Abstract
How is the picture of the visual scene that the eye encodes represented by neural circuits in the brain? In this issue of Cell, Morgan et al. address this question by forming an ultrastructural “con-nectome” of the mouse’s visual thalamus that depicts individual retinal afferents and every contact these form with target relay cells.
How does the eye connect with the brain? In this issue of Cell, Morgan et al. (2016) address this question in a study that is literally cutting-edge. The team slices a substantial chunk of the murine lateral geniculate nucleus (LGN) of the thalamus into a tissue tape from which they form a connectome—an ultrastructural 3D reconstruction of retinal afferents and the thalamic relay cells they target. Specifically, the authors identify all the retinal axons presynaptic to two “seed” relay cells, as well as other relay cells innervated by the same set of axons, and then characterize the morphology of each connection with unprecedented detail. Moreover, the dataset is publically accessible and will surely offer reward for years to come.
The connectome generated suggests a level of retinogeniculate convergence (many afferents innervate a single relay cell) far greater than that inferred from physiological studies. There is commensurate divergence (one afferent contacts multiple relay cells) too. Surprisingly, as the authors note, stereotyped patterns of connectivity fail to emerge, even as statistical tests show that the contacts between retinal axons and thalamic cells are not randomly distributed. This novel dataset suggests that experience may ultimately select which synapses are maintained within a seemingly haphazard circuit and also raises questions about how a complex interconnected network at an early stage of sensory processing allows downstream regions to resolve specific aspects of the stimulus.
To understand the results in a broader context, it is important to note that there are substantial differences in the visual system across taxonomic orders (Figure 1). In highly visual mammals, there are pronounced parallel, stream-specific channels that begin in the retina and continue, with varying degrees of crosstalk, to cortex. For example, in macaque, the axons of magnocellular and parvocellular retinal ganglion cells innervate separate layers of the LGN, which, in turn, project to different strata in the primary visual cortex (Nassi and Callaway, 2009). A variation on this theme is demonstrated in tree shrews, for which ON and OFF ganglion cells target discrete thalamic and, subsequently, cortical layers (Fitzpatrick, 1996). Carnivores, such as cat and ferret, offer additional, though perhaps less crystalline, examples of stream specificity (Sur et al., 1987). The visual system of the mouse is strikingly different. Certain types of feature-selective ganglion cells that are abundant in the murine retina are rare, if present, in primates and carnivores. Also, in mouse, the LGN is parceled into shell and core zones (Bickford et al., 2015) rather than discrete layers. Further, response properties in the rodent cortex have a dispersed “salt and pepper,” versus columnar, organization. Thus, the extent to which different types of retinal inputs mix it up in the LGN is likely to vary across species.
Figure 1. Organization of Visual Streams in Different Species.
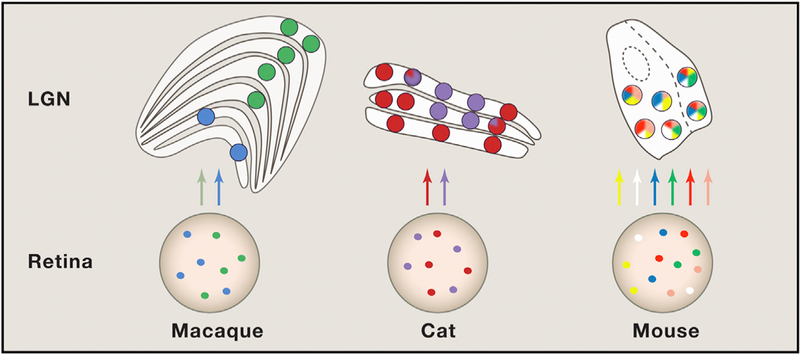
Colored circles represent different retinal ganglion cell (RGC) types and their target relay neurons in the lateral geniculate nucleus (LGN). Mixing of stream s rarely occurs in m acaque and, to a limited extent, in cats. However, the results of the current study suggest that many more RGCs converge on individual relay cells in mouse LGN. Whether these RGCs are different functional subtypes remains unknown. Structures are not drawn to scale.
Previous physiological estimates of retinogeniculate convergence are much lower than the values reported for the connectome. Thus, one wonders about the relative influence of each retinal ganglion cell on its target. Do one or a few afferents dominate, while others play an auxiliary role? To address this topic, it is necessary to estimate the weight of each input. At present, the authors provide exquisite detail regarding the variety of retinogeniculate bouton sizes and their synaptic arrangements on the dendritic compartments of individual cells and cell groups. The dataset might be further mined to examine the potential strength of identified inputs by assessing the ultra-structural features of active zones, such as the volume of the postsynaptic densities and the numbers of their associated vesicle pools. Analyses of this kind offer a unique opportunity to correlate structure and function at an unprecedented fine scale. Justifiably, the current study assumes that each retinal axon segment that exits the sample volume derives from a unique ganglion cell. However, retinal axons sometimes branch deep in the optic tract (Sur et al., 1987), and collaterals from the same trunk can be separated by very long distances (Dhande et al., 2011). Therefore, continued study of the branching patterns of retinogeniculate axons should help refine the organization principles revealed by the connectome.
Morgan, Lichtman, and colleagues (Morgan et al., 2016) analyze tissue from a 32-day-old animal, a stage at which substantial experience-dependent synapse remodeling of retinogeniculate connectivity occurs (Hooks and Chen, 2006). Could some boutons in the connectome be remnants of the maturation process or, perhaps, latent synapses silently waiting to be called to action? This snapshot of the retinogeniculate circuit raises the interesting possibility that changes in the relative weight of these contacts, for example, by insertion and removal of postsynaptic receptors, could dynamically alter network connectivity. Does high structural convergence last throughout life or decrease with age? A companion study at a time point when the murine LGN has matured fully (after postnatal day 60) would provide further insight.
Finally, this study raises questions regarding the potential advantages of high convergence and divergence for vision. Work in cats suggests that convergence might improve signal-to-noise ratios in thalamic circuits, potentially enhancing perceptual acuity (Martinez et al., 2014). Whether the patterns of convergence revealed by the connectome can impart this advantage is a possibility worth considering. A potential role for divergence, on the other hand, is to synchronize network activity and thereby influence the propagation of visual signals to cortex. Also, different patterns of convergence and divergence can lead to different functional out-comes—variously permitting the faithful relay of information from stage to stage or giving rise to novel, emergent properties. For example, some cells in the murine retina and thalamus are direction or orientation selective (Piscopo et al., 2013). Does the LGN inherit these response properties from the eye, or are they generated de novo via mixture of afferent inputs? In macaque, the borders of some thalamic receptive fields are so precise that they fall between adjacent photoreceptors (Sincich et al., 2009). How does divergence and convergence influence receptive field structure in the murine LGN? This connectome may provide insights into coding strategies that mice, and potentially other species, use to see.
All told, Morgan et al. (2016) elegantly accomplish an astonishingly difficult task and provide the community with the first connectome of the LGN. Their exciting results raise questions that touch on the relationship between ultrastructure and function, developmental mechanisms that guide synapse specificity, and how studies of visual processing in mouse might inform our understanding of other species, perhaps including humans.
REFERENCES
- Bickford ME, Zhou N, Krahe TE, Govindaiah G, and Guido W (2015). J. Neurosci 35, 10523–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, and Crair MC (2011). J. Neurosci 31, 3384–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D (1996). Cereb. Cortex 6, 329–341. [DOI] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2006). Neuron 52, 281–291. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Molano-Mazón M, Wang X, Sommer FT, and Hirsch JA (2014). Neuron 81, 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Berger DR, Wetzel AW., and Lichtman JW (2016). Cell 165, this issue, 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, and Callaway EM (2009). Nat. Rev. Neurosci 10, 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscopo DM, El-Danaf RN, Huberman AD, and Niell CM (2013). J. Neurosci 33,4642–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, and Roorda A (2009). Nat. Neurosci 12, 967–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Esguerra M, Garraghty PE, Kritzer MF, and Sherman SM (1987). J. Neurophysiol 58, 1–32 [DOI] [PubMed] [Google Scholar]


