Abstract
BACKGROUND
Combination antiplatelet therapy with clopidogrel and aspirin may reduce the rate of recurrent stroke during the first 3 months after a minor ischemic stroke or transient ischemic attack (TIA). A trial of combination antiplatelet therapy in a Chinese population has shown a reduction in the risk of recurrent stroke. We tested this combination in an international population.
METHODS
In a randomized trial, we assigned patients with minor ischemic stroke or high-risk TIA to receive either clopidogrel at a loading dose of 600 mg on day 1, followed by 75 mg per day, plus aspirin (at a dose of 50 to 325 mg per day) or the same range of doses of aspirin alone. The dose of aspirin in each group was selected by the site investigator. The primary efficacy outcome in a time-to-event analysis was the risk of a composite of major ischemic events, which was defined as ischemic stroke, myocardial infarction, or death from an ischemic vascular event, at 90 days.
RESULTS
A total of 4881 patients were enrolled at 269 international sites. The trial was halted after 84% of the anticipated number of patients had been enrolled because the data and safety monitoring board had determined that the combination of clopidogrel and aspirin was associated with both a lower risk of major ischemic events and a higher risk of major hemorrhage than aspirin alone at 90 days. Major ischemic events occurred in 121 of 2432 patients (5.0%) receiving clopidogrel plus aspirin and in 160 of 2449 patients (6.5%) receiving aspirin plus placebo (hazard ratio, 0.75; 95% confidence interval [CI], 0.59 to 0.95; P = 0.02), with most events occurring during the first week after the initial event. Major hemorrhage occurred in 23 patients (0.9%) receiving clopidogrel plus aspirin and in 10 patients (0.4%) receiving aspirin plus placebo (hazard ratio, 2.32; 95% CI, 1.10 to 4.87; P = 0.02).
CONCLUSIONS
In patients with minor ischemic stroke or high-risk TIA, those who received a combination of clopidogrel and aspirin had a lower risk of major ischemic events but a higher risk of major hemorrhage at 90 days than those who received aspirin alone. (Funded by the National Institute of Neurological Disorders and Stroke; POINT ClinicalTrials.gov number, NCT00991029.)
The risk of ischemic stroke ranges from 3 to 15% in the 90 days after a minor ischemic stroke or a transient ischemic attack (TIA).1–5 In several trials, aspirin has been shown to reduce the risk of recurrent stroke by approximately 20%.6–10 Clopidogrel blocks platelet aggregation through the P2Y12-receptor pathway, a mechanism that is synergistic with aspirin in platelet-aggregation assays. The combination of the two drugs has been more effective than aspirin alone in reducing the risk of ischemic events in patients with acute coronary syndromes.11
We conducted the Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial to evaluate clopidogrel plus aspirin, as compared with aspirin alone, in an international population of patients who had a minor ischemic stroke or TIA. The Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial, which was initiated after the POINT trial and was completed before the termination of the POINT trial, showed a 32% lower risk of stroke recurrence among Chinese patients who were treated within 24 hours after a minor ischemic stroke or TIA with a combination of clopidogrel and aspirin than among those who were treated with aspirin alone, with no increase in the risk of hemorrhagic complications.12 The restricted ethnic population and patterns of care in that trial limited the generalizability of the results, and the combination of clopidogrel and aspirin has not been recommended routinely in guidelines for the treatment of stroke.6,10
METHODS
TRIAL DESIGNS AND OVERSIGHT
We enrolled patients in this randomized, double-blind, placebo-controlled trial from May 28, 2010, to December 19, 2017, at 269 sites in 10 countries in North America, Europe, Australia, and New Zealand, with the majority of the patients (82.8%) enrolled in the United States. The trial was approved by the ethics committee at each participating site. The trial protocol is available with the full text of this article at NEJM.org, as is the Supplementary Appendix, which provides lists of trial committees, sites, and investigators. Details regarding the trial rationale, design, and methods have been described previously.13
An executive committee was responsible for the design, interpretation, and supervision of the trial, including the development of the protocol and protocol amendments. The trial was sponsored by the National Institute of Neurological Disorders and Stroke (NINDS). Sanofi provided the clopidogrel and matching placebo for 75% of the patients enrolled in the trial and provided comments on an earlier version of the manuscript. There was no other industry involvement in the trial and no confidentiality agreement between the authors and Sanofi. The authors vouch for the accuracy and completeness of the data and reporting of adverse events and for the adherence of the trial to the protocol.
An independent data and safety monitoring board provided recommendations to NINDS and guidance to the executive committee, along with monthly assessments of safety and study conduct and oversight of the interim analyses. The members of an independent clinical-event committee who were unaware of group assignments adjudicated primary and secondary efficacy outcomes and major and minor bleeding events.
TRIAL POPULATION
Patients who were at least 18 years of age were enrolled if they could undergo randomization within 12 hours after having an acute ischemic stroke with a score of 3 or less on the National Institutes of Health Stroke Scale (NIHSS) (scores range from 0 to 42, with higher scores indicating greater stroke severity) or a high-risk TIA with a score of 4 or more on the ABCD2 scale14 (which estimates the risk of recurrent stroke after a TIA on the basis of age, blood pressure, clinical features, duration of symptoms, and presence of diabetes; scores ranges from 0 to 7, with higher scores indicating a greater risk of stroke). They were also required to undergo computed tomography or magnetic resonance imaging to rule out intracranial bleeding or other conditions that could explain the neurologic symptoms or detect any contraindications to a trial treatment. Patients with TIA and minor, nondisabling ischemic stroke are generally not considered to be candidates for thrombolysis or endovascular therapy.10 Additional details regarding the inclusion and exclusion criteria are provided in the protocol.13
Patients were ineligible if the symptoms of the initial TIA were limited to isolated numbness, isolated visual changes, or isolated dizziness or vertigo or if they had received any thrombolytic therapy within 1 week before the event. Patients were also ineligible if they were candidates for thrombolysis, endovascular therapy, or endarterectomy; had planned use of antiplatelet therapy or anticoagulation therapy (including those with presumed atrial fibrillation or cardiovascular disease, in whom anticoagulation would be indicated); had a contraindication to aspirin or clopidogrel; or had anticipated use of a nonsteroidal antiinflammatory drug for more than 7 days during the trial period. Written informed consent was required before the performance of any trial procedure.
TRIAL TREAMENTS
Patients were randomly assigned in a 1:1 ratio to receive either clopidogrel plus aspirin or placebo plus aspirin, with stratification according to trial site, with the use of an interactive Web-based system. Patients in the group receiving clopidogrel plus aspirin were given a 600-mg loading dose of clopidogrel, followed by 75 mg per day from day 2 to day 90, and a dose of open-label aspirin that ranged from 50 mg to 325 mg per day. Patients in the aspirin-only group received placebo that matched the appearance and taste of the clopidogrel tablets and the same range of aspirin doses. In the two groups, the dose of aspirin was selected by the treating physician. A dose of 162 mg daily for 5 days followed by 81 mg daily was recommended, consistent with guidelines.6,10 The first dose of trial medication was to be given as soon after randomization as possible. Patients were to be followed for 90 days (with a window of ±14 days) after randomization.
OUTCOMES
The primary outcome was the risk of a composite of ischemic stroke, myocardial infarction, or death from ischemic vascular causes (major ischemic events) on the basis of standard definitions.13 The primary safety outcome was the risk of major hemorrhage, which was defined as symptomatic intracranial hemorrhage, intraocular bleeding causing vision loss, transfusion of 2 or more units of red cells or an equivalent amount of whole blood, hospitalization or prolongation of an existing hospitalization, or death due to hemorrhage.15,16
Key secondary efficacy end points were each component of the primary efficacy outcome, a composite of the primary efficacy outcome and major hemorrhage, and the total number of ischemic and hemorrhagic strokes. Secondary safety outcomes included hemorrhagic stroke, symptomatic intracerebral hemorrhage, other symptomatic intracranial hemorrhage, major hemorrhage other than intracranial hemorrhage, minor hemorrhage that included asymptomatic intracranial hemorrhage, and death from any cause.13 Additional prespecified secondary and tertiary outcomes are provided in the statistical analysis plan in the Supplementary Appendix.
STASTISTICAL ANALYSIS
We determined that a sample of 4150 patients would provide the trial with a power of 90% to detect a hazard ratio of 0.75 with a two-sided alpha level of 0.05 on the basis of an event rate of 15% in the aspirin-only group. The sample was inflated to account for two interim analyses of the primary efficacy outcome with the use of an O’Brien–Fleming spending function. The spending-function approach allowed for additional efficacy interim analyses to be conducted at the request of the data and safety monitoring board while maintaining the type I error rate. On the basis of the observed event rate in the aspirin-only group at the first interim analysis, the sample was increased to 5840 patients to provide the trial with a power of 80% with other variables remaining unchanged in the calculation.
We performed the analyses according to the intention-to-treat principle, using adjudicated outcomes and data from all the patients who had undergone randomization. Data for patients who did not have a 90-day assessment were censored on the 7-day assessment date, on the date of an event, or on the date of death, whichever came later. We performed a secondary, as-treated analysis of the primary outcome that included patients who had received at least one dose of a trial regimen, with data censored 1 day after permanent discontinuation of trial medication.
We used the log-rank test to compare the time from randomization to the first occurrence of any given end point and a Cox proportionalhazards model to estimate the hazard ratio and 95% confidence intervals. There was no adjustment for baseline covariates or for the aspirin dose in the primary efficacy or safety analyses. Interactions between treatment assignment and prespecified subgroups were evaluated in the Cox model. A P value for interaction of less than 0.05 was considered to indicate statistical significance. We used a Cox model stratified according to time point to perform a secondary analysis of the primary efficacy and primary safety outcomes comparing the treatment effect during four time periods: days 0 to 7 versus days 8 to 90 and days 0 to 30 versus days 31 to 90. Secondary efficacy outcome analyses were not adjusted for multiple comparisons and are considered to be exploratory. A post hoc Bonferroni calculation was made for reference purposes to derive an adjusted threshold for P values to account for multiple comparisons of secondary outcomes.
RESULTS
Trial Discontinuation
In August 2017, the prespecified boundary for a safety signal of major hemorrhage was exceeded. Because of the small number of patients with hemorrhage, it was decided to follow these events until a planned meeting of the data and safety monitoring board in December 2017. At that meeting, the board recommended halting enrollment to the trial because of confirmation of a significant excess in the number of patients with major hemorrhage in the combined antiplatelet group, and a planned analysis determined that a treatment effect had crossed the significance boundary for efficacy. A summary of the board’s decision to halt the trial early is provided in the Supplementary Appendix.
PATIENTS
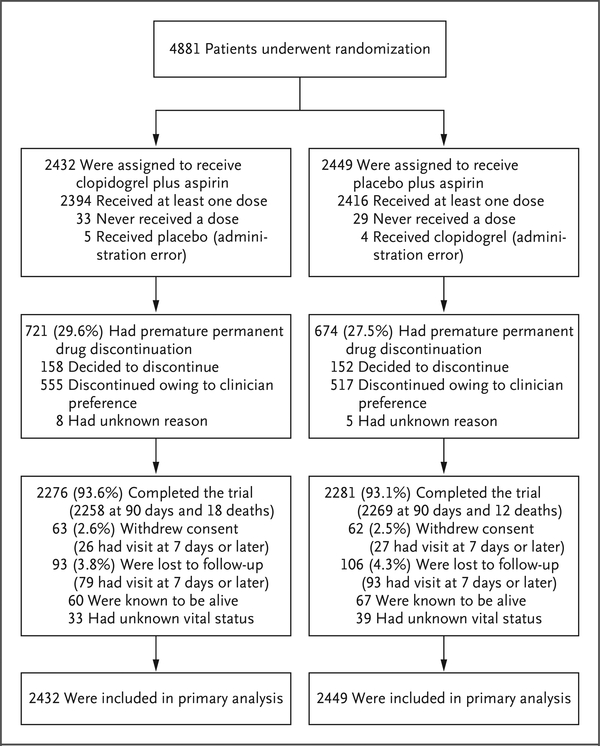
At the time that the trial was halted, 4881 patients had been enrolled, which represented 83.6% of the anticipated number of patients. Of these patients, 4782 (98.0%) had been followed for at least 7 days, and 4557 (93.4%) had completed the 90-day trial visit or had died (Fig. 1). Discontinuation of a trial medication occurred in 29.6% of the patients in the group receiving clopidogrel plus aspirin and in 27.5% of those receiving aspirin alone; rates of withdrawal from the trial or loss to follow-up were 6.4% in the group receiving clopidogrel plus aspirin and 6.8% in the aspirin group. There were no significant differences in the baseline characteristics between the two groups (Table 1, and Table S5 in the Supplementary Appendix).
Figure 1. Enrollment and Outcomes (Intention-to-Treat Analysis).
Patients who discontinued a trial drug were included in the intention-to-treat analysis, as were patients who withdrew consent or were lost to follow-up. In the as-treated analysis, data for patients who received a trial drug were censored at the time of discontinuation.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | Clopidogrel plus Aspirin (N = 2432) | Aspirin (N = 2449) |
|---|---|---|
| Median age (IQR) — yr | 65.0 (55.0–74.0) | 65.0 (56.0–74.0) |
| Female sex — no. (%) | 1097 (45.1) | 1098 (44.8) |
| Race — no./total no. (%)† | ||
| White | 1774/2360 (75.2) | 1781/2378 (74.9) |
| Black | 473/2360 (20.0) | 493/2378 (20.7) |
| Asian | 77/2360 (3.3) | 67/2378 (2.8) |
| Other | 36/2360 (1.5) | 37/2378 (1.6) |
| Hispanic ethnic group — no./total no. (%)† | 144/2320 (6.2) | 146/2328 (6.3) |
| Region — no. (%) | ||
| United States | 2014 (82.8) | 2029 (82.9) |
| Other countries | 418 (17.2) | 420 (17.1) |
| Medical history — no./total no. (%) | ||
| Ischemic heart disease | 257/2426 (10.6) | 240/2443 (9.8) |
| Hypertension | 1693/2423 (69.9) | 1680/2437 (68.9) |
| Diabetes mellitus | 678/2425 (28.0) | 662/2447 (27.1) |
| Medication use at presentation — no. (%) | ||
| Aspirin | 1417 (58.3) | 1397 (57.0) |
| Clopidogrel | 48 (2.0) | 42 (1.7) |
| Time from presentation to randomization | ||
| Mean time (±SD) — hr | 7.4±3.0 | 7.3±2.9 |
| Interval — no./total no. (%) | ||
| <6 hr | 755/2431 (31.1) | 789/2449 (32.2) |
| ≥6 hr | 1676/2431 (68.9) | 1660/2449 (67.8) |
| Qualifying event — no. (%) | ||
| TIA | 1056 (43.4) | 1052 (43.0) |
| Ischemic stroke | 1376 (56.6) | 1397 (57.0) |
| Median qualifying neurologic score (IQR) | ||
| ABCD2 for TIA‡ | 5.0 (4.0–6.0) | 5.0 (4.0–5.0) |
| NIHSS for ischemic stroke§ | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) |
There were no significant differences in baseline characteristics between the two groups. IQR denotes interquartile range, and TIA transient ischemic attack.
Race or ethnic group was determined by the investigator. Hispanic ethnic group was assessed only in patients in the United States; the denominator excludes 233 patients for whom Hispanic status was unknown.
Among patients with TIA, the qualifying score was 4 or more on the ABCD2 scale, which ranges from 0 to 7, with higher scores indicating a greater risk of stroke. The scale is used to estimate the risk of recurrent stroke after a TIA on the basis of age, blood pressure, clinical features, duration of symptoms, and presence of diabetes. Scores were available for 2104 of the 2108 patients with TIA (1055 patients in the clopidogrel group and 1049 in the aspirin group).
A mong patients with ischemic stroke, the qualifying score was 3 or less on the National Institutes of Health Stroke Scale (NIHSS), which ranges from 0 to 42, with higher scores indicating a greater stroke severity. Scores were available for 2750 of the 2773 patients with ischemic stroke (1365 patients in the clopidogrel group and 1385 in the aspirin group).
Primary and Secondary Efficacy Outcomes
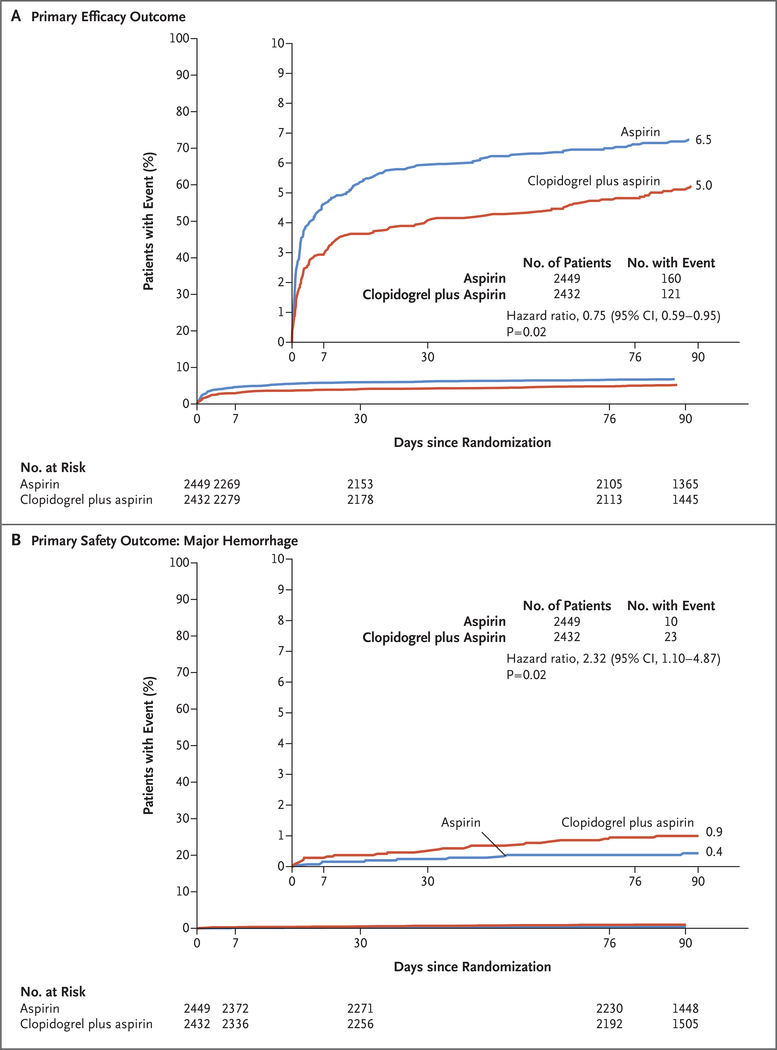
The composite primary efficacy outcome occurred in 121 patients (5.0%) receiving clopidogrel plus aspirin and in 160 patients (6.5%) receiving aspirin alone (hazard ratio, 0.75; 95% confidence interval [CI], 0.59 to 0.95; P = 0.02) (Table 2 and Fig. 2A). The secondary outcome of ischemic stroke occurred in 112 patients (4.6%) receiving clopidogrel plus aspirin and in 155 patients (6.3%) receiving aspirin alone (hazard ratio, 0.72; 95% CI, 0.56 to 0.92; P = 0.01). Except for stroke, there were no significant differences between treatment groups in the other components of the composite primary efficacy outcome (Table 2). The risk of total ischemic or hemorrhagic stroke was lower with clopidogrel plus aspirin than with aspirin alone (hazard ratio, 0.74; 95% CI, 0.58 to 0.94; P = 0.01). A post hoc Bonferroni-corrected P value that incorporates five main secondary outcome comparisons is shown in Table 2 for reference.
Table 2.
Efficacy and Safety Outcomes.
| Outcome | Clopidogrel plus Aspirin (N = 2432) | Aspirin (N = 2449) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| number (percent) | ||||
| Primary efficacy outcome | ||||
| Composite of ischemic stroke, myocardial infarction, or death from ischemic vascular causes | 121 (5.0) | 160 (6.5) | 0.75 (0.59–0.95) | 0.02 |
| Secondary efficacy outcomes | ||||
| Ischemic stroke | 112 (4.6) | 155 (6.3) | 0.72 (0.56–0.92) | 0.01* |
| Myocardial infarction | 10 (0.4) | 7 (0.3) | 1.44 (0.55–3.78) | 0.46* |
| Death from ischemic vascular causes | 6 (0.2) | 4 (0.2) | 1.51 (0.43–5.35) | 0.52* |
| Ischemic or hemorrhagic stroke | 116 (4.8) | 156 (6.4) | 0.74 (0.58–0.94) | 0.01* |
| Composite of ischemic stroke, myocardial infarction, death from ischemic vascular causes, or major hemorrhage | 141 (5.8) | 167 (6.8) | 0.84 (0.67–1.05) | 0.13* |
| Primary safety outcome | ||||
| Major hemorrhage | 23 (0.9) | 10 (0.4) | 2.32 (1.10–4.87) | 0.02 |
| Other safety outcomes | ||||
| Hemorrhagic stroke | 5 (0.2) | 3 (0.1) | 1.68 (0.40–7.03) | 0.47 |
| Symptomatic intracerebral hemorrhage | 2 (0.1) | 2 (0.1) | 1.01 (0.14–7.14) | 0.99 |
| Other symptomatic intracranial hemorrhage | 2 (0.1) | 0 | 0.16 | |
| Major hemorrhage other than intracranial hemorrhage | 17 (0.7) | 7 (0.3) | 2.45 (1.01–5.90) | 0.04 |
| Minor hemorrhage | 40 (1.6) | 13 (0.5) | 3.12 (1.67–5.83) | <0.001 |
| Death from any cause | 18 (0.7) | 12 (0.5) | 1.51 (0.73–3.13) | 0.27 |
Post hoc correction for multiple testing of five secondary end points by the Bonferroni method resulted in a P value of 0.01 to indicate a significant difference between groups.
Figure 2. Primary Efficacy and Safety Outcomes.
Shown are the percentages of patients with the primary efficacy outcome (a composite of ischemic stroke, myocardial infarction, or death from ischemic vascular causes) (Panel A) and the primary safety outcome of major hemorrhage (Panel B). Inset graphs show the same data on an expanded y axis.
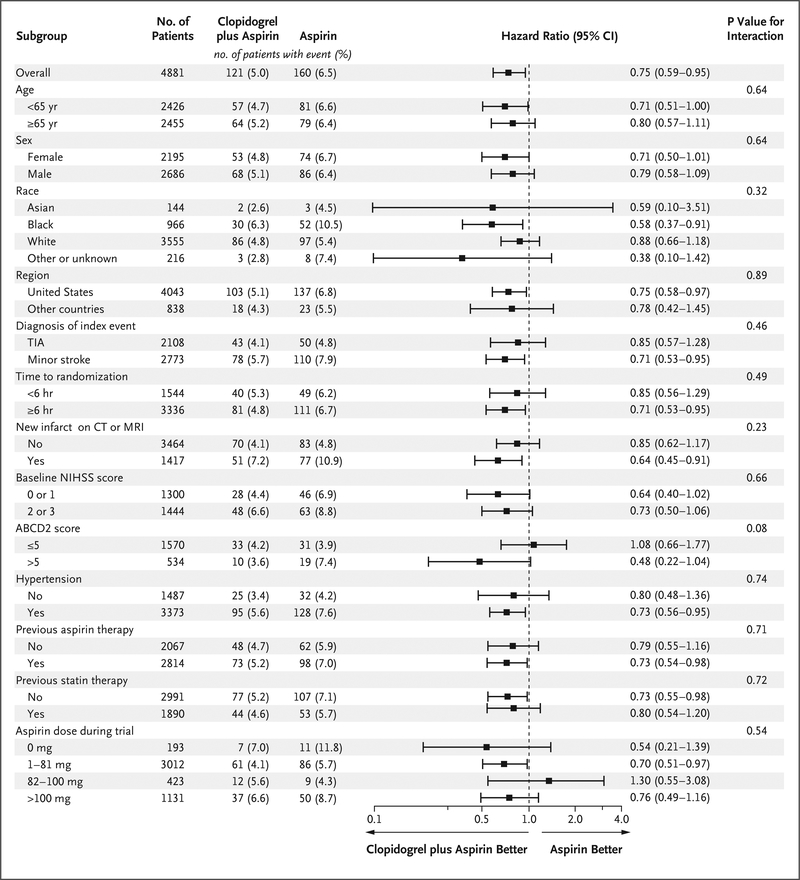
There were no significant treatment-by-subgroup interactions in prespecified subgroups, but the number of patients with available data for analysis limited the power to determine interactions (Fig. 3). There was no difference in treatment effect according to the predominant daily aspirin dose received during the trial period.
Figure 3. Primary Efficacy Outcome, According to Predefined Subgroup.
Race was determined by the investigator. Among patients with ischemic stroke, the qualifying score for participation in the trial was 3 or less on the National Institutes of Health Stroke Scale (NIHSS), which ranges from 0 to 42, with higher scores indicating greater stroke severity. The NIHSS score was missing at baseline for 23 patients, and 6 patients had an NIHSS score above 3 and were excluded from the subgroup analysis of NIHSS score (score of 0 or 1 vs. score of 2 or 3). Among patients with transient ischemic attack (TIA), the qualifying score was 4 or more on the ABCD2 scale, which is used to estimate the risk of recurrent stroke on the basis of age, blood pressure, clinical features, duration of symptoms, and presence of diabetes, with scores ranging from 0 to 7, with higher scores indicating a greater risk of stroke. CT denotes computed tomography, and MRI magnetic resonance imaging.
The proportional-hazard assumption of the treatment effect over a period of 90 days did not hold for the primary efficacy outcome. In a secondary analysis of the treatment effect according to time period, the benefit of clopidogrel plus aspirin was greater in the first 7 days and in the first 30 days than at 90 days (P = 0.04 for days 0 to 7 and P = 0.02 for days 0 to 30), whereas the risk of hemorrhage with clopidogrel plus aspirin versus aspirin alone was greater during the period from 8 to 90 days than during the first 7 days (P = 0.04 for days 8 to 90 and P = 0.34 for days 0 to 7) (Table S4 in the Supplementary Appendix).
The outcome of ischemic stroke, myocardial infarction, death from ischemic vascular causes, or major hemorrhage occurred in 141 patients (5.8%) receiving clopidogrel plus aspirin and in 167 patients (6.8%) receiving aspirin alone (hazard ratio, 0.84; 95% CI, 0.67 to 1.05; P = 0.13). Additional secondary and tertiary analyses are shown in Table S6 in the Supplementary Appendix.
PRIMARY AND SECONDARY SAFETY OUTCOMES
The primary safety outcome of major hemorrhage occurred in 23 of 2432 patients (0.9%) receiving clopidogrel plus aspirin and in 10 of 2449 patients (0.4%) receiving aspirin alone (hazard ratio, 2.32; 95% CI, 1.10 to 4.87; P = 0.02) (Table 2 and Fig. 2B). In analyses of secondary safety outcomes, there were no significant differences between groups in the rates of hemorrhagic stroke, symptomatic intracerebral hemorrhage, or other symptomatic intracranial hemorrhage considered separately (Table 2). Death from hemorrhagic vascular causes occurred in 3 patients receiving clopidogrel plus aspirin and in 2 patients receiving aspirin alone (0.1% in each group). Nonfatal, nonintracranial hemorrhage accounted for most of the major hemorrhages (16 in patients receiving clopidogrel plus aspirin and 7 in those receiving aspirin alone). Minor hemorrhage occurred in 40 patients (1.6%) receiving clopidogrel plus aspirin and in 13 (0.5%) receiving aspirin alone (hazard ratio, 3.12; 95% CI, 1.67 to 5.83; P = 0.002).
Serious adverse events other than components of the primary efficacy outcome were similar in the two groups, except that more patients receiving clopidogrel plus aspirin had events included in the Medical Dictionary for Regulatory Activities, version 15, coding designation “general disorders and administration-site conditions” (e.g., fever, fatigue, and edema) than those receiving aspirin alone (19 vs. 5, P = 0.004) (Tables S1 and S2 in the Supplementary Appendix). Reasons for early discontinuation of a trial drug are provided in Table S3 in the Supplementary Appendix.
AS-TREATED ANALYSES
In the as-treated analysis, major ischemic events occurred in 102 of 2398 patients (4.3%) treated with clopidogrel plus aspirin and in 141 of 2421 patients (5.8%) treated with aspirin alone (hazard ratio, 0.73; 95% CI, 0.56 to 0.94; P = 0.01). Major hemorrhage occurred in 21 patients (0.9%) treated with clopidogrel plus aspirin and in 6 patients (0.2%) treated with aspirin alone (hazard ratio, 3.57; 95% CI, 1.44 to 8.85; P = 0.003). Data regarding outcomes and adverse events in the as-treated population are provided in Tables S2 and S6 in the Supplementary Appendix.
DISSCUSSION
In this international, multicenter, randomized trial, we found that patients with minor ischemic stroke or high-risk TIA who received a combination of clopidogrel and aspirin had a lower risk of major ischemic events but a higher risk of major and minor hemorrhage than did those receiving aspirin alone. Ischemic stroke accounted for most of the composite events of the primary efficacy outcome, and the effect of dual antiplatelet treatment was attributable to a reduction in the rate of these strokes. It is not possible to make direct comparisons between clinical and safety outcomes because disability due to each of the outcomes cannot be ascertained, but we estimate that for every 1000 patients who are treated with clopidogrel plus aspirin during a period of 90 days, such treatment would prevent approximately 15 ischemic events and would cause 5 major hemorrhages.
The results of our trial broaden the results of the CHANCE trial involving Chinese patients to more diverse populations and care settings.12 In the CHANCE trial, there was a rate of moderate-to-severe bleeding of 0.3% in both the combined-antiplatelet group and the aspirin group. The results in the two trials apply to patients with stroke who were not appropriate candidates for anticoagulation, which thereby excluded those with stroke caused by presumed cardioembolism and those who were not candidates for treatment by intravenous thrombolysis or endovascular thrombectomy because their strokes were too mild to justify the use of these two treatments. The CHANCE trial tested a different combination of clopidogrel and aspirin than was used in our trial (two medications combined for the first 21 days, followed by clopidogrel alone with an initial loading dose of 300 mg, as compared with 600 mg of clopidogrel, followed by 75 mg per day, for the duration of our trial).12 The smaller loading dose or limited duration of combined clopidogrel plus aspirin may have reduced the risk of hemorrhage in the CHANCE trial, a hypothesis that is consistent with our finding that the benefit of clopidogrel plus aspirin was concentrated in the first month of the trial, whereas the risk of hemorrhage remained relatively constant throughout the trial. In addition, polymorphisms in the gene encoding CYP2C19 that are associated with incomplete metabolism of clopidogrel into its active form are common among persons of Asian ancestry.17
The international SOCRATES (Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes) trial compared ticagrelor, a P2Y12 inhibitor, with aspirin in an international population and found no between-group difference in the risk of major vascular events.18 The combination of ticagrelor and aspirin is being tested in the THALES (Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA [acetylsalicylic acid] for Prevention of Stroke and Death) trial (ClinicalTrials .gov number, NCT03354429). Blockade of platelet activity beyond what is achieved by clopidogrel and aspirin may lead to excess hemorrhage. In the TARDIS (Triple Antiplatelets for Reducing Dependency after Ischemic Stroke) trial, investigators compared a combination of clopidogrel, aspirin, and dipyridamole with either clopidogrel alone or aspirin plus dipyridamole administered within 48 hours after the onset of ischemic stroke or TIA.19 They found that patients who received the triple combination had no benefit with regard to the incidence and severity of recurrent stroke but had a higher rate of hemorrhage than those who received fewer medications.
Our trial has limitations. Patients with moderate-to-severe stroke, those with cardioembolic stroke, and those who are candidates for thrombolysis or thrombectomy were not represented in the trial, so results cannot be generalized to these groups. Entry criteria also resulted in a limited number of patients with symptomatic carotid atherosclerosis, a group that may benefit from platelet inhibition.20 A trial drug was discontinued permanently in 29% of the patients before trial follow-up was complete. However, rates of discontinuation were similar in the two treatment groups, and reasons for discontinuation were similar. Furthermore, most outcome events occurred early, before the majority of the discontinuations had occurred, and results in an as-treated analysis were similar to those in the intentionto-treat analysis. The overall event rates in our trial were lower than expected,1–4 particularly among the patients in the TIA group who had low ABCD2 scores, which suggests that some patients may not have had a TIA and would have been unlikely to benefit from treatment. The aspirin dose varied in the two treatment groups, which reflected the clinical practices of local investigators; however, in a potentially underpowered analysis, no difference in treatment effect was shown across aspirin doses.
In conclusion, in patients from diverse countries with minor ischemic stroke or high-risk TIA, those who received a combination of clopidogrel and aspirin had a lower risk of a composite of ischemic stroke, myocardial infarction, or death from ischemic vascular causes but had a higher risk of major hemorrhage than patients who received aspirin alone during the 90-day trial period.
Supplementary Material
Acknowledgments
Supported by grants (U01 NS062835, U01 NS056975, and U01 NS059041) from the National Institute of Neurological Disorders and Stroke. Sanofi provided clopidogrel and placebo for 75% of the patients in the trial.
Dr. Johnston reports receiving grant support from AstraZeneca; Dr. Easton, receiving grant support from AstraZeneca and consulting fees from Boehringer Ingelheim; Ms. Farrant, receiving grant support from AstraZeneca; and Dr. Kim, receiving grant support from SanBio and fees for serving as a member of a data and safety monitoring board from Neuravi. No other potential conflict of interest relevant to this article was reported.
Footnotes
REFERENCES
- 1.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency-department diagnosis of transient ischemic attack. JAMA 2000;2 84: 2901–6. [DOI] [PubMed] [Google Scholar]
- 2.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004; 328: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6: 1063–72. [DOI] [PubMed] [Google Scholar]
- 4.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007; 167: 2417–22. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke and major cardiovascular events after transient ischemic attack or minor ischemic stroke. N Engl J Med 2016; 374: 1533–42. [DOI] [PubMed] [Google Scholar]
- 6.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45:2 160–236. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997; 349: 1641–9. [PubMed] [Google Scholar]
- 9.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997; 349:1 569–81. [PubMed] [Google Scholar]
- 10.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49(3):e46–e110. [DOI] [PubMed] [Google Scholar]
- 11.Bowry AD, Brookhart MA, Choudhry NK. Meta-analysis of the efficacy and safety of clopidogrel plus aspirin as compared to antiplatelet monotherapy for the prevention of vascular events. Am J Cardiol 2008; 101: 960–6. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wang Y, Zhao X, et al. Clopi-dogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369:1 1–9. [DOI] [PubMed] [Google Scholar]
- 13.Johnston SC, Easton JD, Farrant M, et al. Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial: rationale and design. Int J Stroke 2013;8 : 479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:2 83–92. [DOI] [PubMed] [Google Scholar]
- 15.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients. J Thromb Haemost 2005; 3: 692–4. [DOI] [PubMed] [Google Scholar]
- 16.Diener HC, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008; 7:8 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Zhao X, Lin J, et al. Associa-tion between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 2016; 316:7 0–8. [DOI] [PubMed] [Google Scholar]
- 18.Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016; 375: 35–43. [DOI] [PubMed] [Google Scholar]
- 19.Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet 2018; 391: 850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amarenco P, Albers GW, Denison H, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 301–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.