Abstract
Problem:
GDM has been associated with disturbances in iron homeostasis and exaggerated immune activation. We sought to investigate the extent to which placental iron storage and macrophage accumulations were altered in GDM.
Method of Study:
We conducted a retrospective, case-control study of archived placental tissues obtained from 22 pregnancies complicated by GDM and 22 unaffected controls. Controls were matched to cases based on maternal age, gestational age at birth, and method of delivery. Placental tissues were assessed for altered histology and CD68 and CD163 staining. Tissue iron was assessed using Prussian blue staining.
Results:
Maternal hematocrit levels were higher in GDM participants compared to controls (P = 0.02). The presence of meconium-laden macrophages was significantly greater within the amnion of GDM cases (adjusted odds ratio (OR) 12.51). Although the total abundance of CD68-expressing macrophages was not significantly different between groups, we detected a significantly greater abundance of CD163 expression within the chorion and decidua of cases. The total area staining positive for iron was 24% (95% confidence intervals of 2%−46%) greater in GDM placentae versus controls.
Conclusion:
GDM is associated with altered placental histology and increases in meconium-laden macrophages. Greater iron stores within the placentae of women with GDM is consistent with reports that iron excess is associated with an increased risk for GDM. The higher level of expression of CD163 on macrophage-like cells of the chorion and decidua in GDM suggests an increase in M2-like macrophages. Overall, our results add to growing evidence that GDM has direct effects on placental structure.
Keywords: CD163, gestational diabetes, histology, iron, macrophages, placenta
1 |. INTRODUCTION
Gestational diabetes mellitus (GDM) is the most common metabolic disorder during pregnancy with a mean prevalence in North America and the Caribbean of 7%.1 It is defined as glucose intolerance newly diagnosed in the second or third trimester of pregnancy.2 GDM significantly increases the risk of pregnancy complications, including fetal macrosomia, neonatal hypoglycemia and hypocalcemia, preeclampsia, premature labor, and Cesarean delivery.2 It also increases the risk of postpartum complications in mother and child including late-onset diabetes and cardiovascular disease.2 The risk of GDM is increased significantly by concomitant obesity, which is itself an increasing problem globally, and is directly associated with pre-pregnancy BMI.3 In the United States alone, approximately 55% of women of childbearing age are either overweight or obese.4 Compared to women with normal pre-pregnancy BMI, the odds of developing GDM increase 1.97 for overweight women, 3.01 for those classified as obese, and 5.55 for morbidly obese women.5 A major barrier to reducing the complications of GDM is a lack of clarity regarding the underlying pathophysiological mechanisms contributing to the impact of the disease, particularly on offspring.
The placenta is a transient, but complex organ, acting as the link between the mother and the developing fetus. It is increasingly appreciated to be a target organ of GDM. It plays important roles throughout pregnancy, mediating the selective exchange of nutrients, waste, gases, and other modulatory factors across the maternal/fetal interface.6 The placenta also orchestrates and maintains maternal/fetal immune tolerance and acts in an endocrine fashion by producing key modulatory hormones while separating the fetus from a possibly adverse or harmful maternal environment.6 Maternal hyperglycemia, as observed in GDM, alters placental morphometric characteristics, changes the structural integrity of syncytiotrophoblasts, and provokes major changes in placental gene expression related to chronic stress and inflammation.7–9
Elevated biomarkers of systemic maternal inflammation have been described in women with GDM, including an increase in circulating levels of interleukin (IL)-6, IL-8, IL-18, and C-reactive protein (CRP) in conjunction with decreased levels of IL-10.10 T-cell directed immunoregulation is skewed in patients with GDM, as evidenced by reduced development and suppressive capacity of regulatory CD4+ T-cell (Treg) populations.11,12 Multiple studies have shown that in conjunction with an increase in maternal circulating inflammatory mediators, GDM is also associated with pro-inflammatory changes within the placenta.8,10,13,14 In particular, macrophages, the primary phagocyte in the healthy placenta, appear to be impacted by this endocrine disorder.15–17
Macrophages function as a first line of antimicrobial defense, modulate tissue homeostasis, and are abundant in the placenta throughout pregnancy.15 Placental macrophages consist of both fetally derived macrophages, known as Hofbauer cells (HBC), which are found in the villous core, and maternally derived decidual macrophages, which reside in the maternal decidua.16 HBCs have been characterized as having an anti-inflammatory (M2) profile, characterized by high cell surface expression of the receptors CD163, CD209, and CD206, and increased secretion of IL-10 and transforming growth factor (TGF)-β.18–20 Furthermore, the DNA methylation pattern of HBCs is consistent with an anti-inflammatory phenotype.21 The documented anti-inflammatory phenotype and importance of placental macrophages in maintaining immune tolerance suggest that these cells play a regulatory role within the placenta. Diabetes during pregnancy has been shown to induce a pro-inflammatory phenotype in placental macrophages, as characterized by an increase in the expression of IL-1β and CCR7 and a decrease in the anti-inflammatory markers CD163, CD209, and IL-10.16 A recent study, however, concluded that HBCs maintain their anti-inflammatory phenotype even in the presence of GDM.22 Thus, there is some controversy regarding the impact of GDM on placental macrophages and the inflammatory state of the placenta.
An identifying characteristic of placental macrophages is a high level of the anti-inflammatory marker CD163, which is involved in the clearance of hemoglobin-haptoglobin complexes and iron uptake.19,23 The role of CD163 in iron recycling and handling is especially of interest in GDM as iron-containing proteins, iron availability, and levels of CD163 differ between women with GDM and non-diabetic pregnancies.24–29 Circulating levels of hemoglobin and iron are higher in GDM patients compared to controls.24 Elevated iron stores, as estimated by increased maternal serum hepcidin and ferritin levels, may be involved with the development of GDM.25,27,29 Two independent studies have shown that antenatal iron supplementation, leading to increased levels of maternal iron, increased the risk of GDM and was associated with glucose impairment and hypertension throughout gestation suggesting a link between imbalanced iron homeostasis and GDM.26,28
Given the relationships among GDM, placental macrophage activation, and iron homeostasis, we sought to conduct a retrospective, case-control study of archived human placental tissue from women with or without GDM to better define placental pathology, placental iron stores, and both macrophage density and CD163 expression in situ.
2 |. MATERIALS AND METHODS
2.1 |. Study design
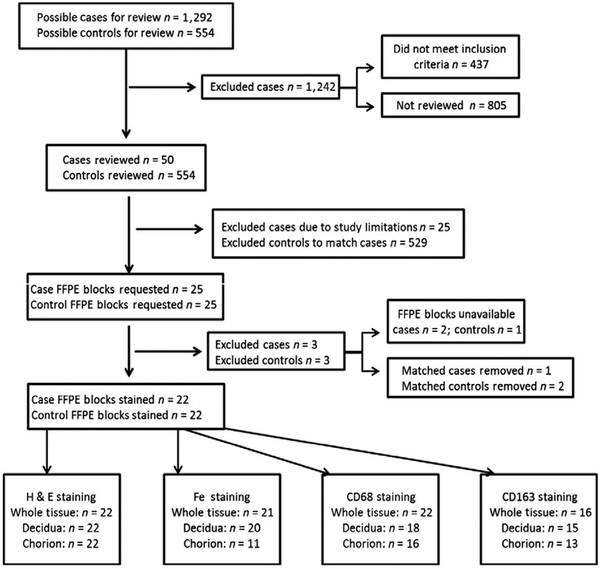
This study was reviewed and approved by the Vanderbilt University Institutional Review Board (protocol #150498). This study was a retrospective conceptualized and completed in collaboration with the Vanderbilt Institute for Clinical and Translational Research (VICTR) at Vanderbilt University Medical Center. Placental tissue specimens were identified through the Synthetic Derivative (SD), a queryable, real-time, deidentified electronic medical record (EMR).30 The SD allowed us to identify patient medical records that were linked to archived formalin-fixed paraffin embedded (FFPE) placental tissue blocks obtained from the same patients. Placentae had been sent for histopathological review at the discretion of the attending obstetrician. The SD was queried to identify possible cases of women with GDM as well as healthy controls who also had placental FFPE blocks available. The databases were searched for key words including GDM, abnormal glucose, and International Statistical Classification of Diseases and Related Health Problems (ICD) 9 codes suggesting GDM including, Personal History of Gestational Diabetes (V12.21), Diabetes Mellitus Complicating Pregnancy Childbirth or the Puerperium (648.0), Gestational Diabetes, Antepartum (648.83). To identify unaffected controls, we required the absence of these key words and codes.
2.2 |. Participants
Our initial query identified 1292 possible GDM cases and 554 possible control cases (Figure 1). The number of participants in this study was limited to 25 patients with GDM and 25 matched controls. The study design and sample distribution are outlined in Figure 1. From possible cases, we selected 25 cases, in which were able to confirm a diagnosis of GDM according to prespecified inclusion and exclusion criteria. Inclusion criteria included an abnormal oral glucose tolerance test (OGTT, defined below) and having access to a full FFPE placental cross section, record of gestational age at birth, maternal age, and delivery method. The presence of GDM was established if the woman had at least 2 values of the 100 g 3-hour OGTT above normal. Normal 100 g OGTT levels were defined as 95 mg/dL at fasting, 180 mg/dL at 1 hour, 155 mg/dL at 2 hours, and 140 mg/dL at 3 hours. Women were excluded from the study if they were diagnosed with preeclampsia, had previously diagnosed diabetes, or had tested positive for illicit drug use. We also excluded women who had active signs of infection during pregnancy or at the time of delivery based on placental pathology notes and noted fetal abnormalities during pregnancy or at delivery. Controls were then matched to cases on the basis of gestational age at delivery, maternal age, and method of delivery.
FIGURE 1.
Study Design and Sample Distribution. Workflow diagram depicting study design and distribution of investigated population including the numbers of patients included and excluded from the study
2.3 |. Data collection
Patient demographic information, vital statistics, and laboratory results including age, race/ethnicity, indication for delivery, body mass index (BMI), placental weight and volume, fasting glucose and 3-hour OGTT results, and maternal hematocrit were obtained through the SD. Not all patient records provided data on BMI or maternal hematocrit levels. We were able to collect data for calculating BMI for 27.2% of participants and maternal hematocrit for 86.3% of participants at time of delivery. Due to the nature of our study design, we could not recover all missing data points for these variables and have listed the missing number of data points where appropriate (Table 1).
TABLE 1.
Characteristics of study participants by case status
| Case n = 22 | Control n = 22 | ||||
|---|---|---|---|---|---|
| Categorical characteristics | n | % | n | % | P-valuea |
| Race | |||||
| White, non-Hispanic | 12 | 55 | 12 | 55 | 0.45 |
| Black, non-Hispanic | 4 | 18 | 3 | 14 | |
| Hispanic | 2 | 9 | 0 | 0 | |
| Asian | 2 | 9 | 5 | 23 | |
| Other | 1 | 5 | 0 | 0 | |
| Unknown/Missing | 1 | 5 | 2 | 9 | |
| Method of delivery | |||||
| Cesarean section | 14 | 64 | 14 | 64 | 1.00 |
| Vaginal | 8 | 36 | 8 | 36 | |
| Continuous variables | n | Mean (SD) | n | Mean (SD) | P-value |
| Maternal age at delivery (y) | 22 | 29.3 (5.8) | 22 | 27.0 (5.5) | 0.19 |
| Gestational age at delivery (weeks) | 22 | 37.2 (1.8) | 22 | 37.5 (2.1) | 0.61 |
| Pre-pregnancy BMI (kg/m2) | 7 | 25.5 (4.2) | 2 | 23.5 (4.0) | 0.58 |
| 1st Trimester BMI | 8 | 26.8 (4.1) | 4 | 27.6 (3.4) | 0.78 |
| Placental weight (g) | 22 | 509.5 (184.0) | 22 | 465.7 (102.5) | 0.34 |
| Placental volume (cm3) | 22 | 761.4 (326.2) | 22 | 685.1 (243.7) | 0.38 |
| Glucose tolerance test, 50 g | 21 | 157.4 (21.3) | 11 | 110.5 (23.5) | <0.001 |
| Fasting glucose | 22 | 90.0 (16.0) | 0 | – | |
| Oral glucose tolerance, 1 h | 22 | 190.7 (27.9) | 0 | – | |
| Oral glucose tolerance, 2 h | 22 | 186.8 (28.6) | 0 | – | |
| Oral glucose tolerance, 3 h | 22 | 140.6 (40.5) | 0 | – | |
| Maternal Hematocrit | 20 | 35.3 (2.5) | 18 | 33.0 (3.1) | 0.02 |
| Maternal hemoglobin | 10 | 12.1 (0.7) | 10 | 11.3 (1.2) | 0.12 |
SD: standard deviation. Significant values are bolded.
P-values derived from Pearson’s chi-squared for categorical variables and from two-sample t test for continuous variables.
2.4 |. Immunohistochemistry and pathological analysis
A total of 50 FFPE blocks were requested from Pathlink, the Vanderbilt Tissue Biobank which accrues patient samples and is linked to a deidentified version of data extracted from an EMR system, in which all personal identifiers have been removed for analysis.31 FFPE blocks containing cross-sectional human placenta were sectioned and stained for analysis by the Translational Pathology Shared Resource (TPSR) at Vanderbilt University Medical Center. In some instances, full-thickness cross sections containing the decidua, placental disk, and chorion were unavailable. For these patients, pairs of blocks jointly forming a full-thickness cross section were obtained for analysis. Serial sections were prepared from the FFPE blocks and were stained with hematoxylin and eosin (H&E), Prussian blue, CD163, and CD68 at TPSR using standard protocols. Some requested samples were unavailable or in poor quality and, thus, were excluded from the study. While other samples, once cut from the FFPE blocks and stained, were missing sections of the decidua, chorion, or both. When this occurred, we excluded the corresponding tissue section in the matched sample. In total, 22 FFPE blocks from cases and 22 FFPE blocks from controls were available for analysis (Figure 1).
All H&E-stained slides were reviewed by a pathologist (JAG) blinded to GDM status and prior diagnostic findings using a custom data entry form. Amnion, villous disk, and decidual surface were systematically evaluated for multiple abnormalities including meconium-laden macrophages, acute inflammation, infarcts, hydrops, villous maturity, and decidual and fetal vessel maturation. This allowed for uniformity and comparability in description. Histological findings were determined using standard clinical criteria. Representative images were captured under bright-field illumination with automated exposure.
2.5 |. Slide imaging and digital quantification
Slides were digitally scanned at 20 × magnification using a Leica SCN400 Slide Scanner by the Digital Histology Shared Resource (DHSR) at Vanderbilt University. Digital analysis and quantification was performed using Leica Biosystems Digital analysis software. For quantification of CD68, CD163, and iron within placental tissue, digital annotations were made segregating the chorion and decidua from the remainder of the placental disk (villous core). Cellular staining and iron staining were quantified for the entire placental tissue and individually for the chorionic and decidual annotated sections. The whole specimen encompasses all tissue on the slide including chorion and decidua if present as well as the villous core. CD68 and CD163 were quantified as the percentage of positively staining cells per total cells. Iron staining was quantified as the percentage of positively staining area of total tissue within the given region. When either the decidua or chorion was absent from a patient sample, the corresponding region was excluded from analysis for its matched control or case.
2.6 |. Statistical analysis
Pearson’s chi-squared and two-sample t tests were used to assess variation in categorical and continuous study participant characteristics, respectively. We used beta regression to predict the marginal effects of gestational diabetes for histological features that were measured as the proportion of cells with a given characteristic (proportion of cells to stain for CD68, CD163, and iron moderately, strongly, and overall). Beta regression accounts for a continuous outcome variable being bounded [0, 1], unlike linear regression. We used logistic regression to calculate the odds ratio (OR) of placental tissue from cases having abnormalities that were either present or absent as compared to controls. For histological characteristics with more than two levels, we used polytomous regression to calculate ORs of the placental tissue from cases having the feature as compared to controls. We provide crude estimates and estimates adjusted for maternal hematocrit. In our supplemental analysis, we used linear regression to estimate the expected change in absorbance and intensity for CD68 and CD163 staining in cases compared to controls.
3 |. RESULTS
3.1 |. Study participant characteristics
Study participant characteristics and data gathered from the SD are summarized in Table 1. The average maternal age for controls was 27.0 while cases had an average maternal age of 29.3 (SD for controls and cases was 5.5 and 5.8, respectively). Average gestational age at time of delivery for controls was 37.5 weeks and 37.2 for cases (SD for controls and cases was 2.1 and 1.8, respectively). The retrospective nature of this study limited our ability to collect BMI information on all subjects. The pre-pregnancy BMI and first trimester BMI information collected did not reveal significant difference between controls and cases. Placental weight and placental volume did not differ between cases and matched controls. The 50 g OGTT was elevated in cases compared to controls, as expected. All control subjects had a normal 50 g OGTT; therefore, 3-hour OGTT was neither clinically indicated nor performed. Maternal hematocrit levels at delivery were significantly elevated in GDM women compared to controls at 35.3% vs 33% for controls (P = 0.02). In light of hematocrit differences between cases and controls, and given the relationship between iron and hemoglobin, we adjusted analyses for differences in maternal hematocrit levels.
3.2 |. GDM placentae exhibit an increase in abnormal histology
Meconium-laden macrophage levels within the amnion of GDM placentae were significantly higher at 36.4% compared to 4.6% of controls. Correspondingly, a greater percentage of GDM placentae showed amnion epithelial reactive changes suggestive of meconium exposure - 68.2% of GDM placentae versus 36.4% of controls (Table 2, Figure 2). These differences remained statistically significant after adjustment for maternal hematocrit.
TABLE 2.
Differences in histological characteristics by gestational diabetes status
| Variable | N | % of cases | % of controls | OR | 95% CI | Adjusted ORa | 95% CI |
|---|---|---|---|---|---|---|---|
| Amnion | |||||||
| Abnormal | 44 | 68.2 | 36.4 | 3.75 | 1.08, 13.07 | 4.05 | 1.04, 15.83 |
| Reactive changes | 44 | 50.0 | 36.4 | 1.75 | 0.52, 5.84 | 1.56 | 0.43, 5.69 |
| Meconium-laden macrophages |
44 | 36.4 | 4.6 | 12.00 | 1.35, 106.80 | 12.51 | 1.26, 124.24 |
| Squamitization | 44 | 13.6 | 4.6 | 3.32 | 0.32, 34.65 | 7.99 | 0.49, 130.65 |
| Fetal vessels | |||||||
| Abnormal | 44 | 22.7 | 13.6 | 1.86 | 0.39, 8.99 | 1.50 | 0.28, 8.08 |
| Inflammation | 44 | 4.6 | 0 | – | – | – | – |
| Cushion lesions | 44 | 4.6 | 4.6 | 1.00 | 0.06, 17.07 | – | – |
| Erythroblastosis | 44 | 9.1 | 4.6 | 2.10 | 0.18, 25.01 | 1.91 | 0.13, 27.07 |
| Villi | |||||||
| Chorangiosis | 44 | 4.6 | 4.6 | 1.00 | 0.06, 17.07 | – | – |
| Hydrops | 44 | 4.6 | 9.1 | 0.48 | 0.04, 5.67 | 0.48 | 0.03, 6.81 |
| Chronic villitis | 44 | 4.6 | 4.6 | 1.00 | 0.06, 17.07 | – | – |
| Intervillositis | 44 | 0.0 | 4.6 | – | – | – | – |
| Abnormal size | 44 | 77.3 | 45.5 | 4.08 | 1.11, 15.02 | 3.17 | 0.71, 14.10 |
| Abnormal vessel | 43 | 63.6 | 52.4 | 1.59 | 0.47, 5.39 | 1.03 | 0.24, 4.42 |
| Hemosiderin-laden | |||||||
| Macrophages | 44 | 18.2 | 22.7 | 0.76 | 0.17, 3.29 | 0.52 | 0.10, 2.70 |
| Any Infarct | 44 | 40.9 | 40.9 | 1.00 | 0.30, 3.33 | 1.25 | 0.28, 5.48 |
| Other | |||||||
| Calcifications | 44 | 63.6 | 27.3 | 4.67 | 1.30, 16.76 | 3.70 | 0.86, 16.89 |
| Fibrin deposits | 43 | 19.1 | 18.2 | 1.06 | 0.23, 4.92 | 0.88 | 0.14, 5.36 |
| Maternal infarct | 41 | 4.5 | 15.0 | 0.28 | 0.03, 2.98 | 0.13 | 0.01, 3.13 |
| Villous Sizeb | |||||||
| Normal | 44 | 22.7 | 54.6 | 1.00 | (referent) | 1.00 | (referent) |
| Immature | 22.7 | 18.2 | 3.00 | 0.56, 16.07 | 3.24 | 0.48, 21.77 | |
| Accelerated maturation |
40.9 | 18.2 | 5.40 | 1.12, 26.04 | 3.80 | 0.61, 23.54 | |
| Hypermature | 13.6 | 9.1 | 3.60 | 0.45, 28.56 | 1.93 | 0.17, 21.66 | |
| Villous vesselsb | |||||||
| Intermediate | 43 | 36.4 | 47.6 | 1.00 | (referent) | 1.00 | (referent) |
| Immature | 27.3 | 9.5 | 3.54 | 0.59, 23.87 | 1.11 | 0.29, 4.21 | |
| External | 36.4 | 42.9 | 5.74 | 0.45, 72.30 | 0.56 | 0.11, 2.80 | |
| Villous infarctb | |||||||
| None | 44 | 59.1 | 59.1 | 1.00 | (referent) | 1.00 | (referent) |
| Small (<4 × field) | 13.6 | 31.8 | 0.43 | 0.09, 2.03 | 3.00 | 0.51, 17.71 | |
| Large (>4 × field) | 26.3 | 9.1 | 0.44 | 0.06, 3.11 | 3.36 | 0.46, 24.51 | |
OR: odds ratio; CI: confidence interval. Significant values are bolded.
Adjusted for maternal hematocrit.
Odds ratios for histological features for more than two levels were calculated using polytomous regression. All other odds ratios are from logistic regression models.
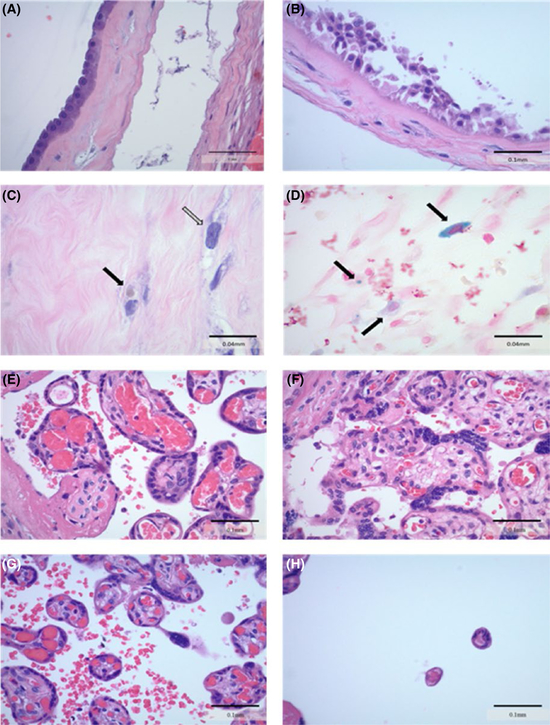
FIGURE 2.
Histological Characteristics of Placenta from Women with GDM and Healthy Controls. Histological characteristics were identified on H & E-stained slides by a clinical pathologist blinded to gestational diabetes status. A, Image of normal amnion tissue from a control placenta (40 × ) B, Amnion displaying example of reactive changes suggestive of meconium in a placenta from a mother with GDM (40 × ) C, Arrows indicating examples of meconium-laden macrophages within the amnion of a GDM placenta (100 × ) D, Prussian blue stained slide representing iron in blue with arrows indicating hemosideran-laden macrophages in a GDM placenta (100 × ) E-H, Representative H & E images showing differing levels of villous maturation in placental villi (40 × ). Normal villi from control, large villi from GDM, accelerated maturation villi from control, and hypermature villi from control, respectively
GDM placentae exhibited abnormal villous size compared to controls with the GDM placentae showing signs of accelerated maturation for gestational age. When adjusted for maternal hematocrit levels, however, the difference was no longer significant. The presence of calcifications was significantly increased in GDM placentae in the crude, but not adjusted analysis (Table 2).
3.3 |. CD163 and iron storage are increased in GDM placentae
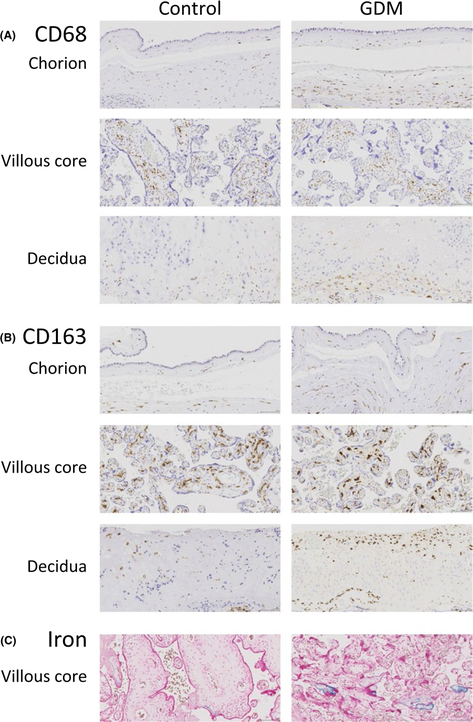
To examine the impact of GDM on placental macrophages and iron stores, we stained full placental cross sections for CD68, CD163, and iron. The chorion of GDM placentae contained a significantly higher number of moderately stained CD163-positive cells compared to controls (Figure 3). The total number of positive cells as well as the total number of moderately staining CD163-positive cells was greater in the decidua of GDM placentae compared to controls. The higher expression of CD163 in the placentae of GDM mothers remained significant after adjustment for maternal hematocrit (Table 3).
FIGURE 3.
Differences in CD68, CD163, and Iron Staining by Gestational Diabetes Status. FFPE blocks of placental specimens from women with GDM and control were stained for (A) CD68 (B) CD163 (C) Prussian blue staining for iron. Slides were then digitally scanned and imaged for digital quantification purposes. Representative images were captured at 20 ×
TABLE 3.
Differences in CD68, CD163, and iron staining by gestational diabetes status
| Variable | N | Mean % case | Mean % control | Change in %a | 95% CI | Adjusted change in %b |
95% CI |
|---|---|---|---|---|---|---|---|
| CD68 | |||||||
| Whole specimen | |||||||
| Positive | 44 | 8.59 | 8.84 | −0.24 | −2.90, 2.41 | −0.27 | −2.96, 2.41 |
| Moderate | 44 | 1.87 | 1.63 | 0.24 | −0.38, 0.86 | 0.17 | −0.10, 0.44 |
| Strong | 44 | 6.72 | 7.20 | −0.48 | −2.90, 1.93 | −0.44 | −2.94, 2.06 |
| Chorion | |||||||
| Positive | 32 | 7.02 | 7.65 | −0.63 | −5.27, 4.01 | −0.84 | −5.34, 3.67 |
| Moderate | 32 | 1.89 | 1.42 | 0.47 | −1.26, 0.32 | 0.37 | −0.35, 1.10 |
| Strong | 32 | 5.13 | 6.23 | −1.10 | −5.35, 3.15 | −1.09 | −4.77, 2.60 |
| Decidua | |||||||
| Positive | 36 | 16.98 | 16.50 | 0.48 | −5.73, 6.68 | 1.27 | −5.96, 8.49 |
| Moderate | 36 | 3.73 | 3.75 | −0.02 | −2.03, 1.99 | 0.73 | −0.95, 2.42 |
| Strong | 36 | 13.93 | 12.50 | 1.44 | −3.83, 6.70 | 1.67 | −5.10, 8.43 |
| CD163 | |||||||
| Whole specimen | |||||||
| Positive | 38 | 20.2 | 18.0 | 2.23 | −1.05, 5.51 | 3.29 | −0.73, 7.31 |
| Moderate | 38 | 11.79 | 10.3 | 1.49 | −0.53, 3.51 | 2.04 | −0.44, 4.53 |
| Strong | 38 | 8.62 | 7.72 | 0.90 | −1.05, 2.84 | 1.25 | −0.91, 3.41 |
| Chorion | |||||||
| Positive | 30 | 20.83 | 14.83 | 6.00 | −0.37, 12.36 | 7.51 | −1.97, 16.98 |
| Moderate | 30 | 15.96 | 11.25 | 4.71 | 0.20, 9.22 | 5.51 | 0.04, 10.97 |
| Strong | 30 | 4.86 | 3.57 | 1.29 | −1.43, 4,01 | 2.03 | −3.65, 7.71 |
| Decidua | |||||||
| Positive | 36 | 24.83 | 18.31 | 6.52 | 0.58, 12.48 | 9.29 | 1.96, 16.61 |
| Moderate | 36 | 16.75 | 11.97 | 4.77 | 0.59, 8.96 | 6.45 | 1.39, 11.51 |
| Strong | 36 | 8.08 | 6.46 | 1.62 | −0.79, 4.04 | 2.60 | −0.75, 51.61 |
| Iron | |||||||
| Whole Specimen |
42 | 0.82 | 0.59 | 0.23 | 0.04, 0.42 | 0.24 | 0.02, 0.46 |
| Chorion | 22 | 0.50 | 0.51 | −0.02 | −0.40, 0.36 | 0.00 | −0.40, 0.40 |
| Decidua | 40 | 0.40 | 0.30 | 0.11 | −0.12, 0.32 | 0.07 | −0.26, 0.40 |
CI: confidence interval. Significant values are bolded.
Change in % represents the change in expect proportion of cells to have the histological characteristic when comparing cases to controls using beta regression.
Change in % represents the change in expect proportion of cells to have the histological characteristic when comparing cases to controls adjusted for maternal hematocrit.
We examined the amount of iron storage within the placenta by Prussian blue staining. The difference in Prussian blue stained positive area was not significantly different in the chorion or decidual areas independently of the villous core (Table 3). However, we observed a significant increase in iron-positive area within the entire tissue, indicating that the increase in iron staining was localized to the fetally derived villous core. The difference remained significant after adjustment for the maternal hematocrit levels.
4 |. DISCUSSION
A growing body of evidence suggests and supports an association between GDM and altered histological and physiological characteristics within the placenta, an increase in maternal and placental inflammation, and an increase in maternal iron status7–12,15–17,24,25,27,29,32 In this study, we used a novel approach to retrieve archived placental tissue linked to deidentified patient records to investigate differences in placental histology, placental macrophage number, distribution, CD163 expression, and iron storage within the placenta to gain a better understanding of how GDM affects these placental characteristics. Indeed, we found an increase in abnormal placental histological characteristics, and increase in CD163 expression, and an increase in iron storage within the placenta of women with GDM. The findings presented here shed light on specific pathological and cellular changes that occur within the placenta as a result of GDM. These changes may mediate the pre- and postnatal complications associated with GDM and confirm the need to look further into the role of iron and iron supplementation during pregnancy in women at risk for GDM.
4.1 |. GDM and maternal hematocrit
Our study identified an association between GDM and an increase in maternal hematocrit level, which is consistent with reports in pregnant and non-pregnant persons with diabetes. Associations between hematocrit levels and diseases of insulin resistance and metabolic syndrome have been reported; however, associations vary depending on study design and population.33–37 Lao reported women with GDM have higher hemoglobin, red blood cell count, and hematocrit levels in the third trimester, but not earlier in gestation.38 Another study reported no increase in hematocrit levels during pregnancy in cases of GDM; however, they described an increase in maternal hemoglobin levels throughout the duration of pregnancy and have a high hemoglobin and hematocrit level at baseline.39 Elevated hematocrit levels have been reported to be an independent risk factor for the development of type 2 diabetes and impaired glucose tolerance.34 Hyperinsulinemia and insulin resistance are features of metabolic syndrome, type 2 diabetes, and GDM. Hematocrit levels increase as a result of increased erythropoiesis, which might explain the increase observed in GDM patients. Previous studies have provided evidence for a relationship between hyperinsulinemia and an increase in erythropoiesis.33 Insulin resistance is associated with increased levels of insulin-like growth factor-1, which may lead to an increase in erythropoiesis, hence, increasing hematocrit levels within GDM patients. This phenomenon has been demonstrated previously in rats.40 In healthy individuals, an increase in hematocrit levels would suggest superior oxygen delivery throughout the body. Alternatively, it is possible that GDM causes worse oxygen delivery to the placenta and the combination of elevated hematocrit levels and accelerated villous maturation that we report here are compensatory responses to an increased need for oxygen within the placenta. Future studies are needed to confirm our findings and assess these possible mechanisms.
4.2 |. Placental histopathological changes in GDM
The histopathological changes identified in the placentae of women with GDM were heterogeneous and varied in their severity. Our analysis discovered a significant increase in the presence of meconium-laden macrophages and epithelial changes presumed reactive to meconium within the amnion of GDM placentae. Meconium is fetal stool composed of bile acid, phospholipases, and other components. While the passage of meconium prior to 32–34 weeks is rare, 15%–20% of placentas are affected by the passage of meconium at delivery. The passage of meconium in utero may be indicative of fetal stress, typically hypoxic or ischemic stress, although not always present in cases of fetal stress. Although the exact implications of increased numbers of meconium-laden macrophages with the amnion are unknown, the uptake of meconium by alveolar macrophages has been shown to decrease macrophage phagocytosis and increase the release of TNF-α from macrophages.41,42
Villi arborize and mature throughout gestation, with more mature villi showing a smaller cross section and peripheral capillaries.43 These changes can be expected to maximize surface area and thus oxygen diffusion. Villous maturation varies in pathologic circumstances—immature villi have been repeatedly reported in GDM, while precocious maturity has been reported in conditions with impaired maternal-fetal circulation.32,43,44 In clinical practice, as in this study, villous maturation is based on a gestalt impression, rather than systematic measurement. Perhaps surprisingly, this gestalt impression has been shown to correlate with maturation-based gene expression changes.45 It is unclear why our results differ from previously reported studies in GDM. There are several minor methodologic differences; however, the most likely cause is the use of a retrospective case: control design with matching by maternal and gestational age in the present study as compared to the prospective cohort-based designs in other studies. As noted in the results, the difference in villous maturation was no longer apparent when controlling for hematocrit. This suggests a unifying mechanism wherein GDM impairs oxygen delivery to the placenta, thus, inducing the compensatory responses of accelerated villous maturation and increased red cell mass.
The presence of villous calcifications was elevated in GDM placentae compared to controls prior to adjustment for maternal hematocrit levels, with a fairly large effect size (unadjusted odds ratio of 4.67 (95% confidence intervals of 1.30–16.76, Table 2). Calcifications are indicative of an aging placenta.46 The clinical significance of such calcifications is not well defined although calcification is likely due to an increase in available calcium and phosphate within the placenta.46 Prominent in late-term and post-term placentae, they can occur in the absence of other placental pathologies, particularly in peripheral areas. However, the mineralization process through which calcifications occur may be accelerated in some disorders of pregnancy. The early appearance of calcifications may represent accelerated placental maturation and or senescence brought about as a result of fetal stress and maternal complications such as maternal hypertensive disorders.47 Quinlin and colleagues first noted that placentae from pregnancies complicated by diabetes develop calcifications earlier in gestation than non-complicated pregnancies and may represent early placental dysfunction.47 Although significance was lost after adjustment for maternal hematocrit, the effect size was 3.70 (95% confidence intervals of 0.86–16.89), suggesting that the small samples size might have played a role in the lack of significance. A difference in placental calcifications indicates that GDM may influence nutrient availability within the placenta as well as leading to accelerated placental maturation and cellular senescence. The hypothesis that GDM may accelerate placental maturation is further strengthened by our reported finding that we observed an increase in villous maturation within the GDM cohort compared to control. The mechanism driving the accelerated maturation which could be linked to an increase in villous calcifications is yet to be defined.
4.3 |. Placental macrophages and GDM
CD68 is a pan-macrophage marker found on monocytes and macrophages.48 We utilized this marker as way to localize and enumerate macrophages within the placenta. Our study identified no significant difference in the number or distribution of placental CD68 + cells between cases and controls. Another study which looked for CD68 expression levels within placental tissue of GDM and control patients found no significant difference in the levels of CD68 transcript levels.49 These findings contradict other reports where increases in the mRNA expression of CD68, in conjunction with increased expression levels of IL-6 and TNF-α, were observed in pregnancies complicated with GDM.14 Mrizak et al also reported an increase in transcript levels of CD68 and CD14, both common human monocyte/macrophage markers, within placenta recovered from women with GDM.12 That study, however, only collected placentae from women diagnosed with GDM whose child was born with macrosomia. The presence of macrosomia as an inclusion criterion may have biased the results for more severe cases of GDM than we included. Other studies reported conflicting results regarding the level of CD68 mRNA in gene expression analysis studies. The discrepancies in results regarding the expression of CD68 mRNA or the enumeration of CD68 + stained cells via microscopy between GDM and control placentae are not yet explained. The infiltration or expression of CD68 within placental tissue may depend on multiple factors, not just the presence or absence of GDM. The severity of insulin resistance, the amount of circulating inflammatory factors, and the degree to which glucose levels are controlled during pregnancy may all influence macrophage numbers and distribution within the placenta.
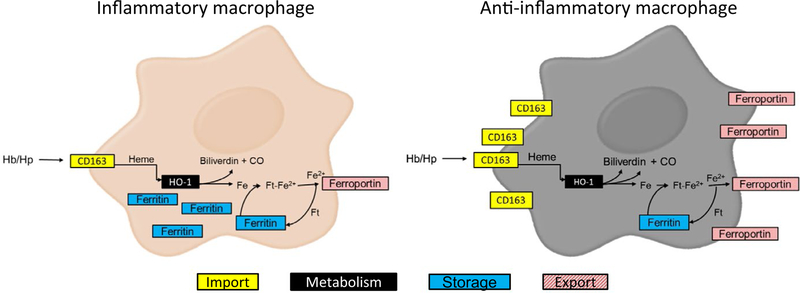
A hemoglobin scavenger receptor that mediates the endocytosis of hemoglobin-haptoglobin complexes, CD163, is exclusively expressed on macrophages and is often utilized as a marker for placental macrophages.20,23 We identified an increase in CD163 + cells within the decidua of GDM placentae and an increase in the amount of moderately stained CD163 + cells within the chorion. Expression of CD163 has been associated with an anti-inflammatory (M2) macrophage phenotype.49,50 The putative relationship of CD163 to iron homeostasis and macrophage polarization is summarized in Figure 4. Anti-inflammatory mediators such as IL-10 promote the expression of CD163 while pro-inflammatory mediators such as IFN-γ and TNF-α have been shown to decrease its expression.51 CD163 also exists in soluble form, sCD163.52 Similar to TNF-α, the ectodomain of CD163 is cleaved from the surface of macrophages in an inflammation-driven fashion.53 Levels of circulating sCD163 increase in mothers with GDM in conjunction with increases in circulating TNF-α and IL-6.54 Placental tissue, as well as adipose tissue, from GDM mothers is a source of increased sCD163 circulating in maternal serum as described by placental and adipose tissue explant studies.54 GDM appears to not only increase the expression of CD163 but also increase levels of sCD163 in maternal serum. These findings are in agreement with previous findings of elevated sCD163 in GDM.54 The molecular mechanisms leading to the cleavage of CD163 from placental macrophages have yet to be elucidated in the case of GDM, so future attention should be paid to discovering the mechanisms involved in this phenomenon.
FIGURE 4.
Macrophage Iron Metabolism is Coupled to Inflammatory Status. The expression of iron regulatory proteins such as CD163, a hemoglobin scavenger receptor, ferritin (Ft), an iron storage protein, and ferroportin, an iron export protein, are affected by the inflammatory status of the macrophage. High levels of intracellular iron stores in the form of ferritin and low levels of CD163 and ferroportin expression are characteristic of M1 or pro-inflammatory macrophages. This phenotype allows for the sequestration of iron from the extracellular environment. M2 or anti-inflammatory macrophages store less intracellular iron and demonstrate increased expression of CD163 and ferroportin, permitting for increased uptake and degradation of hemoglobin/haptoglobin (Hb/Hp) complexes and the recirculation of iron to the extracellular environment. CO, carbon monoxide
4.4 |. Placental iron load increases in GDM
Placental iron load for the tissue as a whole was significantly increased in GDM. However, close examination of both the decidua and chorion independently revealed no difference, indicating that the increase in iron load within the placenta is found primarily within the fetally derived villous tissue. The opposite was found to be true for the presence of CD163. CD163 showed significance within the chorion and decidua individually, but not the tissue as a whole. The villous core, the majority of the placental specimen, is primarily fetally derived tissue, bathed in maternal blood, and acts as the site of exchange between maternal and fetal circulation. As this area is saturated in maternal blood, it makes sense that the villous core stores iron at a higher level than within the chorion or decidua. Iron enters and exits macrophages in many different forms through many different pathways, including CD163.55,56 If macrophage polarization is impacted in cases of GDM, the relative expression of CD163 as well as iron storages within the macrophage may be altered in response. As reviewed by Cairo et al,57 macrophage iron metabolism is influenced by the local inflammatory environment. If GDM influences the inflammatory environment, CD163 expression and iron storage within the placenta may be effected as well. Multiple other cell types within the placenta are involved in iron homeostasis. Our findings of increased iron stores within GDM placentae correspond with previously reported data indicating that women with GDM have a significantly increased iron stores, as measured by hemoglobin, ferritin, and transferrin saturation.24 The physiological mechanism driving the increase in stored iron and more information regarding the cells in which the iron is stored needs further investigation and may shed light onto the apparent link between GDM, iron, and possible mechanisms driving adverse phenotypes.
4.5 |. Study limitations
Our study has important limitations, which may reflect the retrospective nature of, and small number of subjects included in this study. Our pilot study was relatively small and designed to be inclusive of a large range of maternal ages and gestational ages while representing diverse ethnicities. This inclusivity may have introduced variability into our results. Future studies should be designed to account for variability due to gestational age, maternal age, and ethnicity. The previously archived placental tissues included in this study were sent to pathology at the discretion of the obstetrician present at delivery. While there are recommended indications for placental examination, they are inconsistently followed. This could introduce difficult to measure confounders, as normal-appearing placentas from normal pregnancies are rarely sent for pathology. This study was likely underpowered to confirm a previously reported association between increased placental weight and GDM58. Although both placental weight and volume were higher in cases than in controls in our study, neither difference reached statistical significance. This may also reflect more aggressive treatment of GDM than in the time period when prior studies were conducted.59
Our study lacked data on iron status in the mothers, a result of the retrospective nature of the study. This precluded incorporation of such knowledge into our analyses, potentially obscuring important relationships between maternal iron loads and placental pathology. Future studies should examine comprehensively maternal iron status, including total iron, soluble transferrin receptor, erythropoietin levels, ferritin, and hepcidin values. Pre-pregnancy obesity is a risk factor for GDM, but we did not have pre-pregnancy height and weight measurements on most of the subjects in this study. Because gestational weight gain is an important factor in inflammatory processes and the development of GDM, this will need to be better analyzed in future studies.
Although we matched our GDM samples to our controls for variables such as maternal age and gestational age at birth, differences in other patient characteristics might have introduced variability. Differences in some of our measured parameters may be evident in some subgroups for which this pilot study is undersized. The nature of FFPE placental tissue and the age of some of the blocks themselves did not allow us to gather quantifiable information on all sections of the placenta due to quality and loss of tissue.
4.6 |. Conclusion
In conclusion, this study demonstrates that the presence of GDM influences multiple iron-related parameters including an increase in maternal hematocrit levels, an increase in CD163 within the chorion and decidua, and an increase in iron stores within the fetally derived villous core. Our results also highlight GDM’s influence on multiple placental physiological parameters such as meconium-laden macrophages, placental villous size and maturation, and an increase in the presence of villous calcifications. Taken together, the alterations exhibited by the placenta in cases of GDM may provide insight into the role of the placenta in the complications associated with this common metabolic disorder of pregnancy. However, future studies are needed to further elucidate the role of placental iron and inflammation in the development, progression, and postnatal complications associated with GDM.
ACKNOWLEDGMENTS
We would like to thank the Digital Histology Shared Resource and the Translational Pathology Shared Resource at Vanderbilt University Medical Center, and the Vanderbilt Institute for Clinical and Translational Research (VICTR, supported by a Clinical and Translational Science Award from the National Center for Advancing Translational Sciences at the National Institutes of Health), for their assistance in this work. The authors have no conflict of interests to report.
Funding information
Funding for this work (for T.L.B.) was provided by the National Institutes of Health-National Institute of Diabetes & Digestive & Kidney Diseases, Grant/Award Number: F31DK108652–02. The Vanderbilt Medical Student Research Training Program (support for A.P.R.) is supported by the Vanderbilt Short Term Research Training Program for Medical Students (NIH grant DK007383), Vanderbilt Research Training in Diabetes and Endocrinology (NIH grant T32 DK007061), and the Vanderbilt Diabetes Research and Training Center (NIH grant DK20593). This work utilized the core(s) of the Vanderbilt Diabetes Research and Training Center funded by the grant DK020593 from the National Institute of Diabetes and Digestive and Kidney Disease. Stipend support for ACS was provided in part by the National Institute of General Medical Studies award for the Vanderbilt Medical-Scientist Training Program (T32GM07347) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (F30HD094345).
REFERENCES
- 1.Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–s24. [DOI] [PubMed] [Google Scholar]
- 3.Lo JO, Frias AE. Trends in obesity and implications for the fetus In: Rajendram R, Preedy VR, Patel VB, eds. Diet, Nutrition, and Fetal Programming. Cham: Springer International Publishing; 2017:159–170. [Google Scholar]
- 4.Institute of M, National Research Council Committee to Reexamine IOMPWG. The national academies collection: reports funded by National Institutes of Health In: Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US) National Academy of Sciences; 2009:1–868. [PubMed] [Google Scholar]
- 5.Torloni MR, Betrbn AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. [DOI] [PubMed] [Google Scholar]
- 6.McNanley T, Woods J. Placental physiology. Glob. Lib. Wom. Med 2008:659–669. [Google Scholar]
- 7.Calderon IM, Damasceno DC, Amorin RL, Costa RA, Brasil MA, Rudge MV. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res Clin Pract. 2007;78:65–71. [DOI] [PubMed] [Google Scholar]
- 8.Radaelli T, Varastehpour A, Catalano P, Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. [DOI] [PubMed] [Google Scholar]
- 9.al-Okail MS, al-Attas OS. Histological changes in placental syncytiotrophoblasts of poorly controlled gestational diabetic patients. Endocrine J. 1994;41:355–360. [DOI] [PubMed] [Google Scholar]
- 10.Kuzmicki M, Telejko B, Zonenberg A, et al. Circulating pro- and anti-inflammatory cytokines in Polish women with gestational diabetes. Hormone Metab Res. 2008;40:556–560. [DOI] [PubMed] [Google Scholar]
- 11.Pendeloski KP, Mattar R, Torloni MR, Gomes CP, Alexandre SM, Daher S. Immunoregulatory molecules in patients with gestational diabetes mellitus. Endocrine. 2015;50:99–109. [DOI] [PubMed] [Google Scholar]
- 12.Schober L, Radnai D, Spratte J, et al. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin Exp Immunol. 2014;177:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara Cde C, Franca EL, Fagundes DL, et al. Characterization of natural killer cells and cytokines in maternal placenta and fetus of diabetic mothers. J Immunol Res. 2016;2016:7154524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6:650–659. [PMC free article] [PubMed] [Google Scholar]
- 15.Mrizak I, Grissa O, Henault B, et al. Placental infiltration of inflammatory markers in gestational diabetic women. Gen Physiol Biophys. 2014;33:169–176. [DOI] [PubMed] [Google Scholar]
- 16.Sisino G, Bouckenooghe T, Aurientis S, Fontaine P, Storme L, Vambergue A. Diabetes during pregnancy influences Hofbauer cells, a subtype of placental macrophages, to acquire a pro-inflammatory phenotype. Biochem Biophys Acta. 2013;1832:1959–1968. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Zhou Y, Gui J, Li AZ, Su XL, Feng L. Assessment of the number and function of macrophages in the placenta of gestational diabetes mellitus patients. J Huazhong Univ Sci Technolog Med Sci. 2013;33:725–729. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson C, Mjosberg J, Matussek A, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z, Tadesse S, Norwitz E, Mor G, Abrahams VM, Guller S. Isolation of hofbauer cells from human term placentas with high yield and purity. Am J Reprod Immunol. 2011;66:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Romero R, Tarca AL, et al. Methylome of fetal and maternal monocytes and macrophages at the feto-maternal interface. Am J Reprod Immunol. 2012;68:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schliefsteiner C, Peinhaupt M, Kopp S, et al. Human placental hofbauer cells maintain an anti-inflammatory M2 phenotype despite the presence of gestational diabetes mellitus. Front Immunol. 2017;8:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. [DOI] [PubMed] [Google Scholar]
- 24.Afkhami-Ardekani M, Rashidi M. Iron status in women with and without gestational diabetes mellitus. J Diabet Complicat. 2009;23:194–198. [DOI] [PubMed] [Google Scholar]
- 25.Amiri FN, Basirat Z, Omidvar S, Sharbatdaran M, Tilaki KH, Pouramir M. Comparison of the serum iron, ferritin levels and total iron-binding capacity between pregnant women with and without gestational diabetes. J Nat Sc Biol Med. 2013;4:302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo S, Menato G, Villois P, et al. Iron supplementation and gestational diabetes in midpregnancy. Am J Obst Gynecol. 2009;201:158.e151–158.e156. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care. 2006;29:1077–1082. [DOI] [PubMed] [Google Scholar]
- 28.Helin A, Kinnunen TI, Raitanen J, Ahonen S, Virtanen SM, Luoto R. Iron intake, haemoglobin and risk of gestational diabetes: a prospective cohort study. BMJ Open 2012;2:e001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawal S, Hinkle SN, Bao W, et al. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia. 2017;60:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudge MV, Lima CP, Damasceno DC, et al. Histopathological placental lesions in mild gestational hyperglycemic and diabetic women. Diabetol Metab Syndr. 2011;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbieri M, Ragno E, Benvenuti E, et al. New aspects of the insulin resistance syndrome: impact on haematological parameters. Diabetologia. 2001;44:1232–1237. [DOI] [PubMed] [Google Scholar]
- 34.Capoglu I, Unuvar N, Bektas Y, Yilmaz O, Kaya MD. The effects of high haematocrit levels on glucose metabolism disorders. J Int Med Res. 2002;30:433–437. [DOI] [PubMed] [Google Scholar]
- 35.Nebeck K, Gelaye B, Lemma S, et al. Hematological parameters and metabolic syndrome: findings from an occupational cohort in Ethiopia. Diabetes Metab Syndr. 2012;6:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wannamethee G, Shaper AG. Haematocrit: relationships with blood lipids, blood pressure and other cardiovascular risk factors. Thromb Haemost. 1994;72:58–64. [PubMed] [Google Scholar]
- 37.Wannamethee SG, Perry IJ, Shaper AG. Hematocrit and risk of NIDDM. Diabetes. 1996;45:576–579. [DOI] [PubMed] [Google Scholar]
- 38.Lao TT, Ho LF. Gestational diabetes and maternal third-trimester blood count. J Reprod Med. 2002;47:309–312. [PubMed] [Google Scholar]
- 39.Tan PC, Chai JN, Ling LP, Omar SZ. Maternal hemoglobin level and red cell indices as predictors of gestational diabetes in a multi-ethnic Asian population. Clin Exp Obstet Gynecol. 2011;38:150–154. [PubMed] [Google Scholar]
- 40.Kurtz A, Zapf J, Eckardt KU, Clemons G, Froesch ER, Bauer C. Insulin-like growth factor I stimulates erythropoiesis in hypophysectomized rats. Proc Natl Acad Sci USA. 1988;85:7825–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig S, Lopez A, Hoskin D, Markham F. Meconium inhibits phagocytosis and stimulates respiratory burst in alveolar macrophages. Pediatr Res. 2005;57:813–818. [DOI] [PubMed] [Google Scholar]
- 42.Lally KP, Mehall JR, Xue H, Thompson J. Meconium stimulates a pro-inflammatory response in peritoneal macrophages: implications for meconium peritonitis. J Pediatr Surg. 1999;34:214–217. [DOI] [PubMed] [Google Scholar]
- 43.Khong TY, Mooney EE, Ariel I, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. [DOI] [PubMed] [Google Scholar]
- 44.Daskalakis G, Marinopoulos S, Krielesi V, et al. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87:403–407. [DOI] [PubMed] [Google Scholar]
- 45.Leavey K, Benton SJ, Grynspan D, Bainbridge SA, Morgen EK, Cox BJ. Gene markers of normal villous maturation and their expression in placentas with maturational pathology. Placenta. 2017;58:52–59. [DOI] [PubMed] [Google Scholar]
- 46.Jeacock MK. Calcium content of the human placenta. Am J Obstet Gynecol. 1963;87:34–40. [DOI] [PubMed] [Google Scholar]
- 47.Quinlan RW, Cruz AC, Buhi WC, Martin M. Changes in placental ultrasonic appearance. I. Incidence of Grade III changes in the placenta in correlation to fetal pulmonary maturity. Am J Obstet Gynecol. 1982;144:468–470. [PubMed] [Google Scholar]
- 48.Pulford KA, Sipos A, Cordell JL, Stross WP, Mason DY. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2:973–980. [DOI] [PubMed] [Google Scholar]
- 49.Abumaree MH, Al Jumah MA, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013;9:620–641. [DOI] [PubMed] [Google Scholar]
- 50.Tang Z, Niven-Fairchild T, Tadesse S, et al. Glucocorticoids enhance CD163 expression in placental Hofbauer cells. Endocrinology. 2013;154:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 52.Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. 2010;88:1201–1205. [DOI] [PubMed] [Google Scholar]
- 53.Etzerodt A, Rasmussen MR, Svendsen P, et al. Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-alpha in macrophages. J Biol Chem. 2014;289:778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bari MF, Weickert MO, Sivakumar K, et al. Elevated soluble CD163 in gestational diabetes mellitus: secretion from human placenta and adipose tissue. PLoS One. 2014;9:e101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. [DOI] [PubMed] [Google Scholar]
- 56.Soe-Lin S, Apte SS, Andriopoulos B Jr, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci USA. 2009;106:5960–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cairo G, Recalcati S, Mantovani A, Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 2011;32:241–247. [DOI] [PubMed] [Google Scholar]
- 58.Taricco E, Radaelli T. Nobile de Santis MS, Cetin I: Foetal and placental weights in relation to maternal characteristics in gestational diabetes. Placenta. 2003;24:343–347. [DOI] [PubMed] [Google Scholar]
- 59.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]