Daqu fermentations select for mobile genetic elements conferring heat resistance in Enterobacteriaceae and bacilli. The locus of heat resistance (LHR), a genomic island conferring heat resistance in Enterobacteriaceae, and the spoVA2mob operon, conferring heat resistance on bacterial endospores, were enriched 3- to 5-fold during Daqu fermentation and maturation. It is therefore remarkable that the LHR and the spoVA2mob operon are accumulated in the same food fermentation. The presence of heat-resistant Kosakonia spp. and Bacillus spp. in Daqu is not of concern for food safety; however, both genomic islands are mobile and transferable to pathogenic bacteria or toxin-producing bacteria by horizontal gene transfer. The identification of the LHR and the spoVA2mob operon as indicators of fitness of Enterobacteriaceae and bacilli in Daqu fermentation provides insights into environmental sources of heat-resistant organisms that may contaminate the food supply.
KEYWORDS: heat resistance, Daqu, locus of heat resistance, spoVA2mob operon, Cronobacter, Bacillus
ABSTRACT
Daqu is a spontaneous solid-state cereal fermentation used as saccharification and starter culture in Chinese vinegar and liquor production. The evolution of microbiota in this spontaneous fermentation is controlled by the temperature profile, which reaches temperatures from 50 to 65°C for several days. Despite these high temperatures, mesophilic Enterobacteriaceae (including Cronobacter) and bacilli are present throughout Daqu fermentation. This study aimed to determine whether Daqu spontaneous solid-state fermentation selects for heat-resistant variants of these organisms. Heat resistance in Enterobacteriaceae is mediated by the locus of heat resistance (LHR). One LHR-positive strain of Kosakonia cowanii was identified in Daqu, and it exhibited higher heat resistance than the LHR-negative K. cowanii isolated from malted oats. Heat resistance in Bacillus endospores is mediated by the spoVA2mob operon. Out of 10 Daqu isolates of the species Bacillus licheniformis, Brevibacillus parabrevis, Bacillus subtilis, Bacillus amyloliquefaciens, and Bacillus velezensis, 5 did not contain spoVA2mob, 3 contained one copy, and 2 contained two copies. The presence and copy number of the spoVA2mob operon increased the resistance of spores to treatment with 110°C. To confirm the selection of LHR- and spoVA2mob-positive strains during Daqu fermentation, the copy numbers of these genetic elements in Daqu samples were quantified by quantitative PCR (qPCR). The abundance of LHR and the spoVA2mob operon in community DNA relative to that of total bacterial 16S rRNA genes increased 3-fold and 5-fold, respectively, during processing. In conclusion, culture-dependent and culture-independent analyses suggest that Daqu fermentation selects for heat-resistant Enterobacteriaceae and bacilli.
IMPORTANCE Daqu fermentations select for mobile genetic elements conferring heat resistance in Enterobacteriaceae and bacilli. The locus of heat resistance (LHR), a genomic island conferring heat resistance in Enterobacteriaceae, and the spoVA2mob operon, conferring heat resistance on bacterial endospores, were enriched 3- to 5-fold during Daqu fermentation and maturation. It is therefore remarkable that the LHR and the spoVA2mob operon are accumulated in the same food fermentation. The presence of heat-resistant Kosakonia spp. and Bacillus spp. in Daqu is not of concern for food safety; however, both genomic islands are mobile and transferable to pathogenic bacteria or toxin-producing bacteria by horizontal gene transfer. The identification of the LHR and the spoVA2mob operon as indicators of fitness of Enterobacteriaceae and bacilli in Daqu fermentation provides insights into environmental sources of heat-resistant organisms that may contaminate the food supply.
INTRODUCTION
Thermal processing is widely used to kill unwanted vegetative cells and endospores in foods (1). The heat resistance of vegetative cells or endospores of the same species varies substantially (2, 3). Due to the variety of heat resistance in target organisms, heat-resistant strains may tolerate thermal food preservation processes that are lethal to a majority of strains of the same species (4, 5). Comparative genomic analyses have identified transferable genomic islands that confer heat resistance to bacterial endospores (6) and Enterobacteriaceae (7). Knowledge regarding the genetic determinants of bacterial heat resistance not only allows for the rapid identification of heat-resistant strains but also facilitates the identification of environmental conditions that select for heat resistance.
Heat resistance of endospores is enhanced by the spoVA2mob operon (6). The spoVA2mob operon has previously been identified in Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefaciens, Bacillus cereus, and Bacillus thermoamylovorans; cloning of the spoVA2mob operon increased spore heat resistance, with the effect increasing with the copy number of the operon (8, 9). spoVA2mob-mediated heat resistance of bacterial endospores relates to the uptake of dipicolinic acid during sporulation (10, 11). Heat resistance of Enterobacteriaceae, including Escherichia coli, Salmonella, Cronobacter, and Enterobacter cloacae, is mediated by a genomic island termed the locus of heat resistance (LHR) (7, 12). The LHR increases the D60 value (decimal reduction time [i.e., the time required to kill 90% of the microorganisms] at 60°C) relative to that of LHR-negative strains of the same species 10- to 100-fold (13). LHR-encoded proteins include heat shock proteins that prevent or repair protein misfolding and aggregation (14, 15). Both the LHR and the spoVA2mob operon are mobile genetic elements and may transfer to pathogenic bacteria or spoilage organisms; therefore, heat resistance provided by the LHR and the spoVA2mob operon challenges the control of pathogens in food system by thermal processing (13, 16).
The reservoir and selective pressure for LHR-positive or spoVA2mob-positive microorganisms remain unknown; to date, conclusions on environmental conditions selecting for LHR-positive E. coli were based solely on the frequency of LHR-positive isolates in specific ecosystems (12, 17, 18). Ecosystems that maintain a temperature of 60°C for an extended period may select for heat-resistant bacteria. One example is Daqu, a traditional starter culture used for production of spirits and vinegar from cereals. Daqu is produced from cereals by a spontaneous solid-state fermentation which includes three phases: shaping (fermentation stage), ripening (maturation stage), and drying (19). Daqu fermentation supports growth of amylolytic fungi and bacilli; amylolytic enzymes produced in Daqu support starch saccharification and ethanol production in subsequent fermentation stages (20). The temperature is a key process parameter to control the evolution of microbiota (21). Depending on the type of fermentation, the temperature increases during the fermentation stages to reach a maximum ranging from 45 to 65°C and declines to ambient temperature during maturation and drying stages (22). In traditional Daqu fermentations, temperature control is achieved by forced ventilation and by manually turning over the Daqu blocks (19). Mesophilic organisms, including fungi, bacilli, Enterobacteriaceae, E. cloacae, and lactic acid bacteria, are dominant representatives of the Daqu fermentation microbiota (21, 22). Daqu comprising Enterobacteriaceae and bacilli provides a good model for studying the effect of temperature on the selection for heat-resistant strains containing the LHR or the spoVA2mob operon.
Previous studies mainly demonstrated the mechanisms of functional genes mediating heat resistance (6, 7, 14, 23), but the environmental selective pressure for heat resistance genes in natural and human-made ecosystems remains unclear. Therefore, this study aimed to investigate the environmental selective pressure for heat-resistant organisms in food fermentation. LHR-positive and spoVA2mob-positive bacteria were screened for in the Daqu matrix, followed by verification of the heat resistance of each isolate. The cell counts of Enterobacteriaceae and sporeformers were also assessed during Daqu production, as well as the copy numbers of the LHR and the spoVA2mob operon. Moreover, the influence of temperature on the selection for heat-resistant bacteria was determined by evaluating the copy numbers of the LHR and the spoVA2mob operon during Daqu processing.
RESULTS
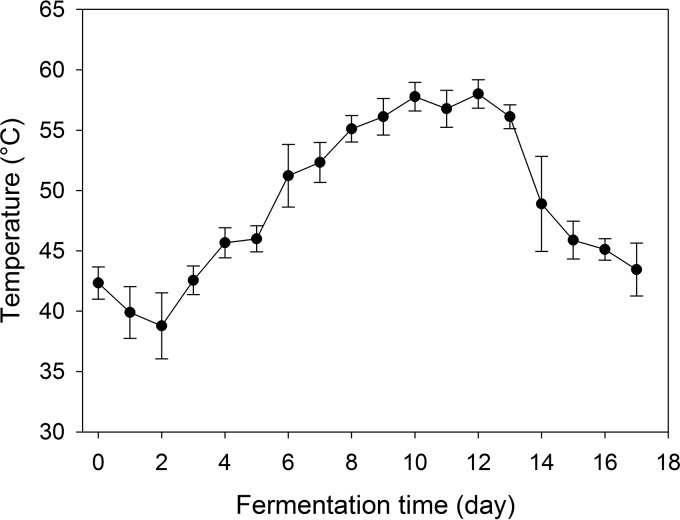
Temperature profile of Daqu piles.
To determine the temperature profile of the Daqu fermentation from which isolates were obtained, the temperature profile in replicate batches of Daqu was monitored during fermentation and maturation (Fig. 1). Microbial activity increased the Daqu temperature to more than 45°C after 4 days of fermentation. The fermentation stage consists of the thermophilic and cooling stages. At the thermophilic stage, the temperature increased to about 60°C, followed by a decrease to 45°C at the cooling stage and a 13-day maturation stage at room temperature. A temperature of more than 55°C, which is typically sufficient to kill Enterobacteriaceae within minutes or hours (13, 24), was maintained for more than 4 days.
FIG 1.
Temperature profile of Daqu piles during the fermentation stage (day 1 to day 17). The temperature remained at ambient temperature throughout the maturation and drying stages (data not shown). Data represent means ± SDs from four fermentation rooms in triplicate.
Identification of bacilli and coliform bacteria from Daqu.
To characterize isolates with respect to their heat resistance, two Daqu samples were obtained from the fermentation and maturation stages. Plate counts were determined on Luria-Bertani (LB) and violet red bile glucose (VRBG) agars (Table 1), and representative isolates were identified at the species level (Tables 2 and 3). Samples were analyzed after several months of storage at −20°C and using protocols for selective enumeration; therefore, cell counts may not fully reflect the counts at the time of fermentation. Endospore-forming organisms increased after 13 days of maturation. Isolates of endospore-forming organisms belonged to B. licheniformis, Brevibacillus parabrevis, B. subtilis, B. amyloliquefaciens, and Bacillus velezensis (Table 2). Cell counts of coliforms increased from 5.48 log CFU/g to 6.64 log CFU/g by the end of maturation. Eleven isolates of Enterobacteriaceae were identified as Kosakonia cowanii, Cronobacter sakazakii, Enterobacter hormaechei, and Pantoea calida (Table 3).
TABLE 1.
Culture-dependent analysis of Daqu microbiota at fermentation stage (day 17) and maturation stage (day 30)
| Bacteria or medium | Fermentation stage |
Maturation stage |
||
|---|---|---|---|---|
| Cell count (CFU/g)a | No. of isolates | Cell count (CFU/g)a | No. of isolates | |
| Total plate count | 5.48 × 107 | 2 | 4.06 × 107 | 6 |
| Endosporesb | 2.24 × 103 | 5 | 2.02 × 105 | 5 |
| VRBGc agar | 3.02 × 105 | 5 | 4.38 × 106 | 6 |
Data are means from two technical replicates.
Endospores were enumerated after heating to 80°C for 30 min.
VRBG, violet red bile glucose.
TABLE 2.
Identification and copy number of the spoVA2mob operon in 10 isolates from Daqu
| Stage | Closest type strain | Strain | Type strain | Identity (%) to type strain | spoVA2mob copy no./genomea,b | spoVA2mob copy no./genomec |
|---|---|---|---|---|---|---|
| Fermentation | Bacillus licheniformis | FUA2146 | ATCC 14580 | 99.7 | —d | 0 |
| Brevibacillus parabrevis | FUA2147 | IFO 12334T | 98.7 | — | 0 | |
| Bacillus subtilis | FUA2148 | DSM 10 | 99.4 | — | 0 | |
| Bacillus amyloliquefaciens | FUA2149 | DSM 7 | 100.0 | 0.71 ± 0.04 | 1 | |
| Bacillus subtilis | FUA2150 | DSM 22148 | 99.2 | 0.69 ± 0.08 | 1 | |
| Maturation | Bacillus licheniformis | FUA2151 | ATCC 14580 | 99.7 | — | 0 |
| Brevibacillus parabrevis | FUA2152 | IFO 12334T | 100.0 | — | 0 | |
| Bacillus amyloliquefaciens | FUA2153 | DSM 7 | 98.6 | 0.89 ± 0.12 | 1 | |
| Bacillus amyloliquefaciens | FUA2154 | NBRC 15535 | 100.0 | 1.42 ± 0.17 | 2 | |
| Bacillus velezensis | FUA2155 | CBMB 205 | 99.6 | 1.27 ± 0.31 | 2 |
Means ± standard deviations in triplicate.
Measured copy numbers.
Adjusted copy numbers.
—, not detected.
TABLE 3.
Identification and presence of LHR of 11 isolates from Daqu
| Stage | Closest type strain | Strain | Type strain | Identity (%) to type strain | Presence of LHRa |
|---|---|---|---|---|---|
| Fermentation | Kosakonia cowanii | FUA10116 | CIP 107300 | 100.0 | − |
| Cronobacter sakazakii | FUA10117 | ATCC 29544 | 99.1 | − | |
| Cronobacter sakazakii | FUA10118 | ATCC 29544 | 100.0 | − | |
| Enterobacter hormaechei | FUA10119 | CIP 103441 | 99.3 | − | |
| Pantoea calida | FUA10120 | 1400/07 | 99.1 | − | |
| Maturation | Kosakonia cowanii | FUA1601 | CIP 107300 | 100.0 | + |
| Kosakonia cowanii | FUA10121 | CIP 107300 | 99.9 | − | |
| Cronobacter sakazakii | FUA10122 | ATCC 29544 | 98.9 | − | |
| Cronobacter sakazakii | FUA10123 | ATCC 29544 | 98.8 | − | |
| Enterobacter hormaechei | FUA10124 | CIP 103441 | 95.3 | − | |
| Pantoea calida | FUA10125 | 1400/07 | 99.6 | − |
−, negative; +, positive.
The LHR and the spoVA2mob operon screening of isolates.
PCR was performed to identify the presence of the LHR and the spoVA2mob operon in all isolates of Enterobacteriaceae and spore-forming bacteria, respectively. Two and three isolates from the fermentation stage and the maturation stage, respectively, were spoVA2mob positive (Table 2). K. cowanii FUA1601 from the fresh Daqu samples was LHR positive (Table 3).
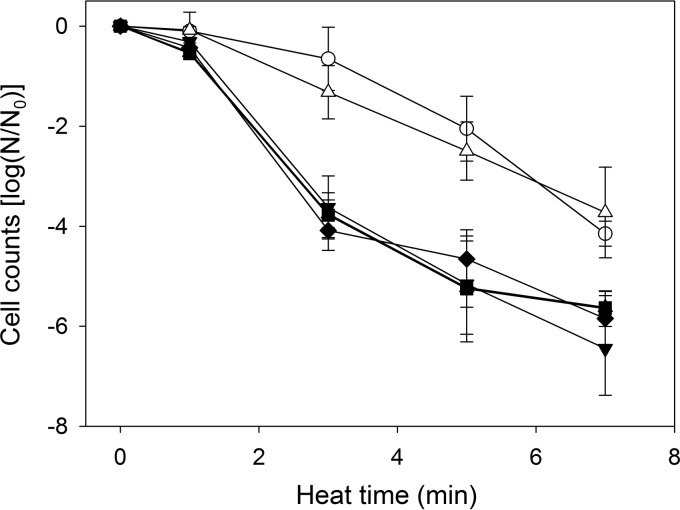
The LHR confers heat resistance on K. cowanii.
To determine whether the LHR confers heat resistance on K. cowanii, the survival of LHR-positive K. cowanii FUA1601 at 60°C was compared to survival of LHR-negative K. cowanii FUA1341, FUA1348, and FUA1349 (Fig. 2). The survival rate of three LHR-negative K. cowanii isolates was significantly lower than that of LHR-positive K. cowanii FUA1601 (P < 0.05). To further confirm the role of LHR in heat resistance, the entire LHR sequence was cloned to pRK767 to form pLHR (7), and pLHR was transferred into LHR-negative K. cowanii FUA10121. The heat resistance of the transformant K. cowanii FUA10121(pLHR) was equivalent to that of the LHR-positive wild-type strain (Fig. 2).
FIG 2.
Cell counts after treatment at 60°C for six strains of Kosakonia cowanii. ○, LHR-positive K. cowanii FUA1601 isolated from Daqu; △, LHR-negative K. cowanii FUA10121(pLHR) isolated from Daqu; ▲, LHR-negative K. cowanii FUA10121 isolated from Daqu; ▼, LHR-negative K. cowanii FUA1341 isolated from malted oats; ■, LHR-negative K. cowanii FUA1348 isolated from malted oats; ◆, LHR-negative K. cowanii FUA1349 isolated from malted oats.
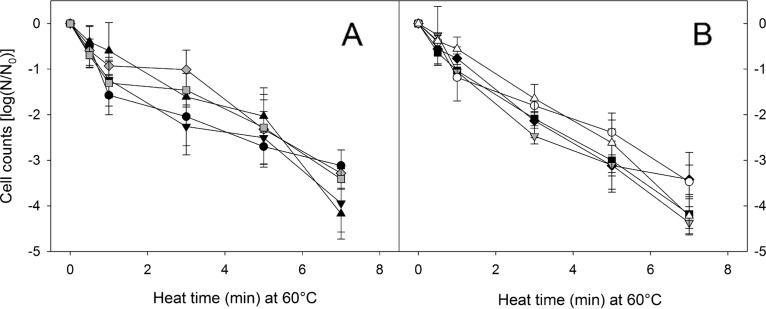
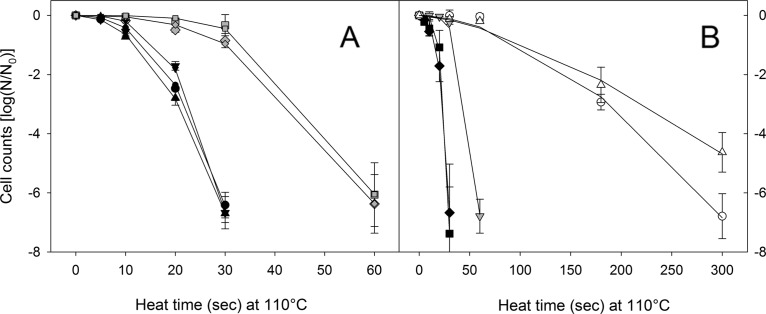
Heat resistance of endospores is dependent on the copy number of the spoVA2mob operon.
To determine heat resistance of vegetative cells and endospores of bacilli possessing the spoVA2mob operon, survival was assessed at 60°C for vegetative cells (Fig. 3) and at 110°C for spores (Fig. 4). Vegetative cells of bacilli were heat sensitive irrespective of the presence of the spoVA2mob operon (Fig. 3). In contrast, the presence of the spoVA2mob operon increased the heat resistance in spores (Fig. 4). Cell counts of spoVA2mob-negative strains were reduced by approximately 7 log CFU/ml after 30 s at 110°C, while cell counts of spoVA2mob-positive strains were reduced by less than 1 log CFU/ml (Fig. 4).
FIG 3.
Cell counts of Bacillus and Brevibacillus isolates after treatment of vegetative cells at 60°C. (A) Heat treatment of vegetative cells of 5 isolates from the fermentation stage. Black circles, B. licheniformis FUA2146; black triangles, Br. parabrevis FUA2147; black inverted triangles, B. subtilis FUA2148; gray diamonds, B. amyloliquefaciens FUA2149; gray squares, B. subtilis FUA2150. (B) Heat treatment of vegetative cells of 5 isolates from the maturation stage. Black diamonds, B. licheniformis FUA2151; black squares, Br. parabrevis FUA2152; gray inverted triangles, B. amyloliquefaciens FUA2153; white circles, B. amyloliquefaciens FUA2154; white triangles, B. velezensis FUA2155. Black, gray, and white symbols represent strains with 0, 1, and 2 copies of the spoVA2mob operon per genome (Table 3). Data are means ± SDs from three independent experiments.
FIG 4.
Cell counts of Bacillus and Brevibacillus isolates after treatment of spore preparations at 110°C. (A) Heat treatment of spores of 5 isolates from the fermentation stage. Black circles, B. licheniformis FUA2146; black triangles, Br. parabrevis FUA2147; black inverted triangles, B. subtilis FUA2148; gray diamonds, B. amyloliquefaciens FUA2149; gray squares, B. subtilis FUA2150. (B) Heat treatment of spores of 5 isolates from the maturation stage. Black diamonds, B. licheniformis FUA2151; black squares, Br. parabrevis FUA2152; gray inverted triangles, B. amyloliquefaciens FUA2153; white circles, B. amyloliquefaciens FUA2154; white triangles, B. velezensis FUA2155. Black, gray, and white symbols represent strains with 0, 1, and 2 copies of the spoVA2mob operon per genome (Table 3). Lines represent the curve fit obtained by nonlinear regression to the Weibull model. Data are means ± SDs from three independent experiments.
Because heat resistance of spores was reported to depend on the copy number of the spoVA2mob operon (6), we also determined the copy number of the spoVA2mob operon in Daqu isolates (Table 2). The lowest copy number of the spoVA2mob operon in B. subtilis FUA2150 was calculated as 0.69; however, because the number of copies per genome must be an integer, the deviation from 1 was assumed to represent experimental error. The copy numbers of the spoVA2mob operon in B. amyloliquefaciens FUA2149 and FUA2153 were comparable to that of B. subtilis FUA2150 and were similarly assumed to be 1. The copy numbers of the spoVA2mob operon of B. amyloliquefaciens FUA2154 and B. velezensis FUA2155 were determined experimentally as 1.42 and 1.27, respectively. These numbers are twice as high as the copy number in B. subtilis FUA2150 and likely represent two spoVA2mob operons per genome.
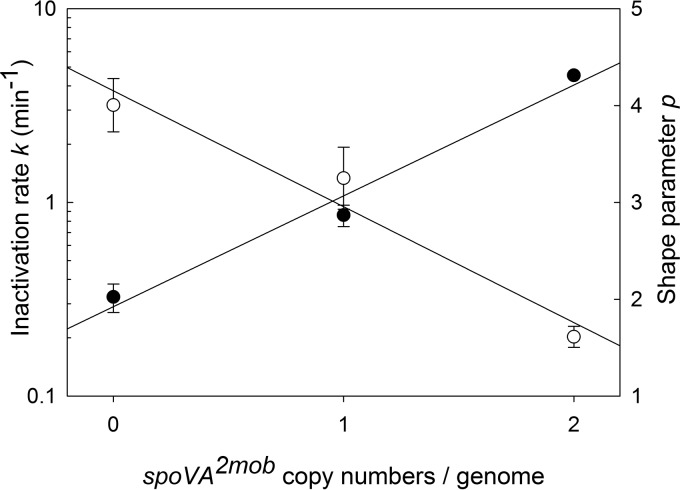
The survival of bacterial endospores at 110°C clearly related to the copy number of the spoVA2mob operon (Fig. 4). To determine whether a quantitative relationship exists between the number of spoVA2mob copies per genome and the heat resistance, survivor curves at 110°C were fitted to the Weibull model (Fig. 4). The use of the nonlinear Weibull model was chosen because all thermal death time curves exhibited pronounced tailing. The relationship between the inactivation rate k, the shape parameter p, and the spoVA2mob copy number is shown in Fig. 5. The inactivation rate of spores of the five strains lacking the spoVA2mob operon was approximately 0.32 min at 110°C. The average inactivation rates of strains carrying one or two spoVA2mob copies were 0.86 and 4.5 min at 110°C, respectively; plotting the gene copy number versus the inactivation rate revealed a log-linear relationship with a correlation coefficient (r2) of 0.96 (Fig. 5). Remarkably, the shape factor, which indicates tailing or shoulders, was also dependent on the copy number of the spoVA2mob operon. The shoulder effect decreased with increasing copy numbers of the spoVA2mob operon (Fig. 4); the shape factor p was correlated to the operon copy number, with an r2 of 0.96 (Fig. 5).
FIG 5.
Weibull parameters k and p of 10 Bacillus and Brevibacillus isolates from Daqu. The values obtained for the inactivation rate k (●) and the shape parameter p (○) at 110°C were averaged for all isolates with 0 (5 strains), 1 (three strains), or 2 (two strains) copies of the spoVA2mob operon per genome. Lines represent log-linear regression (k) and linear regression (p) of copy numbers and the parameters of the Weibull model.
Increase of copy numbers of the LHR and the spoVA2mob operon during the Daqu fermentation process.
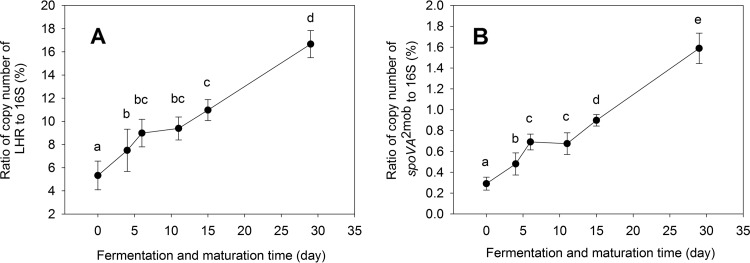
A high frequency of the LHR and the spoVA2mob operon in isolates indicates selection for heat-resistant strains during fermentation. To provide further evidence that Daqu fermentation selects for LHR- and spoVA2mob-positive Enterobacteriaceae and bacilli, respectively, we quantified the copy numbers of these two genes relative to total bacterial 16S rRNA throughout the fermentation process. The copy numbers of the LHR, the spoVA2mob operon, and 16S rRNA genes were measured at 6 time points of Daqu fermentation (Fig. 6). The abundance relative to 16S rRNA genes of both the LHR and the spoVA2mob operon increased during Daqu fermentation. The increase of the LHR and the spoVA2mob operons was significant after only 4 and 6 days of fermentation (Fig. 6), corresponding to increases of fermentation temperature to 45°C and 52°C (Fig. 1), respectively. In the entire process, the relative abundance of the LHR increased more than 3-fold; the relative abundance of the spoVA2mob operon increased more than 5-fold (Fig. 6).
FIG 6.
Relative abundance of gene copies of the LHR and spoVA2mob operon in Daqu community DNA during fermentation and maturation. (A) Copy numbers of the LHR to relative to the copy number of bacterial 16S rRNA genes. (B) Copy number of the spoVA2mob operon relative to the copy number of bacterial 16S rRNA genes. Data represent means ± SDs from four replicate samples. Values in the same panel that do not share a letter are significantly different (P < 0.05).
Frequency of spoVA2mob-positive bacilli in genomes deposited in the NCBI database.
To determine whether the frequency of the spoVA2mob operon in bacilli isolated from Daqu is higher than the frequency of other strains of the same species, we identified the frequency of spoVA2mob-positive BLAST results with the spoVA2mob operon as a query sequence. For the Bacillus spp. of interest, 432 genome sequences are available; of these, 6 of 74 genome sequences of B. licheniformis, 0 of 1 genome sequence of Br. parabrevis, 4 of 185 genome sequences of B. subtilis, 4 of 53 genome sequences of B. amyloliquefaciens, and 2 of 118 genome sequences of B. velezensis were spoVA2mob positive. In 23 out of the 24 finished genomes of Bacillus spp. that contained spoVA2mob, the operon was carried on a chromosome.
DISCUSSION
The LHR and the spoVA2mob operon confer heat resistance on Enterobacteriaceae and bacilli, respectively (6, 7). This study quantified the LHR and the spoVA2mob operon in a Daqu fermentation, demonstrating that the temperature profile of Daqu fermentation can provide selective pressure for these genomic islands and related heat-resistant strains.
Samples used in this study were dried and stored frozen to isolate community DNA for culture-independent analysis of the presence of genes coding for heat resistance. In addition, a total of 29 microbial strains were isolated to verify that LHR- or spoVA2mob-positive Enterobacteriaceae and bacilli, respectively, have a higher resistance to heat than negative isolates of the same species and origin. Plant-associated Enterobacteriaceae, including C. sakazakii, K. cowanii, and Enterobacter hormaechei, were frequently isolated. K. cowanii was classified as Enterobacter until 2013 (25, 26). The diversity of isolates thus conforms to prior reports that plant-associated Enterobacteriaceae are abundant representatives of Daqu microbiota, comparable to other Daqu fermentations from different locations under same temperature profile (20, 27, 28). Of note, plant-associated Enterobacteriaceae and particularly C. sakazakii are also opportunistic pathogens. C. sakazakii and K. cowanii persist for extended periods in low-moisture foods, including infant formula, and can cause nosocomial infections in preterm neonates (29, 30).
This study additionally demonstrated that Daqu fermentation selects for heat-resistant and LHR-positive K. cowanii. The heat resistance of LHR-positive Kosakonia from Daqu is comparable to previously published heat resistance of LHR-positive Enterobacter spp. (12) and confirms that the LHR is a reliable marker for heat resistance (7, 12). The relative abundance of Enterobacteriaceae in Daqu, about 25% of total isolates (27), matches the relative abundance of the LHR copies (this study), indicating that a substantial proportion of Enterobacteriaceae carry the LHR. Even when considering that multiple copies of the LHR may be present in one strain, this study demonstrates that an increase of the fermentation temperature exerts a strong selective pressure for LHR-positive Enterobacteriaceae. A high frequency of LHR-positive Escherichia coli organisms has been previously found for meat (13), cheese (31), and wastewater (17); however, the LHR has not been identified in spontaneous solid-state fermentation or in K. cowanii. The temperature of Daqu ranged from 55 to 60°C for 6 days; at this temperature range, even LHR-positive Kosakonia and Enterobacter spp. are usually killed within minutes of exposure (Fig. 3) (12). A reduction of the water activity induces accumulation of compatible solutes and enhances the heat resistance of LHR-positive Enterobacteriaceae (32–34). The low moisture content of Daqu may thus enhance the selective advantage of LHR-mediated heat resistance of Kosakonia during fermentation.
Bacillus spp. constitute a significant portion of the microbial population in Daqu (19, 21, 22); their endospores survive under low-moisture conditions and at high temperatures (35). This study demonstrated that the count of spores increased during fermentation; the taxonomic identification of spore-forming bacteria matched prior reports on Daqu microbiota (Tables 1 and 2) (22, 36). More surprisingly, 50% of the isolates of sporeformers were spoVA2mob positive. The frequency of spoVA2mob-operon positive bacilli isolated from Daqu is more than 10-fold higher than the proportion of spoVA2mob-positive isolates for which genome sequence data have been accessible in the GenBank genome database. Moreover, most spoVA2mob-positive bacilli and all isolates with two spoVA2mob copies per genome were obtained from the maturation stage. Heat-resistant and spoVA2mob-positive bacilli were identified as B. subtilis, B. amyloliquefaciens, and B. velezensis.
The high proportion of spoVA2mob-positive isolates (Tables 1 and 2) together with the strong increase of the relative abundance of the spoVA2mob operon in community DNA (Fig. 6) indicates a strong selective pressure for the operon during fermentation. This selective pressure is likely exerted on spore survival. The spoVA2mob operon is controlled by a sporulation-specific promoter, and the genes in this operon are specifically expressed during sporulation (37); accordingly, the operon impacts heat resistance of spores but not of vegetative cells (Fig. 3 and 4). The Daqu fermentation temperature is substantially below the temperature range that allows rapid inactivation of spoVA2mob-negative endospores (Fig. 3) (6, 38, 39). The 4- to 5-fold increase of the proportion of spoVA2mob-positive bacterial endospores during Daqu fermentation suggests that the operon also improves long-term survival of Bacillus endospores at the temperature range of 50 to 60°C, which is not well documented in the experimental literature.
The heat resistance of Bacillus endospores depended not only on the presence but also on the copy number of the spoVA2mob operon, consistent with a prior report (8). Kinetic modeling of spore inactivation in combination with quantification of the spoVA2mob copy numbers allowed establishment of quantitative relationships. The inactivation rate was strongly influenced by the spoVA2mob copy numbers, as indicated by the log-linear relationship (Fig. 5). Remarkably, the shape parameter p was also dependent on the presence and copy number of the spoVA2mob operon. For log-linear thermal death time curves, the shape parameter is 1; tailing is represented by a shape parameter below 1, and values for p that are higher than 1 indicate a shoulder (40, 41). Physiological mechanisms that relate to shouldering and tailing phenomena are poorly investigated (42). The spoVA2mob operon strongly impacted the shoulder effect in thermal death time curves (Fig. 4 and 5); this phenomenon warrants further investigation.
In conclusion, the Daqu fermentation analyzed in this study appears to have selected for mobile genetic elements conferring heat resistance in Enterobacteriaceae and bacilli. Both the LHR and the spoVA2mob operon were enriched by the end of Daqu processing, and the relative abundance of the two increased approximately 3 and 5 times, respectively. Bacillus endospores exhibit a much higher resistance to heat than Enterobacteriaceae; it is therefore remarkable that the LHR and the spoVA2mob operon, which confer resistance on Enterobacteriaceae and Bacillus spores, respectively, are enriched in the same food fermentation. Current knowledge allows identification of ecosystems which select for LHR-positive Escherichia coli; however, the selective pressure for other Enterobacteriaceae is unknown. All current isolates of spoVA2mob-positive bacilli were obtained from commercial food products (39); data on natural habitats that provide selective pressure for this operon are unavailable. This study extends prior knowledge by indicating that heat resistance may contribute to ecological fitness for K. cowanii and Bacillus spp. in food fermentations and may account for their abundance in fermentation microbiota. Daqu is one of only a few food fermentations for which fermentation temperatures reach or exceed 60°C; moreover, it is also one of only a few fermentations dominated by bacilli and Enterobacteriaceae (43). The selective pressure for heat-resistant Enterobacteriaceae and bacilli in food fermentations is therefore unprecedented. The identification of the LHR and the spoVA2mob operon as likely indicators of fitness of Enterobacteriaceae and bacilli in Daqu fermentation may provide insight into environmental sources of heat-resistant spoilage organisms. The presence of heat-resistant Kosakonia spp. and Bacillus spp. in Daqu is not a food safety concern; however, both genomic islands are mobile and transferable to pathogenic bacteria or toxin-producing bacteria by horizontal gene transfer. Our study may contribute to the identification of the source of heat-resistant spoilage organisms and pathogens that may contaminate the food supply.
MATERIALS AND METHODS
Sample collection.
Daqu samples were obtained by Li et al. (20) from two independent industry-scale fermentations. The stacked layers of Daqu blocks were manually turned every 2 days during the fermentation stage to allow adequate aeration and to control the pile temperature. During the 17-day fermentation, the temperature of the blocks was strictly controlled (20) according to traditional solid-state fermentation techniques such as stacking and opening windows and doors, with a small variation in the Daqu core temperature (pile temperature) between the different fermentation rooms; afterwards, for the 13-day maturation, samples were dried and cooled to room temperature. In enumeration and isolation, samples were collected on day 17 (the end of fermentation stage) and day 30 (the end of maturation stage) and were analyzed. For quantitative PCR (qPCR) analysis, samples were collected separately at day 0 (40°C to ∼45°C) and days 4 (42°C to ∼47°C), 6 (46°C to ∼51°C), 11 (53°C to ∼58°C), 15 (45°C to ∼50°C), and 30 (<25°C) based on the temperature control during the fermentation process (Fig. 1). Samples were collected from two replicate fermentation rooms at each time point, and two samples per fermentation room were obtained. Two technical replicates were analyzed by qPCR. Daqu samples were ground to powder in a sterile grinder and then transferred into a sterile stomacher bag (Stomacher Lab System, London, UK) to obtain about 500 g of sample. All samples were dried and frozen at −20°C immediately for further analysis.
Enumeration and isolation.
Dry samples (10 g) were mixed with 90 ml of buffered peptone water (Oxoid), soaked at 4°C for 30 min, and homogenized (Stomacher Lab Blender 400; Seward Medical, London, UK) for 2 min. Duplicate counting plates were prepared using appropriate dilutions. For plating, 50 μl of the dilution was spread on the surface of a dried plate with a spiral plater (Don Whitley Scientific, Shipley, UK). The total bacterial count was determined on Luria-Bertani (LB) (Difco) plates after incubation at 37°C for 24 h. For the estimation of bacterial spores, a 10% (wt/vol) sample suspension was heated at 80°C for 30 min and enumerated as described above. Serial dilutions of homogenate were surface plated on violet red bile glucose (VRBG) agar (Oxoid) and incubated at 44°C for 18 h, separately. After incubation, the colonies appearing on the selected plates were counted and calculated as CFU per gram of dry Daqu sample. Three representative colonies of each morphology were isolated and stored in glycerol at −80°C.
Identification of isolates.
For sequencing analysis, genomic DNA of bacteria was extracted from pure cultures of isolates using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The genomic DNA was then used as a template for PCR to amplify the bacterial 16S rRNA using the primers 27F/1492R (44) and Phusion high-fidelity DNA polymerase (Fisher Scientific). Amplified 16S rRNA fragments were analyzed on 1% (wt/vol) agarose gels stained with SYBRsafe (Invitrogen, Burlington, Ontario, Canada). After purification with a GeneJET Gel Extraction and DNA Cleanup Micro kit (Thermo Fisher Scientific, Waltham, MA), the 16S rRNAs were sequenced with Sanger sequencing by the Molecular Biology Service Unit of the University of Alberta (Canada). The 16S rRNA sequences were compared with sequences of type strains using the sequence match tool of the Ribosomal Database Project (https://rdp.cme.msu.edu/seqmatch/seqmatch_intro.jsp).
PCR screening of Daqu isolates for the spoVA2mob operon and LHR.
All isolates were screened for heat resistance with primers targeting the last gene of the spoVA2mob operon and three fragments of the LHR in Escherichia coli AW1.7. Primers used in this study are shown in Table 4. To detect the presence of the spoVA2mob operon, primers were designed to selectively amplify the last gene of the spoVA2mob operon of B. amyloliquefaciens DSM7 (GenBank accession no. FN597644.1).
TABLE 4.
Sequences of primers for PCR
| Primer | Sequence (5′→3′) | Tm (°C) | Product size (kb) | Reference |
|---|---|---|---|---|
| 27F | AGAGTTTGATCCTGGCTCAG | 58 | 1.5 | 44 |
| 1492R | GGCTACCTTGTTACGACTT | |||
| Det-hyp-2mob-F | GTGCCTGAATGGTTAGATATAGC | 68 | 0.86 | This study |
| Det-hyp-2mob-R | TTATCCTTTTAAAATAGGGGTCACTTTATC | |||
| Lhr2-F | TACAAGATTGCCCTGGAAGT | 60 | 0.20 | 14 |
| Lhr2-R | CTTGATCGAATCCTGGTTGG | |||
| HR-F1 | TTAGGTACCGCTGTCCATTGCCTGA | 62 | 1.8 | 7 |
| HS-R1 | AGACCAATCAGGAAATGCTCTGGACC | |||
| HR-F2.2 | GAGGTACCTGTCTTGCCTGACAACGTTG | 64 | 2.8 | 7 |
| HR-R2 | TATCTAGAATGTCATTTCTATGGAGGCATGAATCG | |||
| HS-F1 | GCAATCCTTTGCCGCAGCTATT | 64 | 2.8 | 7 |
| HR-R3 | GTCAAGCTTCTAGGGCTCGTAGTTCG |
Preparation of vegetative cells and spores of bacilli.
Vegetative cells of Bacillus and Brevibacillus were prepared for heat testing at 60°C. All strains were grown in LB broth at 37°C with agitation at 200 rpm for 12 h. After being subcultured at a 1% dilution three times, cultures were examined by microscopic observation under bright field to verify that more than 99.9% of the cells had not sporulated. Spore suspensions of 10 isolates from Daqu in water were prepared as described previously (5).
Determination of heat resistance of bacilli and K. cowanii.
Vegetative cells of bacilli were treated at 60°C for 1, 3, 5, or 7 min as previously described (4). After heating, appropriate dilutions were plated on LB agar and incubated at 37°C for 24 h. After incubation, CFU were calculated.
Spore suspensions were transferred to 25 μl of Dade Accupette pipettes (P4518-25). The glass capillaries were heat sealed, placed in an oil bath at 110°C for 0, 5, 10, 20, 30, 60, 180, or 300 s, and rapidly cooled in water. The cell counts of heat-treated spore suspensions were determined as described above.
To confirm the contribution of the LHR in the heat resistance of K cowanii, pLHR was transformed into LHR-negative FUA10121 by electroporation. Transformants were plated on LB agar containing 15 mg/liter of tetracycline hydrochloride. The construction of pLHR was described previously (7). Four strains of K. cowanii (FUA1601, FUA1341, FUA1348, and FUA1349) were treated at 60°C for 1, 3, 5, or 7 min. The cell counts of heat-treated culture were determined as described above.
Nonlinear model of spores for thermal inactivation.
A modified model originating from the Weibull frequency distribution was proposed to describe nonlinear survival curves of spores (45):
where N presents the number of surviving cells after a duration of heat treatment time t, while N0 is the initial population. For a given temperature (110°C), parameter distributions are k and p, and these two values were determined by nonlinear curve fit procedure in Sigma Plot 12.5. Initial parameters of k and p are 1 and −0.01, respectively.
Quantification of copy number of the LHR and the spoVA2mob operons.
qPCR was used for determining the copy number of the spoVA2mob operon, LHR, and 16S rRNA in Daqu community DNA. Community DNA was isolated from 1 g of Daqu powder using the E.Z.N.A. soil DNA kit (Omega Bio-Tek, Doraville, GA). The 7500 fast real-time PCR instrument and 7500 software v 2.0.5 (Applied Biosystems) were used for qPCR amplification and detection. qPCR samples were prepared in duplicates of 25 μl of reaction mixture in MicroAmp optical 96-well reaction plates and sealed with optical adhesive film (Applied Biosystems). Each reaction well contained 50 ng of template DNA, 12.5 μl of 2 × SYBR green PCR master mix (Qiagen, Hilden, Germany), and 0.5 mmol each of forward and reverse primers. Nuclease-free water was used as the negative control. The thermal cycling protocol was as follows: initial denaturation for 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at melting temperature (Tm), and specific extension time at 72°C. The fluorescence signal was measured at the end of each extension step at 72°C. After the amplification, a melting-curve analysis with a temperature gradient of 0.1°C/s from 70 to 95°C was performed to confirm that only the specific products were amplified. Finally, the samples were cooled down to 4°C. Table 4 shows the sizes of the amplified products and their melting temperatures, for determination of specific PCR product amplification. The standard curves for the spoVA2mob operon, LHR, and 16S rRNA ranged from 1 × 103 to 1 × 109 copies/μl. All curves were linear in the range tested (r2 > 0.999) by the duplicate reactions. A high amplification efficiency (0.98) was determined for all three target genes.
Determination of copy number of the spoVA2mob operon per genome.
DNA was isolated from each strain using a Qiagen DNeasy blood and tissue kit (Qiagen). The qPCR assay for determination of spoVA2mob operons is described above. The genome copies can be calculated as follows:
where n is genome size (in base pairs) and m is amount of genomic DNA in one PCR (in nanograms). The average genome sizes of B. amyloliquefaciens, B. velezensis, and B. subtilis were determined with completed genomes of these species that were available in the National Center for Biotechnology Information (NCBI) database in November 2017. The average genome sizes (numbers of genomes) were as follows: B. amyloliquefaciens, 3.97 ± 0.10 Mb (53); B. subtilis, 4.11 ± 0.28 Mb (166); and B. velezensis, 4.00 ± 0.11 Mb (97).
Frequency of the spoVA2mob operon in genome-sequenced bacilli.
The sequence of the spoVA2mob operon (6) was BLAST searched against all completed genomes of species matching spoVA2mob-positive isolates obtained in the present study. The BLAST search was performed on 4 May 2018 using the NCBI nucleotide BLAST against 74 genomes of B. licheniforms, 1 genome of Br. parabrevis, 185 genomes of B. subtilis, 54 genomes of B. amyloliquefaciens, and 118 genomes of B. velezensis (total of 432 genomes).
Statistical analysis.
Data were subjected to analysis of variance (ANOVA) using SPSS 21.0 (SPSS Inc., Chicago, IL) software. The least significant difference (LSD) was used to test the difference between means. Differences between means were evaluated as significant at a P value of <0.05.
ACKNOWLEDGMENTS
We acknowledge financial support from Canada Research Chairs, Alberta Agriculture and Forestry, and China Scholarship Council.
We are grateful to Mandi Hoke for isolating strains from malted oats.
REFERENCES
- 1.Doyle MP, Buchanan RL (ed). 2012. Food microbiology: fundamentals and frontiers. ASM Press, Washington, DC. [Google Scholar]
- 2.Liu Y, Gill A, McMullen L, Gänzle MG. 2015. Variation in heat and pressure resistance of verotoxigenic and nontoxigenic Escherichia coli. J Food Prot 78:111–120. doi: 10.4315/0362-028X.JFP-14-267. [DOI] [PubMed] [Google Scholar]
- 3.Esty J, Meyer K. 1922. The heat resistance of the spores of B. botulinus and allied anaerobes. XI. J Infect Dis 36:650–664. doi: 10.1093/infdis/31.6.650. [DOI] [Google Scholar]
- 4.Dlusskaya E, McMullen L, Gänzle M. 2011. Characterization of an extremely heat-resistant Escherichia coli obtained from a beef processing facility. J Appl Microbiol 110:840–849. doi: 10.1111/j.1365-2672.2011.04943.x. [DOI] [PubMed] [Google Scholar]
- 5.Margosch D, Ehrmann MA, Buckow R, Heinz V, Vogel RF, Gänzle MG. 2006. High-pressure-mediated survival of Clostridium botulinum and Bacillus amyloliquefaciens endospores at high temperature. Appl Environ Microbiol 72:3476–3481. doi: 10.1128/AEM.72.5.3476-3481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berendsen EM, Boekhorst J, Kuipers OP, Wells-Bennik MH. 2016. A mobile genetic element profoundly increases heat resistance of bacterial spores. ISME J 10:2633–2642. doi: 10.1038/ismej.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer RG, Zheng J, Garcia-Hernandez R, Ruan L, Gänzle MG, McMullen LM. 2015. Genetic determinants of heat resistance in Escherichia coli. Front Microbiol 6:932. doi: 10.3389/fmicb.2015.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berendsen EM, Koning RA, Boekhorst J, de Jong A, Kuipers OP, Wells-Bennik MH. 2016. High-level heat resistance of spores of Bacillus amyloliquefaciens and Bacillus licheniformis results from the presence of a spoVA operon in a Tn1546 transposon. Front Microbiol 7:1912. doi: 10.3389/fmicb.2016.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krawczyk AO, Berendsen EM, de Jong A, Boekhorst J, Wells-Bennik MH, Kuipers OP, Eijlander RT. 2016. A transposon present in specific strains of Bacillus subtilis negatively affects nutrient-and dodecylamine-induced spore germination. Environ Microbiol 18:4830–4846. doi: 10.1111/1462-2920.13386. [DOI] [PubMed] [Google Scholar]
- 10.Kort R, O'Brien AC, Van Stokkum IH, Oomes SJ, Crielaard W, Hellingwerf KJ, Brul S. 2005. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl Environ Microbiol 71:3556–3564. doi: 10.1128/AEM.71.7.3556-3564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovar-Rojo F, Chander M, Setlow B, Setlow P. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J Bacteriol 184:584–587. doi: 10.1128/JB.184.2.584-587.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercer RG, Walker BD, Yang X, McMullen LM, Gänzle MG. 2017. The locus of heat resistance (LHR) mediates heat resistance in Salmonella enterica, Escherichia coli and Enterobacter cloacae. Food Microbiol 64:96–103. doi: 10.1016/j.fm.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Gänzle M. 2016. Some like it hot: heat resistance of Escherichia coli in food. Front Microbiol 7:1763. doi: 10.3389/fmicb.2016.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer R, Nguyen O, Ou Q, McMullen L, Gänzle MG. 2017. Functional analysis of genes comprising the locus of heat resistance in Escherichia coli. Appl Environ Microbiol 83:e01400-17. doi: 10.1128/AEM.01400-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Franke KB, Kamal SM, Kim H, Lünsdorf H, Jäger J, Nimtz M, Trček J, Jänsch L, Bukau B. 2018. Stand-alone ClpG disaggregase confers superior heat tolerance to bacteria. Proc Natl Acad Sci U S A 115:E273–E282. doi: 10.1073/pnas.1712051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadiq FA, Li Y, Liu T, Flint S, Zhang G, Yuan L, Pei Z, He G. 2016. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int J Food Microbiol 238:193–201. doi: 10.1016/j.ijfoodmicro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhi S, Banting G, Li Q, Edge TA, Topp E, Sokurenko M, Scott C, Braithwaite S, Ruecker NJ, Yasui Y. 2016. Evidence of naturalized stress-tolerant strains of Escherichia coli in municipal wastewater treatment plants. Appl Environ Microbiol 82:5505–5518. doi: 10.1128/AEM.00143-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma A, Chui L. 2017. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J Microbiol Methods 133:87–89. doi: 10.1016/j.mimet.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Xiu L, Kunliang G, Hongxun Z. 2012. Determination of microbial diversity in Daqu, a fermentation starter culture of Maotai liquor, using nested PCR-denaturing gradient gel electrophoresis. World J Microbiol Biotechnol 28:2375–2381. doi: 10.1007/s11274-012-1045-y. [DOI] [PubMed] [Google Scholar]
- 20.Li P, Lin W, Liu X, Wang X, Luo L. 2016. Environmental factors affecting microbiota dynamics during traditional solid-state fermentation of Chinese Daqu starter. Front Microbiol 7:1237. doi: 10.3389/fmicb.2016.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao C, Lu ZM, Zhang XJ, Wang ST, Ao L, Shen CH, Shi JS, Xu ZH. 2017. Bio-heat is a key environmental driver shaping the microbial community of medium-temperature Daqu. Appl Environ Microbiol, 83:e01550-17. doi: 10.1128/AEM.01550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HY, Gao YB, Fan QW, Xu Y. 2011. Characterization and comparison of microbial community of different typical Chinese liquor Daqus by PCR-DGGE. Lett Appl Microbiol 53:134–140. doi: 10.1111/j.1472-765X.2011.03076.x. [DOI] [PubMed] [Google Scholar]
- 23.Krawczyk AO, de Jong A, Omony J, Holsappel S, Wells-Bennik MH, Kuipers OP, Eijlander RT. 2017. Spore heat activation requirements and germination responses correlate with sequences of germinant receptors and with the presence of a specific spoVA2mob operon in foodborne strains of Bacillus subtilis. Appl Environ Microbiol 83:e03122-16. doi: 10.1128/AEM.03122-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazarowec-White M, Farber JM. 1997. Thermal resistance of Enterobacter sakazakii in reconstituted dried-infant formula. Lett Appl Microbiol 24:9–13. doi: 10.1046/j.1472-765X.1997.00328.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan Q, Fanning S. 2015. Strategies for the identification and tracking of Cronobacter species: an opportunistic pathogen of concern to neonatal health. Front Pediatr 3:38. doi: 10.3389/fped.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tall BD, Gangiredla J, Gopinath GR, Yan Q, Chase HR, Lee B, Hwang S, Trach L, Park E, Yoo Y, Chung T. 2015. Development of a custom-designed, pan genomic DNA microarray to characterize strain-level diversity among Cronobacter spp. Front Pediatr 3:36. doi: 10.3389/fped.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Aflakpui FWK, Yu H, Luo L, Lin WT. 2015. Characterization of activity and microbial diversity of typical types of Daqu for traditional Chinese vinegar. Ann Microbiol 65:2019–2027. doi: 10.1007/s13213-015-1040-2. [DOI] [Google Scholar]
- 28.Li P, Liang H, Lin WT, Feng F, Luo L. 2015. Microbiota dynamics associated with environmental conditions and potential roles of cellulolytic communities in traditional Chinese cereal starter solid-state fermentation. Appl Environ Microbiol 81:5144–5156. doi: 10.1128/AEM.01325-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaradat ZW, Al Mousa W, Elbetieha A, Al Nabulsi A, Tall BD. 2014. Cronobacter spp.—opportunistic food-borne pathogens. A review of their virulence and environmental-adaptive traits. J Med Microbiol 63:1023–1037. [DOI] [PubMed] [Google Scholar]
- 30.Mardaneh J, Soltan-Dallal MM. 2014. Isolation and identification of E. cowanii from powdered infant formula in NICU and determination of antimicrobial susceptibility of isolates. Iran J Pediatr 24:261. [PMC free article] [PubMed] [Google Scholar]
- 31.Marti R, Muniesa M, Schmid M, Ahrens CH, Naskova J, Hummerjohann J. 2016. Heat-resistant Escherichia coli as potential persistent reservoir of extended-spectrum β-lactamases and Shiga toxin-encoding phages in dairy. J Dairy Sci 99:8622–8632. doi: 10.3168/jds.2016-11076. [DOI] [PubMed] [Google Scholar]
- 32.Feeney A, Johnston CD, Govender R, O'Mahony J, Coffey A, Sleator RD. 2014. Analysis of the role of the Cronobacter sakazakii ProP homologues in osmotolerance. Gut Pathog 6:15. doi: 10.1186/1757-4749-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breeuwer P. 2014. Adaptation of pathogenic microorganisms to dry conditions: the microbiological safety of low water activity foods and spices, p 37–48. In Gurtler JB, Doyle MP, Kornacki JL (ed), Food microbiology and food safety. Springer, New York, NY. [Google Scholar]
- 34.Pleitner A, Zhai Y, Winter R, Ruan L, McMullen LM, Gänzle MG. 2012. Compatible solutes contribute to heat resistance and ribosome stability in Escherichia coli AW1.7. Biochim Biophys Acta 1824:1351–1357. doi: 10.1016/j.bbapap.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Bond WW, Favero MS. 1977. Bacillus xerothermodurans sp. nov., a species forming endospores extremely resistant to dry heat. Int J Syst Bacteriol 27:157–160. [Google Scholar]
- 36.Liang H, Zhang C, Shao C, Peng X, Liang L, Su J, Li C. 2015. A DGGE marker-mediated fast monitoring of bacterial diversity and comprehensive identification of high-temperature Daqu starter. J Food Sci 80:1519–1525. doi: 10.1111/1750-3841.13144. [DOI] [PubMed] [Google Scholar]
- 37.den Besten HM, Berendsen EM, Wells-Bennik MH, Straatsma H, Zwietering MH. 2017. Two complementary approaches to quantify variability in heat resistance of spores of Bacillus subtilis. Int J Food Microbiol 253:48–53. doi: 10.1016/j.ijfoodmicro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Esteban MD, Conesa R, Huertas JP, Palop A. 2015. Effect of thymol in heating and recovery media on the isothermal and non-isothermal heat resistance of Bacillus spores. Food Microbiol 48:35–40. doi: 10.1016/j.fm.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Berendsen EM, Zwietering MH, Kuipers OP, Wells-Bennik MH. 2015. Two distinct groups within the Bacillus subtilis group display significantly different spore heat resistance properties. Food Microbiol 45:18–25. doi: 10.1016/j.fm.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Weibull W. 1951. A statistical distribution function of wide applicability. J Appl Mech 18:293–297. [Google Scholar]
- 41.Peleg M, Cole M. 2000. Estimating the survival of Clostridium botulinum spores during heat treatments. J Food Prot 63:190–195. doi: 10.4315/0362-028X-63.2.190. [DOI] [PubMed] [Google Scholar]
- 42.Mathys A, Heinz V, Schwartz FH, Knorr D. 2007. Impact of agglomeration on the quantitative assessment of Bacillus stearothermophilus heat inactivation. J Food Eng 81:380–387. doi: 10.1016/j.jfoodeng.2006.11.012. [DOI] [Google Scholar]
- 43.Gänzle MG. 2015. Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2:106–117. doi: 10.1016/j.cofs.2015.03.001. [DOI] [Google Scholar]
- 44.Eden PA, Schmidt TM, Blakemore RP, Pace NR. 1991. Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction-amplified 16S rRNA-specific DNA. Int J Syst Bacteriol 41:324–325. [DOI] [PubMed] [Google Scholar]
- 45.Mafart P, Couvert O, Gaillard S, Leguérinel I. 2002. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int J Food Microbiol 72:107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]