Abstract
Roflumilast, a selective inhibitor for PDE4, is approved by FDA as an anti-inflammation drug for treatment of chronic obstructive pulmonary disease (COPD). This study investigates the effects of roflumilast on cerebral inflammation in the rat SAH model. Here, we show that subcutaneous administration of roflumilast (3 mg/kg) significantly improved the neurological deficits. Measurement of evans blue extravasation and brain water content revealed a significant reduction of blood-brain barrier permeability and brain edema. Importantly, roflumilast treatment remarkably decreased levels of IL-1β, IL-6, and TNF-α and the number of apoptotic neurons in the brain after SAH. These results indicate that roflumilast is effective in treating cerebral inflammation following SAH.
Keywords: subarachnoid hemorrhage, roflumilast, cerebral inflammation
INTRODUCTION
Subarachnoid hemorrhage (SAH) is a form of stroke, mainly caused by rupture of intracranial blood vessels leading often to long-lasting neurological impairment or death. Inflammation is known to contribute to brain edema, blood-brain barrier (BBB) permeability, cerebral vasospasm, and neuronal apoptosis following SAH [1, 2]. In patients, numerous studies have shown that levels of inflammatory cytokines increased not only within cerebrospinal fluid (CSF) but also in blood after SAH [3–5] and associated with vasospasm, hyperthermia, and unfavorable outcome [3, 6, 7]. Noticeably, this systemic increase of inflammatory cytokines following SAH is related to a late inflammatory response and a predictive marker for poor outcome [1]. Animal studies show elevated serumal cytokine concentration, accumulation of vascular neutrophils and increase of adhesion molecules within hours following SAH, and these responses are associated with early brain injury [8]. It is therefore possible that anti-inflammatory therapy in peripheral blood may suppress serum inflammatory mediators from entering the brain parenchyma.
Phosphodiesterase-4 (PDE-4) is the predominant enzyme that degrades the second messenger cAMP in many immune cells. It is expressed in the cortex and hippocampus. Inhibition of PDE-4 yields beneficial effects in animal models of neurological diseases and injuries where inflammation plays an essential role, such as spinal cord injury [9], traumatic brain injury [10], Alzheimer’s disease [11], major depressive disorder [12], multiple sclerosis [13], and ischemic stroke [14, 15]. Roflumilast, a highly selective PDE-4 inhibitor, has been approved by the Food and Drug Administration (FDA) of USA as an anti-inflammation drug for peripheral inflammatory disorders, severe chronic obstructive pulmonary disease (COPD) associated with chronic bronchitis [16, 17]. In vitro, roflumilast N-oxide inhibits PDE-4 in a variety of cells, including neutrophils, endothelial cells, monocytes/macrophages, Cd4+ and CD8+ T cells, and smooth muscle cells [18]. In vivo, roflumilast mitigates the tobacco smoke-induced lung inflammation, pulmonary vascular remodeling and hypertension [18]. Roflumilast is the brain penetrant; its concentration in the brain increases in a dose-dependent manner [19, 20]. It is effective on hypertension-induced impairment of learning and >memory [19] and the cognitive deficit in rodents at non-emetic doses [20].
The present study tests the hypothesis that roflumilast decreases proinflammatory cytokines, blood-brain barrier permeability, and neuronal apoptosis following SAH in rats.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley (SD) rats (12 weeks old, body weight 250–350 g) were purchased from the Shandong Experimental Animal Center (Jinan, China) and were housed in cage on a 12-h light and 12-h dark cycle with free access to water and food. All animals were treated according to the National Institute of Health guidelines for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Taishan Medical University. They were randomly divided into the sham, SAH, and SAH+roflumilast group.
Rat SAH Model and Drug Administration
Rat SAH model was produced using the single blood injection model with modifications, as described previously [21]. Briefly, rats were anesthetized with chloral hydrate (400 mg/kg body weight) by intraperitoneal injection and fixed to the stereotactic frame with the 30 °C head down. Then, a 1-ml syringe with a 25-gauge needle was lowered into the cistern magna after exposing of the atlanto-occipital membrane. 0.3 ml volume of non-heparinized autologous blood from the femoral artery was injected into the cistern magna for 3 min with a syringe pump. Rats were allowed to recover 30 min after SAH.
Rats received vehicle (1 ml, saline, containing 3% DMSO) or roflumilast (3 mg/kg body weight) through subcutaneous injection at 2, 24, and 48 h after the surgery. The dose of roflumilast (3 mg/kg) is equivalent to that used for the treatment of cognitive deficits in mice [20].
Neurological Score
Three behavioral tests (appetite, activity, and deficits) (Table 1) were performed at 48 and 72 h after SAH as described previously [21]. Two “blinded” investigators tested the neurological scoring.
Table 1.
Behavior Scores
| Category | Behavior | Score |
|---|---|---|
| Appetite | Finished meal | 0 |
| Left meal unfinished | 1 | |
| Scarcely ate | 2 | |
| Activity | Active, walking, barking, or standing | 0 |
| Lying down, walk and stand with some stimulations | 1 | |
| Almost always lying down | 2 | |
| Deficits | No deficits | 0 |
| Unstable walk | 1 | |
| Impossible to walk and stand | 2 |
Measurement of Brain Water Content
The brain water content of the sham, SAH, and SAH+roflumilast group was measured at 48 and 72 h after SAH according to the wet/dry method. In brief, after anesthesia with chloral hydrate, the hemisphere of brain was taken and weighed immediately to determine the wet weight, then dried completely and to determine the dry weight. The brain water content was expressed as (wet weight – dry weight)/wet weight × 100%.
Blood-Brain Barrier Permeability
The BBB permeability was measured by evans blue (E8010, Solaribio) dye extravasation at 48 and 72 h following SAH as reported previously [22]. In brief, after anesthesia with chloral hydrate, rats in each group received 2% EB (5 ml/kg) through intravenous injection. After 60 min, rats were perfused via left ventricle with 50 ml PBS to remove remaining intravascular EB dye. Then, a hemisphere of the brain was weighed and homogenized using handheld homogenizers. After centrifugation, the homogenates mixed with an equal volume of trichloroacetic acid in ethanol (1:3) and incubated for 12 h. Then, the supernatant and the standards (0, 0.5, 1, 2, 4, 6, 8, and 10 μg/ml) were detected at (Exλ = 620 nm, Emλ = 680 nm) by a SpectraMax M5e microplate reader. The evans blue content of each group was calculated according to the standard curve and represented as microgram per gram.
Immunofluorescence and TUNEL Staining
Immunofluorescence and TUNEL staining were performed at 48 and 72 h following SAH according to our previous study [23]. In brief, after anesthesia with chloral hydrate, rats in the sham, SAH and SAH+roflumilast group were perfused transcardially via left ventricle with PBS followed by 4% paraformaldehyde in PBS. The brains were carefully removed and postfixed overnight in PBS containing 4% paraformaldehyde.
For frozen sectioning, brains were placed in PBS containing 30% sucrose at 4 °C until buoyancy was lost coronal sections (10 μm thick) were cut on a Leica CM1950 cryostat (Leica, Germany), permeabilized with 1% Triton X-100 for 5 min on ice, blocked in 5% goat serum for 2 h at room temperature, and then incubated with one of the primary antibodies [IL-1β (1:200, sc-7884), IL-6 (1:200, sc-1265), TNF-α (1:200, sc-1351), Santa Cruz Biotechnology; cleaved caspase-3 (1:200, ab2302), Abcam, USA; NeuN (1:200, MABN140, Millipore, USA)] overnight at 4 °C. Sections were washed with PBS, and incubated with an appropriate secondary antibody [anti-Rabbit IgG-FITC (1: 200, F9887), anti-mouse IgG-TRITC (1:200, T5393), anti-goat-IgG-TRITC (1:200, T7028), Sigma] for 2 h at room temperature. Sections were finally washed with PBS buffer and cover-slipped with the anti-fading medium. In addition, TUNEL staining of sections were performed using the in situ Cell Death Detection Kit with Fluorescein (Cat #11684795910, TUNEL, Roche, Germany) according to the manufacturer’s instruction. All images were captured on a fluorescence microscope (Olympus BX51, Japan).
Enzyme-Linked Immunosorbent Assay
After anesthesia with chloral hydrate, 1 ml of blood was drawn from the femoral vein in animals of the sham, SAH, and SAH+roflumilast group using a syringe without any anticoagulant. The blood was centrifuged for 10 min at 4 °C at 3000 rpm and the supernatant stored in a −80 °C refrigerator. One hemisphere of the brain was taken and weighed immediately, and homogenized in PBS using a handheld homogenizer. The homogenate was centrifuged for 10 min at 4 °C at 13000 rpm and the protein concentration of the supernatants was determined using the Protein Assay kit (Tiangen, China). Levels of IL-1β, IL-6, and TNF-α were measured using an enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer’s instructions (MultiSciences, China) and normalized to that of the sham group.
Statistical Analysis
All data obtained were expressed as mean ± SD (standard deviation). Comparisons among the sham, SAH, and SAH+roflumilast group were made by two-way analysis of variance (ANOVA) using the GraphPad Software Prism 6.0. Statistical significance was considered at the 95% confidence interval.
RESULTS
Roflumilast Improved Neurological Deficits Following SAH
To evaluate possible neuroprotective role of roflumilast on experimental SAH, neurological assessments were conducted at 48 and 72 h after SAH using a battery of three behavioral tests (Table 1). As shown in Fig. 1, the neurological score in the SAH group was obviously increased when compared to the sham group, while neurological score in the SAH+roflumilast group significantly reduced as compared with the SAH group. The mortalities of the sham, SAH, and SAH+roflumilast at 72 h were 0% (0 of 24 mice), 22.5% (7 of 31), and 20.0% (6 of 30), respectively.
Fig. 1.
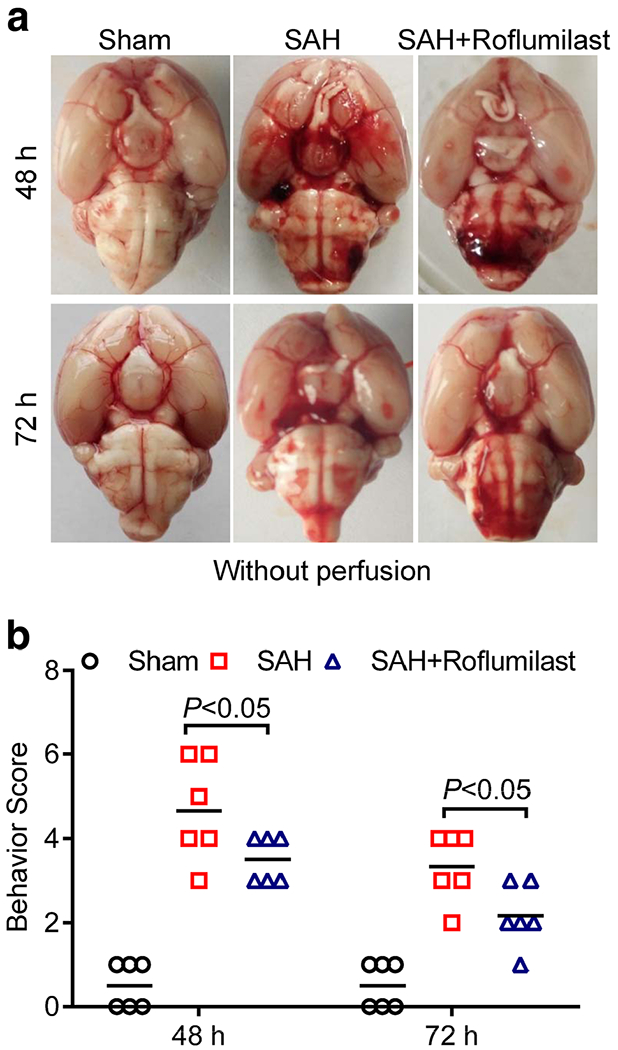
a Representative images of brain in the sham, SAH, and SAH+roflumilast group at 48 and 72 h. b The neurological score was performed at 48 and 72 h in the sham, SAH, and SAH+roflumilast group. Values were expressed as mean ± SD (P < 0.05; two-way ANOVA with Tukey’s multiple comparison test; n = 6 in each group).
Roflumilast Inhibited Blood-Brain Barrier Permeability and Brain Edema Following SAH
Next, we examined the effect of roflumilast on BBB permeability and brain edema at 48 and 72 h after experimental SAH. The evans blue dye extravasation and brain water content were higher in the SAH group than in the sham group, while SAH-induced increase of BBB permeability and brain edema were reduced back to lower level in the roflumilast-treated SAH group (Fig. 2).
Fig. 2.
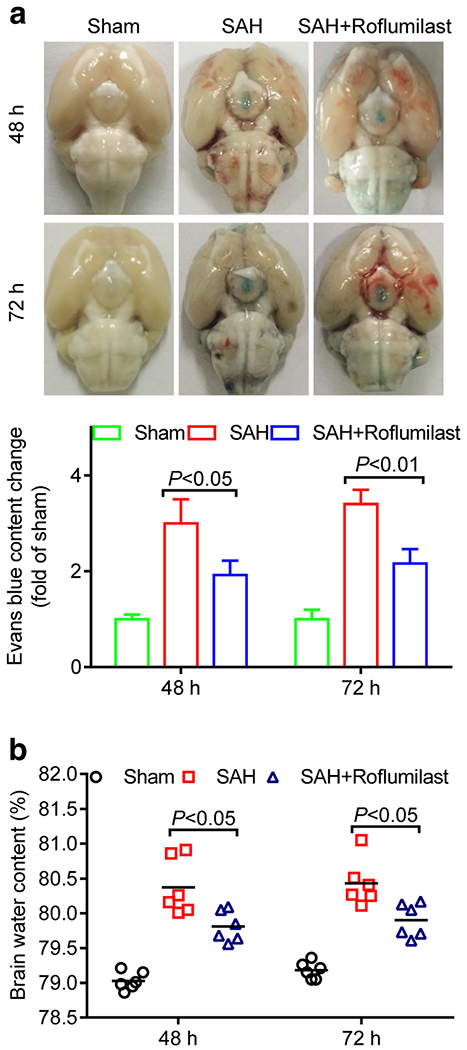
a Representative images showed rat brain with perfusion after Evans blue injection in the sham, SAH, and SAH+roflumilast group at 48 and 72 h. a Evans blue content as indices of blood-brain barrier permeability, and the brain water content (b) was measured at 48 and 72h in the sham, SAH, and SAH+roflumilast group. Values were expressed as mean ± SD (P < 0.05 or 0.01; two-way ANOVA with Tukey’s multiple comparison test; n = 3 (a) or n = 6 (b) in each group).
Roflumilast Suppressed the Level of IL-1β, IL-6, and TNF-α Following SAH
We also examined the effect of roflumilast on the expression of IL-1β, IL-6, and TNF-α at 48 and 72 h after SAH. Immunofluorescence staining results were recorded using fluorescence microscope. Statistics results showed that the number of IL-1β, IL-6, and TNF-α-positive cells in the SAH group were significantly increased when compared to the sham group, while administration of roflumilast significantly reduced these positive cells as compared with the SAH group (Fig. 3). Next, ELISA was used to detect the cortical or serumal level of IL-1 β, IL-6, and TNF-α at 48 and 72 h after SAH induction. As shown in Fig. 4, our results revealed that the cortical or serumal level of IL-1β, IL-6, and TNF-α obviously increased in the SAH group when compared to the sham group. However, this phenomenon was partially offset by administration of roflumilast. The cortical and serumal expression of IL-1 β, IL-6, and TNF-α, which were increased by SAH injury, were decreased in the roflumilast-treated SAH group (Fig. 4).
Fig. 3.
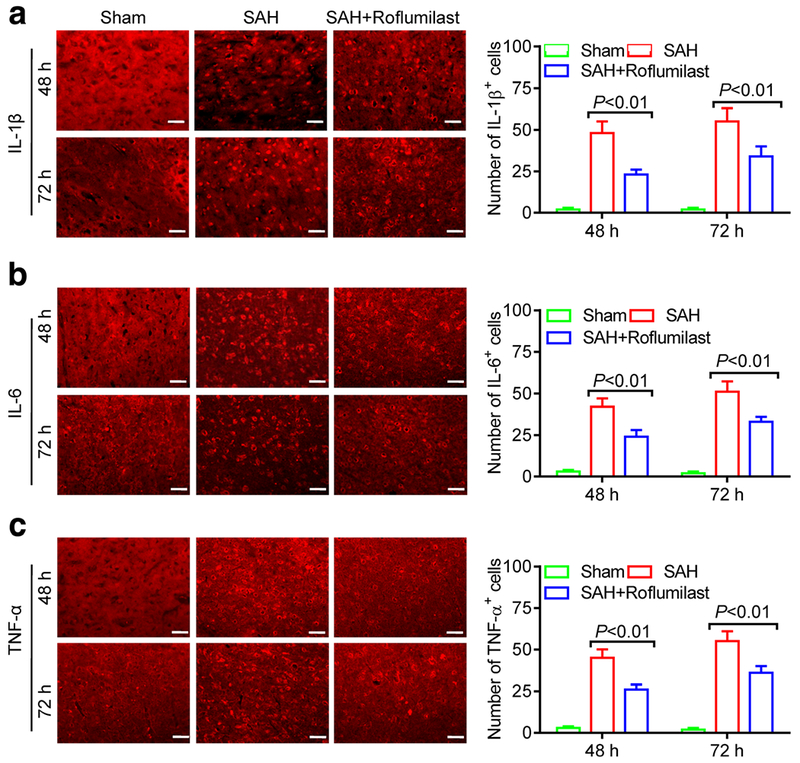
Representative immunofluorescence staining slices of a IL-1 β, b IL-6, or c TNF-α in the sham, SAH, and SAH+roflumilast group at 48 and 72 h. Scale bar is 20 μm Quantitative analysis of the a IL-1 β, b IL-6, or c TNF-α positive cells were expressed as mean ± SD(P < 0.01; two-way ANOVA with Tukey’s multiple comparison test; n = 3 in each group).
Fig. 4.
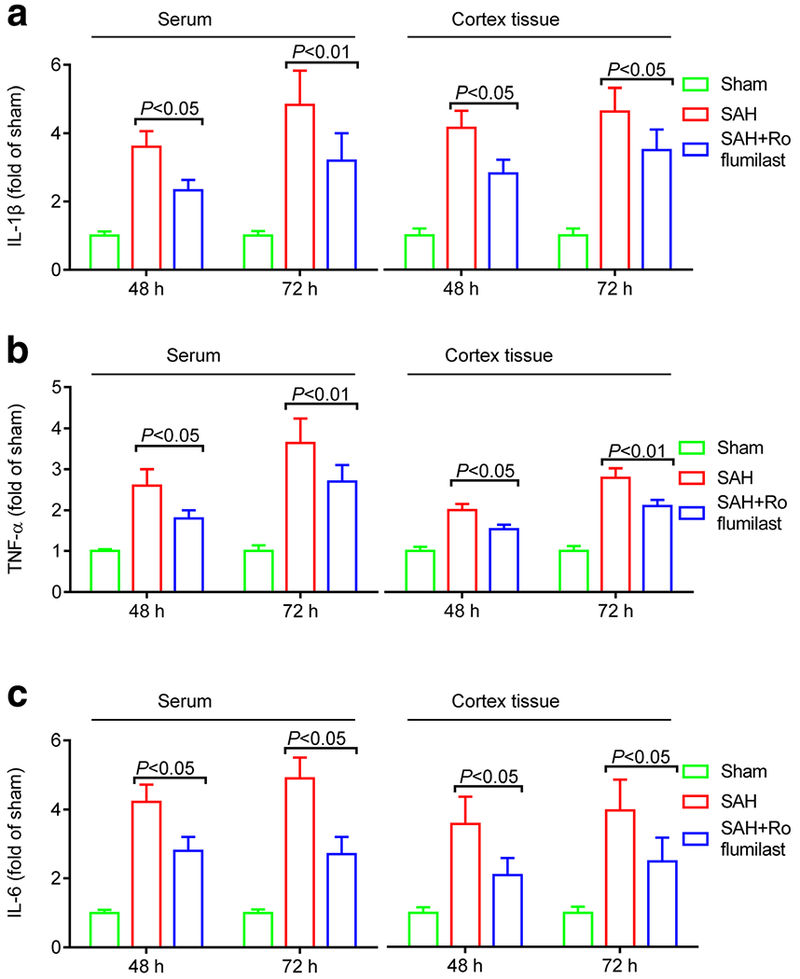
The cortical or serumal level of IL-1β (a), IL-6 (b), or TNF-α (c) in the sham, SAH, and SAH+roflumilast group at 48 and 72 h. Values were expressed as mean ± SD (P < 0.05 or 0.01; two-way ANOVA with Tukey’s multiple comparison test; n = 3 in each group).
Roflumilast Inhibited Neuronal Apoptosis Following SAH
We quantified cellular apoptosis by using the active caspase-3/NeuN (a general neuronal marker) immunofluorescence staining and TUNEL/DAPI staining at 48 and 72 h after SAH induction. Statistics results showed that numerous active caspase-3 positive cells were obviously increased in cortex of the SAH group when compared to the sham group. However, quantification showed that active caspase-3 positive cells were significantly reduced with roflumilast as compared with the SAH group (Fig. 5). Similarly, the TUNEL-positive cells were significantly reduced in the SAH+roflumilast group when compared to the SAH group (Fig. 6).
Fig. 5.
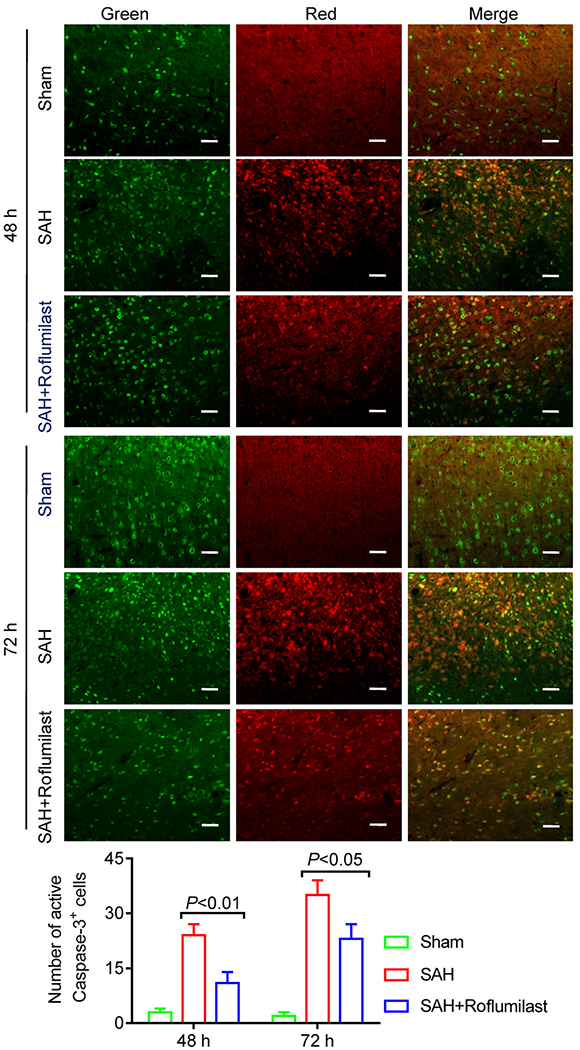
Representative immunofluorescence staining slices of active caspase-3 plus NeuN in the sham, SAH, and SAH+rofumilast group at 48 and 72 h. Scale bar is 20 μm. Quantitative analysis of the active caspase-3 staining positive cells were expressed as mean ± SD (P < 0.01; two-way ANOVA with Tukey’s multiple comparison test; n = 3 in each group).
Fig. 6.
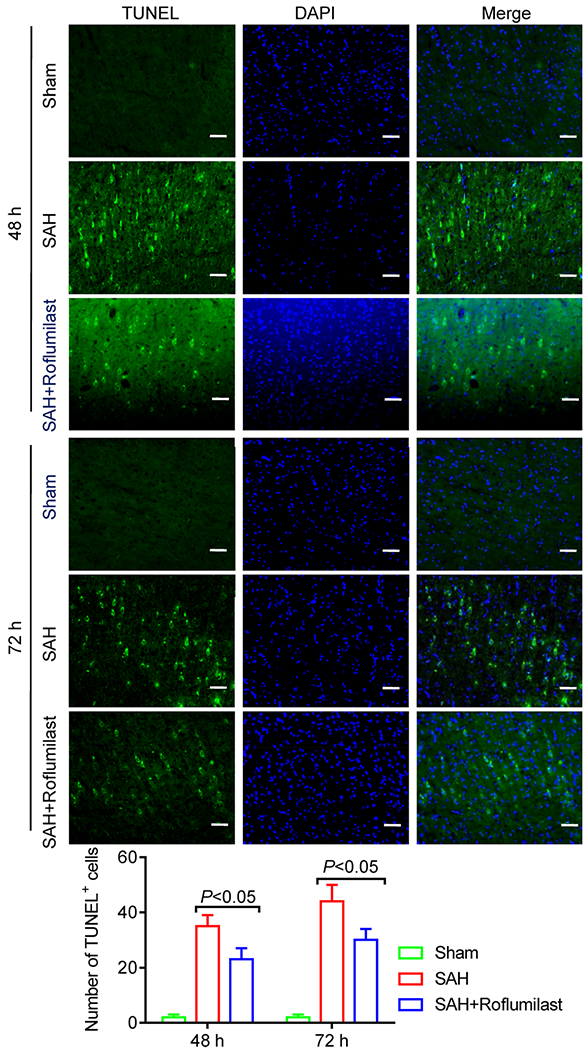
Representative immunofluorescence staining slices of TUNEL staining in the sham, SAH, and SAH+roflumilast group at 48 and 72 h. Scale bar is 20 μm. Quantitative analysis of the TUNEL staining positive cells were expressed as mean ± SD (P < 0.01; two-way ANOVA with Tukey’s multiple comparison test; n = 3 in each group).
DISCUSSION
In this study, we show that subcutaneous administration of roflumilast ameliorated brain edema and BBB permeability, decreased levels of cortical and serumal proinflammatory cytokines and reduced neuronal apoptosis 48 and 72 h after SAH. These results suggest that roflumilast is neuroprotective during early brain injury after SAH through an anti-inflammatory mechanism.
Neurological deficit and brain edema were important indicators for evaluating the outcome following SAH. Our results indicate that administration of roflumilast improved the neurological deficit and reduced brain edema after SAH, which is in good accordance with previous studies in ischemic stroke [14, 15]. Our observation in the SAH model is in accordance with previous study describing PDE4 inhibition-induced attenuation of blood-brain barrier permeability after ischemic stroke, an effect that may attribute to decrease of inflammation [14]. It is noteworthy to mention that roflumilast is clearly brain penetrant; plasma and brain concentration of roflumilast and its metabolite (roflumilast-N-oxide) showed linear increase after oral or subcutaneous administration [19, 20].
PDE4 inhibition has been shown to reduce the neutrophils invasion and the expression of proinflammatory cytokines IL-1β and TNF-α after ischemic stroke in mice [14]. Our observations show that the PDE4 inhibitor roflumilast protects neurons in hemorrhagic stroke in rat by reducing the cortical and serumal level of IL-1 β, IL-6, or TNF-α, which are known to aggravate brain injury. It is unclear how roflumilast reduces cerebral cytokines. It is possible that roflumilast inhibits peripheral inflammation and thus reduces the amount of inflammation cytokines entering the brain. Roflumilast may reduce the neutrophils invading the brain after SAH. Further investigation is warranted to explore the mechanism of roflumilast on cerebral inflammation after SAH.
PDE4 inhibition was also found to attenuate the neuronal apoptosis and increase cell proliferation and survival rate through the CREB signaling pathway in peri-infarct region after ischemic stroke [15]. Our results found that roflumilast administration obviously decreases the number of TUNEL-positive cells and active caspase-3/NeuN-positive cells after SAH. Even with the limitations of the present study, our results provide information about the effects of roflumilast against SAH-induced cerebral inflammation. Our findings suggested that roflumilast may be an available therapy for treating SAH that inflammation plays an important and direct role in disease progression.
CONCLUSION
The present study shows for the first time that roflumilast against cerebral inflammation in experimental SAH. Subcutaneous administration of roflumilast (3 mg/kg) significantly improved the neurological deficit, reduced the BBB permeability, the level of proinflammatory cytokines IL-1β, IL-6, and TNF-α, and neuronal apoptosis at 48 and 72 h in rat SAH model. These findings indicate that anti-inflammation strategy of using roflumilast may provide neuroprotection after SAH.
ACKNOWLEDGMENTS
This work was supported by funds from the National Natural Science Foundation of China (Grant No. 81471212, 81271275, 81070947 to Baoliang Sun; 81671141 to Zongyong Zhang) and the Natural Science Foundation of Shandong, China (Grant No. ZR2012HZ006 to Baoliang Sun).
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest. The authors declare that they have no conflict of interest
Qingjian Wu and Lifeng Qi are co-first authors.
REFERENCES
- 1.Miller BA, Turan N, Chau M, and Pradilla G. 2014. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. BioMed Research International 2014: 384342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradilla G, Chaichana KL, Hoang S, Huang J, and Tamargo RJ. 2010. Inflammation and cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery Clinics of North America 21 (2): 365–379. [DOI] [PubMed] [Google Scholar]
- 3.Kaynar MY, Tanriverdi T, Kafadar AM, Kacira T, Uzun H, Aydin S, Gumustas K, Dirican A, and Kuday C. 2004. Detection of soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in both cerebrospinal fluid and serum of patients after aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 101 (6): 1030–1036. [DOI] [PubMed] [Google Scholar]
- 4.Horstmann S, Su Y, Koziol J, Meyding-Lamade U, Nagel S, and Wagner S. 2006. MMP-2 and MMP-9 levels in peripheral blood after subarachnoid hemorrhage. Journal of the Neurological Sciences 251 (1–2): 82–86. [DOI] [PubMed] [Google Scholar]
- 5.Mocco J,Mack WJ, Kim GH, Lozier AP, Laufer I,Kreiter KT, Sciacca RR, Solomon RA, Mayer SA, and Connolly ES Jr. 2002. Rise in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery 97 (3): 537–541. [DOI] [PubMed] [Google Scholar]
- 6.Mathiesen T, Edner G, Ulfarsson E, and Andersson B. 1997. Cerebrospinal fluid interleukin-1 receptor antagonist and tumor necrosis factor-alpha following subarachnoid hemorrhage. Journal of Neurosurgery 87 (2): 215–220. [DOI] [PubMed] [Google Scholar]
- 7.Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF, and Lee KS. 2003. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 53 (1): 123–133 discussion 133-125. [DOI] [PubMed] [Google Scholar]
- 8.Sehba FA, Pluta RM, and Zhang JH. 2011. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Molecular Neurobiology 43 (1): 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaal SM, Garg MS,Ghosh M, Lovera L, Lopez M, Patel M, Louro J, Patel S, Tuesta L, Chan WM, and Pearse DD. 2012. The therapeutic profile of rolipram, PDE target andmechanism of action as a neuroprotectant following spinal cord injury. PloS One 7 (9): e43634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins CM, Oliva AA Jr., Alonso OF, Pearse DD, Bramlett HM, and Dietrich WD. 2007. Modulation of the cAMP signaling pathway after traumatic brain injury. Experimental Neurology 208 (1): 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong B, Vitolo OV, Trinchese F, Liu S, Shelanski M, and Arancio O. 2004. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. The Journal of Clinical Investigation 114 (11): 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HT 2009. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Current Pharmaceutical Design 15 (14): 1688–1698. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Garcia C, Bravo B, Ballester A, Gomez-Perez R, Eguiluz C, Redondo M, Martinez A, Gil C, and Ballester S. 2013. Comparative assessment of PDE 4 and 7 inhibitors as therapeutic agents in experimental autoimmune encephalomyelitis. British Journal of Pharmacology 170 (3): 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraft P, Schwarz T, Gob E, Heydenreich N, Brede M, Meuth SG, and Kleinschnitz C. 2013. The phosphodiesterase-4 inhibitor rolipram protects from ischemic stroke in mice by reducing blood-brain-barrier damage, inflammation and thrombosis. Experimental Neurology 247: 80–90. [DOI] [PubMed] [Google Scholar]
- 15.Hu S, Cao Q, Xu P,Ji W,Wang G, and Zhang Y. 2016. Rolipram stimulates angiogenesis and attenuates neuronal apoptosis through the cAMP/cAMP-responsive element binding protein pathway following ischemic stroke in rats. Experimental and Therapeutic Medicine 11 (3): 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabe KF 2011. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. British Journal of Pharmacology 163 (1): 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong J, Poole P, Leung B, and Black PN. 2011. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 5: CD002309. [DOI] [PubMed] [Google Scholar]
- 18.Hatzelmann A, Morcillo EJ,Lungarella G, Adnot S, Sanjar S, Beume R, Schudt C, and Tenor H. 2010. The preclinical pharmacology of roflumilast—a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulmonary Pharmacology & Therapeutics 23 (4): 235–256. [DOI] [PubMed] [Google Scholar]
- 19.Jabaris SG, Sumathy H, Kumar RS, Narayanan S, Thanikachalam S, and Babu CS. 2015. Effects of rolipram and roflumilast, phosphodiesterase-4 inhibitors, on hypertensioninduced defects in memory function in rats. European Journal of Pharmacology 746: 138–147. [DOI] [PubMed] [Google Scholar]
- 20.Vanmierlo T, Creemers P, Akkerman S, van Duinen M, Sambeth A, De Vry J, Uz T, Blokland A, and Prickaerts J. 2016. The PDE4 inhibitor roflumilast improves memory in rodents at nonemetic doses. Behavioural Brain Research 303: 26–33. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZY, Yang MF, Wang T, Li DW, Liu YL, Zhang JH, and Sun BL. 2015. Cysteamine alleviates early brain injury via reducing oxidative stress and apoptosis in a rat experimental subarachnoid hemorrhage model. Cellular and Molecular Neurobiology 35 (4): 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang ZY, Jiang M, Fang J,Yang MF, Zhang S, Yin YX, Li DW, Mao LL, Fu XY, Hou YJ, Fu XT, Fan CD, and Sun BL. 2017. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Molecular Neurobiology 54 (1): 1–14. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZY, Sun BL, Liu JK, Yang MF, Li DW, Fang J, Zhang S, Yuan QL, and Huang SL. 2015. Activation of mGluR5 attenuates microglial activation and neuronal apoptosis in early brain injury after experimental subarachnoid hemorrhage in rats. Neurochemical Research 40 (6): 1121–1132. [DOI] [PubMed] [Google Scholar]


