not learned. Trends Anaesth Crit Care. 2013;3:316–319. [Google Scholar]
- 251.Martí-Carvajal A J, Solà I, Gluud C, Lathyris D, Cardona A F. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lai P S, Matteau A, Iddriss A, Hawes J C, Ranieri V, Thompson B T. An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock. Minerva Anestesiol. 2013;79(01):33–43. [PMC free article] [PubMed] [Google Scholar]
- 253.Nadel S, Goldstein B, Williams M Det al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial Lancet 2007369(9564):836–843. [DOI] [PubMed] [Google Scholar]
- 254.Minnich D J, Moldawer L L. Anti-cytokine and anti-inflammatory therapies for the treatment of severe sepsis: progress and pitfalls. Proc Nutr Soc. 2004;63(03):437–441. doi: 10.1079/pns2004378. [DOI] [PubMed] [Google Scholar]
- 255.Aziz M, Jacob A, Yang W L, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93(03):329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kell D B. Finding novel pharmaceuticals in the systems biology era using multiple effective drug targets, phenotypic screening and knowledge of transporters: where drug discovery went wrong and how to fix it. FEBS J. 2013;280(23):5957–5980. doi: 10.1111/febs.12268. [DOI] [PubMed] [Google Scholar]
- 257.Weinberg E D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64(01):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- 258.Xia Y, Farah N, Maxan A, Zhou J, Lehmann C. Therapeutic iron restriction in sepsis. Med Hypotheses. 2016;89:37–39. doi: 10.1016/j.mehy.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 259.Chapin J C, Hajjar K A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29(01):17–24. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cornet A D, Smit E G, Beishuizen A, Groeneveld A B. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98(03):579–586. [PubMed] [Google Scholar]
- 261.Wang C, Chi C, Guo L et al. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: a systematic review and meta-analysis. Crit Care. 2014;18(05):563. doi: 10.1186/s13054-014-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zarychanski R, Abou-Setta A M, Kanji S et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43(03):511–518. doi: 10.1097/CCM.0000000000000763. [DOI] [PubMed] [Google Scholar]
- 263.van Roessel S, van der Laan A M, de Pont A-CJM. What is the best heparin to treat sepsis with? Crit Care Med. 2015;43(06):e212–e213. doi: 10.1097/CCM.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 264.Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care. 2016;4:23. doi: 10.1186/s40560-016-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rosenberg V A, Buhimschi I A, Lockwood C J et al. Heparin elevates circulating soluble fms-like tyrosine kinase-1 immunoreactivity in pregnant women receiving anticoagulation therapy. Circulation. 2011;124(23):2543–2553. doi: 10.1161/CIRCULATIONAHA.111.046821. [DOI] [PubMed] [Google Scholar]
- 266.Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Kadota K, Sato K. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med Exp. 2015;3(01):36. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wiedermann C J, Kaneider N C. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul Fibrinolysis. 2006;17(07):521–526. doi: 10.1097/01.mbc.0000245302.18010.40. [DOI] [PubMed] [Google Scholar]
- 268.Iba T, Saitoh D, Gando S, Thachil J. The usefulness of antithrombin activity monitoring during antithrombin supplementation in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2015;135(05):897–901. doi: 10.1016/j.thromres.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 269.Warren B L, Eid A, Singer P et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 270.Iba T, Yamada A, Hashiguchi N, Nagaoka I. New therapeutic options for patients with sepsis and disseminated intravascular coagulation. Pol Arch Med Wewn. 2014;124(06):321–328. doi: 10.20452/pamw.2299. [DOI] [PubMed] [Google Scholar]
- 271.Yamakawa K, Umemura Y, Hayakawa M et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(01):229. doi: 10.1186/s13054-016-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chien J Y, Jerng J S, Yu C J, Yang P C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med. 2005;33(08):1688–1693. doi: 10.1097/01.ccm.0000171183.79525.6b. [DOI] [PubMed] [Google Scholar]
- 273.Levels J HM, Geurts P, Karlsson H et al. High-density lipoprotein proteome dynamics in human endotoxemia. Proteome Sci. 2011;9(01):34. doi: 10.1186/1477-5956-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Catapano A L, Pirillo A, Bonacina F, Norata G D. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103(03):372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 275.Bandeali S, Farmer J. High-density lipoprotein and atherosclerosis: the role of antioxidant activity. Curr Atheroscler Rep. 2012;14(02):101–107. doi: 10.1007/s11883-012-0235-2. [DOI] [PubMed] [Google Scholar]
- 276.Tabet F, Rye K A. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond) 2009;116(02):87–98. doi: 10.1042/CS20080106. [DOI] [PubMed] [Google Scholar]
- 277.Guo L, Ai J, Zheng Z et al. High density lipoprotein protects against polymicrobe-induced sepsis in mice. J Biol Chem. 2013;288(25):17947–17953. doi: 10.1074/jbc.M112.442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Contreras-Duarte S, Varas P, Awad F, Busso D, Rigotti A.Protective role of high density lipoproteins in sepsis: basic issues and clinical implications[in Spanish].Rev Chilena Infectol 2014310134–43. [DOI] [PubMed] [Google Scholar]
- 279.Pirillo A, Catapano A L, Norata G D. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. 2015;224:483–508. doi: 10.1007/978-3-319-09665-0_15. [DOI] [PubMed] [Google Scholar]
- 280.Almog Y, Shefer A, Novack V et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(07):880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 281.Dobesh P P, Olsen K M. Statins role in the prevention and treatment of sepsis. Pharmacol Res. 2014;88:31–40. doi: 10.1016/j.phrs.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 282.Harvey M, Cave G. Co-administration of phospholipid emulsion with first dose bacteriocidal antibiotic may retard progression of the sepsis response in gram negative septicaemia. Med Hypotheses. 2014;83(05):563–565. doi: 10.1016/j.mehy.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 283.Saito H, Maruyama I, Shimazaki S et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5(01):31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 284.Levi M, Van Der Poll T. Thrombomodulin in sepsis. Minerva Anestesiol. 2013;79(03):294–298. [PubMed] [Google Scholar]
- 285.Mimuro J, Takahashi H, Kitajima I et al. Impact of recombinant soluble thrombomodulin (thrombomodulin alfa) on disseminated intravascular coagulation. Thromb Res. 2013;131(05):436–443. doi: 10.1016/j.thromres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 286.Vincent J L, Ramesh M K, Ernest D et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(09):2069–2079. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- 287.Eguchi Y, Gando S, Ishikura H et al. Post-marketing surveillance data of thrombomodulin alfa: sub-analysis in patients with sepsis-induced disseminated intravascular coagulation. J Intensive Care. 2014;2(01):30. doi: 10.1186/2052-0492-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shirahata A, Mimuro J, Takahashi H et al. Recombinant soluble human thrombomodulin (thrombomodulin alfa) in the treatment of neonatal disseminated intravascular coagulation. Eur J Pediatr. 2014;173(03):303–311. doi: 10.1007/s00431-013-2155-8. [DOI] [PubMed] [Google Scholar]
- 289.Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T, Shimazu T. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J Thromb Haemost. 2015;13(04):508–519. doi: 10.1111/jth.12841. [DOI] [PubMed] [Google Scholar]
- 290.Yoshimura J, Yamakawa K, Ogura H et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care. 2015;19:78. doi: 10.1186/s13054-015-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Levi M. Recombinant soluble thrombomodulin: coagulation takes another chance to reduce sepsis mortality. J Thromb Haemost. 2015;13(04):505–507. doi: 10.1111/jth.12868. [DOI] [PubMed] [Google Scholar]
- 292.Hayakawa M, Yamakawa K, Saito S et al. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115(06):1157–1166. doi: 10.1160/TH15-12-0987. [DOI] [PubMed] [Google Scholar]
- 293.Iba T, Gando S, Saitoh D, Wada H, Di Nisio M, Thachil J. Antithrombin supplementation and risk of bleeding in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2016;145:46–50. doi: 10.1016/j.thromres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 294.Wada H, Thachil J, Di Nisio M, Kurosawa S, Gando S, Toh C H; Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis.Harmonized guidance for disseminated intravascular coagulation from the International Society on Thrombosis and Haemostasis and the current status of anticoagulant therapy in Japan: a rebuttal J Thromb Haemost 201311112078–2079. [DOI] [PubMed] [Google Scholar]
- 295.Iba T, Thachil J. Is antithrombin III for sepsis-associated disseminated intravascular coagulation really ineffective? Intensive Care Med. 2016;42(07):1193–1194. doi: 10.1007/s00134-016-4288-5. [DOI] [PubMed] [Google Scholar]
- 296.Dellinger R P, Levy M M, Rhodes A et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(02):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12(07):1010–1019. doi: 10.1111/jth.12596. [DOI] [PubMed] [Google Scholar]
- 298.Oda S, Aibiki M, Ikeda T et al. The Japanese guidelines for the management of sepsis. J Intensive Care. 2014;2(01):55. doi: 10.1186/s40560-014-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wada H, Asakura H, Okamoto K et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125(01):6–11. doi: 10.1016/j.thromres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 300.Wada H, Okamoto K, Iba T et al. Addition of recommendations for the use of recombinant human thrombomodulin to the “Expert consensus for the treatment of disseminated intravascular coagulation in Japan”. Thromb Res. 2014;134(04):924–925. doi: 10.1016/j.thromres.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 301.Krishnan R, Tsubery H, Proschitsky M Y et al. A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies. J Mol Biol. 2014;426(13):2500–2519. doi: 10.1016/j.jmb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 302.Barber M, Tait R C, Scott J, Rumley A, Lowe G D, Stott D J. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J Thromb Haemost. 2004;2(11):1873–1878. doi: 10.1111/j.1538-7836.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- 303.Murthy S B, Jawaid A, Qureshi S U, Schulz P E, Schulz P E. The apolipoprotein 2 allele in Alzheimer's disease: suggestions for a judicious use of antiplatelet and anticoagulant medications. J Am Geriatr Soc. 2009;57(06):1124–1125. doi: 10.1111/j.1532-5415.2009.02284.x. [DOI] [PubMed] [Google Scholar]
- 304.Nilsson K P, Lindgren M, Hammarström P. A pentameric luminescent-conjugated oligothiophene for optical imaging of in vitro-formed amyloid fibrils and protein aggregates in tissue sections. Methods Mol Biol. 2012;849:425–434. doi: 10.1007/978-1-61779-551-0_29. [DOI] [PubMed] [Google Scholar]
- 305.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148(06):1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tycko R, Wickner R B. Molecular structures of amyloid and prion fibrils: consensus versus controversy. Acc Chem Res. 2013;46(07):1487–1496. doi: 10.1021/ar300282r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Langkilde A E, Morris K L, Serpell L C, Svergun D I, Vestergaard B.The architecture of amyloid-like peptide fibrils revealed by X-ray scattering, diffraction and electron microscopy Acta Crystallogr D Biol Crystallogr 201571(Pt 4):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Bembde A S. A study of plasma fibrinogen level in type-2 diabetes mellitus and its relation to glycemic control. Indian J Hematol Blood Transfus. 2012;28(02):105–108. doi: 10.1007/s12288-011-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


