Several knowledge gaps exist in the vector competence of various geographical populations of O. turicata that transmit B. turicatae. A western population of this tick is distributed from California to Texas, and an eastern population exists in Florida. Utilizing western and eastern populations of the vector, we studied acquisition and transmission of two B. turicatae isolates. Regardless of the isolate used, infection frequencies were poor in mice after the eastern population feeding on them. Since salivary gland colonization is essential for B. turicatae transmission, these tissues were further evaluated. Interestingly, the salivary glands from the two populations were similarly colonized with B. turicatae. These findings suggest the role of tick saliva in the establishment of infection and that the salivary glands may be a bottleneck for successful transmission.
KEYWORDS: Ornithodoros turicata, relapsing fever, Borrelia turicatae, vector competence, vector competency
ABSTRACT
Vector competence refers to the ability of an arthropod to acquire, maintain, and successfully transmit a microbial pathogen. Tick-borne relapsing fever (TBRF) spirochetes are globally distributed pathogens, and most species are transmitted by argasid ticks of the genus Ornithodoros. A defining characteristic in vector competence is an apparent specificity of a species of TBRF spirochete to a given tick species. In arid regions of the southern United States, Borrelia turicatae is the primary cause of TBRF. Interestingly, there are two populations of the tick vector distributed throughout this region. Ornithodoros turicata is a western population that ranges from California to Texas. There is a gap through Louisiana, Mississippi, and Alabama where the tick has not been identified. An isolated eastern population exists in Florida and was designated a subspecies, O. turicata americanus. A knowledge gap that exists is the poor understanding of vector competence between western and eastern populations of ticks for B. turicatae. In this study, we generated uninfected colonies of O. turicata that originated in Texas and Kansas and of O. turicata americanus. B. turicatae acquisition, maintenance through the molt, and subsequent transmission were evaluated. Our findings revealed significant differences in murine infection after feeding infected O. turicata and O. turicata americanus ticks on the animals. Interestingly, the salivary glands of both tick populations were colonized with B. turicatae to similar densities. Our results suggest that the salivary glands of the tick colonies assessed in this study impact vector competence of the evaluated B. turicatae isolates.
IMPORTANCE Several knowledge gaps exist in the vector competence of various geographical populations of O. turicata that transmit B. turicatae. A western population of this tick is distributed from California to Texas, and an eastern population exists in Florida. Utilizing western and eastern populations of the vector, we studied acquisition and transmission of two B. turicatae isolates. Regardless of the isolate used, infection frequencies were poor in mice after the eastern population feeding on them. Since salivary gland colonization is essential for B. turicatae transmission, these tissues were further evaluated. Interestingly, the salivary glands from the two populations were similarly colonized with B. turicatae. These findings suggest the role of tick saliva in the establishment of infection and that the salivary glands may be a bottleneck for successful transmission.
INTRODUCTION
Vector competence of tick-borne pathogens encompasses acquisition, maintenance, and subsequent successful transmission back into a vertebrate host during blood feeding (1). Tick-borne relapsing fever (TBRF) spirochetes are an example of pathogens that have adapted to colonize argasid and ixodid ticks and vertebrates. During mammalian infection, TBRF spirochetes attain high densities in the blood. As an antibody response is generated against the primary population in the blood, the pathogens undergo antigenic variation of the variable large and small membrane proteins (Vlps and Vsps, respectively), and a new variant emerges (2). The dynamics between host immunity and pathogen can occur for several months, providing multiple opportunities for tick acquisition of spirochetes.
Once TBRF spirochetes enter the tick, two physical barriers that impact vector competence are the midgut and salivary glands. During initial entry into the midgut, the spirochetes must adapt to the physiologically and immunologically new environment and are maintained transstadially and transovarially (1). While TBRF spirochetes are transmitted by ixodid and argasid ticks, the pathogen's life cycle in argasids, specifically members of the genus Ornithodoros, is better understood. TBRF spirochetes will establish a long-term colonization of the salivary glands of Ornithodoros species and are subsequently transmitted within seconds of a tick bite (3).
A notable feature of vector biology is the specificity of a given TBRF species for a specific Ornithodoros species (4, 5). For example, in the United States, the following specificity is observed: Borrelia hermsii with Ornithodoros hermsi, Borrelia parkeri with Ornithodoros parkeri, and Borrelia turicatae with Ornithodoros turicata (4). While O. turicata is distributed throughout the southern United States and into regions of Latin America, within North America, there are two geographically isolated populations. A western population of the tick exists in California, Arizona, Colorado, Utah, New Mexico, Kansas, Oklahoma, and Texas (6, 7). Currently, there is an ecological gap between the population in Texas and the eastern population in Florida (8). Beck and colleagues considered the eastern population to be a subspecies and resurrected the name O. turicata americanus because of the tick's distribution and ecological and biological characteristics that differed from the western population (9).
With the observed vector specificity of TBRF spirochetes, the intricacies of vector colonization and transmission of B. turicatae between the western and eastern populations of ticks remain unclear. The potential for TBRF in Florida exists given the isolation of the Florida canine Borrelia (FCB) from the blood of a clinical dog (10). Through multilocus sequencing, FCB had over 98% DNA identity with B. turicatae isolates that originated from Texas (11). However, infected ticks have not been reported from Florida, and the competence of O. turicata americanus for B. turicatae is unknown.
In this study, we generated uninfected colonies of O. turicata from Texas and Kansas and O. turicata americanus from Florida and evaluated their competence for two B. turicatae isolates that originated in Texas and Florida. Since Ornithodoros species possess upwards of six nymphal stages, we infected third-stage O. turicata and O. turicata americanus ticks by feeding cohorts of ticks on mice infected with a Texas or Florida isolate of B. turicatae. After molting, transmission bloodmeals were conducted at the fourth and fifth nymphal stages, and we evaluated B. turicatae infection frequencies in mice. B. turicatae colonization of the salivary glands of O. turicata and O. turicata americanus was assessed using the Texas strain producing the green fluorescent protein (GFP). Spirochetes were also quantified in the salivary glands of the ticks used in this study. Interestingly, while differences in murine infection frequencies were observed after feeding O. turicata and O. turicata americanus, the salivary glands of both ticks remained similarly populated with B. turicatae. These findings indicate the importance of defining the molecular mechanisms that facilitate vector colonization and transmission of TBRF spirochetes.
RESULTS
Determination of natural infection from O. turicata colonies.
O. turicata ticks that originated from Kansas were previously determined to be uninfected (3, 12). Since tick colonies that originated from Texas and Florida were from recent field collections (8), their infectivity statuses were determined. Feeding of 10 male and female ticks on mice and evaluating murine blood for 10 consecutive days for microscopic detection of spirochetes indicated that the animals were not infected. An assessment of the serological responses 30 days after tick feeding was also negative (data not shown). These results indicated that O. turicata ticks originating from Texas and O. turicata americanus ticks were likely uninfected with B. turicatae.
As an additional measure to determine the infectivity statuses of the Texas and Florida tick colonies, we evaluated their offspring because of reported transovarial transmission of B. turicatae (13). First- and second-stage nymphs (∼50 ticks) were fed on mice, and we failed to detect spirochetes in the blood by dark-field microscopy for 10 consecutive days. The animals also failed to seroconvert when evaluated 30 days after the bloodmeal (data not shown). Furthermore, a PCR for the B. turicatae bipA gene using genomic DNA extracted from a cohort of 10 to 20 second-stage nymphs failed to detect spirochete DNA in the ticks (data not shown). Given these results and previous work reporting that five O. turicata ticks provide a sufficient infectious dose to mice (3), we considered the Texas colony of O. turicata and O. turicata americanus ticks to be free of B. turicatae.
Acquisition of B. turicatae by O. turicata and O. turicata americanus.
In this study, the two B. turicatae isolates used originated from Texas and Florida and were previously designated 91E135 and Florida canine Borrelia (FCB) (11), respectively. For simplicity, in our study, these isolates were designated Bt-TX and Bt-FL, respectively. Mice that were needle inoculated with the Bt-TX or Bt-FL were spirochetemic the following day. Both isolates of B. turicatae attained ∼1 × 107 spirochetes per ml of blood when ∼70 third-stage O. turicata or O. turicata americanus nymphs were fed on each infected mouse. All ticks engorged to repletion. After feeding, five ticks from each group were dissected and the midgut ruptured, and spirochetes were visualized by dark-field microscopy, indicating that spirochetes were imbibed. Evaluation of the ticks after the molt indicated that they were four-stage nymphs.
Transmission of Bt-TX and Bt-FL isolates from O. turicata and O. turicata americanus.
In all experiments, 10 ticks were given the opportunity to feed on a mouse, and at least four fed to repletion (Table 1). In murine infection studies using O. turicata ticks originating from Texas and Kansas, Bt-TX and Bt-FL were detected in murine blood by microscopy or quantitative PCR (qPCR) within 5 days after tick feeding. In mice evaluated by qPCR, spirochetes attained similar densities (Table 1), and the animals relapsed within the 10-day sampling period. The animals infected by tick bite also seroconverted (Fig. 1). Additionally, similar murine infection frequencies were observed regardless of whether ticks were infected with Bt-TX or Bt-FL (Table 1).
TABLE 1.
Summary of Bt-TX and Bt-FL murine infection frequencies after feeding O. turicata from Texas and Kansas and O. turicata americanus
| Treatmenta | No. of fed ticks | No. of infected animals/total no. of animals as determined by: |
Spirochetemia × 106 (avg ± SD)c | ||
|---|---|---|---|---|---|
| qPCR | Microscopy | Immunoblotting | |||
| Ot-TX::Bt-FL | 9 | 10/10 | 10/10 | 10/10 | 1.05 ± 1.86 |
| Ot-TX::Bt-TX | 4 | 5/5 | 4/5 | 5/5 | 1.38 ± 1.2 |
| Ot-KS::Bt-FL | 10 | 9/10 | 9/10 | 9/10 | 0.16 ± 0.16 |
| Ot-KS::Bt-TXb | 10 | NA | NA | 10/10 | NA |
| Ota-FL::Bt-FL | 10 | 1/10 | 0/10 | 4/10 | 2.38 ± 0.0 |
| Ota-FL::Bt-TX | 8 | 1/10 | 0/10 | 1/10 | 1.00 ± 0.0 |
Ot, O. turicata; Ota, O. turicata americanus.
Infection was only detected by evaluating seroconversion. NA, not applicable.
Values are the peak spirochetemia in the group of infected animals, as determined by qPCR.
FIG 1.
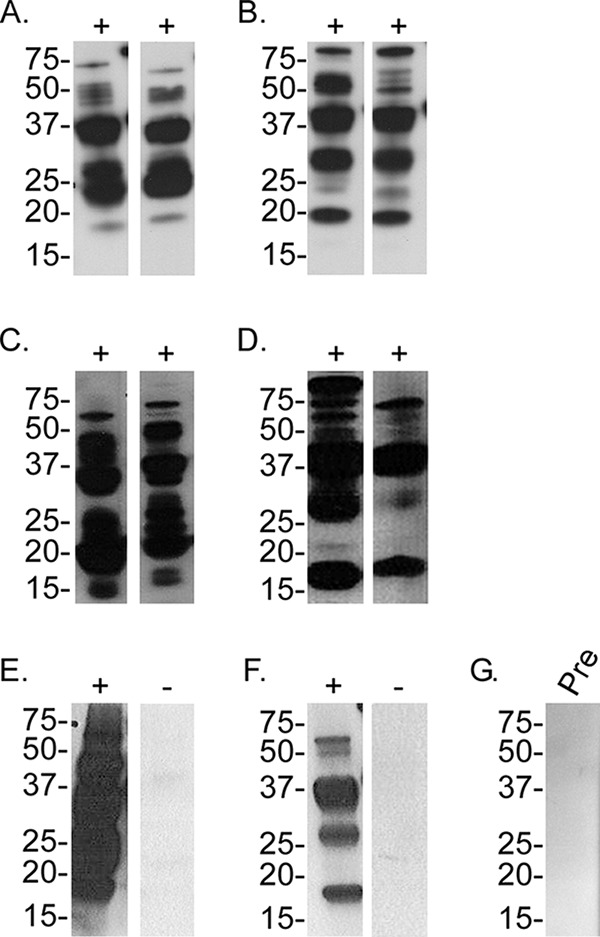
Assessment of serological responses to B. turicatae (Bt) protein lysates from mice fed upon by infected O. turicata and O. turicata americanus ticks. Immunoblots from two mice are representative of the remaining animals in each treatment group. Serum samples were used from mice that were fed upon by the following O. turicata (Ot) or O. turicata americanus (Ota)::B. turicatae isolate combinations: Ot-TX::Bt-TX (A), Ot-TX::Bt-FL (B), Ot-KS::Bt-TX (C), Ot-KS::Bt-FL (D), Ota::Bt-TX (E), and Ota::Bt-FL (F). A preinfection (Pre) serum sample is shown (G) and represents the remaining animals. Molecular masses are shown on the left of the immunoblots in kilodaltons.
Infection frequencies in mice that were fed upon by O. turicata americanus were lower than in the Texas and Kansas colonies of O. turicata. After the transmission feeding of O. turicata americanus colonized with Bt-FL, infection was detected in one of 10 animals by qPCR, while spirochetes were not detected by microscopy. In total, four of 10 mice seroconverted (Table 1 and Fig. 1), indicating that infection in three animals was below the limit of qPCR detection. Also, the qPCR-positive animal failed to relapse within the 10-day sampling period. After feeding O. turicata americanus ticks colonized with Bt-TX, one of 10 animals became infected (Table 1 and Fig. 1), as determined by qPCR and immunoblotting. This animal relapsed within the 10-day sampling period. These results suggest that while murine infection by O. turicata americanus feeding was lower than that by O. turicata, the Florida population of ticks was still colonized with B. turicatae.
Evaluation of colonization of O. turicata americanus ticks by B. turicatae-gfp.
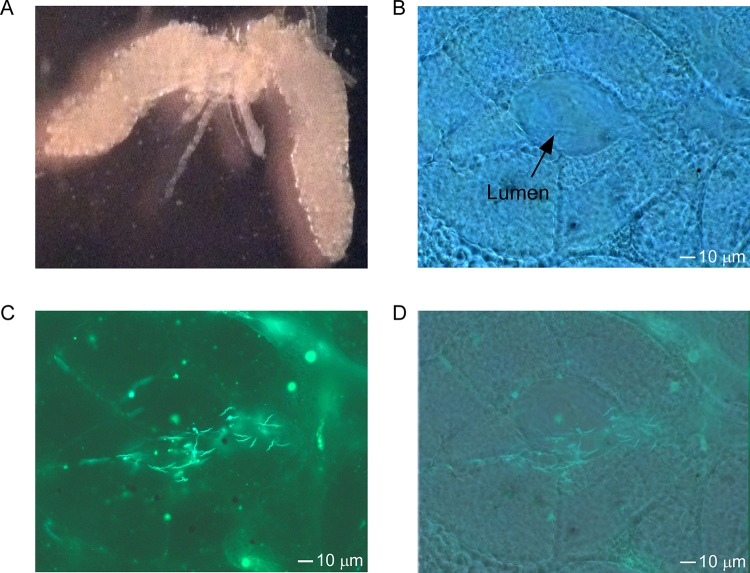
To further assess the vector colonization of O. turicata americanus, salivary glands were evaluated using the Bt-TX isolate producing GFP (14). Interestingly, the salivary glands were heavily colonized with fluorescing spirochetes for at least 8 months after acquisition (Fig. 2). Furthermore, the spirochetes were localized in the lumen of type two granular acini (Fig. 2), indicating they were positioned for transmission upon feeding. In total, Bt-TX producing GFP was detected in the salivary glands of 14 of 15 O. turicata americanus ticks. These infection frequencies were similar to those by O. turicata ticks that originated from Texas and Kansas.
FIG 2.
Demonstration of O. turicata americanus salivary gland colonization by B. turicatae-gfp. (A) Intact salivary glands were excised from O. turicata americanus ticks that were infected with B. turicatae-gfp. A dark-field image shows the structural outline of the acinus (B), and an image of fluorescing B. turicatae-gfp localizes the spirochetes to the acinus lumen (C). (D) Dark-field and fluorescent images were also overlaid. (B to D) A scale bar is shown in the bottom right.
Quantification of B. turicatae in tick salivary glands.
Since B. turicatae producing GFP was qualitatively detected in the salivary glands of the tick vector, a qPCR assay was developed to quantify spirochete densities in these tissues. Prior to performing a duplex qPCR assay with tick tissues, a series of control qPCR experiments were conducted with B. turicatae flaB and O. turicata β-actin gene primers and probes. qPCR was performed with individual primer sets with each plasmid, and nonspecific binding was not observed (data not shown). Both primer sets were also assayed with a single plasmid, which indicated that primer inhibition was not occurring. Also, qPCR using flaB primers with genomic DNA from uninfected ticks and β-actin gene primers with B. turicatae genomic DNA was performed, further indicating the specificities of the primers for their respective genes. We also confirmed that the β-actin gene primers and probe were compatible with O. turicata americanus genomic DNA. Generating a standard curve using 1 × 105 to 1 × 101 copies of pCR2.1::flaB and pCR2.1::β-actin plasmids indicated the compatibility of primers in duplex qPCR assays and that identical gene copies were used (Fig. S1).
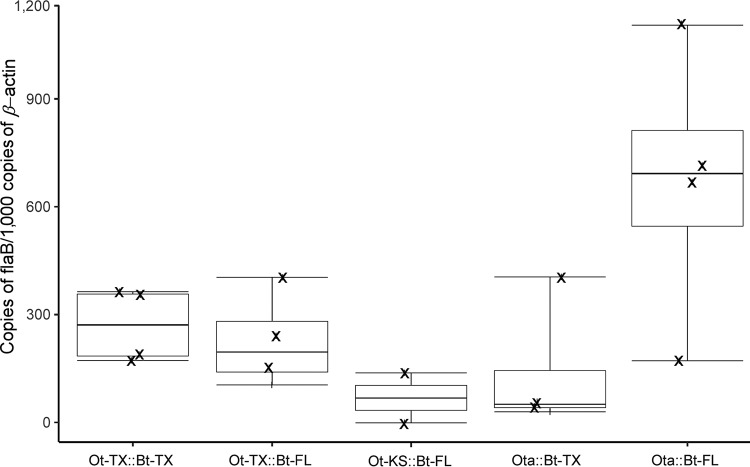
To quantify differences in spirochete loads between O. turicata and O. turicata americanus, qPCR was performed with genomic DNA isolated from the salivary glands of the tick cohorts used in the infection studies (Fig. 3). Interestingly, isolating genomic DNA from a pool of salivary glands from five ticks failed to detect a statistically significant difference in average copies of flaB between Texas, Kansas, and Florida ticks. These results indicated that while murine infection frequencies varied between O. turicata and O. turicata americanus, B. turicatae colonized the salivary glands of the ticks used in this study to similar densities.
FIG 3.
Quantification of Bt-TX and Bt-FL in the salivary glands of O. turicata and O. turicata americanus ticks. Each gDNA sample consisted of salivary glands from five ticks, and two to four samples were evaluated per group. gDNA from salivary gland samples of O. turicata-B. turicatae or O. turicata americanus-B. turicatae isolate groups are as follows: Ot-TX::Bt-TX, Ot-TX::Bt-FL, Ot-KS::Bt-FL, Ota::Bt-TX, and Ota::Bt-FL.
DISCUSSION
The current study identified significant differences in colonization, maintenance, and subsequent successful transmission of B. turicatae from O. turicata and its geographically isolated subspecies O. turicata americanus. Both isolates of B. turicatae were able to adapt to two barriers that affect vector competence, the midgut and salivary glands. However, the infection frequencies in mice after feeding by O. turicata and O. turicata americanus were significantly different. The Bt-TX and Bt-FL isolates of B. turicatae successfully infected mice at similar frequencies when the animals were fed on by O. turicata ticks that originated from Texas and Kansas. Conversely, the infection frequencies of Bt-TX and BT-FL in mice after tick bites by O. turicata americanus were 10% and 40%, respectively.
To further understand the observed vector competence, we evaluated salivary gland acini. The salivary glands of argasid ticks are composed of type I and II acini (15). Type I acini directly attached to the main salivary duct and consist of a primary central cell and multiple pyramidal cells surrounding the short acinar duct (15). Moreover, type I acini are agranular, and secretory vacuoles are absent. Type II acini are granular and function in saliva secretion (15), indicating their potential significance in pathogen transmission. Supporting the role of type II acini in pathogenesis, Policastro and colleagues reported that B. hermsii was only detected in granular acini of post-transmission-fed ticks (16). In the assessment of O. turicata americanus salivary glands, utilization of B. turicatae producing GFP indicated that the pathogens were also localized in the lumen of type II acini. Moreover, salivary glands of O. turicata and O. turicata americanus ticks were colonized to similar densities, as determined by qPCR. These findings indicated that B. turicatae was positioned for transmission, and the low infection frequencies in mice after blood feeding by O. turicata americanus were not likely due to spirochete colonization of type I acini.
Argasid-borne TBRF spirochetes display an apparent vector competence that is restricted to single Ornithodoros species. Early studies described the vector specificity of Borrelia mazzottii that was recovered in Mexico (5). The pathogen could be acquired and maintained by Ornithodoros talaje ticks that originated from Mexico and Guatemala, but Ornithodoros species from other Latin American countries failed to maintain and transmit B. mazzottii. Schwan further investigated vector specificity by feeding cohorts of O. hermsi, O. turicata, and O. parkeri on mice infected with B. hermsii (4). He demonstrated that B. hermsii colonized the midgut of all tick species, but only O. hermsi successfully transmitted the pathogen to mice. Interestingly, B. hermsii was visualized in the central ganglia of the nontransmitting tick species but was undetectable in the salivary glands. This suggests that penetration of the basal membrane of the salivary glands may be a restrictive barrier.
The phenomenon of vector competence has been overlooked for O. turicata and O. turicata americanus, and our findings suggest that the salivary glands likely have one or more of the following impacts on the transmission of B. turicatae. First, physiological pressures in the salivary glands of O. turicata americanus may limit the B. turicatae diversity in these tissues. Second, the infectious doses delivered by the two tick subspecies may vary. Third, the molecular differences in saliva compositions between O. turicata and O. turicata americanus may impact the establishment of infection. The inability of B. turicatae to infect most mice by O. turicata americanus transmission suggests the impact of saliva in the transmission of B. turicatae. Future work will focus on comparative sialomics between O. turicata and O. turicata americanus to define the molecular mechanisms of saliva-facilitated transmission.
The establishment of infection is also likely dependent on the infectious dose delivered during feeding. While the number of spirochetes that enter the host through tick feeding remains vague, previous work suggests that the infectious dose is marginal. Infection frequencies in mice after O. turicata ticks attached for 15 s were equivalent to those of engorged ticks (3). Additionally, evaluation of the salivary glands of O. turicata after the bloodmeal indicated that the tissues remained heavily populated with B. turicatae (14). A constraint in capturing the infectious dose delivered by Ornithodoros species has been the challenge of saliva collection. The salivary glands of Ornithodoros species undergo few morphological changes during feeding (15, 17), and saliva production is likely minimal. In our study, infection frequencies after O. turicata americanus feedings were evaluated at the fourth and fifth nymphal stages, and it remains unclear whether there are stage-specific differences in transmission. Additional work will continue to address this challenge to determine the infectious dose delivered by O. turicata and O. turicata americanus.
Detecting similar densities of B. turicatae in O. turicata and O. turicata americanus salivary glands, yet lower infection frequencies in mice, suggested that the tissues of O. turicata americanus may have limited the population diversity of spirochetes entering the vertebrate host. In total, five of 20 mice became infected after feeding by O. turicata americanus, and B. turicatae was detected in only two animals by qPCR during the 10-day sampling period. The remaining three mice were considered infected by an assessment of serological responses to B. turicatae spirochetes. In these animals, detectable levels of infection may have occurred after the 10-day sampling period, which could be due to the limited diversity of B. turicatae spirochetes entering the mammal, resulting in a delay in the establishment of infection. Alternatively, the infection in these animals may have been transient and quickly cleared by the murine immune response. Collectively, these findings suggest that physiological pressures within O. turicata americanus salivary glands may have limited the diversity of spirochetes entering the mice.
The ecology and disease burden of B. turicatae are undefined, and additional work is needed to understand the pathogen's maintenance in nature. The spirochetes have been isolated from clinical domestic dogs (10, 11), which suggests the role of canids in the pathogen's natural maintenance. While we observed poor transmission frequencies to mice, it remains unknown whether canids are a more competent host for infection of B. turicatae by O. turicata americanus transmission. Moreover, little is known regarding the transmission dynamics of B. turicatae from O. turicata americanus, and additional studies are needed to define the intricacies of transmission. For example, work should focus on establishing colonies of O. turicata americanus that originate throughout Florida and on evaluating the transmission of other B. turicatae isolates. Additionally, while there is no evidence for coxal fluid transmission of B. turicatae from O. turicata, this mode of transmission has not been thoroughly evaluated for O. turicata americanus. Future studies will evaluate coxal fluid transmission as an alternative route of infection in the maintenance of B. turicatae. As the mechanisms of B. turicatae transmission are further defined, clarity will be obtained regarding the pathogen's ecology and spillover into the human population.
MATERIALS AND METHODS
Ethics statement.
Transmission studies performed using mice were approved by the Institutional Care and Use Committee (IACUC) at Baylor College of Medicine, with protocol numbers AN-6563 and AN-6580, and their laboratory animal program complies with standards and guidance that were established by the Association for Assessment and Accreditation of Laboratory Animal Care and the National Institution of Health of Laboratory Animal Welfare. The veterinary staff and animal care were provided by animal husbandry.
Tick colonies and B. turicatae isolates used in the study.
Two populations of O. turicata ticks were used in this study, western and eastern populations. The western populations of O. turicata originated from Kansas and Texas. The Kansas colony was uninfected and was obtained from the Rocky Mountain Laboratories, NIAID, NIH, and maintained at the Baylor College of Medicine. The Texas population of O. turicata was originally collected from coyote dens in La Salle County, TX (8). The eastern population of the tick, O. turicata americanus, was collected from Gopher tortoise dens in Marion County, FL (8). Tick colonies were kept separate based on the locality of collection. Ticks were morphologically identified using characteristics described by Cooley and Kohls (6) and housed under previously described conditions (14). The infection status of the ticks was determined by feeding cohorts of ∼10 adults on mice and evaluating infection in the animals by microscopy and seroconversion to B. turicatae protein lysates, as described below. To assess infection of the progeny of O. turicata and O. turicata americanus, PCR for the B. turicatae homologue of the Borrelia immunogenic protein A gene (bipA) was performed, as previously described (18). Moreover, the infectious statuses of first- and second-stage nymphs were evaluated by feeding 20 to 30 ticks on mice and assessing infection by microscopy and seroconversion.
The B. turicatae strains used in the study originated from Texas and Florida. 91E135, the Texas strain, was originally obtained by inoculating mice with dissected tick tissues, and when the animal was spirochetemic, BSK-H medium was inoculated with infected blood (11). The Florida isolate was cultured in BSK-H medium from the blood of an infected dog (10). For the current study, we refer to the Texas and Florida isolates as Bt-TX and Bt-FL, respectively. Additionally, the Bt-TX isolate producing GFP was previously generated (14) and in the current report is used to evaluate salivary gland colonization of O. turicata and O. turicata americanus. All infection studies were used with B. turicatae isolates that were passaged ≤10 times after the original isolation.
Tick acquisition of Bt-TX and Bt-FL and subsequent transmission studies to mice.
Infected cohorts of O. turicata (from Texas and Kansas) and O. turicata americanus were obtained by feeding third-stage nymphs on mice (acquisition bloodmeal) that were needle inoculated with 1 × 107 Bt-TX or Bt-FL. O. turicata and O. turicata americanus feedings were performed in parallel. After the ticks molted, transmission bloodmeals were performed as previously described (14), and groups of five mice were fed upon by four to 10 fourth-stage nymphal ticks per mouse. Upon completion of the bloodmeal, the ticks were removed from the mice, and the number of engorged ticks was noted. Ticks were housed separately in 50-ml conical tubes based on the animal upon which they fed. Transmission studies were repeated using fifth-stage nymphs.
Detection of B. turicatae infection in mice.
The infection frequencies of B. turicatae after transmission bloodmeals were determined by microscopic observation using dark-field microscopy, quantitative PCR (qPCR), and the evaluation of seroconversion, as previously described (3). Blood samples were collected from mice for 10 consecutive days by tail nick. For qPCR, 2.5 μl of blood was collected and added into 47.5 μl of lysis-stabilization buffer (Agilent, Santa Clara, CA, USA). An additional drop (∼2 μl) of blood was collected for microscopic examination using an Axio Imager A2 dark-field microscope (Zeiss, Munich, Germany). For qPCR assays, primers and a probe for the B. turicatae flagellin gene (flaB) were used, and conditions were as previously reported (3). A standard curve for qPCR was generated using 1 × 108 to 1 × 104 in vitro-grown B. turicatae spirochetes per ml.
Serological assessment of infection.
Protein lysates from 1 × 107 B. turicatae spirochetes were separated electrophoretically using Mini-PROTEAN TGX precast gels (Bio-Rad, Hercules, CA, USA). Proteins were transferred onto Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Four weeks after transmission feedings, blood was collected from mice, and serum samples were used to probe immunoblots, as previously reported (18, 19). Mouse serum samples were used at a 1:200 dilution, and Rec-protein G-horseradish peroxidase (Rec-protein G-HRP; Life Technologies, Carlsbad, CA, USA) diluted at 1:4,000 was used as the secondary antibody. Serological reactivity to whole-spirochete lysates was determined by chemiluminescence using ECL Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK).
Tick dissections and assessment of salivary gland colonization by fluorescence microscopy and qPCR.
Ticks were infected with Bt-TX producing GFP, and colonization of O. turicata americanus was evaluated as previously described (14). Tick salivary glands were dissected using an Axio Stemi microscope (Zeiss, Munich, Germany), rinsed with 1× phosphate-buffered saline (PBS), and transferred to a clean slide, and a coverslip was placed on the tissue. Slides were immediately evaluated with an Axio Imager A2 fluorescence microscope (Zeiss), and images were captured and analyzed using the Zen 2012 digital imaging software. An exposure time of 300 to 500 ms was used to capture fluorescent images.
O. turicata and O. turicata americanus ticks were further evaluated to quantify B. turicatae spirochetes in the salivary glands. The ticks were dissected, as previously described (12). Each sample consisted of a pool of salivary gland tissues from five individual ticks. Genomic DNA was extracted from each sample using the DNeasy blood and tissue kit (Qiagen, Hilden, Germany).
Standards for the qPCR assays were developed by targeting the B. turicatae flaB and O. turicata β-actin genes. Full-length flaB (1,005 bases) and a partial sequence of β-actin (1,020 bases) were amplified from B. turicatae and O. turicata genomic DNA, respectively. The amplicons were cloned into the PCR 2.1 vector using the quick one-step TA Cloning kit (Invitrogen, Carlsbad, CA, USA). Cloning reactions were transformed into One Shot TOP10 chemically competent Escherichia coli cells and grown under kanamycin selection. PCR was performed to screen colonies for flaB and β-actin. Plasmid preparations from the PCR-positive colonies were generated and sequenced by Lone Star Labs (Houston, TX, USA) to confirm the sequences of flaB and β-actin. These plasmids were subsequently used to generate standard curves for qPCR assays.
For duplex qPCR assays, primers (Table 2) were used at 400 nM and the probe at 300 nM, and the reaction conditions used were 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. Thirty-five cycles were run, and assays were performed in triplicate using 50 ng of genomic DNA (gDNA). Control reactions included performing the assay with individual primers for each gene to evaluate nonspecific binding. No-template controls were included to determine if any of the qPCR reagents were contaminated with gDNA. Individual reactions with O. turicata, O. turicata americanus, and B. turicatae gDNA templates were run against flaB and β-actin gene primer and probe sets to determine nonspecific binding. flaB and β-actin plasmids were tested with β-actin and flaB gene primer and probe sets, and vice versa, to detect cross-reactivity. qPCR assays were performed using the Applied Biosystems ViiA 7 real-time PCR system (Life Technologies, Carlsbad, CA, USA).
TABLE 2.
Oligonucleotides and probes used for cloning and qPCRa
| Primer or probe | Sequence (5′–3′) |
|---|---|
| Cloning primers | |
| flaB F | ATGATCATAAATCATAATACGTCAGCTATAAATG |
| flaB R | TCTAAGCAATGATAATACATACTGAGGCAC |
| β-Actin F | GGTCAGAAGGACAGCTACGTC |
| β-Actin R | CCGATCCAGACGGAGTACTT |
| qPCR primers and probes | |
| flaB F | ACAGCTGAAGAGCTTGGAATG |
| flaB R | TGATTTGCACCCACATGTACTC |
| flaB probe | YAK-AGCTGGATCACAAGCTTCATGGACA-IBFQ |
| β-Actin F | TATCCACGAGACCACCTACAA |
| β-Actin R | TCTGCATACGATCGGCAATAC |
| β-Actin probe | 6FAM-AAGGACCTGTACGCCAACACTGTC-IBFQ |
F, forward; R, reverse; YAK, Yakima dye; 6FAM, fluorescein amide; IBFQ, Iowa Black FQ quencher.
Statistical analyses.
For qPCR assays on murine blood and tick salivary glands, an analysis of variance was performed to determine statistical significance between different treatment groups. The GraphPad Prism software was used to perform all statistical analyses. A Kruskal-Wallis one-way analysis of variance with the Dunn multiple-comparison test was used to determine significant differences between treatment groups. P values of ≤0.05 were used to denote statistical significance.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH grants AI127377, AI123651, and AI103724 (to J.E.L.).
We declare no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01505-18.
REFERENCES
- 1.Sonenshine DE, Mather TN. 1994. Ecological dynamics of tick-borne zoonoses, vol 1 Oxford University Press, Inc, Oxford, United Kingdom. [Google Scholar]
- 2.Barbour AG. 1990. Antigenic variation of a relapsing fever Borrelia species. Annu Rev Microbiol 44:155–171. doi: 10.1146/annurev.mi.44.100190.001103. [DOI] [PubMed] [Google Scholar]
- 3.Boyle WK, Wilder HK, Lawrence AM, Lopez JE. 2014. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl Trop Dis 8:e2767. doi: 10.1371/journal.pntd.0002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwan TG. 1996. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis 5:167–181. [PubMed] [Google Scholar]
- 5.Davis GE. 1956. A relapsing fever spirochete, Borrelia mazzottii (sp. nov.) from Ornithodoros talaje from Mexico. Am J Hyg 63:13–17. [DOI] [PubMed] [Google Scholar]
- 6.Cooley RA, Kohls GM. 1944. The Argasidae of North America, Central America, and Cuba. The American midland naturalist monogaph no. 1. The University Press, Notre Dame, IN. [Google Scholar]
- 7.Davis GE. 1940. Ticks and relapsing fever in the United States. Public Health Rep 55:2347–2351. doi: 10.2307/4583554. [DOI] [Google Scholar]
- 8.Donaldson TG, Perez de Leon AA, Li AI, Castro-Arellano I, Wozniak E, Boyle WK, Hargrove R, Wilder HK, Kim HJ, Teel PD, Lopez JE. 2016. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): climate variation and host diversity. PLoS Negl Trop Dis 10:e0004383. doi: 10.1371/journal.pntd.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck AF, Holscher KH, Butler JF. 1986. Life cycle of Ornithodoros turicata americanus (Acari: Argasidae) in the laboratory. J Med Entomol 23:313–319. doi: 10.1093/jmedent/23.3.313. [DOI] [PubMed] [Google Scholar]
- 10.Breitschwerdt EB, Nicholson WL, Kiehl AR, Steers C, Meuten DJ, Levine JF. 1994. Natural infections with Borrelia spirochetes in two dogs from Florida. J Clin Microbiol 32:352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS, Breitschwerdt EB, Porcella SF. 2005. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol 43:3851–3859. doi: 10.1128/JCM.43.8.3851-3859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez JE, Wilder HK, Hargrove R, Brooks CP, Peterson KE, Beare PA, Sturdevant DE, Nagarajan V, Raffel SJ, Schwan TG. 2013. Development of genetic system to inactivate a Borrelia turicatae surface protein selectively produced within the salivary glands of the arthropod vector. PLoS Negl Trop Dis 7:e2514. doi: 10.1371/journal.pntd.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis GE. 1941. Ornithodoros turicata: the male; feeding and copulation habits, fertility, span of life, and the transmission of relapsing fever spirochetes. Public Health Rep 56:1799–1802. doi: 10.2307/4583854. [DOI] [Google Scholar]
- 14.Krishnavajhala A, Wilder HK, Boyle WK, Damania A, Thornton JA, Perez de Leon AA, Teel PD, Lopez JE. 2016. Imaging Borrelia turicatae producing green fluorescent protein reveals persistent colonization of Ornithodoros turicata midgut and salivary glands from nymphal acquisition through transmission. Appl Environ Microbiol 83:e2503-16. doi: 10.1128/AEM.02503-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balashov YS. 1972. Bloodsucking ticks (Ixodoidea)—vectors of diseases of man and animals. Misc Publ Entomol Soc Am 8:161–376. [Google Scholar]
- 16.Policastro PF, Raffel SJ, Schwan TG. 2013. Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency. Infect Immun 81:2899–2908. doi: 10.1128/IAI.00542-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonenshine DE, Roe RM. 2014. Biology of ticks, 2nd ed, vol 1 Oxford University Press, New York, NY. [Google Scholar]
- 18.Lopez JE, Wilder HK, Boyle W, Drumheller LB, Thornton JA, Willeford B, Morgan TW, Varela-Stokes A. 2013. Sequence analysis and serological responses against Borrelia turicatae BipA, a putative species-specific antigen. PLoS Negl Trop Dis 7:e2454. doi: 10.1371/journal.pntd.0002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilder HK, Wozniak E, Huddleston E, Tata SR, Fitzkee NC, Lopez JE. 2015. Case report: a retrospective serological analysis indicating human exposure to tick-borne relapsing fever spirochetes in Texas. PLoS Negl Trop Dis 9:e0003617. doi: 10.1371/journal.pntd.0003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.