Abstract
Introduction:
Sexual quality of life (SQoL) is a critical component of sexual health and is understudied in Sub-Saharan African settings with endemic HIV and sexually transmitted infection (STI).
Aim:
We sought to assess SQoL among heterosexual couples in Kisumu, Kenya, and how this was associated with HIV status, STIs, and sexual practices.
Methods:
This was a prospective cohort study of community-recruited couples. SQoL, HIV status, herpes simplex virus (HSV)-2 status, bacterial vaginosis (BV), sexual practices, and sociodemographics were measured at baseline, 6 months, and 12 months. Multivariable linear regression with random intercept was fitted separately for females and males, accounting for partner characteristics.
Main Outcome Measure:
SQoL was assessed with an 18-item female and 11-item male survey.
Results:
From April 2014 through July 2016, 252 couples were enrolled, and followed up through September 2017. At baseline, women were median age 23 years, 10% HIV positive, 53% HSV-2 seropositive, and 22% with BV. At baseline men were median age 26 years, 12% HIV positive, 47% HSV-2 seropositive, and 56% circumcised. Mean SQoL was higher for men (88) than women (78), with consistent scores over time. In multivariable analysis (P < .05 each), SQoL Questionnaire—Female (SQoL-F) score was reduced with: male partner report that sex felt rougher than he would have liked (9.5-point decrease), female HSV-2 seropositivity (5.15-point decrease), female reported having dry vaginal sex (5.27-point decrease); among women with BV, SQoL-F score declined with recent sexual activity (8.27-point decrease) and increasing age (0.75-point decrease per 1 year increase in age). Age and recent sex did not affect SQoL-F for women without BV. SQoL Questionnaire—Male score was decreased 4.99 points if male was employed, 4.52 points if male reported multiple recent sex partners, and 29.5 points for HIV positive men whose female partner reported having sex when not in the mood. Men’s SQoL increased by 0.84 points for each 1-U increase in female partner body mass index and 17.6 points for HIV positive men whose female partner reported recent sex with him.
Clinical Implications:
Within sexual partnerships, men had greater SQoL than women, and the adverse impact of BV and STIs on SQoL was greater for women than men.
Strength & Limitations:
Research is needed to ensure relevant domains are measured in settings where measure of SQoL has not been validated, along with robust measures of physiologic and psychologic correlates.
Conclusion:
More attention to SQoL as an outcome may strengthen interventions aimed at preventing HIV and STIs and improving sexual health holistically.
Keywords: Sexual Quality of Life, Sexually Transmitted Infections, HIV, Kenya, Couples, Bacterial Vaginosis
INTRODUCTION
Sexual health encompasses physical well-being and a positive approach to sexuality and sexual relationships, including pleasurable sexual experiences.1 Sexual quality of life (SQoL) is a multidimensional construct, encompassing physical, social, emotional, and psychological dimensions,2 and is postulated to be more sensitive to evaluate declines in sexual health, than measures of sexual functioning alone.3 Poor SQoL has been associated with concomitant increases in anxiety and depression, and relationship disruptions.4 Further, SQoL is part of, and associated with overall health-related quality of life.5 Factors associated with SQoL have largely been studied among elderly populations and those with illness. Factors associated with decreased sexual functioning and SQoL include physiologic, medical, and psychological factors, such as medication use,4 depression and substance abuse,6 herpes simplex virus (HSV)-2 infection,7,8 and HIV infection.9
In Kenya, HIV prevalence in the general adult population is 5.9%, but 20% in Kisumu County, which borders Lake Victoria in western Kenya.10,11 Following age-related trends in HIV, in western Kenya HSV-2 prevalence increases dramatically from 10% in 13- to 14-year-old girls to 28% in 15- to 19-yearolds.12,13 Bacterial vaginosis (BV) affects over 20% of general population women in Kenya14,15 and up to 40% of women with HIV or in clinic-based settings.16–18 Given the high burden of HIV and sexually transmitted infection (STIs), SQoL may be adversely affected. Relatively little is known about the SQoL and associated factors among heterosexuals in a Sub-Saharan African setting with endemic rates of HIV and STIs. Understanding the impact of HIV, STIs, and sexual practices on SQoL can inform the development and tailoring of counseling messages to reduce stigma and lessen the potential adverse impacts of these infections. We sought to assess SQoL in a cohort of heterosexual couples in Kisumu, Kenya, and hypothesized it would be negatively associated with HIV and STIs infection, and would vary by sexual practices.
METHODS
This study was approved by the institutional review boards of University of Chicago at Illinois, Maseno University (Kisumu, Kenya), and Rush University (Chicago, IL, USA).
Study Setting
Kisumu is positioned 400 km west of Nairobi, adjacent to Lake Victoria. Kisumu town accounts for 40% of the population of Kisumu County, and is the urban center of the province. The population consists mostly of members of the Luo ethnic group. Kisumu is an impoverished area, with 45% of the county’s child population estimated to be socioeconomically deprived.19 The disease burden typifies rural African communities, with mortality in adolescents and young adults primarily attributed to communicable diseases, injuries, and maternal causes. The practice of polygamy is common (~20%) in Nyanza region.20,21 In Kisumu County, women’s median age of first marriage is 19 years, with median age of first birth 19.6 years, and total fertility rate of 3.6.20
Study Design and Participants
This study used data and biological specimens from Afya Jozi, Afya Jamii (Kiswahili for “Healthy Pair, Healthy Community”), a prospective cohort study of heterosexual couples in Kisumu, Kenya, the capital of Kisumu County. Subjects were recruited from public spaces in the community, including bus stops, motorcycle stands, markets, beauty parlors, barber shops, and in central areas of neighborhoods. In public spaces, study staff (usually 1 female and 1 or 2 males, wearing identification badges and collared polo shirts printed with the study name and their employer information) gave brief talks on the importance of HIV and STI counseling and testing. Recruiters then introduced and briefly explained the study. They made themselves available to those who were interested, moving to a more secluded area, away from a crowd. They recorded first names and telephone numbers in their study log books, and then subsequently called those who were interested to schedule screening appointments and arrange transportation. Men and women had to come to the study clinic together for eligibility screening. The eligibility of each member of the couple was assessed separately in a private room. To be eligible, members of couples had to independently confirm they had been in a sexual relationship for at least 6 months’ duration, with no plans to move for the duration of the study, and agree to attend all study visits together. We included men aged 18e35 years and female partners aged 16 years and older. After the baseline visit, couples were scheduled for follow-up at 1 month, 6 months, and 12 months. Each member of eligible couples who consented and enrolled received 400 Kenyan shillings (~4 U.S. dollars) compensation for time and travel for each study visit.
Data Collection
Following written informed consent, at each study visit, participants underwent standardized medical history and physical examination, plus a personal interview to obtain sociodemographic information and to assess sexual practices. Gender-matched clinicians (clinical officer or nurse) trained in research and survey administration interviewed participants in their language of choice (English, DhoLuo, or Kiswahili). Sociodemographic data included age, educational attainment, past month income, and current employment status. Sexual practices that were assessed included condom use, having multiple sex partners, and timing of last sexual activity. The recall period for sexual practices was the past 6 months, unless otherwise specified. Women were asked about the frequency of having sex during menses, and having sex when they were not in the mood; women and men were asked the frequency of having sex when your vagina/your partner’s vagina was dry, and sex feeling rougher than they would have liked. The response categories to these questions were “never,” “rarely,” “sometimes,” “often,” or “always,” and this was dichotomized as ever vs never in analysis. Men’s circumcision status was assessed at each visit during the physical examination. Women underwent urine pregnancy testing at each study visit.
Sample Size and Sampling
Afya Jozi, Afya Jamii was designed to estimate the effect of the penile microbiome on female sex partner’s risk of BV. With an anticipated cumulative event rate of 50% occurring over 12 months, we estimated needing to enroll 204 couples to be able to detect a hazard ratio of 1.35 over a range of exposure prevalences, accounting for 15% loss to follow-up. Over time, loss to followup was higher than anticipated, and sample size goal was increased to 250. Recruitment was stratified on male circumcision status and age, so that 50% of the sample would be circumcised, and 50% would be aged 18–26 years, to maximize the ability to assess differences between circumcised and uncircumcised men, and to ensure a range of age-related behavioral and biological risk in the sample.
Detection of HIV, BV, and HSV-2
Testing for HIV infection was conducted using a serial rapid test protocol that followed the Kenyan national guidelines.22 Subjects who reported themselves as HIV positive at baseline were not required to have confirmatory testing. Men or women testing or reporting HIV positive were referred for care at a nearby health facility if not already in HIV care. Serum specimens were tested for HSV-2 antibody (Kalon HSV-2 IgG enzyme-linked immunosorbent assay; Kalon Biological Limited, Aldershot, United Kingdom), using the manufacturer’s recommended cut-off. At baseline and each follow-up visit, smears prepared from clinician-collected vaginal swabs were Gram stained and evaluated according to Nugent criteria; a score of 7–10 was defined as BV.23 Treatment was provided at point of care and based on detection of 3 or more of Amsel criteria24: amine odor positive, vaginal pH >4.5, vaginal discharge, and detection of clue cells. Women with BV who were not treated at time of presentation were called to return for treatment; treatment was documented for 93% of positive women at baseline, 89% at 6 months, and 85% at 12 months.
Measurement of SQoL
We assessed SQoL at baseline, 6 months, and 12 months using the SQoL Questionnaire—Female (SQoL-F)3 and SQoL Questionnaire—Male (SQoL-M).25 We chose the SQoL Questionnaire as a measurement tool due to parallel assessments for females and males, prior successful use in studies with broad global distribution,26–28 and relatively low participant burden.27 Additionally, SQoL-F has been used successfully in studies with African populations, although not in Kenya.28,29 The SQoL-F (18 items) and SQoL-M (11 items) include a series of questions, each with a 6-point response scale (1 “completely agree” to 6 “completely disagree”). Prior to implementing the study, we conducted pre-testing with 3 women and 3 men who were native Kenyans residing in Kisumu, to maximize understandability and reliability of the questions. Each SQoL item was evaluated with 6 questions relating to comprehension (interpretation and paraphrasing), confidence of judgment (certainty and specificity), retrieval from memory, and decision process for answering. This process led to rewording of some questions (Supplemental Table 1). The pre-testing process also uncovered potential limitations of the surveys. Regarding the question, “I feel that I can talk to my partner about sexual matters,” women and men both felt that men usually initiate this conversation unless there was a doctor’s advice to discuss sexual life. Thus, this question may have less relevance in our population, but we retained all scale items. In our sample, Cronbach alpha for SQoL-F ranged from 0.89–0.92 over the 3 study visits, and 0.92–0.93 for SQoL-M, reflecting superior internal consistency, but potentially redundant items.30 Baseline mean responses for individual SQoL-F and SQoL-M items are shown in Supplemental Table 1.
Statistical Analysis
The outcome for analysis was SQoL. Female and male raw scores were transformed to a standardized scale of 0 to 100 by summing the component items and subtracting the lowest possible score, then dividing this by the possible raw score range.3 The final figure was multiplied by 100. Higher scores imply a greater SQoL. We used observations with complete data (ie, all items answered) because at baseline, only 2 female respondents and 1 male respondent did not answer all items. All items were answered by all respondents at subsequent assessments.
We fit separate models for females and males, and assessed the association of SQoL with index and partner sociodemographics, sexual behaviors and practices, and HIV and STI status. Sociodemographic factors assessed at baseline were age, educational attainment, and duration of relationship; at baseline and each 6-month assessment, current employment status and body mass index (BMI) were assessed. Age and BMI were centered at the overall mean for each gender. Frequency of condom use was assessed and analyzed as “always,” “sometimes,” or “never.” Number of sexual partners in the past 6 months was assessed as continuous variable and dichotomized as 2 or more vs 1 based on the distribution of responses. A low frequency of women (<2%) reported multiple sex partners over time, and thus this was not examined in analyses. Sexual practices, BV, HSV-2 status, and HIV status were analyzed as time-varying covariates.
We modeled SQoL with linear regression, with a random intercept to account for the repeated measurements. Variables were screened by univariate significance P < .10. Pairs of variables with Spearman correlation coefficient >0.30 were subjected to variable selection. We retained variables with the greatest statistical significance as reflected by largest t value in the univariate model for entry in multivariable analysis. In 1 instance, 3 variables were correlated with each other, and we used a proportional Venn diagram (created in Stata 15/SE31) to aid variable selection (Supplemental Figure 1). We then tested 2-way interaction of variables with biological relevance (male circumcision status, HSV-2 serostatus, HIV status, and BV). The selected variables from univariate analysis and interaction terms with P < .10 were entered in a multivariable model, and backward selection was performed until all variables met a P < .05 threshold. Variables that were significant in univariate analysis but did not reach significance in multivariable analysis were removed from the model. Additionally, interactions were required to have at least 10 observations per cell to be maintained in models.32 In the female univariate model, BV interacted with several variables(HIV,HSV-2, age, time since last sex, male partner circumcision status, educational attainment) at the P < .10 level, with coefficients reflecting a poorer than expected SQoL. The interaction of BV with most other covariates suggested stratified analyses should be conducted, but was precluded due to limited number of observations for the BV-positive stratum (n ¼ 139 observations). Therefore, we present results for the sample of women as a whole as the primary analysis, and as a secondary analysis we present results among BV-negative observations (Supplemental Table 2). All reported P values are 2-sided tests of significance. Missing values for BMI (n = 6 female measurements, n = 2 male measurements) were imputed when the subject in question had 1 or more values at other visits by averaging those values. No other missing values were imputed. Analyses were performed in R using package lme4 for regression and package ggplot2 for violin plots.33 R2 for mixed effects linear regression was calculated according to Nakagawa and Schielzeth,34 with R-package MuMIn.33
RESULTS
Sample Characteristics
From April 2014 through July 2016, 252 couples were enrolled (Table 1). Follow-up was 67% for women (n = 169) and 69% for men (n = 175) at 6 months, and 58% for women (n = 147) and 67% for men (n = 161) at 12 months. Paired measures were available for 249 couples at baseline, 165 (65%) couples at 6 months, and 138 (55%) couples at 12 months. Baseline HIV prevalence was 10.5% among women, and 12.4% among men, and 82.6% of couples were seroconcordant HIV negative, 12.1% HIV serodiscordant, and 5.3% seroconcordant HIV positive. Baseline HSV-2 seroprevalence was 56.8% among women and 46.6% among men. BV prevalence was 22% at baseline,with 34% 1-year cumulative incidence, and 42% of all women were diagnosed with BV at some point during the 12 months.
Table 1.
Participant characteristics at baseline
| Females, N = 252 | Males, N = 252 | |
|---|---|---|
| Sociodemographics | ||
| Age, y, median (SD) | 23 (4.1) | 26 (4.0) |
| Duration of relationship, mo, median (IQR) | 36 (24–60) | |
| Highest educational attainment | ||
| Primary or less | 125 (50) | 101 (40) |
| Some secondary/secondary | 93 (37) | 112 (45) |
| Post-secondary | 34 (13) | 39 (15) |
| Currently employed | 123 (49) | 199 (79) |
| Income in past 1 mo, Kenyan shillings [~US$]* | ||
| None | 36 (14) | 3 (1) |
| <2,000 [<$20] | 80 (32) | 15 (6) |
| 2,000-<5,000 [$20-<$50] | 67 (27) | 39 (15) |
| 5,000-<10,000 [$50-<$100] | 44 (17) | 88 (35) |
| 10,000–25,000 [$100-$250] | 18 (7) | 89 (35) |
| >25,000 [>$250] | 4 (2) | 17 (7) |
| Do not know | 3 (1) | 0 (0) |
| Refused | 0 (0) | 1 (0.4) |
| Biological variables | ||
| HIV positive | 26 (10) | 31 (12) |
| HSV-2 seropositive | 143 (57) | 117 (47) |
| Bacterial vaginosis | 54 (22) | |
| Circumcised | 142 (56) | |
| Pregnant | 26 (10) | |
| Median body mass index (IQR) | 21.8 (19.8–24.2) | 20.9 (19.7–22.8) |
| Sexual practices and behaviors† | ||
| ≥2 Sexual partners | 5 (2) | 57 (23) |
| Frequency of condom use | ||
| Always | 20 (8) | 16 (6) |
| Sometimes | 73 (29) | 81 (32) |
| Never | 159 (63) | 155 (62) |
| Had sex during menses, ever vs never | 31 (12) | |
| Had sex when not in the mood | 88 (35) | |
| Sex felt rougher than you would have liked, ever vs never | 60 (24) | 7 (3) |
| Had sex when your vagina was dry, ever vs never | 167 (67) | |
| Last sex with current partner | ||
| Within last 2 d | 131 (52) | 132 (52) |
| 3–7 d | 63 (25) | 82 (33) |
| >7 d ago | 58 (23) | 37 (15) |
| Washed vagina/penis ≤1 h after the last time you had sex, vs >1 h | 72 (29) | 196 (78) |
Values are n (%) unless otherwise specified.
Not all cells sum to N due to missing data.
HSV = herpes simplex virus; IQR = interquartile range.
US$ estimated from Kenyan shillings using an exchange rate of 100 shillings per 1 dollar.
Recall period is past 6 mo, unless otherwise specified
Sexual Quality of Life
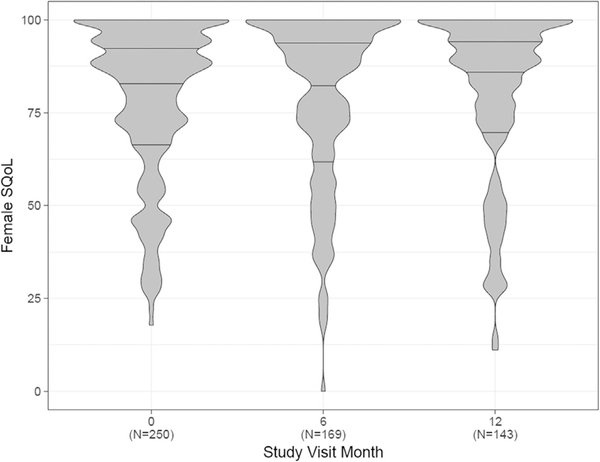
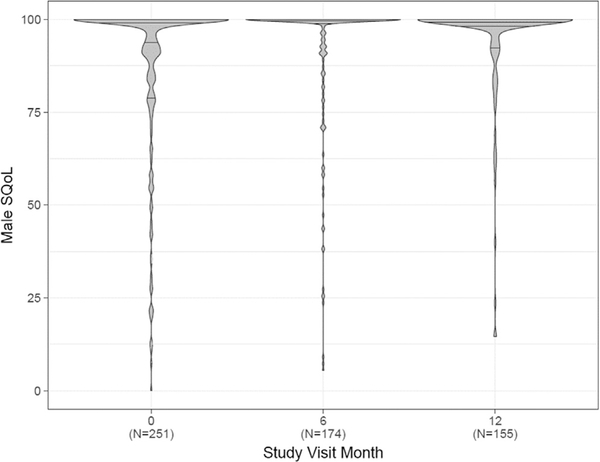
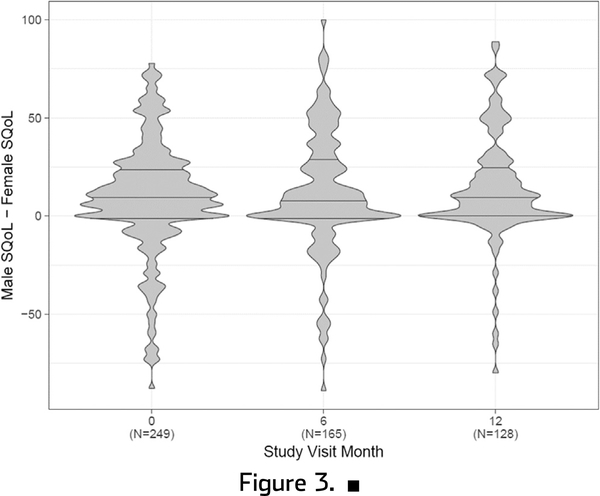
The mean SQoL at baseline was 78.2 (SD 20.6) for women and 87.7 (SD 21.9) for men (Figure 1). The distribution was right skewed for both, though with more variability among women. The mean difference in paired measures of SQoL was 9.39 (SD 28.5). There was no change over time in SQoL of women, men, or the difference between their scores over time (Figures 1 and 2). The correlation between male and female SQoL was weak at baseline (Spearman rho = 0.14) and over time (6 months = 0.06, 12 months = 0.02).
Figure 1.
Violin plots showing distribution of female sexual quality of life (SQoL) over time. Horizontal lines within plots represent median (center lines) and interquartile range (upper line is 75th percentile and lower line is 25th percentile). Width represents number of responses.
Figure 2.
Violin Plots showing distribution of male sexual quality of life (SQoL) over time. Horizontal lines within plots represent median (center lines) and interquartile range (upper line is 75th percentile and lower line is 25th percentile). Width represents number of responses.
Factors Associated With SQoL Among Women
In univariate analysis, numerous factors were associated with SQoL among females at the P < .05 level (Table 2). In multivariable analyses (Table 2), 5 variables remained associated with SQoL-F (P < .05), all with inverse associations (intercept = 85.3): male reporting sex felt rougher than he would have liked (−9.50), female HSV-2 seropositivity (−5.15), female reporting dry sex (−5.27), interaction of BV diagnosis with recent sexual intercourse (−8.27), and interaction of BV diagnosis with increasing age of woman (−0.75 per 1-year increase in age). Among BV negative observations (Supplemental Table 2), in multivariable analysis, 3 variables were associated with SQoL at the P < .05 level (intercept = 83.1): female HSV-2 seropositivity (−5.20 points, P = .02), female reporting dry sex (6.80 points, P < .01), and female reporting recent sex with the male partner (4.98, P = .01).
Table 2.
Factors associated with sexual quality of life among females
| Characteristic | Univariate N = 562, n = 251 Est (95% CI) P value | Multivariable adjusted N = 521, n = 248 Est (95% CI) P value |
|---|---|---|
| Intercept | 85.3 (81.1 to 89.4) <.01 | |
| Male reported sex was rougher than would have liked, ever vs never* | −8.46 (−16.1 to −0.80) .03 | −9.50 (−17.0 to −2.04) .01 |
| Female HSV-2 seropositive | −6.38 (−10.7 to −2.08) <.01 | −5.15 (−9.49 to −0.82) .02 |
| Female reported dry sex, ever vs never* | −3.03 (−6.19 to 0.12) .06 | −5.27 (−8.50 to −2.05) <.01 |
| Interaction of bacterial vaginosis diagnosis with female reported having sex with current partner within the last 2 d, vs >2 d | −6.71 (−11.5 to −1.88) .01 | −8.27 (−14.7 to −1.83) .01 |
| Female reported having sex with current partner within the last 2 d, vs >2 d | −0.17 (−3.26 to 2.92) .92 | 2.91 (−0.65 to 6.47) .11 |
| Interaction of bacterial vaginosis diagnosis with female age in y | −0.86 (−1.60 to −0.13) .02 | −0.75 (−1.50 to −0.01) .05 |
| Female age in y | −0.30 (−0.89 to 0.29) .32 | −0.04 (−0.65 to 0.58) .91 |
| Bacterial vaginosis diagnosis | −2.82 (−6.22 to 0.58) .10 | 0.26 (−3.86 to 4.38) .90 |
| Male partner HIV positive | −8.39 (−15.1 to −1.66) .01 | |
| Female with primary educational attainment, reference is some secondary/secondary | −5.51 (−10.3 to −0.73) .02 | |
| Male partner with primary educational attainment, reference is some secondary/secondary | −5.20 (−10.1 to −0.33) .04 | |
| Male partner with post-secondary education, reference is some secondary/secondary | 5.78 (−0.86 to 12.4) .09 | |
| Male partner BMI | 0.73 (−0.09 to 1.55) .08 | |
| Female partner BMI | 0.52 (−0.09 to 1.13) .09 | |
| R2 | .67 |
Not shown: Variables that were not associated with female sexual quality of life in univariate analysis were male partner sexual quality of life, condom use, circumcision status, relationship duration, employment status of either partner, male partner reporting recent sex, woman HIV status, HSV-2 seropositive partner, woman reported sex during menses, woman reported sex rougher than she would have liked, woman reported sex when not in mood, woman having attained post-secondary education, woman pregnant, male partner reported multiple sex partners, and genital washing practices of either partner. BMI = body mass index; Est = coefficient estimate; HSV = herpes simplex virus; N = number of observations; n = number of individuals.
Recall period is in the last 6 mo.
Factors Associated With SQoL Among Men
Among males, univariate analysis identified numerous factors associated with SQoL at the P < .05 level (Table 3). In multivariable analysis, 3 variables remained inversely associated with male SQoL (P < .05; intercept = 93.0): male is currently employed (−4.99), male reports having 2 or more sex partners (−4.52), the interaction of male HIV positivity with female partner reporting having sex when not in the mood (−29.5). There was a 0.84 increase in male SQoL per unit increase in female partner’s BMI. The interaction of female partner reporting recent sex with male HIV positivity was associated with increased male SQoL (17.6).
Table 3.
Factors associated with sexual quality of life among males
| Characteristic | Univariate N = 580, n = 252 Est (95% CI) P value | Multivariable Adjusted N = 512, n = 244 Est (95% CI) P value |
|---|---|---|
| Intercept | 95.0 (90.9 to 99.1) <.01 | |
| Female partner BMI | 0.75 (0.17 to 1.34) .01 | 0.84 (0.25 to 1.43) .01 |
| Male is currently employed | −5.27 (−8.51 to −2.03) <.01 | −4.99 (−8.56 to −1.42) .01 |
| Male reports having ≥2 sexual partners* | −4.95 (−8.87 to −1.04) .01 | −4.52 (−8.59 to −0.44) .03 |
| Interaction of male HIV positive with female partner reported having sex when not in the mood, ever vs never* | −20.3 (−29.6 to −10.9) <.01 | −29.5 (−41.1 to −17.9) <.01 |
| Female partner reported having sex when not in the mood, ever vs never* | −4.02 (−7.63 to −0.41) .03 | −0.63 (−4.55 to 3.30) .75 |
| Interaction of male HIV positive with female partner reported having sex with current partner within the last 2 d, vs >2 d | 9.89 (−0.15 to 19.9) .05 | 17.6 (5.51 to 29.6) <.01 |
| Female partner reported having sex with current partner within the last 2 d, vs >2 d | −0.30 (−3.51 to 2.91) .85 | −1.30 (−4.67 to 2.08) .45 |
| Male HIV positive | −0.92 (−7.22 to 5.39) .78 | 1.33 (−6.01 to 8.67) .72 |
| Female partner reports sex during menses, ever vs never* | −7.03 (−12.7 to −1.34) .02 | |
| Male partner BMI | 0.81 (0.04 to 1.57) .04 | |
| Female partner reports sex was rougher than would have liked, ever vs never* | −3.52 (−7.38 to 0.34) .07 | |
| Male age in y | −0.46 (−1.01 to 0.09) .10 | |
| Female partner age in y | −0.45 (−0.99 to 0.08) .10 | |
| R2 | .59 |
Not shown: Variables that were not associated with male sexual quality of life in univariate analysis were female partner sexual quality of life, condom use, circumcision status, relationship duration, employment status of female partner, female partner bacterial vaginosis positive, male partner reporting recent sex, either partner HIV positive, either partner herpes simplex viruse-2 seropositive, man reported sex rougher than he would have liked, education status of either partner, woman pregnant, reporting of recent sex by either partner, and genital washing practices of either partner. BMI = body mass index; Est = coefficient estimate; N = number of observations; n = number of individuals.
Recall period is in the last 6 mo.
DISCUSSION
We measured SQoL among a community-recruited sample of 252 heterosexual couples in Kisumu, Kenya, over a 1-year period. SQoL was higher for men (mean 88) than women (mean 78). A validation study of the SQoL-F among women from the United States found mean score 62.7 for 69 women with spinal cord injury (mean age 44.5 years), mean score 59.0 for 65 women with female sexual dysfunction (mean age 38.5 years), and mean score 90.1 for 60 women with normal sexual function (mean age 37 years).3 Comparable measures among African populations and young adults are limited. Among women recruited from an African women’s clinic in London, United Kingdom, women having undergone genital mutilation had a mean SQoL score of 62, compared to 89 for sexually active control women of mean age 34 years from Nigeria or Ghana who had not undergone genital mutilation.28 Thus, the mean score we observed for women (78) may be somewhat lower than expected. Among 306 Canadian male university students of mean age 20 years, the mean raw SQoL-M score was 49.6,35 and 53.8 among 181 Iranian men with mean age 35 years.26 For comparison, the mean raw baseline SQoL-M score in our study was 59.2, much higher than scores derived from other relatively young and healthy male populations.
In keeping with other studies,36,37 HSV-2 seropositivity was associated with decreased SQoL for females. Given the high seroprevalence of HSV-2, it would have improved understanding to assess frequency or severity of outbreaks and impact on SQoL, to help inform clinician recommendations for suppressive therapy and/or enhanced counseling. Suppressive therapy for genital herpes reduces recurrence and improves quality of life,37 but may not be a feasible long-term option for resource-limited individuals. Among U.S. women with recently diagnosed genital herpes, greater patient-centered communication was associated with greater quality-of-life scores.38 Thus for individuals with more symptomatic genital herpes and limited access to suppressive therapy, the provider may have an impactful role in managing psychological distress.
In the male multivariable model, increasing BMI of the female partner was associated with increasing SQoL. In our sample, increasing BMI may reflect better overall health or socioeconomic status. Though BMI was not associated with HIV status of men or women, women’s BMI was positively correlated with her own and her partner’s employment and with the woman’s educational attainment (results not shown). Similarly, male BMIwas positively correlated with employment status and educational attainment, though more strongly with his partner’s educational attainment and employment status than his own, suggesting a potential differential influence of female partner’s factors within the couple.
On average men reported greater SQoL than women. Of 5 factors associated with SQoL-F, only 1 was male partner generated (sex felt rougher than he would have liked). Among men, 3 of 5 factors associated with SQoL were female partner characteristics (BMI, having sex when not in the mood, recent sex reported). Notably, male SQoL was substantially decreased (27 points) if the man was HIV positive and the woman reported having sex when not in the mood, potentially reflecting the male partner’s awareness of the female partner’s perception. Limited data are available regarding sexual functioning, sexual satisfaction, and relationship perspectives from both members of a couple, especially among younger and relatively healthy samples. In a United States-based study of 950 adult heterosexual couples aged 62–90 years, women reported significantly lower scores than men on relationship quality, including measure of sexual relationship agreement.39 In a Canadian study of first-year university students and their sexual partners (58 couples), for men, partner-perceived sexual compatibility was a predictor for measures of sexual satisfaction, sexual depression, and sexual anxiety; for women, male partner’s perceptions were relevant only to sexual satisfaction.40 Moreover, women’s own perceptions were more strongly related to dimensions of their own sexual relationship than were men’s own perceptions related to their own dimension of sexual relationship. Offman and Matheson40 posit this may stem from women self-monitoring more than men, with self-perceptions consequently having greater impact on their own sexual functioning. They suggest that the greater influence of female partner’s perceptions on men’s assessment of their own sexual functioning dimensions may occur when men recognize the female partner’s perception of sexual incompatibility40; that this does not operate the other way (ie, with male partner perceptions strongly affecting the female partner’s assessment of their own sexual quality or functioning) may arise from violation of male gender norms.40 These results and interpretations may not relate directly to our findings as our study population has different cultural and sexual norms and we used different measures of sexual quality. Yet in the absence of comparable data, these studies with similar gender differentials provide plausible hypotheses for future research related to self-monitoring and gender norms.
Two thirds of women reported ever having sex when their vagina was dry during the past 6 months and this was associated with a 5.27-point reduction in female SQoL. We asked women whether they preferred wet sex, dry sex, or no preference; 68% preferred wet sex, 31% preferred dry sex, and 2 women (0.8%) reported “no preference.” Of women who reported last sex was dry, we asked “What do you do to make sex dry?”: 65% reported wiping with a cloth or tissue, 16% reported “limited foreplay,” “starting as soon as partner is ready,” or “going quickly,” and the remainder reported doing “nothing” to make their vagina dry before sex (results not shown). Among women who reported having dry sex, 31% reported sex felt rougher than they would have liked, compared to 7% among women who reported not having dry sex. Similarly, 36% of women who reported dry sex also reported having sex when not in the mood, compared to 20% among women who did not report dry sex. Given the discrepancy in reported practice of dry sex and preference for dry sex, and associations with having sex when not in the mood and sex feeling rougher than would have liked, practice of dry sex may reflect sexual practices that are not mutually agreed upon. From studies conducted in South Africa, acts that violate sexual autonomy may be considered “normal” under dominant sociocultural norms,41–44 yet this constraint of sexual agency within a consensual relationship may manifest as reduced SQoL. Having sex when “not in the mood” was common among women, and associated with decreased SQoL. Male SQoL was also decreased for men who were HIV positive and when the female reported having sex when not in the mood. As demonstrated by Pettifor et al,43 among 15- to 24-year-old women in South Africa, sexual power inequities were associated with increased likelihood of HIV infection, inconsistent condom use, and forced sex. As suggested by Jewkes and Morrell,44 interventions to support women’s sexual agency through relationship equity may lead to reduced violence against women and prevent HIV.
Few males reported sex feeling rougher than they would have liked, ranging from 3–8% over study visits, but this was associated with substantially lower SQoL for both partners. Male report of sex feeling rough was not associated with female reports of dry sex, sex feeling rough, or having sex when not in the mood. Reporting that sex felt rougher than would have liked was not associated with either partner’s HSV-2 or HIV status, but was more common among uncircumcised men (6.4%) than circumcised men (3%) (P = .048, results not shown). Results from a randomized controlled trial of voluntary medical male circumcision show that circumcised men have 30–40% reduced risk of penile coital injuries45; coital injuries may be associated with feeling of rougher sex. Future studies should examine the meaning of sex feeling “rougher than would have liked” and contributing factors for men and especially women, given the high frequency of occurrence among women.
BV was not independently associated with female SQoL, but interacted with recent sexual intercourse and woman’s age antagonistically. A qualitative study of Australian women with recurrent BV found women felt embarrassed and “dirty,” avoided sexual activity, and BV had negative impact on self-esteem.46 In a qualitative study of African American women with recurrent BV, shame and embarrassment were common themes that negatively impacted sexual relationships and women’s quality of life.47 In our sample, the interaction of BV with recent sexual intercourse may reflect exacerbation of the negative feelings associated with having BV or the development of BV soon after sex. The interaction with age may reflect increased awareness or monitoring among women as they age. While comparable data are not available for Sub-Saharan Africa, our data contribute to the growing literature on the broader impacts of BV on SQoL.
We observed that men’s SQoL decreased with being currently employed and report of 2 or more sex partners in the past 6 months. We did not find comparable results in the published literature that are similar to these findings. As a generalization, greater socioeconomic status is on average associated with greater health and quality of life. In our sample, many men with current employment are self-employed (eg, bicycle or motor bike drivers, day laborer, street vendor), which is often unpredictable and low income, and thus may not be representative of greater socioeconomic status.
Limitations
We selected the SQoL survey for several advantages, although it has not been validated in a Kenyan setting, and despite high internal consistency, our pre-testing indicates that some questions may not be culturally relevant. A review of 57 questionnaires measuring sexual function and SQoL finds lack of validation in low- and middle-income countries and general adult populations is a limitation of most assessments.4 More research is needed to ensure relevant domains are measured, along with validity and reliability. In addition to being a relatively young sample, our participants were largely impoverished, limiting generalizability even within the study area. Sexual behaviors were self-reported, and subject to misreporting. Future studies aimed at improving measure and understanding of SQoL in an understudied population such as ours should include robust measures of physiologic and psychologic correlates.4
CONCLUSION
SQoL was higher among men than women in our community-recruited sample of heterosexual couples in Kisumu, Kenya. Partner factors had greater impact for male SQoL than did male factors for female SQoL. The impact of STIs on SQoL was greater for women than men, as HSV-2 and BV were inversely associated SQoL among women, but not among men. More attention to SQoL as an outcome or intermediate variable may strengthen interventions aimed at preventing HIV and STIs.
Supplementary Material
Figure 3.
Violin plots showing distribution of difference in male and female sexual quality of life (SQoL) over time. Horizontal lines within plots represent median (center lines) and interquartile range (upper line is 75th percentile and lower line is 25th percentile). Width represents number of responses.
Acknowledgments
Funding: This study was supported by grant number R01-AI110369 from the NIH, National Institute of Allergy and Infectious Diseases, Division of Microbiology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
Supriya D. Mehta
(b) Acquisition of Data
Walter Agingu; Fredrick Otieno; Winnie Ochieng; Finch Odhiambo
(c) Analysis and Interpretation of Data
Supriya D. Mehta; Rachel K. Nordgren; Walter Agingu; Fredrick Otieno; Robert C. Bailey
Category 2
(a) Drafting the Article
Supriya D. Mehta; Rachel K. Nordgren
(b) Revising It for Intellectual Content
Walter Agingu; Fredrick Otieno; Winnie Ochieng; Finch Odhiambo; Robert C. Bailey
Category 3
(a) Final Approval of the Completed Article
Supriya D. Mehta; Rachel K. Nordgren; Walter Agingu; Fredrick Otieno; Winnie Ochieng; Finch Odhiambo; Robert C. Bailey
UNCITED FIGURE
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jsxm.2018.08.007.
REFERENCES
- 1.World Health Organization. Sexual and reproductive health: defining sexual health. Available at: http://www.who.int/reproductivehealth/topics/sexual_health/sh_definitions/en/. Accessed March 16, 2018.
- 2.Spitzer WO. State of science 1986: quality of life and functional status as target variables for research. J Chronic Dis 1987;40:465–471. [DOI] [PubMed] [Google Scholar]
- 3.Symonds T, Boolell M, Quirk F. Development of a questionnaire on sexual quality of life in women. J Sex Marital Ther 2005;31:385–397. [DOI] [PubMed] [Google Scholar]
- 4.Arrington R, Cofrancesco J, Wu AW. Questionnaires to measure sexual quality of life. Qual Life Res 2004;13:1643–1658. [DOI] [PubMed] [Google Scholar]
- 5.Koole O, Noestlinger C, Colebunders R. Quality of life in HIV clinical trials: why sexual health must not be ignored. PLoS Clin Trial 2007;2:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinke EE, Mosack V, Hill TJ. Depression, quality of life,physical activity, and the impact of drugs on sexual activity in a population-based sample, ages 20e59 years. Issues Ment Health Nurs 2018;39:527–553. [DOI] [PubMed] [Google Scholar]
- 7.Royer HR, Falk EC, Heidrich SM. Genital herpes beliefs: implications for sexual health. J Pediatr Adolesc Gynecol 2013; 26:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Herpes simplex virus [fact sheet]. Available at: http://www.who.int/mediacentre/factsheets/fs400/en/. Accessed March 16, 2018.
- 9.Wilson TE, Jean-Louis G, Schwartz R, et al. HIV infection and women’s sexual functioning. J Acquir Immune Defic Syndr 2011;54:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenya Ministry of Health. Kenya AIDS response progress report 2016. National AIDS Control Council; Available at: http://nacc.or.ke/wp-content/uploads/2016/11/Kenya-AIDS-Progress-Report_web.pdf. [Google Scholar]
- 11.Kenya Ministry of Health. Kenya HIV county profiles 2016. National AIDS and STI control program; Available at: http://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf. [Google Scholar]
- 12.Amornkul PN, Vandenhoudt H, Nasokho P, et al. HIV prevalence and associated risk factors among individuals aged 13e34 years in rural western Kenya. PLoS One 2009; 4:e6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otieno FO, Ndivo R, Oswago S, et al. Correlates of prevalent sexually transmitted infections among participants screened for an HIV incidence cohort study in Kisumu, Kenya. Int J STD AIDS 2015;26:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masha SC, Wahome E, Vaneechoutte M, et al. High prevalence of curable sexually transmitted infections among pregnant women in a rural county hospital in Kilifi, Kenya. PLoS One 2017;12:e0175166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pintye J, Drake AL, Kinuthia J, et al. A risk assessment tool for identifying pregnant and postpartum women who may benefit from preexposure prophylaxis. Clin Infect Dis 2017;64:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukusi EA, Cohen CR, Meier AS, et al. Bacterial vaginosis: risk factors among Kenyan women and their male partners. Sex Transm Dis 2006;33:361–367. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RS, Richardson BA, Graham SM, et al. A prospective study of risk factors for bacterial vaginosis in HIV-1-seronegative African women. Sex Transm Dis 2008; 35:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jespers V, Crucitti T, Menten J, et al. ; Vaginal Biomarkers Study Group. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in Sub-Saharan Africa: a cross-sectional study. PLoS One 2014;9:e109670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenya Interagency Rapid Assessment. Available at: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/Kisumu%20Secondary%20Data%20Review_20141305.pdf. Accessed July 19, 2018.
- 20.National Bureau of Statistics-Kenya and ICF International. Kenya Demographic and Health Survey (KDHS) 2014. Rockville, MD, USA: KNBS and ICF International; Available at: http://dhsprogram.com/pubs/pdf/FR308/FR308.pdf. Accessed July 19, 2017. [Google Scholar]
- 21.Kwena Z, Mwanzo I, Shisanya C, et al. Predictors of extramarital partnerships among women married to fishermen along Lake Victoria in Kisumu County, Kenya. PLoS One 2014;9:e95298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National AIDS and STI Control Program, Ministry of Health,Kenya. Guidelines for HIV testing services in Kenya. Nairobi: NASCOP. Available at: https://aidsfree.usaid.gov/sites/default/files/hts_policy_kenya_2015.pdf. [Google Scholar]
- 23.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- 25.Abraham L, Symonds T, Morris MF. Psychometric validation of a sexual quality of life questionnaire for use in men with premature ejaculation or erectile dysfunction. J Sex Med 2008;5:595–601. [DOI] [PubMed] [Google Scholar]
- 26.Maasoumi R, Lamyian M, Montazeri A, et al. The sexual quality of life-female (SQOL-F) questionnaire: translation and psychometric properties of the Iranian version. Reprod Health 2013;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Symonds T, Abraham L, Bushmakin AG, et al. Sexual function questionnaire: further refinement and validation. J Sex Med 2012;9:2609–2616. [DOI] [PubMed] [Google Scholar]
- 28.Andersson SH, Rymer J, Joyce DW, et al. Sexual quality of life in women who have undergone female genital mutilation: a case-control study. BJOG 2012;119:1606–1611. [DOI] [PubMed] [Google Scholar]
- 29.Owiredu WKBA Owusu AO, Amidu N, et al. Sexual dysfunction and sexual quality of life among the physically challenged in the Kumasi metropolis, Ghana. Health Qual Life Outcomes 2015;13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ 2011;2:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 32.Wilson Van Voorhis CR, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol 2007;3:43–50. [Google Scholar]
- 33.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available at: http://www.R-project.org. [Google Scholar]
- 34.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method Ecol Evol 2013;4:133–142. [Google Scholar]
- 35.Tutino JS, Shaughnessy K, Ouimet AJ. Looking at the bigger picture: young men’s sexual health from a psychological perspective. J Health Psychol 2018;23:345–358. [DOI] [PubMed] [Google Scholar]
- 36.Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs 2009;38:320–326. [DOI] [PubMed] [Google Scholar]
- 37.Brentjens MH, Yeung-Yue KA, Lee PC, Tyring SK. Recurrent genital herpes treatments and their impact on quality of life. Pharmacoeconomics 2003;21:853–863. [DOI] [PubMed] [Google Scholar]
- 38.Ports KA, Reddy DM, Barnack-Tavlaris JL. Sex differences in health care provider communication during genital herpes care and patients’ health outcomes. J Health Commun 2013; 18:1436–1448. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Waite LJ. Relationship quality and shared activity in marital and cohabiting dyads in the National Social Life, Health, and Aging Project, Wave 2. J Gerontol Series B 2014; 69(Suppl):S64–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Offman A, Matheson K. Sexual compatibility and sexual functioning in intimate relationships. Can J Hum Sex 2005; 14:31–39. [Google Scholar]
- 41.Shai NJ, Jewkes R, Nduna M, et al. Masculinities and condom use patterns among young rural South Africa men: a crosssectional baseline survey. BMC Public Health 2012;12:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med 2003;56:125–134. [DOI] [PubMed] [Google Scholar]
- 43.Pettifor AE, Measham DM, Rees HV, et al. Sexual power and HIV risk, South Africa. Emerg Infect Dis 2004;10:1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jewkes R, Morrell R. Sexuality and the limits of agency among South African teenage women: theorizing femininities and their connections to HIV risk practices. Soc Sci Med 2012; 74:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta SD, Krieger JN, Agot K, et al. Circumcision and reduced risk of self-reported penile coital injuries: results from a randomized controlled trial in Kisumu, Kenya. J Urol 2010; 184:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One 2013;8:e74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne SC, Cromer PR, Stanek MK, et al. Evidence of African American women’s frustrations with chronic recurrent bacterial vaginosis. J Am Acad Nurse Pract 2010;22:101–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.