Microbial communities are an increasing problem in medicine but also in industry. Thus, an efficient and rapid removal of biofilms is becoming increasingly important. With the aid of the kINPen09, a radiofrequency plasma jet (RFPJ) instrument, decisive new findings on the effects of plasma on C. albicans biofilms were obtained. This work showed that the inactivation of biofilms takes place mainly on the bottom, which in turn offers new possibilities for the removal of biofilms by other strategies, e.g., mechanical treatment. This result demonstrated that nonthermal atmospheric pressure plasma is well suited for biofilm decontamination.
KEYWORDS: antimicrobial, atomic force microscopy, biological decontamination, confocal laser scanning microscopy, fluorescence microscopy, inactivation, viability, cell viability
ABSTRACT
Microorganisms are predominantly organized in biofilms, where cells live in dense communities and are more resistant to external stresses than are their planktonic counterparts. With in vitro experiments, the susceptibility of Candida albicans biofilms to a nonthermal plasma treatment (plasma source, kINPen09) in terms of growth, survival, and cell viability was investigated. C. albicans strain SC5314 (ATCC MYA-2876) was plasma treated for different time periods (30 s, 60 s, 120 s, 180 s, 300 s). The results of the experiments, encompassing CFU, fluorescence Live/Dead, and 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) assays, revealed a negative influence of the plasma treatment on the proliferation ability, vitality, and metabolism of C. albicans biofilms, respectively. Morphological analysis of plasma-treated biofilms using atomic force microscopy supported the indications for lethal plasma effects concomitant with membrane disruptions and the loss of intracellular fluid. Yielding controversial results compared to those of other publications, fluorescence and confocal laser scanning microscopic inspection of plasma-treated biofilms indicated that an inactivation of cells appeared mainly on the bottom of the biofilms. If this inactivation leads to a detachment of the biofilms from the overgrown surface, it might offer completely new approaches in the plasma treatment of biofilms. Because of plasma's biochemical-mechanical mode of action, resistance of microbial cells against plasma is unknown at this state of research.
IMPORTANCE Microbial communities are an increasing problem in medicine but also in industry. Thus, an efficient and rapid removal of biofilms is becoming increasingly important. With the aid of the kINPen09, a radiofrequency plasma jet (RFPJ) instrument, decisive new findings on the effects of plasma on C. albicans biofilms were obtained. This work showed that the inactivation of biofilms takes place mainly on the bottom, which in turn offers new possibilities for the removal of biofilms by other strategies, e.g., mechanical treatment. This result demonstrated that nonthermal atmospheric pressure plasma is well suited for biofilm decontamination.
INTRODUCTION
Candida albicans is one of the most common pathogens causing mycosis worldwide. It is the eponym of the Candida group, which is a yeast genus that can cause fungal infections in humans and animals. C. albicans is an imperfect fungus that multiplies by budding (1). Typically, C. albicans cells bind to biogenic or abiogenic surfaces and grow as three-dimensional (3D) structures, also designated biofilms (2). Especially foodborne pathogens and spoilage microorganisms like C. albicans prefer biofilm formation on stainless steel, aluminum, glass, polytetrafluoroethylene (PTFE) seals and polyamide (PA) materials, which are typically found in food-processing environments (3–5). The fungus can form mono- or multispecies biofilms, which can be considered densely packed cellular communities embedded in an extracellular matrix (6). These biofilm communities are known to be more resistant to physical and chemical stresses than planktonic cells and can cause major economic loss within industrial sectors (7). Moreover, more than 4 million people in Germany suffer from chronic wounds, which are often caused by resistant biofilm-forming pathogens (8). The treatment costs for these patients amount to up to 4 billion euros per year in Germany alone (9). Catheter-related bloodstream infections (CRBSI), caused by biofilms and their direct access to the bloodstream, are a big problem in the United States. Several studies counted 80,000 CRBSI each year in the United States, leading to costs between $4,888 and $11,591 for each CRBSI event. These facts demonstrate the need for successful treatment of biofilms in medicine.
Candida sp. is responsible for two types of infections: superficial infections like oral and vaginal candidiasis and systemic infections (10, 11). In about 75% of the world's population, C. albicans appears in the oral cavity and is the fourth most common cause of hospital-acquired systemic infections in the United States, with up to 50% mortality (12–15). Due to the increased life span of today's population, there is also an increase in the number of patients who wear dental prostheses, which leads to a concomitant increase in Candida stomatitis (16–18). Dental diseases like periodontitis, gingivitis, caries, oral candidiasis, or periimplantitis are relevant diseases for people of all ages. Depending on the oral food intake, the oral cavity is constantly exposed to a high level of microbial contamination induced by the assimilated food (19). Thus, the germ load of the oral cavity is much higher than for other parts of the human body. Lesions in the oral cavity often lead to diseases such as oral submucous fibrosis or stomatitis (20) and therefore play an important role as a symptom for immunodeficiency. During the infection process, colonization factors such as adhesins, invasins, and hyphae and their thigmotropic properties are of major importance (21). Together with Staphylococcus aureus, C. albicans has a much higher incidence of 11 to 65% for a stomatitis than in a monospecies biofilm (22). At present, the conventional treatment method for biofilms consists of antibiotics. Biofilms have a higher resistance to antimicrobial agents and a higher physicomechanical tolerance than do planktonic cells (23, 24). These resistances are mainly due to complex interactions of the cells in the biofilm and the surrounding extracellular matrix (6, 25–27).
In 243 episodes of candidemia, 45 (19%) had reduced susceptibility and 27 of them were fully resistant to fluconazole, the main antifungal agent for candidemia treatment. Despite 8% of C. albicans, 4% of C. tropicalis, and 4% of C. parapsilosis isolates having reduced susceptibility to fluconazole, these species embrace 36% of the reduced-susceptibility group and 48% of the fully resistant group (28). Thus, there is a much higher probability for fully resistant C. albicans strains than for strains with reduced susceptibility. Antimicrobial resistance will be of increasing interest to society, as it is associated with rising mortality rates and higher medical costs (29). Therefore, alternatives to conventional antibiotic therapies are urgently needed.
An innovative technology addressing these problems is nonthermal atmospheric pressure plasma (NTP). With moderate temperatures, NTPs avoid thermal host tissue damage but have in fact much higher antimicrobial effects than, e.g., UV or hydrogen peroxide treatments (30, 31). In addition, NTPs are very effective against antibiotic-resistant pathogens and there are no antimicrobial resistance mechanisms against plasma known to date (32–34). Therefore, NTPs could be used for the decontamination of dental cavities and a wide range of other applications and devices in industry and medicine (35).
A basic understanding of the mechanistic effects of plasma on prokaryotic and eukaryotic biofilms is a prerequisite for its application. Consequently, this work investigated the inactivation effects of plasma on C. albicans biofilms. For this purpose, biofilm viability, vitality, and metabolism were examined after plasma treatment. The impact of plasma treatment on the three-dimensional biofilm structures was examined by employing epifluorescence and confocal laser scanning microscopy (CLSM); moreover, cell morphological changes were detected with atomic force microscopy (AFM).
RESULTS
The antimicrobial effect of plasma components on biofilm cells might be caused by different mechanisms. To unravel the exact mode of action of the plasma treatment, its effect on C. albicans biofilms was monitored using CFU counting to investigate the proliferation of the surviving cells (36), vitality assays with fluorescence Live/Dead staining, and a metabolism assay using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) assay. In order to exclude negative effects on the biofilms due to increased temperature, thermal imaging measurements were carried out.
Effects of temperature generated by plasma treatment on biofilm cells.
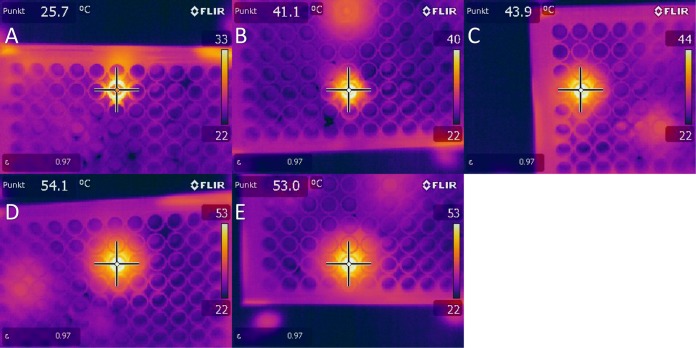
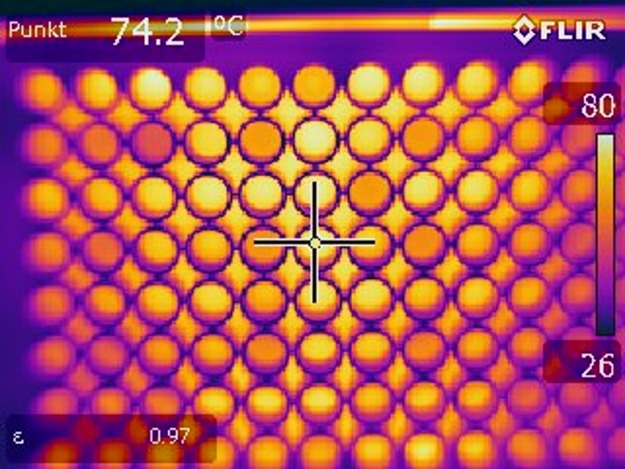
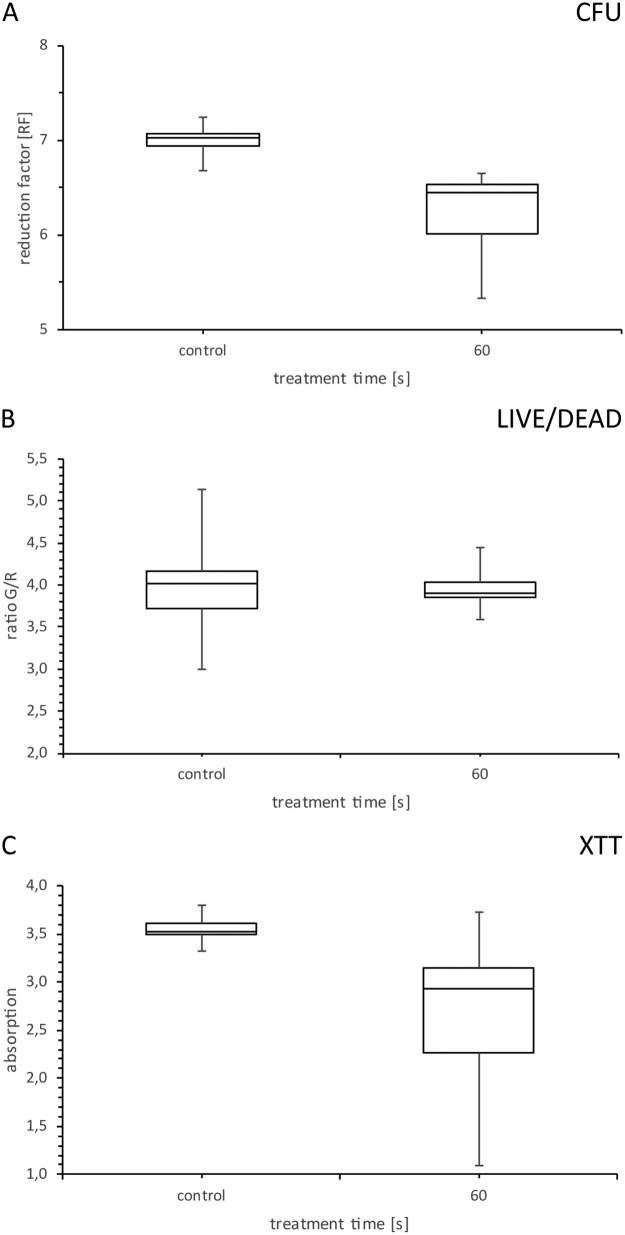
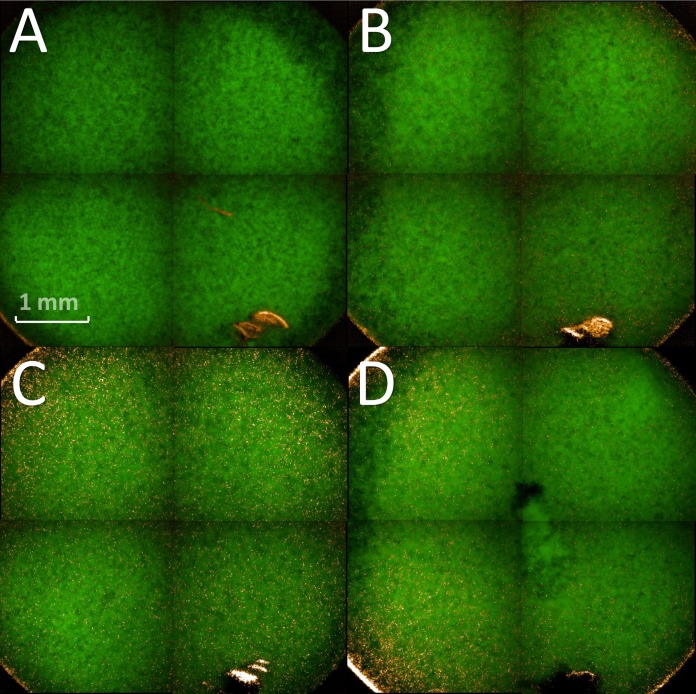
Five different treatment times were measured directly after plasma treatment with regard to the temperatures reached in the biofilm. Temperatures in the range of 25 to 55°C were measured for the selected plasma treatment times (Fig. 1). Because the temperatures were above 37°C, we further analyzed the effect of temperature on the biofilm cells. For this, a 96-well plate with C. albicans biofilms was heated on a plate heater for 60 s at 130°C. A temperature of 74.2°C was reached after this treatment (Fig. 2). We used these biofilms for further analysis with the aid of CFU, fluorescence, and XTT assays to exclude temperature effects in a broader spectrum than we were able to measure at the radiofrequency plasma jet (RFPJ) treatment. The results of the CFU, fluorescence, and XTT measurements of the untreated biofilms were compared with those of the heated biofilms (Fig. 3). We detected a decrease in the RF of 0.5 in the median (Fig. 3A) and a reduction of the absorption of 0.7, which represented a reduction of 24% compared to the untreated biofilms (Fig. 3C). In the Live/Dead assay, no influence of the temperature was detected (Fig. 3B). In addition, fluorescence microscopic images of the heated biofilms were taken to exclude effects of temperature on the biofilms (Fig. 4).
FIG 1.
Thermal image measurements of biofilms taken directly after plasma treatment. Images were taken at a distance of 20 cm with an FLIR thermal imaging camera at plasma treatment times of 30 s (A), 60 s (B), 120 s (C), 180 s (D), and 300 s (E).
FIG 2.
Thermal image measurements of biofilms taken directly after heating. Images were taken at a distance of 20 cm with an FLIR thermal imaging camera. Biofilms were heated 1 min at 130°C on a plate heater.
FIG 3.
Box and whisker plots of CFU, fluorescence, and XTT measurements of biofilms heated on a plate heater. Candida albicans biofilms were heated 1 min at 130°C on a plate heater and used for CFU (A), fluorescence (B), and XTT (C) assays. Biofilms were compared to untreated control biofilms. n = 15.
FIG 4.
Fluorescence microscopy of Candida albicans biofilms after heating on a plate heater. Biofilms were heated 1 min at 130°C on a plate heater. Shown are four different biofilms from different locations of the 96-well plate after heating on a plate heater. Green, living cells; red (orange), dead cells.
Effects of plasma treatment on the proliferation ability of biofilm cells.
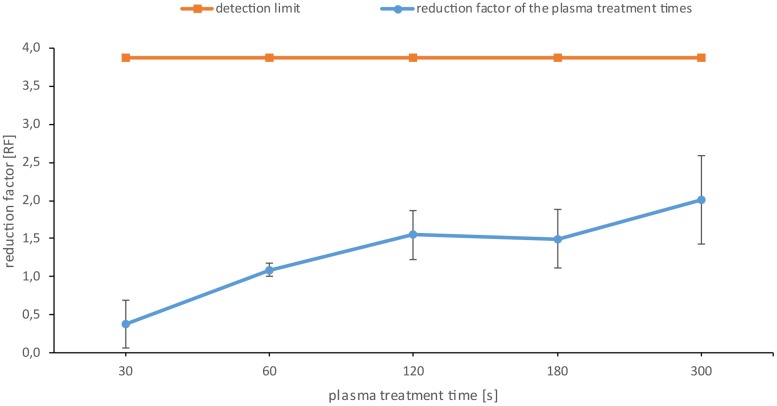
Five different treatment times between 30 and 300 s were selected, which should demonstrate the dynamic range of the plasma effect. Six biofilms were treated with plasma for the respective times in 4-fold repetitions (Fig. 5). The effect of plasma treatment on the biofilm CFU is displayed as reduction factor (RF). After 60 s of plasma treatment time, a significant increase in the RF was detected. The RF reached a maximum of 2.0 after 300 s of plasma treatment. The orange line in Fig. 5 represents the detection limit in the range of which we could detect the CFU.
FIG 5.
CFU assay of the Candida albicans biofilms after plasma treatment. The orange line represents the detection limit up to which representative values could be counted. The blue line shows the reduction factor (RF) of the different plasma treatment times. The error bars were calculated using the propagation of error and weighted error for the controls and the weighted mean value for the samples. The data points are the weighted mean values for the total population of the quadruple repetition. n = 6 for each repetition. P < 0.05.
Effects of plasma treatment on the biofilm vitality.
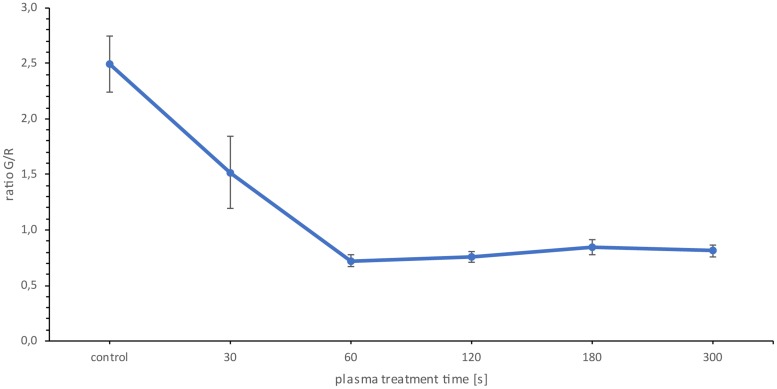
The Live/Dead BacLight assay kit (Thermo Scientific, Waltham, MA, USA) contains the DNA intercalating dyes SYTO9, staining living cells of the biofilm, and propidium iodide, which is membrane impermeable and reduces SYTO9 in cells with damaged cell membranes. After 30 s of plasma treatment, a decrease in the green fluorescence intensity to red fluorescence intensity ratio (G/R) of 0.97 was detected, which corresponded to a 39% reduction compared to the untreated reference biofilms (Fig. 6). After 60 s of plasma treatment, a maximum decrease of 1.78 in G/R was observed, which corresponded to a 71.08% reduction. There was no significant change in the G/R ratio after longer treatment times (Fig. 6). Thus, significant changes in the vitality of the biofilm took place only in the first 60 s of plasma treatment.
FIG 6.
Fluorescence assay of Candida albicans biofilms. The G/R ratio is the quotient of green fluorescence intensity and red fluorescence intensity. The data points are the mean values for the total population of the quadruple repetition. n = 6 for each repetition. P < 0.05.
Effect of plasma treatment on the metabolism of the cells.
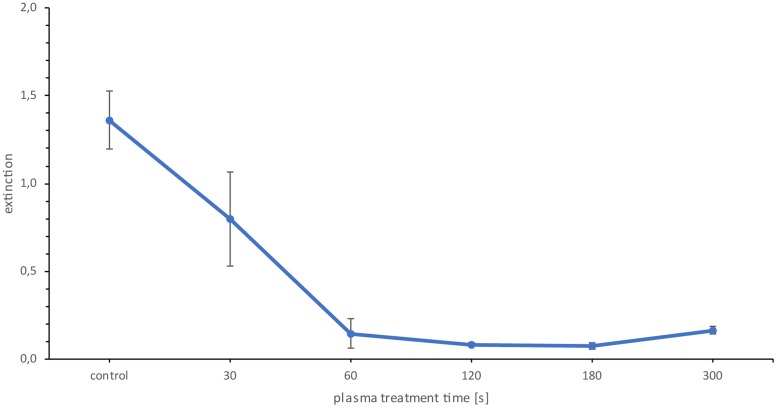
Due to the fact that the CFU and the Live/Dead fluorescence showed antimicrobial effects after plasma treatment, the metabolism of cells was investigated as a third indicator of inactivation. For this, the XTT assay was used. The metabolic activity based on XTT quantification assay was reduced by 41% at 30 s and by 89% at 60 s (Fig. 7). Longer treatment time did not further reduce cell metabolic activity.
FIG 7.
XTT assay of Candida albicans biofilms. The data points are the mean values for the total population of the quadruple repetition. n = 6 for each repetition. P < 0.05.
Confirmation of plasma treatment effects by fluorescence microscopy.
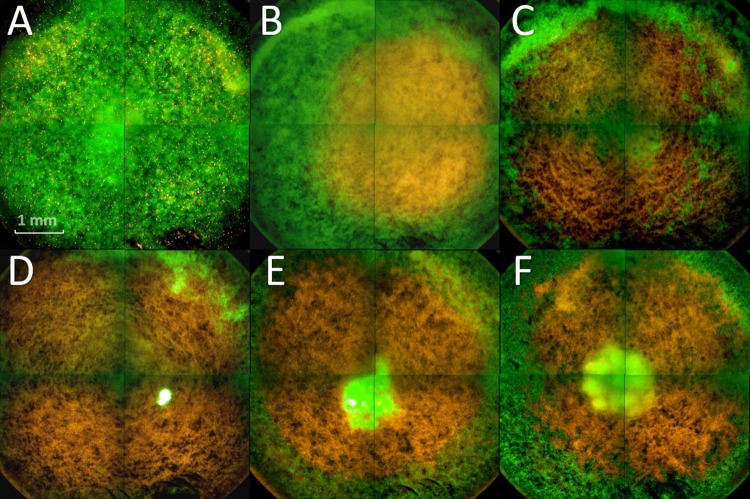
The Live/Dead BacLight staining was also used for an epifluorescent inspection of plasma-treated C. albicans biofilms cultivated in 96-well plates. Bottom view images of the biofilms revealed that 30 s of treatment affected mainly the area of the biofilm that was in the direct range of the plasma effluent (Fig. 8B). This area showed orange/yellow staining, representing dead cells, which was not observed in the untreated control biofilms. With increasing treatment time, the effect could be observed throughout the well (Fig. 8C to F). After 120 s of treatment time, living cells were visible in the center of the biofilm (Fig. 8D). More living cells became visible with longer treatment times. However, this effect did not occur after every plasma treatment, but it was included for a complete display of the results.
FIG 8.
Fluorescence microscopy of plasma-treated biofilms. (A) Control; (B to F) plasma treatment for 30 s (B), 60 s (C), 120 s (D), 180 s (E), and 300 s (F). The biofilms were stained with SYTO9, showing green fluorescence (living cells), and propidium iodide, showing red fluorescence (dead cells).
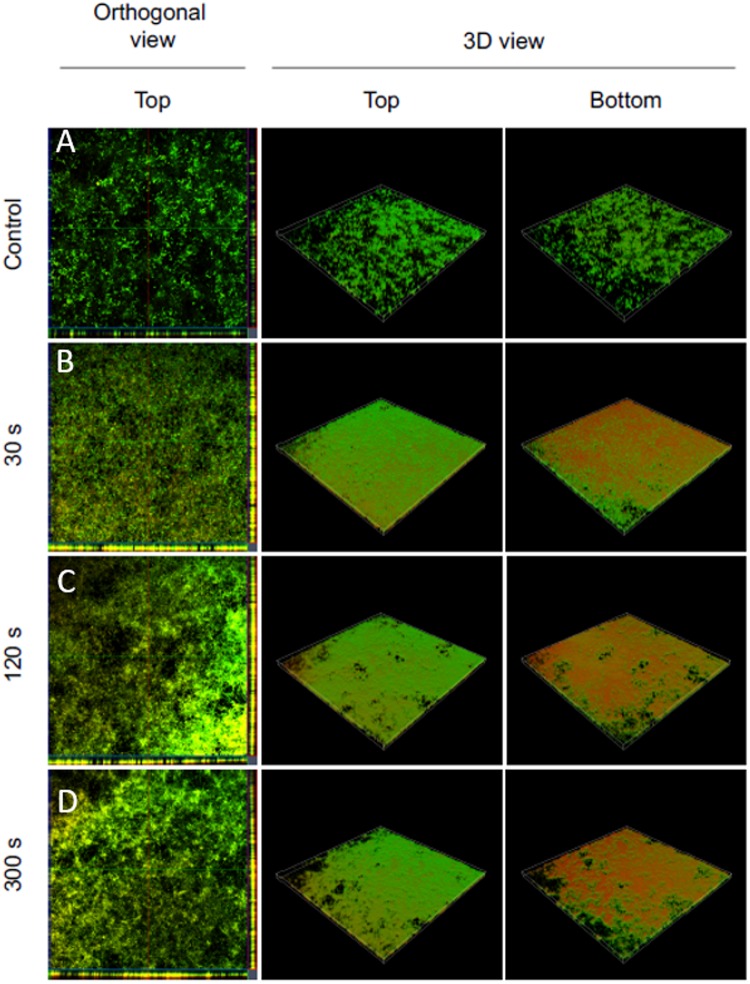
CLSM confirmed cell inactivation being most dominant on the bottom of the biofilms.
Using CLSM, 5.5-μm sections of the treated biofilms were acquired and displayed in orthogonal and 3D views (Fig. 9). All plasma treatments caused an enhanced propidium iodide staining indicating cell death compared to the reference (Fig. 9, orthogonal view). After 30 s, plasma treatment affected mostly the cells located at the bottom of the biofilm, indicated by a strong propidium iodide staining, whereas the top of the biofilm appeared to be less affected (Fig. 9, 3D view).
FIG 9.
Confocal laser scanning microscopy of Live/Dead-stained Candida albicans biofilms after plasma treatment. Left panels show an orthogonal view of the top biofilm layer (horizontal optical sections in the center and vertical optical sections in the flanking pictures). Central and right panels show 3D images with top and bottom views of the biofilms. For each biofilm, an area of 1,272.2 μm by 1,272.2 μm was visualized.
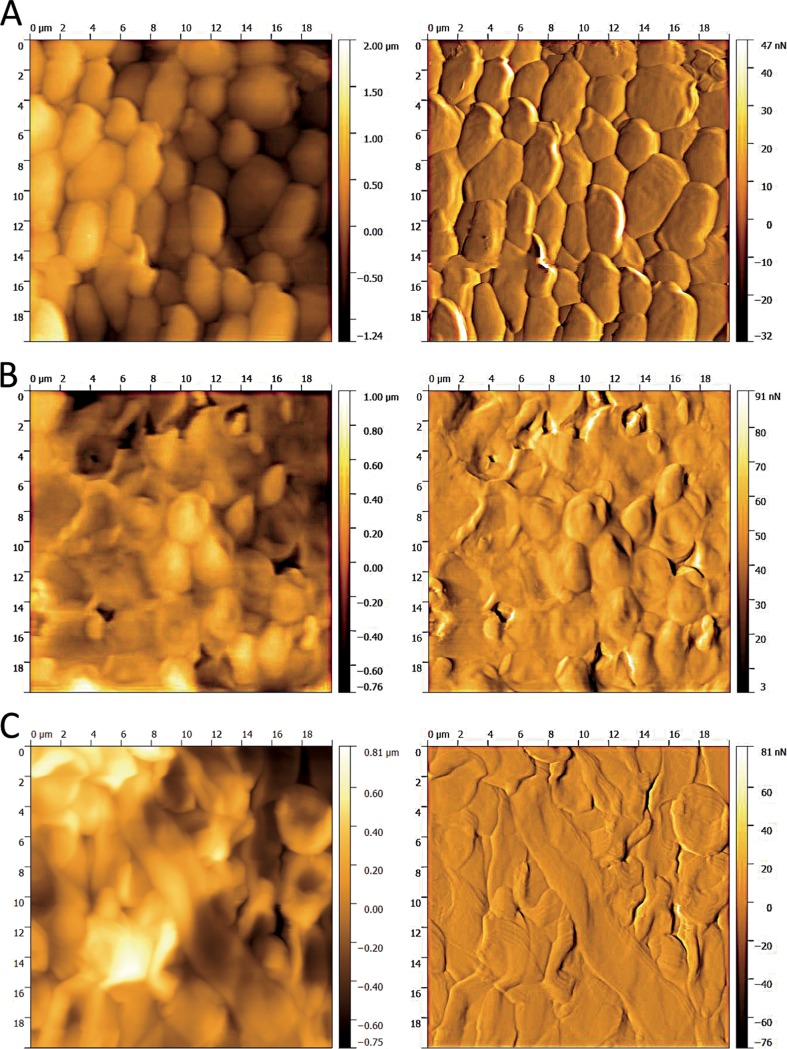
AFM confirmed alterations in the cell morphology of the biofilm after plasma treatment.
AFM is often used to investigate cell morphological alterations in the micrometer range. The AFM images of an untreated biofilm and plasma-treated biofilms for different time periods are shown in Fig. 10. Each image was taken as a topographic image on the left and as an error signal image on the right. In order to detect the alterations caused by the plasma treatment clearly, the average treatment time and the maximum treatment time were examined and recorded. They indicate major changes in the cell morphology even after 120 s of treatment time (Fig. 10). Membrane disruptions and the loss of intracellular fluid could be detected. In contrast, the cells of the untreated biofilm appeared to be vital and without visible damage.
FIG 10.
Atomic-force microscopy images of the plasma-treated biofilms. The left panels show topographical images from the top of the biofilm layer, and the right panels show error signal images of the same section. (A) Control; (B and C) plasma treatment times of 120 s (B) and 300 s (C). The images were taken in contact mode with a cantilever spring constant (k) of 0.1 to 0.6 N/m2 and a frequency of 0.4 Hz, the set point at 8 N/m2, and an area of 20 μm2.
DISCUSSION
Since the invention of nonthermal plasma sources operating at atmospheric pressure, a large number of studies dealing with the effects of plasma itself (direct mode) and its effluents (indirect mode) on pathogens or living tissue have been published (37–41). However, much less is known about the treatment of living biofilms with nonthermal plasma sources. Koban et al. treated 48-h-old C. albicans biofilms on titanium discs with different plasma sources and were able to detect an RF of 0.5 after 300 s of treatment with the RFPJ at a distance of 0.7 mm with an argon gas flow of 5 standard liters per minute (slm) (42). Gorynia et al. used the same experimental setup at a distance of 10 mm for 72-h-grown Streptococcus sanguinis biofilms on titanium discs and could also demonstrate an RF of 0.58 after 180 s of plasma treatment (43). In contrast to these results, we obtained an RF of 1.1 after just 60 s of plasma treatment. Although the same plasma source was used, the experimental setups were different. It is therefore difficult to compare our values with those from the studies previously mentioned. In our study, e.g., no titanium discs were used for cultivation, but C. albicans was grown directly on coated 96-well plates. Furthermore, a distance of 18 mm from the nozzle to the biofilm was selected. Despite the greater distance, we were able to detect a temperature of 54°C directly in the biofilm after 180 s of plasma treatment with the RFPJ in the same experimental setup. However, our results indicated that the increased temperature has no significant influence on the biofilms (Fig. 3 and 4). Moravsky et al. treated C. albicans with a radiofrequency plasma jet and were able to achieve a reduction of 65.2% after 30 s of plasma treatment and up to 99.6% after 60 s of plasma treatment using the XTT assay (44). These results show that the efficiency of our plasma treatment of eukaryotic pathogens is comparable to those reported in other publications. The inactivation of the cells after plasma treatment was proven by different experimental approaches. Counting of CFUs was used to test the proliferation ability of treated cells, fluorescence Live/Dead assay was employed to monitor the vitality of the cells, and an XTT assay was used to investigate the plasma effect on cellular metabolism. Using these three complementary methods ensured that the inactivation of the biofilms by the plasma treatment was indeed reliable (45).
Recent studies dealing with the treatment of C. albicans biofilms with RFPJ demonstrated an inactivation of cells exclusively in the area of plasma effluents. However, most of these studies did not investigate the three-dimensional inactivation effects within the biofilm. Pei et al. reported the inactivation of a 25.5-μm-thick biofilm of Enterococcus faecalis after treatment with a handheld air plasma jet (46). Here, the authors demonstrated a complete inactivation of the whole biofilm after 300 s of treatment. Delben et al. also showed an influence of plasma treatment with an atmospheric pressure plasma jet (APPJ) on three-dimensional biofilms of C. albicans (47). However, both of these studies used a different plasma source and a generally different experimental setup. It is well known that the biofilm thickness, the microorganism, the treatment conditions, and the gas used play a predominant role in microbial inactivation.
Even though a whole series of publications reported the ability to microscopically detect the inactivation of biofilms using plasma treatments, we are not aware of any work that has shown that primarily the bottom of the biofilm was affected by the plasma treatment. This new insight naturally raises the question of how this kind of inactivation was done.
Wilking et al. were able to detect water channels in Bacillus subtilis biofilms for the transport of fluids in biofilms (48). For this, they used an aqueous solution containing a mixture of fluorescent beads to visualize the connectivity of the channels. Interestingly, they were able to detect a dense network of channels in the center of the biofilm, which reached to the surface of the biofilm, extended downwards, and spread over the entire biofilm. Another important aspect of their work was the microscopic proof of the water channel structure. These channels use the surface to which the cells of the biofilm have adhered to form their bottom structure. There is a range of other studies that also detected such water channels in bacterial biofilms (49–51). If such water channels exist in C. albicans biofilms, this could explain the results obtained in our work. Due to these structures and the expulsion rates of the gases through the plasma effluent, it can be assumed that the reactive oxygen species (ROS) will accumulate in the lower part of the biofilm. In addition, deposits of the liquids contained in the lower part of the biofilm are more likely to occur, in which the reactive species dissolve and then have a permanent effect on the cells of the lower part of the biofilm. Because the reactive species react very quickly, the short reaction times with the upper cell layers of the biofilm are probably sufficient to trigger visible inactivation effects.
Pure argon gas (Linde, Pullach, Germany) has a purity of 99.9%. Nevertheless, secondary components of ≤5 mol fractions N2 and ≤2 mol fractions O2 are present in the gas. However, the most important components were the species of ambient air that reacts with the components of the effluent. The energy generated by the RFPJ breaks up the oxygen bonds mainly, in contrast to the triple bonds of the nitrogen components. Shield gas experiments have shown that ambient air can account for up to 15% of the active species of plasma treatment (52). Stewart determined diffusion times of different gases in biofilms (53). Based on those results, a diffusion time of 4.47 s for nitrogen, representing all light gases, could be calculated for the C. albicans biofilms investigated here. These results offered plausible explanations for the effects that were observed in fluorescence microscopy. Another effect that became apparent in fluorescence microscopy was a central spot of living cells, which were visible in the 120-s plasma-treated biofilms. Desiccation effects, caused by the plasma, might cause this phenomenon. Reduced layer thicknesses of the biofilm in the direct treatment area of the plasma effluent were excluded by the 3D models of the CLSM for this phenomenon. Dead cells on the bottom of the biofilm have disrupted membranes (Fig. 10). As the intracellular fluid escapes, cells might have lost their integrity and detached from the biofilm structure. Due to the dehydration, disruptions and dissolutions in the actin filament may occur (54). As a result, the biofilm structure partially dispersed and the next layer of living cells became visible. This occurred primarily in the center of the biofilm, since the plasma nozzle was placed directly over the center of the biofilm during treatment. However, this observation was not made for every plasma treatment, but it was added for the completeness of the results.
Conclusion.
Microbial biofilms are an important and costly problem, not only in medicine, but also in industrial applications. Thus, an efficient and rapid removal of biofilms is becoming increasingly important. This work provides new insights into the successful inactivation of C. albicans biofilms by plasma using kINPen09 (see Materials and Methods). Notably, this study provides evidences that the employed plasma treatment caused a primary inactivation of cells located at the basement of the biofilm, which at the same time facilitates the removal of biofilms by other strategies, e.g., mechanical treatment. This novel insight into plasma inactivation of biofilms indicates that plasma is well suited to fight biofilms growing on various surfaces, since surface detachment of biofilms is an important prerequisite for an effective removal of biofilms, especially in industrial settings.
MATERIALS AND METHODS
Fungal strain and growth conditions.
A fungus strain with primarily vertical biofilm growth named Candida albicans SC5314 (ATCC MYA-2876) was used. The fungus was grown on Sabouraud agar and incubated 24 h at 37°C. A suspension of the fungus in phosphate-buffered saline (PBS; pH 7.2, according to Sörensen) was adjusted to an optical density (OD) at 520 nm of 0.375. One milliliter of this suspension was added to 9 ml RPMI medium (without bicarbonate; Merck, Darmstadt, Germany), which served as an inoculum for biofilm cultivation in microtiter plates. For plasma treatment, a coated Polysterol 96-well plate (Sarstedt, Nümbrecht, Germany) was used. As an exception, a 12-well plate was used for the AFM. Two hundred microliters of the suspension was pipetted per well and incubated 90 min at 37°C and 80 rpm on a rotary shaker to allow cell attachment. After incubation, the medium was removed, the well was washed with 200 μl PBS (pH 7.2 according to Sörensen), and 200 μl RPMI medium was added. The plate was incubated 24 h at 37°C and 80 rpm on a rotary shaker, resulting in well-established C. albicans biofilms for further analysis. This protocol was kindly provided by the research group of Christiane Yumi Koga-Ito, Institute of Science and Technology—UNESP, Department of Oral Sciences and Diagnosis.
Plasma source.
kINPen09 (neoplas GmbH; Greifswald, Germany), a commercial APPJ, was used for plasma treatment. Using a direct current (DC) power supply (system energy, 8 W at 220 V, 50/60 Hz) and 99.99% argon gas connection (Linde, Pullach, Germany) with 5 slm, plasma was generated under atmospheric pressure. The inside of the APPJ handpiece consists of a 1.6-mm-diameter quartz capillary with an embedded electrode of 1-mm diameter (Fig. 11). When the APPJ was in operation, a high-frequency voltage of 1.1 MHz, 2 to 6 kVpp, was applied to the electrode. This generated the plasma at the tip of the electrode and expelled it into the surrounding medium, forming an effluent (55).
FIG 11.
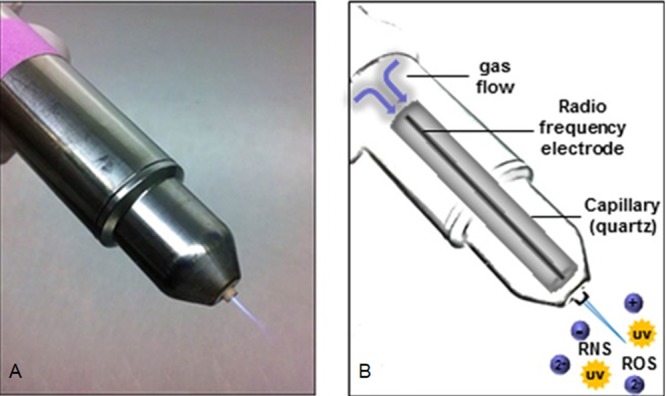
Radiofrequency plasma jet (RFPJ) instrument kINPen09 (Neoplas GmbH; Greifswald, Germany). (A) Photograph of RFPJ; (B) schematic structure of the RFPJ and composition of the physical plasma. (Reprinted from reference 58 with permission.)
Plasma treatment of C. albicans SC5314 biofilms.
Established biofilms were washed with PBS (pH 7.2 according to Sörensen), followed by complete removal of the liquid. Five different time intervals were used at a distance of 18 mm from the plasma effluent tip to the biofilm surface using a computer-controlled XYZ table (CNC Maschinenbau; Geldern, Germany), to which the plasma source was attached. Only one sample group was treated per time point to avoid dehydration effects of the biofilms. For CFU counting, fluorescence Live/Dead assays, and XTT assays, 200 μl PBS (pH 7.2 according to Sörensen) was added per well, the biofilms were mechanically disrupted, and the resulting cell suspension was collected. This step was repeated three times, until the complete biofilm was transferred.
Analytical determinations.
First, the effect of temperature on the biofilms was investigated. Therefore, biofilms were plasma treated and thermal images were taken directly after the treatment with an FLIR thermal imaging camera (FLIR Systems; Frankfurt am Main, Germany) at a distance of 20 cm. To determine the influence of the temperatures reached in the biofilms, they were grown in 96-well plates and incubated for 60 s at 130°C on a heating plate until the desired temperature could be detected at a distance of 20 cm using the FLIR thermal imaging camera. The biofilms were then examined by fluorescence microscopy using the Operetta CLS and further analyzed using CFU, fluorescence, and XTT assays. The CFU assay was used to determine the proliferation rate of the C. albicans biofilms after plasma treatment. Serial dilution of the samples was done by diluting the sample solution after plasma treatment 1:10 with maximum recovery diluent (MRD; 0.85% NaCl, 1% Trypton). The references were finally diluted 1:10,000 and the samples 1:1,000. Each dilution step was plated on Sabouraud agar by pipetting 10 μl per dilution onto the plate, and by means of the tilting technique, the sample was spread out. The plates were then incubated for 24 h at 37°C. The colonies for the respective dilution levels were counted, and the CFU per milliliter values were calculated as follows:
where 10x is the dilution factor for the lowest dilution, v is the volume of diluted cell suspension per plate in milliliters, ∑cy is the total number of colonies on all (ny) plates of the lowest evaluated dilution level, 10−x, and ∑cy + 1 is the total number of colonies on all (ny + 1) plates of the next-highest dilution level evaluated, 10−(x + 1) (56). After calculating the CFU per milliliter, the reduction factor (RF) was determined using the equation RF = MVrlog10 − MVslog10, where MVrlog10 represents the mean value of the CFU per milliliter of the reference group and MVslog10 is the mean value of the CFU per milliliter of the treated samples. For the final illustration, the weighted mean value of the total population was used. The propagation of error of the different experimental days was calculated, and the weighted error was calculated from the sum of the propagation of errors and is displayed as error bars in the illustrations (57).
Fluorescence Live/Dead assay.
The Live/Dead BacLight bacterial viability kit was prepared by mixing reagents A and B at a ratio of 1:1. After the mixing step, 0.9 μl of this solution was pipetted to 300 μl of the sample solution after plasma treatment for each well, followed by incubation for 20 min at room temperature with 80 rpm on a rotary shaker in the dark. In the next step, the 96-well plate was scanned with an excitation wavelength of 470 nm and emission wavelength of 530 nm for green fluorescence and 630 nm for red fluorescence with the Varioskan-Flash device (Thermo Scientific, Waltham, MA, USA). Finally, the G/R ratio was calculated by dividing the fluorescence intensity of the red fluorescence from the fluorescence intensity of the green fluorescence. The experiments were repeated 4 times (n = 6).
XTT assay.
For the XTT assays, the XTT cell proliferation assay kit (Applichem, St. Louis, MO, USA) was used. The activation solution and the XTT solution were mixed 1:50, and 50 μl of the mixture was transferred in a 96-well plate containing 100 μl sample solution after plasma treatment per well. The 96-well plate was incubated for 2 h at 37°C and 80 rpm in the dark. After the incubation time, 96-well plates were scanned at a wavelength of 470 nm using a Varioskan-Flash device. The obtained values were blank corrected. The experiments were repeated 4 times (n = 6).
Fluorescence microscopy.
Biofilms were cultivated as previously described, using a black 96-well plate with a glass bottom (PerkinElmer, Hamburg, Germany). Plasma-treated biofilms were resuspended in 300 μl of 0.85% NaCl after treatment to avoid dehydration of the biofilms. The Live/Dead BacLight bacterial viability kit was prepared by mixing reagents A and B in a ratio of 1:1. Next, 0.9 μl of the fluorescence solution was added to each well and the 96-well plate was incubated at room temperature for 20 min and 80 rpm in the dark. After incubation, the supernatants were removed, and the samples were washed again three times with 0.85% NaCl. Epifluorescence images were acquired using the Operetta CLS high-content imager (PerkinElmer, Hamburg, Germany) with the following objectives (Zeiss, Oberkochen, Germany): 1.25× (air, numerical aperture [NA] = 0.03), 5× (air, NA = 0.16), 20× (air, NA = 0.4), 40× (washing, NA = 1.1). Depending on the experiment, several fields of view were recorded and combined in the software. SYTO9 was excited by 475-nm (110-mW) light-emitting diode (LED), and the fluorescence was collected with a 525- ± 25-nm band pass filter. Propidium iodide was excited by a 550-nm (170-mW) LED, and fluorescence was collected with a 610- ± 40-nm band pass filter. A laser autofocus (785 nm) was available for all measurements. The images were displayed using Harmony 4.6 software.
CLSM.
Biofilms were cultivated, plasma treated, and Live/Dead stained as described above. After the staining and washing procedure, the supernatants were removed and the biofilms were analyzed using a Zeiss LSM 510 microscope (Carl Zeiss, Jena, Germany) equipped with a 10× objective (air, NA = 0.1). Filter and detector settings for monitoring SYTO9 and propidium iodide fluorescence (excitation at 488 nm using an argon laser, emission light of SYTO9 selected with a 505- to 530-nm bandpass filter, emission light of propidium iodide selected with a 650-nm longpass filter). Three-dimensional images were acquired using the ZEN 2009 software (Carl Zeiss, Jena, Germany) with an area of 1,272.2 μm by 1,272.2 μm and z-stack sections of 5.5 μm.
Atomic force microscopy.
For better adhesion of the coverslips (13 mm; Sarstedt, Nümbrecht, Germany) to the surface of the well plates and to avoid growth of the fungus at the bottom of the coverslips, 50 ml Gelrite (Duchefa, Haarlem, Netherlands) was autoclaved and directly used after the autoclaving process due to a rapid thermal curing process. A volume of 200 μl liquid Gelrite was pipetted to each well of a 12-well plate. The coverslips were placed at the surface of the liquid Gelrite and were thermally cured. The biofilms were cultivated as described above, and 1 ml of the RPMI medium was pipetted to each well until the coverslips were topped with the medium. Twelve-well plates were incubated at 37°C and 80 rpm for 1 h. After the incubation time, additional washing steps with 1 ml of 0.85% NaCl in each well were performed. Twenty-four hours later, the cultivation medium was removed and the biofilm-overgrown coverslips were washed with 1 ml 0.85% NaCl again. After washing, samples were treated for 120 s and 300 s by the RFPJ plasma effluent. Dehydration of the coverslips before AFM analysis was avoided by using a humidity chamber. The AFM measurements were carried out on a DI CP II SPM (Veeco, Plainview, NY, USA), which was mounted on a vibration-free object table (TS-150; Table Stable, Zwillikon, Switzerland). The setup was standing on an optical bench encased by an additional acoustic protection. The AFM was equipped with a linearized piezo scanner, on which the coverslips were mounted on a metal sample holder with leading tabs. The samples were measured using cantilevers with nominal spring constants (k = 0.1 to 0.6 N m−2) in contact mode. The pictures were taken by a scanning speed of 0.4 Hz by a picture size of 20 μm2 and a set point of 8 N m−2. Pictures were edited with Gwyddion (Czech Metrology Institute, Brno, Czech Republic).
ACKNOWLEDGMENTS
This work was supported by the Leibniz Institute for Plasma Science and Technology, INP, in Greifswald, Germany, and the DFG-TRR34 (A3 subproject granted to Katharina Riedel). Fluorescence microscopy was supported by the group of Sander Bekeschus within the project “Zentrum für Innovationskompetenz plasmatis, Nachwuchsgruppe: Plasma-Redox-Effekte,” which was financially supported by the German Federal Ministry of Education and Research (BMBF).
REFERENCES
- 1.Nguyen Q, Tudisco F, Gautier A, Hein M. 2017. An efficient multilinear optimization framework for hypergraph matching. IEEE Trans Pattern Analysis Machine Intelligence 39:1054–1075. doi: 10.1109/TPAMI.2016.2574706. [DOI] [PubMed] [Google Scholar]
- 2.Serra E, Hidalgo-Bastida LA, Verran J, Williams D, Malic S. 2017. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens 7:15. doi: 10.3390/pathogens7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herald PJ, Zottola EA. 1988. Attachment of Listeria monocytogenes to stainless-steel surfaces at various temperatures and pH values. J Food Sci 53:1549–1562. doi: 10.1111/j.1365-2621.1988.tb09321.x. [DOI] [Google Scholar]
- 4.Mafu AA, Roy D, Goulet J, Magny P. 1990. Attachment of Listeria monocytogenes to stainless-steel, glass, polypropylene, and rubber surfaces after short contact times. J Food Prot 53:742–746. doi: 10.4315/0362-028X-53.9.742. [DOI] [PubMed] [Google Scholar]
- 5.Notermanns S, Dormans JAMA, Mead GC. 1991. Contribution of surface attachment to the establishment of micro-organisms in food processing plants: a review. J Bioadhesion Biofilm Res 5:21–36. doi: 10.1080/08927019109378226. [DOI] [Google Scholar]
- 6.Steenackers HP, Parijs I, Foster KR, Vanderleyden J. 2016. Experimental evolution in biofilm populations. FEMS Microbiol Rev 40:373–397. doi: 10.1093/femsre/fuw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 8.Debus ES, Winkler MS, Larena-Avellaneda A, Bültemann A, Daum H, Lingenfelder M, Schulenburg B, Gross-Fengels W. 2003. Medizinische und ökonomische Aspekte der Zentrumsbildung in der Wundbehandlung. Gefässchirurgie 8:259–268. [Google Scholar]
- 9.Lauterbach S. 2006. Chronische Wunden - Ulcus cruris, diabetisches Fußsyndrom, Dekubitus, Verbrennungen. PZ Prisma 2:239. [Google Scholar]
- 10.Calderone RA, Clancy C. 2012. Candida and candidiasis, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 11.Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, Diekema DJ. 2010. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 14.Ruhnke M. 2006. Epidemiology of Candida albicans infections and role of non-Candida-albicans yeasts. Curr Drug Targets 7:495–504. doi: 10.2174/138945006776359421. [DOI] [PubMed] [Google Scholar]
- 15.Calderone RA. 2002. Candida and candidiasis. ASM Press, Washington, DC. [Google Scholar]
- 16.Gleiznys A, Zdanaviciene E, Zilinskas J. 2015. Candida albicans importance to denture wearers. A literature review. Stomatologija 17:54–66. [PubMed] [Google Scholar]
- 17.Yasui M, Ryu M, Sakurai K, Ishihara K. 2012. Colonisation of the oral cavity by periodontopathic bacteria in complete denture wearers. Gerodontology 29:e494–e502. doi: 10.1111/j.1741-2358.2011.00506.x. [DOI] [PubMed] [Google Scholar]
- 18.Douglass CW, Shih A, Ostry L. 2002. Will there be a need for complete dentures in the United States in 2020? Prosthet Dent 87:5–8. doi: 10.1067/mpr.2002.121203. [DOI] [PubMed] [Google Scholar]
- 19.Li XJ, Kolltveit KM, Tronstad L, Olsen I. 2000. Systemic diseases caused by oral infection. Clin Microbiol Rev 13:547–558. doi: 10.1128/CMR.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankari SL, Gayathri K, Balachander N, Malathi L. 2015. Candida in potentially malignant oral disorders. J Pharm Bioallied Sci 7:162–164. doi: 10.4103/0975-7406.155902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alalwan H, Rajendran R, Lappin DF, Combet E, Shahzad M, Robertson D, Nile CJ, Williams C, Ramage G. 2017. The anti-adhesive effect of curcumin on Candida albicans biofilms on denture materials. Front Microbiol 8:659. doi: 10.3389/fmicb.2017.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias KD, Barbugli PA, de Patto F, Lordello VB, Penteado LD, Medeiros AI, Vergani CE. 2017. Soluble factors from biofilm of Candida albicans and Staphylococcus aureus promote cell death and inflammatory response. BMC Microbiol 17:146. doi: 10.1186/s12866-017-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steenackers HP, Parijs I, Dubey A, Foster KR, Vanderleyden J. 2016. Experimental evolution in biofilm populations. FEMS Microbiol Rev 40:980–980. doi: 10.1093/femsre/fuw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall CW, Mah TF. 2017. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev 41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 25.Costerton JW. 1995. Overview of microbial biofilms. J Ind Microbiol 15:137–140. doi: 10.1007/BF01569816. [DOI] [PubMed] [Google Scholar]
- 26.Hornemann JA, Lysova AA, Codd SL, Seymour JD, Busse SC, Stewart PS, Brown JR. 2008. Biopolymer and water dynamics in microbial biofilm extracellular polymeric substance. Biomacromolecules 9:2322–2328. doi: 10.1021/bm800269h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadell CD, Xavier JB, Foster KR. 2009. The sociobiology of biofilms. FEMS Microbiol Rev 33:206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 28.Oxman DA, Chow JK, Frendl G, Hadley S, Hershkovitz S, Ireland P, McDermott LA, Tsai K, Marty FM, Kontoyiannis DP, Golan Y. 2010. Candidaemia associated with decreased in vitro fluconazole susceptibility: is Candida speciation predictive of the susceptibility pattern? J Antimicrob Chemother 65:1460–1465. doi: 10.1093/jac/dkq136. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove SE, Carmeli Y. 2003. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 36:1433–1437. doi: 10.1086/375081. [DOI] [PubMed] [Google Scholar]
- 30.Ranjan R, Krishnamraju PV, Shankar T, Gowd S. 2017. Nonthermal plasma in dentistry: an update. J Int Soc Prev Community Dent 7:71–75. doi: 10.4103/jispcd.JISPCD_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Öngel C, Keleş M, Acar E, Birer Ö. 2015. Atmospheric pressure plasma jet treatment of human hair fibers. J Bio- Tribo-Corrosion 1:7. doi: 10.1007/s40735-015-0007-y. [DOI] [Google Scholar]
- 32.Flynn PB, Higginbotham S, Alshraiedeh NH, Gorman SP, Graham WG, Gilmore BF. 2015. Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens. Int J Antimicrobial Agents 46:101–107. doi: 10.1016/j.ijantimicag.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova JM, Murashev AN, Selezneva II, Shimizu T, Sysolyatina EV, Shaginyan IA, Petrov OF, Mayevsky EI, Fortov VE, Morfill GE, Naroditsky BS, Gintsburg AL. 2011. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol 60:75–83. doi: 10.1099/jmm.0.020263-0. [DOI] [PubMed] [Google Scholar]
- 34.Panngom K, Lee SH, Park DH, Sim GB, Kim YH, Uhm HS, Park G, Choi EH. 2014. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS One 9:e99300. doi: 10.1371/journal.pone.0099300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laroussi M. 2005. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Processes Polymers 2:391–400. doi: 10.1002/ppap.200400078. [DOI] [Google Scholar]
- 36.Todar K. 2004. The good, the bad, and the deadly. Science 304:1421. [Google Scholar]
- 37.Guo L, Xu R, Zhao Y, Liu D, Liu Z, Wang X, Chen H, Kong MG. 2018. Gas plasma pre-treatment increases antibiotic sensitivity and persister eradication in methicillin-resistant Staphylococcus aureus. Front Microbiol 9:537. doi: 10.3389/fmicb.2018.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vleugels M, Shama G, Deng XT, Greenacre E, Brocklehurst T, Kong MG. 2005. Atmospheric plasma inactivation of biofilm-forming bacteria for food safety control. IEEE Trans Plasma Sci 33:824–828. doi: 10.1109/TPS.2005.844524. [DOI] [Google Scholar]
- 39.Goree J, Liu B, Drake D, Stoffels E. 2006. Killing of S-mutans bacteria using a plasma needle at atmospheric pressure. IEEE Trans Plasma Sci 34:1317–1324. doi: 10.1109/TPS.2006.878431. [DOI] [Google Scholar]
- 40.Dobrynin D, Fridman G, Friedman G, Fridman A. 2009. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys 11:e115020. doi: 10.1088/1367-2630/11/11/115020. [DOI] [Google Scholar]
- 41.Shashurin A, Keidar M, Bronnikov S, Jurjus RA, Stepp MA. 2008. Living tissue under treatment of cold plasma atmospheric jet. Appl Phys Lett 93:e181501. doi: 10.1063/1.3020223. [DOI] [Google Scholar]
- 42.Koban I, Matthes R, Hubner NO, Welk A, Meisel P, Holtfreter B, Sietmann R, Kindel E, Weltmann KD, Kramer A, Kocher T. 2010. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys 12:e073039. doi: 10.1088/1367-2630/12/7/073039. [DOI] [Google Scholar]
- 43.Gorynia S, Koban I, Matthes R, Welk A, Gorynia S, Hubner NO, Kocher T, Kramer A. 2013. In vitro efficacy of cold atmospheric pressure plasma on S. sanguinis biofilms in comparison of two test models. GMS Hyg Infect Control 8:Doc01. doi: 10.3205/dgkh000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moravsky L, Klas M, Machova E, Pisklova K, Matejcik S. 2015. Influence of a plasma jet on the viability of Candida albicans. Open Chem 13:257–262. [Google Scholar]
- 45.Pantanella P, Valenti P, Natalizi T, Passeri D. 2013. Analytical techniques to study microbial biofilm on abiotic surfaces: pros and cons of the main techniques currently in use. Ann Ig 25:31–42. [DOI] [PubMed] [Google Scholar]
- 46.Pei X, Lu X, Liu J, Liu D, Yang Y, Ostrikov K, Chu PK, Pan Y. 2012. Inactivation of a 25.5 μm Enterococcus faecalis biofilm by a room-temperature, battery-operated, handheld air plasma jet. J Phys D Appl Phys 45:e165205. doi: 10.1088/0022-3727/45/16/165205. [DOI] [Google Scholar]
- 47.Delben JA, Zago CE, Tyhovych N, Duarte S, Vergani CE. 2016. Effect of atmospheric-pressure cold plasma on pathogenic oral biofilms and in vitro reconstituted oral epithelium. PLoS One 11:e0155427. doi: 10.1371/journal.pone.0155427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilking JN, Zaburdaev V, De Volder M, Losick R, Brenner MP, Weitz DA. 2013. Liquid transport facilitated by channels in Bacillus subtilis biofilms. Proc Natl Acad Sci U S A 110:848–852. doi: 10.1073/pnas.1216376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai WL, De La Fuente L, Arias CR. 2013. Biofilm formation by the fish pathogen Flavobacterium columnare: development and parameters affecting surface attachment. Appl Environ Microbiol 79:5633–5642. doi: 10.1128/AEM.01192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 51.Dunne NJW. 2002. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reuter S, Winter J, Schmidt-Bleker A, Tresp H, Hammer MU, Weltmann KD. 2012. Controlling the ambient air affected reactive species composition in the effluent of an argon plasma jet. IEEE Trans Plasma Sci 40:2788–2794. doi: 10.1109/TPS.2012.2204280. [DOI] [Google Scholar]
- 53.Stewart PS. 2003. Diffusion in biofilms. J Bacteriol 185:1485–1491. doi: 10.1128/JB.185.5.1485-1491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Small JV. 1981. Organization of actin in the leading-edge of cultured-cells—influence of osmium-tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol 91:695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weltmann KD, Kindel E, Brandenburg R, Meyer C, Bussiahn R, Wilke C, von Woedtke T. 2009. Atmospheric pressure plasma jet for medical therapy: plasma parameters and risk estimation. Contrib Plasma Phys 49:631–640. doi: 10.1002/ctpp.200910067. [DOI] [Google Scholar]
- 56.Bast E. 2001. Mikrobiologische Methoden: eine Einführung in grundlegende Arbeitstechniken, vol 2, p 429 Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 57.Gränicher WHH. 1994. Messung beendet—was nun?, 2:section 6.2.2./S6-4-6–9 Hochschulverlag AG, Zurich, Switzerland. [Google Scholar]
- 58.Weiss M, Gumbel D, Hanschmann EM, Mandelkow R, Gelbrich N, Zimmermann U, Walther R, Ekkernkamp A, Sckell A, Kramer A, Burchardt M, Lillig CH, Stope MB. 2015. Cold atmospheric plasma treatment induces anti-proliferative effects in prostate cancer cells by redox and apoptotic signaling pathways. PLoS One 10:e0130350. doi: 10.1371/journal.pone.0130350. [DOI] [PMC free article] [PubMed] [Google Scholar]