Abstract
(R,S)-Ketamine produces rapid, robust, and sustained antidepressant effects in major depressive disorder. Specifically, its pharmacological efficacy in treatment refractory depression is considered a major breakthrough in the field. However, the mechanism of action of ketamine’s rapid effect remains to be determined. In order to identify pathways that are responsible for ketamine’s effect, a targeted metabolomic approach was carried out using a double-blind, placebo-controlled crossover design, with infusion order randomized with medication-free patients with treatment-resistant major depressive disorder (29 subjects) and healthy controls (25 subjects). The metabolomic profile of these subjects was characterized at multiple time points, and a comprehensive analysis was investigated between the following: MDD and healthy controls, treatment and placebo in both groups and the corresponding response to ketamine treatment. Ketamine treatment resulted in a general increase in circulating sphingomyelins, levels which were not correlated with response. Ketamine response resulted in more pronounced effects in the kynurenine pathway and the arginine pathway at 4 h post-infusion, where a larger decrease in circulating kynurenine levels and a larger increase in the bioavailability of arginine were observed in responders to ketamine treatment, suggesting possible mechanisms for response to ketamine treatment.
Keywords: Global Arginine Bioavailability Ratio; Sphingomyelins; Kynurenine metabolites; (R,S)-ketamine; Metabolomics
Introduction
Major depressive disorder (MDD) affects approximately 16% of the world population (Kessler et al. 2003) and remains among the leading cause of disability worldwide and number one cause of suicide (Olin et al. 2012). Despite available therapeutic options, many of which target monoaminergic neurotransmission, about a third of patients fail to achieve remission (Rush et al. 2006; Trivedi et al. 2006) and the relapse rate remains high, underscoring the need for new alternative targets in depression. Since the initial discovery that a single administration of a sub-anesthetic dose of glutamatergic modulator (R,S)-ketamine produces rapid and sustained antidepressant effects in MDD patients (Berman et al. 2000), multiple subsequent randomized controlled trials have shown that ketamine has rapid and sustained antidepressant actions on treatment-resistant MDD and bipolar disorder (Zarate et al. 2006; Diazgranados et al. 2010a, b; Zarate et al. 2012a; Iadarola et al. 2015). Although the discovery of ketamine’s antidepressant effects is considered an undisputed breakthrough in the treatment of depression, ketamine’s clinical use is not standardized and limited in part due to its possible risks including dissociation, cognitive impairment, psychotomimetic effects, and potential for abuse liability even at low sub-anesthetic doses (Krystal et al. 1994; Morgan and Curran 2012; Sanacora et al. 2017; Short et al. 2018). While the undesirable side effects of ketamine are attributed largely to N-methyl-D-aspartate receptor (NMDAR) inhibition, the pharmacological target that mediates the antidepressant effect of ketamine is a matter of dispute. This may involve direct effects of ketamine on the NMDAR or other targets (Zanos et al. 2018) and/or actions of ketamine’s metabolites including the hydroxynorketamines (HNKs) (Zarate et al. 2012,b; Zanos et al. 2016).
Metabolomics provides a comprehensive analysis of lipids, amino acids, biogenic amines, and other metabolic products within a given biological subject. Through careful analysis of the metabolic profile of a subject, both before and after treatment, as well as a comparison between healthy and disease states, the biological pathways involved in the pathophysiology of the disease can be elucidated (Gowda et al. 2008). In fact, in a recent study that used metabolomics to compare treatment with ketamine/esketamine vs controls in MDD patients, it was shown that changes in several metabolites, including phospholipids, tyrosine, and tryptophan, were different between responders/non-responders (Rotroff et al. 2016). However, in that study, the patients continued receiving antidepressant medications during the study and no healthy controls were evaluated. Characterizing the metabolic profile that differentiates MDD patients from healthy subjects, understanding the metabolic changes that occur with ketamine treatment within each group and between groups and distinguishing between responders and non-responders may help identify downstream targets relevant to the mechanism of action of ketamine for the treatment of MDD. Here, we report a targeted metabolomic profiling for a randomized controlled trial, which tested the effectiveness of treatment (ketamine and placebo) in MDD patients and healthy controls.
Methods
Subjects
Men and women, ages 18 to 65 years with diagnosis of recurrent major depressive disorder (MDD) without psychotic features as diagnosed using the Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Version (First et al. 2001) and with an age of onset ≤ 40 years, were eligible participants. Potential participants were enrolled if they had a score of ≥ 20 on the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) at screening and before each infusion. In addition, each subject had to have failed to respond to at least one prior adequate antidepressant trial, assessed by the Antidepressant Treatment History Form, and was experiencing a current major depressive episode of at least 4 weeks duration (Sackeim 2001). Subjects were either un-medicated or tapered off medications for a minimum of 2 weeks before randomization (5 weeks for fluoxetine, 3 weeks for aripiprazole). Individuals with a DSM-IV diagnosis of drug or alcohol dependence or abuse within the past 3 months, serious, unstable illness, or uncorrected hypo- or hyperthyroidism were excluded. Additional study details have been previously published (Nugent et al. 2018).
Healthy control subjects consisted of males and females, 18–65 years old with no Axis I disorder as determined by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID)-Patient Version (First et al. 2001) and no family history of Axis I disorders (in first-degree relatives). All subjects were studied at the National Institute of Mental Health (NIMH) Clinical Research Center Mood Disorders Research Unit in Bethesda, Maryland.
The study was approved by the Combined Neuroscience Institutional Review Board (IRB) at the National Institutes of Health (NIH). All subjects provided written informed consent before entry into the study. Informed consents and ongoing study participation for patients with MDD were monitored by the Human Subjects Protection Office at NIH (NCT00088699).
Ketamine administration
Subjects received intravenous infusions of either saline solution or 0.5 mg/kg of ketamine hydrochloride, 2 weeks apart, utilizing a double-blind, placebo-controlled crossover design, with infusion order randomized. The scheme of ketamine infusion was similar to a previously described procedure (Zarate et al. 2006), where patients received a single intravenous infusion of 0.5 mg/kg of (R,S)-ketamine hydrochloride over the course of 40 min. Subjects were rated 60 min prior to each infusion (baseline) and at 40, 80, 120, and 230 min as well as 1, 2, 3, 7, 10, and 11 days after the infusion. Baseline and post-ketamine infusion scores for depression were assessed using MADRS and psychotic symptoms were obtained using the Brief Psychiatric Rating Scale (BPRS) positive and total symptoms subscale (Overall and Gorham 1962). Response was considered > 50% improvement from baseline on the MADRS. Non-response was considered as < 50% improvement from baseline on the MADRS (Zimmerman et al. 2004).
Patient samples
Plasma samples (ACD) were collected using the vacutainer system 60 min prior to the initiation of ketamine infusion, at 40 min (end of the infusion), 80 min, 110 min, and 230 min, and Days 1, 2, and 3 post-infusion. Samples were centrifuged at 3000 rpm at 4 °C for 10 min and stored at − 80 °C until assay.
Targeted metabolomics
Targeted metabolites were extracted and concentrations were assessed using the AbsoluteIDQ kit p180 (Biocrates Life Science AG, Austria), following the manufacturer’s protocol for the API5500 LC-MS/MS System (AB SCIEX, USA) running with Analyst 1.5.2 software equipped with an electrospray ionization source, a Shimadzu CBM-20A command module, LC-20AB pump, and a Shimadzu SIL-20ACHT autosampler and a CTO-10Ac column oven heater, as previously described (Moaddel et al. 2016). Briefly, 10 μl of plasma samples was pipetted onto a 96-well Biocrates kit, and samples were dried for 30 min. Fifty microliters of 5% PITC reagent was added and incubated for 20 min and the plate was dried under nitrogen for 1 h. Three hundred microliters of 5 mM ammonium acetate in methanol was added and incubated at room temperature on a shaker (450 rpm) for 30 min. The plate was centrifuged at 100×g for 2 min; 50 μl of each sample was transferred to a 96-deep-well LC plate and 10 μl of each sample was transferred to the 96-deep-well FIA plate. Four hundred fifty microliters of 40% methanol was added to the LC plate. Four hundred ninety microliters of FIA running solvent was added to the FIA plate. Ten microliters was injected onto the Eclipse XDB C18, 3.5 μm, 3.0 × 100 mm with a Phenomenex C18 Security Guard Cartridge, 3.0 mm ID. The mobile phase consisted ofsolvent A (water containing 0.2% formic acid) and solvent B (acetonitrile containing 0.2% formic acid), with the following gradients: 0–0.5 min 0% B, 5.5 min 95% B; 6.5 min 95% B; 7.0 min 0% B; 9.5 min 0% B for the LC plate. Evaluation of the samples was carried out using the MetIDQ software. The FIA plate was run with 20 μl injection directly onto the MS at a flow of 30 μl/min with water/acetonitrile (1:1) containing 0.2% formic acid as the mobile phase, with the following flow rate program: 0–1.6 min 30 μl/min; 2.4 min 200 μl/min; 2.8 min 200 μl/min; and 3.0 min 30 μl/min. Concentrations were calculated using the Analyst/MetIDQ software and reported in micromolar (Suppl Table 1).
Tryptophan-kynurenine metabolome method
Concentrated stock solutions of standards were prepared at 1000 μg/ml and stored at − 20 °C. Tryptophan (TRP), quinolinic acid (QA), picolinic acid (PA), 3-hydroxy anthranilic acid (3-HAA), and serotonin (5HT) were dissolved in 50:50 methanol:water with 0.1% formic acid. Kynurenine (KYN) and 3-hydroxy kynurenine (3-HK) were dissolved in 0.1% formic acid in methanol. Xanthurenic acid (XA) and kynurenic acid (KA) were dissolved in DMSO. Anthranilic acid (AA) was dissolved in water. The deuterated internal standards were dissolved in the same solvent as their corresponding standard.
Separation of the kynurenines was accomplished using an X-Select HSS C18 guard column (2.1 × 5 mm) and an XSelect HSS C18 column (2.1 × 150 mm, 2.5 μm, Waters) at 40 °C. Mobile phase A consisted of 0.2% aqueous formic acid and mobile phase B was 0.2% formic acid in methanol. The following linear gradient was run for 30 min at a flow rate of 0.3 ml/min: 0–1 min 5% B, 3 min 23% B, 3.1–5 min 70% B, 5.5–20 min 90% B, 20.1 min 10% B, 21 min 5% B. Calibration curves were prepared in 0.1% formic acid in 10:90 methanol:water by a 0.5 serial dilution of standards from 100,000 ng/ml to 195.31 ng/ml for TRP; 5000 ng/ml to 9.77 ng/ml for KYN; 2500 ng/ml to 4.89 ng/ml for 3-HK; 1250 ng/ml to 2.44 ng/ml for PA, 3HAA, and 5HT; 312.50 ng/ ml to 0.61 ng/ml for XA, KA, and AA.
The metabolites were quantified using area ratios calculated using their corresponding deuterated standard. D4-KYN was used as the internal standard for 3-HK, 3-HAA, and AA. The internal standard consisted of 10,000 ng/ml D5-TRP and 500 ng/ml D4-KYN, D4-XA, D5-KA, D4-PA, D3-5HT. A pooled sample of the study subjects was run each day.
To 40 μl plasma, 10 μl internal standard and 10 μl 0.1% formic acid in water were added. Solid phase extraction cartridges (OasisHLB, Waters Corp.)wereconditioned with1 ml methanol, then 1 ml water. The samples were added and washed with 100 μl water. Finally, the metabolites were eluted with 1 ml 0.1% formicacid in 95:5 methanol:water and stream dried under nitrogen. The samples were reconstituted in 100 μl 0.1% formic acid in 10:90 methanol:water and transferred to autosampler vials for analysis.
Data was acquired using a Nexera XR HPLC (Shimadzu) coupled with a QTRAP 6500+ (SCIEX) and was analyzed with Analyst 1.6 (SCIEX). The positive ion mode data was obtained using multiple reaction monitoring (MRM). The instrumental source settings for curtain gas, ion spray voltage, temperature, ion source gas 1, and ion source gas 2 were 30 psi, 5500 V, 500 °C, 40 psi, and 50 psi, respectively. The collision-activated dissociation was set to medium and the entrance potential was 10 V. The standards (Supplemental Table 1A) and internal standards (Supplemental Table 1B) were characterized using the MRM ion transitions, declustering potentials (DP), collision energies (CE), and collision cell exit potentials (CXP) listed in Supplemental Table 1.
Statistical methods
Statistical analyses were performed using R statistical analysis software version 3.2.0 (http://www.r-project.org). Prior to analysis, metabolites were log2 transformed, centered at the transformed minimum, and scaled by the transformed range to ensure that all means were constrained between 0 and 1 and that all mean differences were constrained between − 1 and 1 to allow comparisons of effect sizes between metabolites.
Baseline metabolite concentrations between MDD and healthy controls were compared using t tests. Differences in changes in metabolite concentrations by treatment group (ketamine vs placebo) and disease status (MDD and healthy control) were compared using generalized estimating equations to account for within-participant correlation over time (baseline, 230 min, 1 day, and 3 days). Each metabolite concentration was regressed on treatment, disease status, time, all two- and three-way interactions of the aforementioned factors, and period (whether participants were randomized to receive ketamine in the first or second period to account for the cross-over design) and computed robust empirical standard errors. Treatment effects were computed as signed Euclidean distance measures, namely the estimated mean squared differences in changes from baseline, which form the numerator of an F test, were averaged across each follow-up time point. The sign indicated whether ketamine treatment was associated with more positive changes (e.g., larger increases or smaller decreases) than placebo.
Changes in metabolites between responders (> 50% improvement from baseline on the MADRS) and non-responders were compared, separately at both 230 min and 1 day by computing metabolite change scores (concentration at follow-up minus concentration at baseline) and regressing changes on responder status and baseline metabolite values.
The Benjamini-Hochberg approach to computing q-values was utilized, which measures the proportion of false positives, to address multiple comparisons (Benjamini and Hochberg, 1995). Unadjusted p values were also reported. Statistical significance was defined as q-value < 0.05. We also note when results have marginal significance (q-value between 0.05 and 0.10), or when q-value > 0.10, but p value < 0.05.
Results
Characteristics of the population
The subject population in this study included 29 MDD subjects who met DSM-IV TR criteria and 25 healthy controls, with an age range 18–65 (Nugent et al. 2018). There were no significant differences in demographic characteristics between healthy controls and patients with MDD.
Plasma analysis
Plasma samples from 54 subjects (29 MDD and 25 Healthy Controls) were analyzed for 186 distinct metabolites, including amino acids, biogenic amines, acyl carnitines, phosphatidyl cholines, lysophosphatidyl cholines, and sphingomyelins. Of these, 58 metabolites fell below the lower level of quantitation in > 20% of samples and were excluded from analysis. Of the biogenic amines and amino acid metabolites, the majority were at or above the limit of quantitation. Of the lipids, sphingomyelins (SM), lysophosphatidyl cholines (LPC), and phosphatidyl cholines (PC) were analyzed. For the PCs, “aa” indicates that both moieties at the sn-1 and sn2 positions are fatty acids and bound to the glycerol backbone via ester bonds—”ae” denotes that one of the moieties, either in the sn-1 or at sn-2 position, is a fatty alcohol and bound via an ether bond. Sphingolipids contain a sphingoid base attached to an acyl group such as a fatty acid (Dinoff et al. 2017) and are the most common form of lipid in brain cell membranes.
MDD vs control at baseline
The initial comparisons carried out were aimed at identifying potential biomarkers for MDD vs control subjects. At baseline, no metabolite significantly differed between medication-free MDD and controls at a q-value < 0.1, although a small number of metabolites differed with p value < 0.05 (Table 1; Suppl Fig. 1). Kynurenine/tryptophan ratio (Kyn/Trp) was higher in MDD than in controls (p = 0.007), and serine (Ser) and Trp were higher in controls than in MDD (77.8 ± 21.9 μM vs 62.9 ± 14.6 μM; p = 0.008) and (43.24 ± 10.7 μM vs 37.32 ± 10.8 μM) (p = 0.049), respectively (Table 1; Suppl Fig. 1). Kyn did not significantly differ between participants with MDD and controls at baseline (p = 0.94). Threonine, citrulline, and SMC26:1 were all also found to be higher in controls relative to MDD (all p < 0.05) (Table 1; Suppl Fig. 1). The lower circulating levels of threonine (82.5 ± 22.5 μM vs 96.7 ± 27.4 uM) and citrulline (19.2 ± 5.2 μM vs 23.8 ± 7.2 μM) in MDD patients relative to healthy controls are consistent with those of previous reports (Baranyi et al. 2015; Hess et al. 2017).
Table 1.
Baseline mean values of metabolites that were significantly different between MDD vs healthy controls (Student t test, p value < 0.05)
| Metabolites | Control (μM) | MDD (μM) |
|---|---|---|
| Citrulline | 23.80 ± 7.22 | 19.25 ± 5.17 |
| Serine | 77.81 ± 21.86 | 62.93 ± 14.62 |
| Threonine | 96.70 ± 27.43 | 82.47 ± 22.54 |
| Tryptophan | 43.24 ± 10.74 | 37.32 ± 10.79 |
| Alpha-amino adipidic acid | 0.75 ± 0.59 | 0.68 ± 0.56 |
| Kynurenine | 1.32 ± 0.43 | 1.31 ± 0.36 |
| Serotonin | 0.17 ± 0.16 | 0.19 ± 0.19 |
| SM C26:1 | 0.21 ± 0.08 | 0.16 ± 0.06 |
| Kynurenine/Trp | 0.03 ± 0.01 | 0.035 ± 0.008 |
Treatment effects
Treatment effects: Healthy control
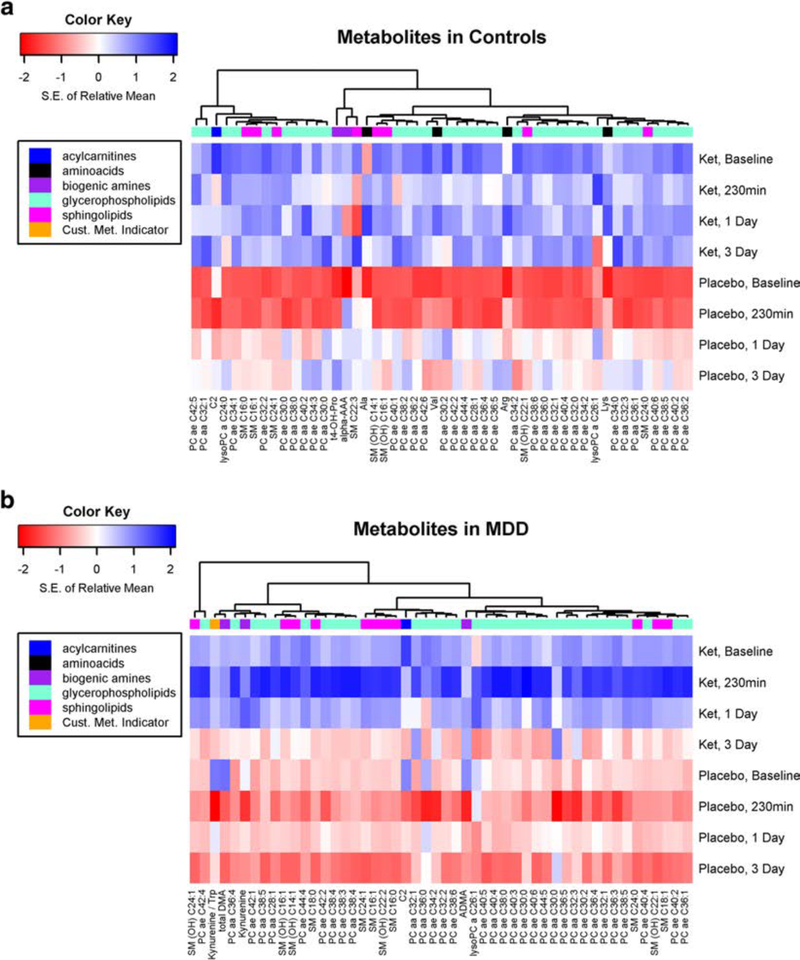
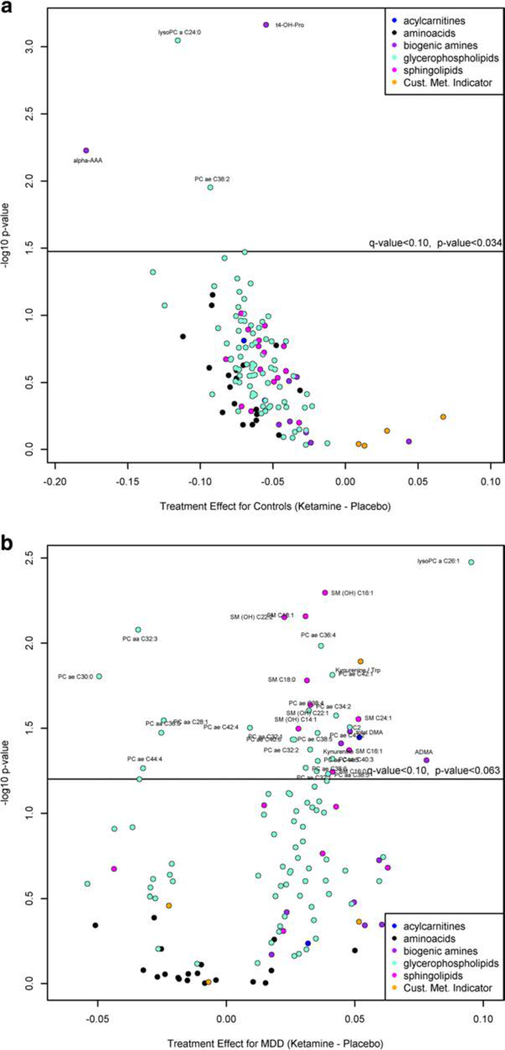
A heat map (Fig. 1a) shows the 50 most significantly different changed metabolites between ketamine treatment and placebo in healthy controls. Of these metabolites, the metabolites that show the most significant changes over time after ketamine administration relative to placebo in healthy controls with a q-value < 0.05 are displayed in Fig. 2a (Suppl Table 2A). Alpha-amino adipidic acid (α-AAA) decreased from baseline to day 1 following ketamine treatment, while in placebo it increased to 230 min and then slightly declined (p = 0.006). A decrease in circulating levels of α-AAA would be beneficial, as it is a known mGluR2 agonist (Guldbrandt et al. 2002), which has been reported to be an astrocytic toxin that can induce depressive-like behavior (Domin et al. 2014). However, as the circulating levels were near the limit of quantitation, these findings need to be replicated. Trans-4-hydroxy proline (t4-OHPro) changed little after ketamine but significantly increased after placebo (p= 0.0007). LPC C24:0 decreased with maximal effects observed atday 3, whilein the placebo group, circulating levels increased with maximal effects at day 3 (p= 0.0009),with baseline circulating levels similar between healthy controls and MDD subjects and previously reported values (0.18 ± 0.04 μM vs 0.19 ± 0.05 uM) (Psychogios et al. 2011; Rotroff et al. 2016). There were no significant differences in changes in Kyn/Trp or Kyn in ketamine vs placebo among controls.
Fig. 1.
a Heat map shows the top 50 most significantly differently changed metabolites between ketamine and placebo over time among healthy controls (without major depressive disorder). Rows indicate standardized mean of log-transformed metabolite by treatment group and time. The dendrogram from hierarchical clustering indicates groups of metabolites with similar treatment and time profiles. b Heat map shows the top 50 most significantly differently changed metabolites between ketamine and placebo over time among participants with major depressive disorder. Rows indicate standardized mean of log-transformed metabolite by treatment group and time. The dendrogram from hierarchical clustering indicates groups of metabolites with similar treatment and time profiles
Fig. 2.
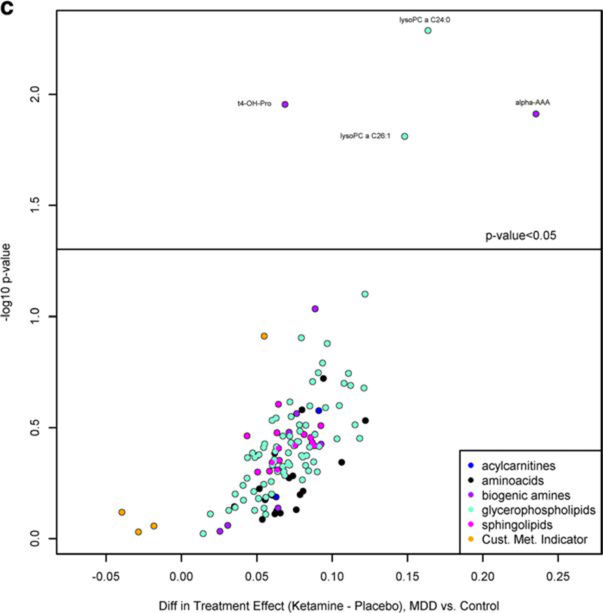
Volcano plot for ketamine treatment effect relative to placebo in a healthy controls (q-value < 0.05), b in MDD patients (q-value < 0.10), and c in MDD patients relative to healthy controls (q-value < 0.10). A positive value indicates that either (1) metabolite tended to increase more with ketamine than placebo over time, or (2) metabolite tended to decrease less with ketamine than placebo over time. A negative value indicates either that the metabolite tended to (1) increase less with ketamine than placebo over time, or (2) decrease more with ketamine than placebo over time. The specific pattern can be determined by plotting the time course of the metabolite
Treatment effects: MDD
A heat map (Fig. 1b) shows the 50 most significantly different changed metabolites between ketamine treatment and placebo in MDD patients. In this group, no metabolites were differentially affected by ketamine treatment (q-value < 0.05); however (Fig. 2b; Suppl Table 2A), 6 metabolites and 32 lipids (PCs and SMs) had marginally significant differences (q< 0.10) with no significant difference observed for any LPC (q ≥ 0.10), similar to a previous study that demonstrated no change in any circulating LPC levels in MDD subjects receiving ketamine or esketamine treatment (Rotroff et al. 2016). The metabolites that had q-value <0.10 and a p value <0.01 were the PC aa C32:3, LPC a C26:1, SM (OH) C16:1, SM C18:1, and SM (OH) C22:2. PCs tended to be lowest at the 230-min time point in the placebo group, whereas in the ketamine-treated groups, PCs tended to be the lowest at day 3, with marginal significance. While the vast majority of metabolites increased more with ketamine over placebo over time, C2 (acetyl carnitine), SM(OH) C14:1, PCaa C32:1, PC ae C34:2, and PC ae C42:4 had a smaller decrease in the ketamine-treated group relative to placebo. Furthermore, ketamine administration was followed by changes in Kyn/Trp and Kyn by this criterion (p= 0.013 and 0.039, respectively). After ketamine, Kyn/Trp increased slightly at 230 min then steadily dropped through day 3, whereas after placebo, Kyn/Trp sharply dropped at 230 min, increased at 1 day, then slightly dropped at day 3 (Suppl. Fig. 2). Following ketamine, Kyn changed little from baseline to day 1, then dropped at day 3, whereas on placebo, Kyn decreased sharply at 230 min, increased at day 1, and decreased slightly on day 3.
Treatment effects: MDD vs healthy control
Comparing the ketamine vs placebo treatment effects in MDD relative to controls, no metabolites were differentially affected by ketamine in MDD compared to controls at a q-value < 0.05. However, at a q-value < 0.10 (Fig. 2c; Suppl Table 2C), LPC a C24:0, LPCa C26:1, α-AAA, and t4-OHPro were significantly different between MDD and control. A decrease in circulating levels of LPCa, C24:0, and α-AAA was observed in healthy controls with ketamine treatment, with both metabolites increasing in the placebo group over time. In MDD patients, circulating LPC C26:1 levels increased for the first day post-ketamine treatment returning to baseline at day 3.
Responders vs non-responders
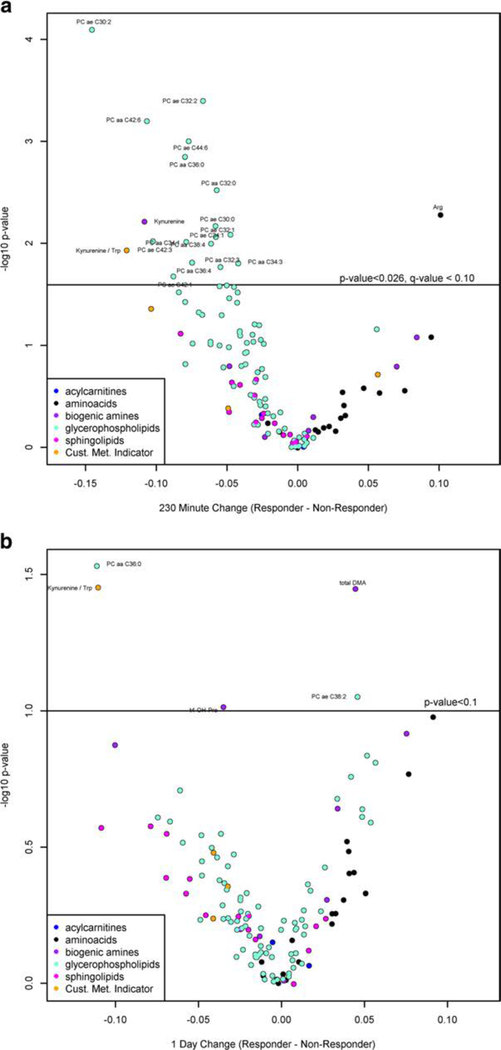
The MDD patients were also studied based on their response to ketamine treatment (responders/non-responders) at 230 min (8/ 21) or day 1 (10/19), determined by the improvement of the MADRS scores. At 230 min, no metabolite changes significantly differed among responders compared to non-responders after adjusting for baseline metabolites at the q-value < 0.05 level; however, multiple metabolites differed at a marginally significant q-value < 0.10 (Fig. 3a; Suppl Table 3A). Of note, Kyn and Kyn/Trp ratios were lower among responders (p = 0.006 and 0.012, respectively), while arginine was higher among responders (p = 0.005). Similarly, no metabolite changes were significantly different among responders compared to non-responders after adjusting for base line at the q-value< 0.10level at day 1. However, at a p value < 0.05, Kyn/Trp continued to be lower in responders (p = 0.035). In addition, total DMA was higher among responders (p = 0.036). Sixteen PCs were identified to be lower in responders than non-responders at 230 min post-infusion including two (PC aa 32:3; PC ae C30:0) which were previously identified as metabolites that decreased more in ketamine-treated vs placebo in MDD patients and 3 PCs (PC ae 32:1, PC ae 32:2; PC ae 42:1) that were shown to increase more in MDD subjects with ketamine treatment vs placebo. The remaining PCs (PC aa 32:0; PC aa 34:1, PCaa34:3; PC aa 36:4; PC aa 38:4; PC aa 42:6; PC ae 30:2; PC ae 34:1; PC ae 36:0; PC ae 42:3; PC ae 44:6) were not previously identified in our analysis. At 1-day post-infusion (Fig. 3b; Suppl Table 3B), only PC aa C36:0 remained significantly different between responder and non-responder, where it was shown to decrease more with ketamine treatment vs placebo in MDD subjects at day 1 (not significant, p value < 0.10, but q-value > 0.10) and PC ae C38:2 was also shown to be higher in responders vs non-responders at day 1.
Fig. 3.
Volcano plot for the determination of metabolites that were significantly different among responders compared to non-responders after adjusting for baseline metabolites at the q-value < 0.10 level based on the improvement of the MADRS scores at a 230 min (8 responders/21 non-responders) and b day 1 (10 responders/19 non-responders). Positive values indicate that either (1) responders had a larger increase than non-responders, or responders had a smaller decrease than non-responders
Kynurenine metabolites
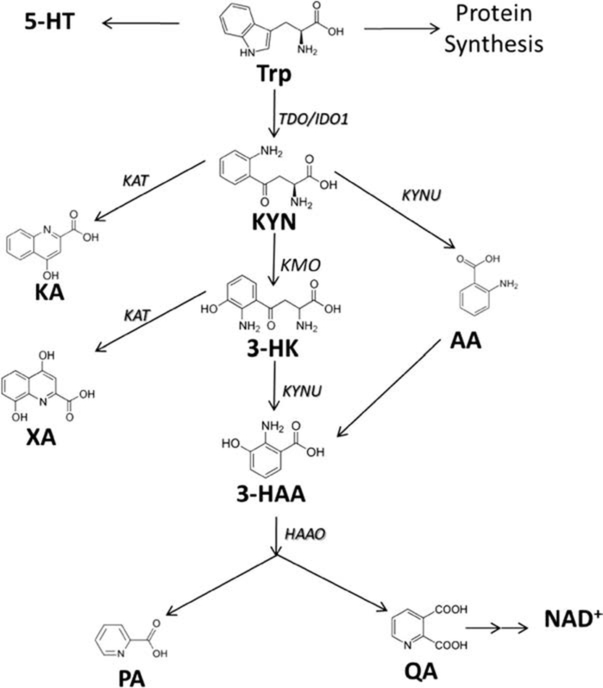
Changes in Trp, Kyn, and/or the Kyn/Trp ratio were observed across several comparisons; as a result, eight metabolites from the Kyn metabolome (Scheme 1)were quantified using a newly developed LC-MS/MS method in healthy controls and MDD subjects. Each Kyn pathway metabolite was log2 transformed, scaled, normalized (value at 230 min minus baseline), and regressed on responder status adjustment for baseline. Of the eight metabolites tested (Suppl Table 4), anthranilic acid and picolinic acid were higher in responders vs non-responders at 230 min (p = 0.004 and 0.13, respectively).
Scheme 1.
Kynurenine metabolome
Discussion
In this study, a targeted metabolomic analysis was carried out comparing patients with MDD and healthy controls, ketamine treatment effects on MDD patients and healthy controls, and changes in the metabolic profile between responders and non-responders. Comparing patients with MDD and healthy controls at baseline, no metabolite was significantly different (q-value < 0.1). A contributing factor to difference observed from previous studies that demonstrated statistically significant differences for certain metabolites between MDD subjects and healthy controls (Steffens et al. 2010, Baranyi et al. 2015, Hess et al. 2017) may be that in our study the MDD patients were both treatment resistant and not on medication during the study. In addition, the lack of differences in circulating amino acids was also consistent with a report by Maes et al., where the authors also did not observe changes in serum amino acid levels (Maes et al. 1998). Marginal significance (p < 0.05) was observed, however, with healthy controls having higher levels of serine, Trp, threonine, citrulline, and SMC26:1 and MDD patients having a higher Kyn/Trp ratio. Replication in a larger sample of a similar patient population is needed.
The effects of ketamine on healthy controls and MDD patients demonstrated that in healthy controls, α-AAA, t4OHPro, and LPC24:0 were significantly affected with ketamine treatment, while in MDD patients, PCs and SMs (Fig. 2), as well as changes in Kyn/Trp ratio and circulating Kyn levels, were marginally affected. When comparing treatment effects between MDD and healthy controls, only LPC a C24:0, LPCa C26:1, α-AAA, and t4-OHPro were affected. The stratification of MDD patients into responders/non-responders identified Kyn and Kyn/Trp ratios (lower among responders) and arginine (higher among responders) as potential prognostic biomarkers for ketamine response. Based on these results, the Kyn and arginine pathways, as well as lipids, will be discussed in greater detail, as several metabolites in each group were identified across multiple comparisons.
Kynurenine pathway
The role of the kynurenine pathway in the pathophysiology of depression has been well established (Reus et al. 2015). Trp is a substrate for the generation of several bioactive compounds with various important physiological roles including anabolic processes, yet the majority of Trp is metabolized along the Kyn pathway (Cervenka et al. 2017). This pathway is critical as it generates a range of metabolites that are involved in the interface of inflammatory/immune response and glutamatergic neurotransmission (Miller 2013). The two most important rate-limiting enzymes involved in the catabolism of Trp to Kyn are 2,3-dioxygenase (TDO), mainly expressed in the liver, and indoleamine 2,3-dioxygenase (IDO), with IDO1 mainly expressed in the blood, placenta, lungs, and brain and IDO2 mainly expressed in the brain (Moaddel et al. 2016). IDO is upregulated during pro-inflammatory states induced by cytokines (IL-1β, IL-6, IFN-α, TNF-α) (Heisler and O’Connor 2015) and is a therapeutic target in depression (Dobos et al. 2012). In our study, there was no statistically significant difference in the Kyn levels but a higher circulating Trp level in healthy control subjects when compared to MDD patients (p = 0.049) was observed. This is consistent with a previous study where a decrease in Trp levels in depressed patients was reported (Maes et al. 2011), indicating a shunted “metabolism of tryptophan away from serotonin production towards kynurenine production”(Oxenkrug 2013). Further, the baseline Kyn/Trp ratio was found to be higher in MDD patients than in that in controls (p = 0.007). This is similar to the observation seen in a mouse model of neuroinflammation-induced depression, where an increase in the Kyn/Trp ratio was observed and was abated by the addition of an IDO inhibitor (Dobos et al. 2012). Other studies have shown mixed results concerning the metabolites from the Kyn pathway, with some having reported higher circulating Kyn levels in MDD patients (Sublette et al. 2011, Allen et al. 2018), while others reported no difference between controls and depressed patients for Trp, Kyn or the Kyn/Trp ratio (Sorgdrager et al. 2017). In this study, circulating levels of Trp, Kyn, and the Kyn/Trp ratio were examined based on responder status at 230 min and day 1 and Kyn and Kyn/Trp ratio were lower among responders. This difference was maintained up to day 1 for the Kyn/Trp ratio (p = 0.035), similar to previous reports where they also found lower Kyn concentrations in responders (Allen et al. 2018). Based on these results, the study was expanded to examine the metabolites of Kyn and their relation to response to ketamine treatment. Kyn is converted to a series of metabolites (Scheme 1), including several neuroprotectants (kynurenic acid, picolinic acid) and neurotoxins (3-hydroxykynurenine, quinolinic acid). In this study, of all the Kyn metabolites analyzed, only anthranilic acid was significantly higher in responders (p = 0.004) at 230 min (Suppl Table 2). Recent clinical data demonstrated changes in 3-hydroxy anthranilic acid and anthranilic acid levels in patients with a range of neurological and other disorders, including depression (Darlington et al. 2010), with depressed patients presenting a decrease in 3-hydroxyanthranilic acid levels and an increase in anthranilic acid levels.
Arginine pathway
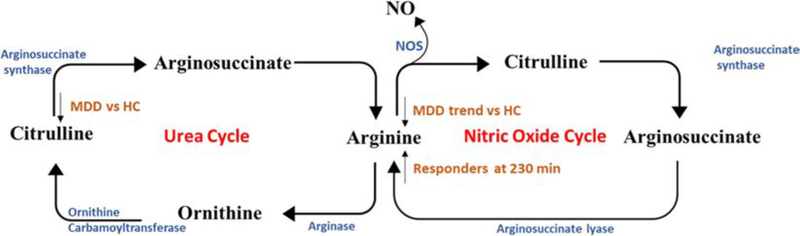
Arginine is a substrate for protein synthesis and a precursor for several metabolites including polyamines, proline, ornithine and glutamate (Morris 2006). Arginine is hydrolyzed by arginase to ornithine and urea in the urea cycle, where it is further oxidized to citrulline. Arginine can also be converted to nitric oxide and citrulline by nitric oxide synthases (Wegener and Volke 2010), in the nitric oxide cycle (Scheme 2). Consequently, changes in arginine levels have the potential to disrupt many cellular and organ functions.
Scheme 2.
Arginine pathways
In our study, lower circulating levels of citrulline were observed in MDD patients relative to healthy controls (19.2 ± 5.2 μM vs 23.8 ± 7.2 μM), and lower average circulating levels of arginine were observed in MDD subjects, albeit not significant. These results are consistent with previous reports (Baranyi et al. 2015; Hess et al. 2017, Ali-Sisto et al. 2018), where the authors showed decreased circulating levels of arginine and citrulline in MDD patients. No differences were observed for citrulline or arginine when comparing treatment with ketamine to placebo in healthy controls or MDD patients. However, in the MDD group, comparing responders against non-responders, arginine levels were shown to significantly increase in responders, with an increase of 16.6 ± 8.8 μM in responders vs an increase of 7.1 ± 10.7 μM in non-responders at 230 min (p = 0.005). The increase in arginine levels is consistent with a recent study that also found a similar increase with esketamine treatment (Rotroff et al. 2016). Other antidepressants have also been shown to have an effect in this pathway, with duloxetine resulting in an increase in the circulating levels of citrulline in a study with 12 MDD patients, while bupropion was associated with higher arginine levels in a study with 6 MDD patients (AliSisto et al. 2018). The results of the study clearly demonstrate that ketamine is modulating the bioavailability of arginine in responders to ketamine treatment and that the nitric oxide cycle may be a potential mechanism of action of ketamine in responders to ketamine treatment. Several groups have shown significantly lower circulating levels of nitric oxide (NO) in MDD groups compared to healthy controls (Chrapko et al. 2004), and have shown that the antidepressant paroxetine increased circulating NO levels in healthy controls (Lara et al. 2003) and in MDD patients (Chrapko et al. 2006). NO has been shown to serve as a messenger molecule in a number of physiological processes linked to the major psychiatric diseases, including depression (Wegener and Volke 2010). Multiple factors affect NO levels including arginase activity, amino acid levels, NOS activity, and the presence of specific endogenous NOS inhibitors, such as asymmetric dimethylarginine (ADMA). Arginase activity, determined by the arginine/ ornithine ratio, had higher changes with responders relative to non-responders (p = 0.044) at 230 min when adjusted for base-line levels, indicating the increased bioavailability of arginine may result from decreased arginase activity, similar to what was found in a recent study, where they also observed a decrease in arginase activity after first improvement of depression (Baranyi et al. 2015; Hess et al. 2017). A novel approach for a more precise measurement of the bioavailability of arginine and NO production is the Global Arginine Bioavailability Ratio (GABR) which is calculated as arginine/(citrulline + ornithine) (Baranyi et al. 2015, Ali-Sisto et al. 2018). Lower GABR has been associated with increased risk in cardiovascular disease (Sourij et al. 2011), has been shown to be lower in PTSD subjects (Bersani et al. 2016) and MDD subjects (Ali-Sisto et al. 2018), and was negatively associated with PTSD symptom severity and was inversely correlated with inflammation markers IL-6 and TNF-α (Bersani et al. 2016). In our study, the GABR increased more at 230 min for responders relative to non-responders (0.34 vs 0.17, p = 0.027), but not at 1 day (p = 0.244), consistent with the increased arginine levels at 230 min in responders. This result is not surprising, as it was previously demonstrated that GABR increased with first improvement of depression in a separate study (Baranyi et al. 2015). NO has been shown to serve as a messenger molecule in a number of physiological processes linked to the major psychiatric diseases, including depression (Wegener and Volke 2010). NO is generated by NMDA-receptor coupled neuronal nitric oxide synthase (nNOS) (Wegener and Volke 2010), and has been associated with synaptic plasticity, memory, long-term potentiation, and the regulation of blood pressure (Forstermann and Sessa 2012). Whether NO is directly acting on NOS or through other metabolites is unknown. ADMA, a known NOS inhibitor (Wegener and Volke 2010), was also studied and in our study no difference was observed with baseline ADMA levels between any group (Harraz and Snyder 2017).
Ketamine’s mechanism of action, may therefore, be elicited through the arginine pathway and the nitric oxide cycle, as responders had a greater increase in circulating arginine levels in response to ketamine treatment relative to non-responders and that response was related to the bioavailability of arginine.
Lipids
Of the lipids analyzed in MDD subjects (Fig. 2b), 11/15 PCs and 8/9 SMs were previously identified as metabolites that were negatively associated with ketamine response (Rotroff et al. 2016) using a similar platform. In that study, the circulating metabolite levels increased with decreasing MADRS scores. In this analysis, similar trends were observed in the ketamine treatment group, with the majority having little change in the placebo group. Sphingomyelins (SM (OH) C16:1, SM C18:1, and SM (OH) C22:2) were found to have a significant increase in MDD patients treated with ketamine relative to placebo (p < 0.05).Further, SMC26:1 was shown to be present in higher concentrations in healthy controls vs MDD subjects (0.21 ± 0.08 μM vs 0.16 ± 0.06 μM, p = 0.01). These data are consistent with previous reports of increased acidic sphingomyelinase activity in peripheral blood mononuclear cells in subject with major depression (Kornhuber et al. 2005), where MDD patients had lower circulating levels of SMs. Acid sphingomyelinase catalyzes the hydrolysis of sphingomyelin to phosphorylcholine and ceramide (Gracia-Garcia et al. 2011; Mühle et al. 2013). Ceramide is further degraded to sphingosine via ceramidase (Mühle et al. 2013), where sphingosine is phosphorylated to sphingosine-1-phosphate (S1P) via sphingosine kinase (SphK)1/2, which acts through S1P1-receptors (Anderson and Maes 2014), and has been shown to result in increased levels of BDNF and neurogenesis (Anderson and Maes 2014). The balance between these two sphingolipids is referred to as the “sphingolipid-rheostat” as increased ceramide levels can initiate apoptosis and increased S1P concentrations generally promote cell survival and proliferation (van Brocklyn and Williams 2012). Previous studies have suggested that sphingolipid metabolism may be a potential novel therapeutic target in depression (Kornhuber et al. 2005; Dinoff et al. 2017), as higher circulating levels of ceramides C16:0, C18:0, C20:0, C24:1, and C26:1 (Gracia-Garcia et al. 2011) and lower circulating levels of SMs have been reported in patients with MDD. In our study, ketamine induced a significant increase in circulating concentration of SMs in MDD patients relative to placebo, suggesting that ketamine may be having an effect on acid sphingomyelinase activity, and as a result decreasing circulating ceramide levels; however, further studies in this pathway are needed to confirm these findings. When comparing responders and non-responders, no SMs were identified at 230 min to be different between responder and non-responders (Fig. 3a), suggesting that response to ketamine treatment is not dependent on changes in circulating SM levels.
Conclusions
Inflammation, including increased levels of pro-inflammatory cytokines and changes in the nitric oxide cycle, has been shown to play a key role in the pathogenesis of MDD (Kiecolt-Glaser et al. 2015). In our study, we demonstrated that ketamine has effects on the kynurenine pathway and arginine pathway, both of which are upregulated under inflammatory conditions. Our data shows that ketamine treatment resulted in an immediate (230 min) decrease in the Kyn/Trp ratio and a decrease in circulating Kyn levels, as well as a more pronounced increase in the GABR in responders. The data suggest that response to ketamine treatment may be dependent on the ability of ketamine to elicit a pronounced effect on these pathways. In addition, the SM pathway was also identified as a pathway that was affected by ketamine treatment but did not distinguish between responders and non-responders.
This study had several strengths. Notably, subjects were well characterized and hospitalized for several weeks before and after the infusion, and were drug free prior to the experiment. Nevertheless, several limitations should be noticed; while the results are intriguing, they need to be replicated on a larger sample cohort. Further, the expansion of the study to an untargeted approach may offer additional insights into the mechanism of action of ketamine. In addition, we aim that in the future studies, we may include the effect of ketamine repeat dose treatment for treatment-resistant depression patients over a longer period of time.
Supplementary Material
Acknowledgments
Funding This research was supported by the Intramural research Program of the National Institutes of Health, National institute on Aging and National Institute on Mental Health and NCATS.
Footnotes
Compliance with ethical standards
The study was approved by the Combined Neuroscience Institutional Review Board (IRB) at the National Institutes of Health (NIH). All subjects provided written informed consent before entry into the study. Informed consents and ongoing study participation for patients with MDD were monitored by the Human Subjects Protection Office at NIH (NCT00088699).
Conflict of interest C.A.Z. is listed as a co-inventor on a patent application for the use of ketamine in major depression. R.M. and C.A.Z. are listed as co-inventors on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro- and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. R.M., P.M., C.J.T., T.D.G., and C.A.Z are listed as co-inventors on a patent application for the use of (2R, 6R)-hydroxynorketamine and (2S, 6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. R.M., P.M., C.J.T., and C.A.Z have assigned their patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. T.D.G has assigned their patent rights to the University of Maryland Baltimore but will share a percentage of any royalties that may be received by the University of Maryland Baltimore.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00213–018-4992–7) contains supplementary material, which is available to authorized users.
References
- Ali-Sisto T, Tolmunen T, Viinamaki H, Mantyselka P, Valkonen-Korhonen M, Koivumaa-Honkanen H, Honkalampi K, Ruusunen A, Nandania J, Velagapudi V, Lehto SM (2018) Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J Affect Disord 229:145–151 [DOI] [PubMed] [Google Scholar]
- Allen AP, Naughton M, Dowling J, Walsh A, O’Shea R, Shorten G, Scott L, McLoughlin DM, Cryan JF, Clarke G, Dinan TG (2018) Kynurenine pathway metabolism and the neurobiology of treatment-resistant depression: comparison of multiple ketamine infusions and electroconvulsive therapy. J Psychiatr Res 100:24–32 [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M (2014) Reconceptualizing adult neurogenesis: role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol Disord Drug Targets 13(1):126–136 [DOI] [PubMed] [Google Scholar]
- Baranyi A, Amouzadeh-Ghadikolai O, Rothenhausler HB, Theokas S, Robier C, Baranyi M, Koppitz M, Reicht G, Hlade P, Meinitzer A (2015) Nitric oxide-related biological pathways in patients with major depression. PLoS One 10(11):e0143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4):351–354 [DOI] [PubMed] [Google Scholar]
- Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, Makotine I, Abu-Amara D, Coy M, Reus VI, Epel ES, Marmar C, Mellon SH (2016) Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun 52:153–160 [DOI] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, Ruas JL (2017) Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357(6349):eaaf9794. [DOI] [PubMed] [Google Scholar]
- Chrapko W, Jurasz P, Radomski MW, Lara N, Archer SL, Le Melledo J-M (2004) Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psych 56:129–134 [DOI] [PubMed] [Google Scholar]
- Chrapko W, Jurasz P, Radomski MW, Archer SL, Newman SC, Baker G, Lara N, Le Melledo J-M (2006) Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology 31(6):1286–1293 [DOI] [PubMed] [Google Scholar]
- Darlington LG, Forrest CM, Mackay GM, Smith RA, Smith AJ, Stoy N, Stone TW (2010) On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res 3:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010a) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67(8): 793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr (2010b) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71(12):1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoff A, Saleem M, Herrmann N, Mielke MM, Oh PI, Venkata SLV, Haughey NJ, Lanctot KL (2017) Plasma sphingolipids and depressive symptoms in coronary artery disease. Brain Behav 7(11): e00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos N, de Vries EF, Kema IP, Patas K, Prins M, Nijholt IM, Dierckx RA, Korf J, den Boer JA, Luiten PG, Eisel UL (2012) The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis 28(4): 905–915 [DOI] [PubMed] [Google Scholar]
- Domin H, Szewczyk B, Woźniak M, Wawrzak-Wleciał ŚM (2014) Antidepressant-like effect of the mGluR5 antagonist MTEP in an astroglial degeneration model of depression. Behav Brain Res 273: 23–33 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams AR (2001). Structured clinical interview for DSM-IV TR axis I disorders, research version, patient edition (SCID-I/P).
- Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837 837a–837d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D (2008) Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn 8(5):617–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Garcia P, Rao V, Haughey NJ, Bandaru VV, Smith G, Rosenberg PB, Lobo A, Lyketsos CG, Mielke MM (2011) Elevated plasma ceramides in depression. J Neuropsychiatry Clin Neurosci 23(2): 215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldbrandt M, Johansen TN, Frydenvang K, Brauner-Osborne H, Stensbol TB, Nielsen B, Karla R, Santi F, Krogsgaard-Larsen P, Madsen U (2002) Glutamate receptor ligands: synthesis, stereochemistry, and enantiopharmacology of methylated 2-aminoadipic acid analogs. Chirality 14(4):351–363 [DOI] [PubMed] [Google Scholar]
- Harraz MM, Snyder SH (2017) Antidepressant actions of ketamine mediated by the mechanistic target of rapamycin, nitric oxide, and Rheb. Neurotherapeutics 14(3):728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler JM, O’Connor JC (2015) Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 50: 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S, Baker G, Gyenes G, Tsuyuki R, Newman S, Le Melledo JM (2017) Decreased serum L-arginine and L-citrulline levels in major depression. Psychopharmacology 234(21):3241–3247 [DOI] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA Jr (2015) Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis 6(3):97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289(23):3095–3105 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Derry HM, Fagundes CP (2015) Inflammation: depression fans the flames and feasts on the heat. Am J Psych 172(11). 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Medlin A, Bleich S, Jendrossek V, Henkel AW, Wiltfang J, Gulbins E (2005) High activity of acid sphingomyelinase in major depression. J Neural Transm 112(11):1583–1590 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3):199–214 [DOI] [PubMed] [Google Scholar]
- Lara N, Archer SL, Baker GB, Le Melledo J-M (2003) Paroxetine-induced increase in metabolic end products of nitric oxide. J Clin Psychopharm 23(6):641–645 [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R (2011) The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35(3): 702–721 [DOI] [PubMed] [Google Scholar]
- Miller AH (2013) Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology 38(9):1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Fabbri E, Khadeer M, Carlson OD, Gonzalez-Freire M, Zhang P, Semba RD, Ferrucci L (2016) Plasma biomarkers of poor muscle quality in older men and women from the Baltimore longitudinal study of aging. J Gerontol Ser A 71(10):1266–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389 [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV, Independent Scientific Committee on Drugs (2012) Ketamine use: a review. Addiction 107:27–38 [DOI] [PubMed] [Google Scholar]
- Morris SM (2006) Arginine: beyond protein. Am J Clin Nutr 83(2): 508S–5512S [DOI] [PubMed] [Google Scholar]
- Mühle C, Huttner HB, Walter S, Reichel M, Canneva F, Lewczuk P, Gulbins E, Kornhuber J (2013) Characterization of acid sphingomyelinase activity in human cerebrospinal fluid. Plos One. 10.1371/journal.pone.0062912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA Jr (2018) Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Psychiatry, Mol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin B, Jayewardene AK, Bunker M, Moreno F (2012) Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS One 7(10):e48002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812 [Google Scholar]
- Oxenkrug G (2013) Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets 14(5): 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J (2015) Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res 68:316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotroff DM, Corum DG, Motsinger-Reif A, Fiehn O, Bottrel N, Drevets WC, Singh J, Salvadore G, Kaddurah-Daouk R (2016) Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl Psychiatry 6(9):e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163(11):1905–1917 [DOI] [PubMed] [Google Scholar]
- Sackeim HA (2001) The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62(Suppl 16):10–17 [PubMed] [Google Scholar]
- Sanacora G, Heimer H, Hartman D, Mathew SJ, Frye M, Nemeroff C, Robinson Beale R (2017) Balancing the promise and risks of ketamine treatment for mood disorders. Neuropsychopharmacology 42(6):1179–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5(1):65–78 [DOI] [PubMed] [Google Scholar]
- Steffens DC, Jiang W, Ranga K, Krishnan R, Karoly ED, Mitchell MW, O’Connor CM, Kaddurah-Daouk R (2010) Metabolomic differences in heart failure patients with and without major depression. J Geriatr Psychiatry Neurol 23(2):138–146. 10.1177/0891988709358592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgdrager FJH, Doornbos B, Penninx BWJH, de Jonge P, Kema IP(2017) The association between the hypothalamic pituitary adrenal axis and tryptophan metabolism in persons with recurrent major depressive disorder and healthy controls. J Affect Disord 222:32–39 [DOI] [PubMed] [Google Scholar]
- Sourij H, Meinitzer A, Pilz S, Grammer TB, Winkelmann BR, Boehm BO, Marz W (2011) Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 218(1):220–225 [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT (2011) Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun 25(6):1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SDS (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163(1):28–40 [DOI] [PubMed] [Google Scholar]
- van Brocklyn JR, Williams JB (2012) The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: oxidative stress and the seesaw of cell survival and death. Comp Biochem Physiol B Biochem Mol Biol 163(1):26–36 [DOI] [PubMed] [Google Scholar]
- Wegener G, Volke V (2010) Nitric oxide synthase inhibitors as antidepressants. Pharmaceuticals (Basel) 3(1):273–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604):481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD (2018) Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32(3):197–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8):856–864 [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012a) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71(11):939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012b) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72(4):331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I (2004) Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. J Psychiatr Res 38(6):577–582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.