Abstract
Background:
Health care facilities are considering the use of reusable respiratory protective devices (RPDs) to mitigate a potential N95 filtering facepiece respirator shortage caused by an influenza pandemic. US regulators are also considering stockpiling reusable RPDs for pandemic preparedness, but limited data exist on the effectiveness of cleaning and disinfection of these devices. This study defines reprocessing protocols and evaluates their effectiveness against a pandemic influenza strain in a laboratory setting.
Methods:
Five half-mask elastomeric respirator models and 3 powered air-purifying respirator models were contaminated with influenza virus and artificial skin oil on multiple surfaces. RPDs were then manually treated with 1 of 2 methods: cleaned or cleaned and disinfected. Presence of viable influenza was determined via swab sampling and a median tissue culture infectious dose assay.
Results:
Across 41 RPD surfaces, a mean log reduction in viable influenza of 4.54 ±0.97 log10 median tissue culture infectious dose was achieved for all treated surfaces, which included both cleaned and cleaned and disinfected surfaces.
Conclusions:
The methods defined as part of this study are effective for eliminating viable influenza in the presence of artificial skin oil on most of the RPD surfaces tested. Material type and RPD design should be considered when implementing RPD reprocessing protocols.
Keywords: N95, Respiratory protection, Disinfection, Cleaning infection control, Reuse, Mucin, Sebum
Influenza transmission in health care settings is a substantial safety concern that places patients, health care workers (HCWs), and other staff at risk for infection.1 Given the potential severity of health consequences (ie, illness and death) associated with pandemic influenza, a comprehensive pandemic influenza preparedness plan should address airborne transmission in addition to contact and droplet transmission to ensure that HCWs are protected against all potential routes of exposure.2 The use of a particulate respirator that is at least as protective as a National Institute of Occupational Safety and Health-approved N95 filtering facepiece respirator (FFR) is listed by the Occupational Safety and Health Administration (OSHA) as a recommendation for pandemic influenza preparedness.2 The Centers for Disease Control and Prevention (CDC) issued guidance calling for the use of N95 respirators for HCW protection during the initial stages of the 2009 H1N1 pandemic.3 Because HCWs are at risk of exposure to airborne infectious agents, including influenza,2 an adequate supply of respiratory protection devices (RPDs) must be available for the HCW population. The supply of single-use N95 FFRs during an influenza pandemic or a widespread outbreak of other infectious respiratory illnesses may be inadequate,2,4,5 which could potentially result in shortages for health care facilities, as was observed during the early part of the 2009 H1N1 pandemic.6–9 Reusable RPDs, such as half-mask elastomeric respirators (HMERs) and powered air-purifying respirators (PAPRs), have been identified as an option to mitigate a potential FFR shortage.4,6,10
Even when used properly, personal protective equipment (PPE) has the potential to become fomites via handling, aerosol deposition of respiratory secretions, or other transmission route.11,12 As opposed to single-use N95 FFRs, which are intended to be disposed of after each use, reusable RPDs require reprocessing (cleaning and disinfecting) to maintain sanitary conditions as often as necessary or before being used by a different individual according to OSHA13; manufacturer’s guidance may vary. The requirement to clean and disinfect respirators necessitates the establishment of reprocessing protocols for HCWs to follow. According to CDC guidance, cleaning refers to the removal of visible soil from objects and surfaces and normally is accomplished manually or mechanically using water with detergents or enzymatic products. Disinfection is defined as a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects usually through the use of liquid chemicals or wet pasteurization.14
OSHA requires reprocessing procedures to be included in an employer’s respiratory protection program for all worksites where respirator use is required.13 According to OSHA, an employer must use either the cleaning and disinfecting procedures recommended by OSHA or the procedures recommended by the respirator manufacturer, as long as the procedures are equivalent in effectiveness to the OSHA method.13 Other disinfection or sterilization methods, such as ethylene oxide exposure or steam autoclaving, are generally not compatible with HMERs or PAPRs.15 Ultimately, clear and specific instructions should be provided to HCWs in such a way that they can easily understand and follow to reprocess reusable RPDs in a safe and effective manner. Yet, depending on the source, guidance for cleaning and disinfecting respirators does not always provide the same type of information necessary to perform these procedures.
Currently, guidance for HMER reprocessing varies between manufacturers in regard to the level of detail provided to the user.16–20 For example, 3M (St Paul, MN) defines the cleaning agent for their 6200/7502 HMER models as a 3M respirator wipe in addition to a warm cleaning solution not exceeding 120°F and the disinfecting agent as a 0.4% bleach solution with an undefined contact time.16,17 Honeywell (Morris Plains, NJ) defines the cleaning agent for their North 7700 model simply as a cleaner sanitizer solution to be used according to its instructions.20 Briefly, OSHA’s cleaning guidance recommends the use of a mild detergent with water at a 110°F maximum temperature followed by rinsing and draining. For disinfection, OSHA defines 2 disinfecting agents and provides appropriate concentrations and contact times.13 A 2015 study performed by Bessesen et al21 evaluated reprocessing procedures provided by HMER manufacturers. As part of this study, 6 subjects tested manufacturers’ instructions for use (IFUs) for cleaning and disinfecting an HMER; all participants made multiple errors during the HMER reprocessing. Out of 66 attempts, 31 errors were made using the manufacturers’ IFUs. Observations made by the study’s authors include that there was no mention of PPE, the difficulty of reading the IFUs due to small print, and no contact time specified for disinfecting solutions for the HMER models used in this study.21
PAPR reprocessing can be even more complicated due to the various PAPR components having their own separate guidance on reprocessing.22–24 There is also variability in the level of detail provided in reprocessing guidance between manufacturers and device models from the same manufacturer. For example, to properly clean all of the components of a 3M Air-Mate PAPR system, there are at least 5 different protocols to follow: blower unit, breathing tube, belt, hood, and battery, not all of which have recommended cleaning steps provided by the manufacturer.22 Based on the product manuals, a disinfection protocol is provided for the 3M Breathe Easy model, but not for the 3M Air-Mate or Syntech MAXAIR 78SP SeriesPAPR (Stilwell, KS) models.22–24 To further complicate the task of establishing PAPR reprocessing protocols, OSHA’s recommended practices for RPD reprocessing cannot be used with several PAPR parts due to their electrical components, leaving guidance gaps that may hinder HCWs from being able to effectively reprocess PAPRs.13
The US Food and Drug Administration (FDA) uses the Spaulding classification scheme of medical devices as critical, semicritical, or noncritical according to the degree of risk for infection involved in use of the items and accordingly recommends the appropriate microbicidal processes for each category.25 Critical devices are introduced directly into the bloodstream or contact a normally sterile tissue during use and must be cleaned and sterilized after each use. Semicritical devices contact intact mucous membranes or nonintact skin and must be cleaned and either sterilized or treated with a highlevel disinfection process. Noncritical devices contact intact skin only (without penetration) and must be cleaned and treated with either an intermediate- or low-level disinfection process depending on the level of contamination.
Currently, reusable RPDs are not cleared by the FDA for use in hospitals, yet there are health care institutions using the devices as part of their respiratory protection program.21,26 The Veterans Health Administration has stockpiled 3 models of reusable HMERs as a means to meet demand for respiratory protection during an influenza or other large-scale aerosol transmissible outbreak.21 FDA clearance would likely require data supporting the effectiveness of reprocessing protocols, but few studies assessing the effectiveness of cleaning and disinfection protocols for HMERs and PAPRs have been published. In 2014, Subhash et al27 performed a study evaluating the effectiveness of common health care disinfectant wipes against H1N1 influenza on HMERs. Using viable assays, they determined quaternary ammonium/isopropyl alcohol and bleach detergent wipes eliminated live virus, whereas 70% isopropyl alcohol alone was ineffective, albeit based on only 1 surface per respirator and 1 HMER model. Other limitations of this study were the inoculum titer used in the study is unknown and the highest viable recovery was only 73 plaque-forming units, capping the maximum demonstrable effectiveness at <1-log reduction in viability. Additionally, the influenza virus was applied in the absence of protective factors such as soiling agents. In general, very few data are available from viral decontamination studies on HMERs and PAPRs using soiling agents. The spread of viruses is expected to occur primarily via large droplets or contact, indicating that the presence of soiling agents like skin oil or saliva is likely. Soiling agents can shield viruses from environmental factors (eg, temperature, humidity, and ultraviolet light) as well as physical and chemical decontaminants.14
The objectives of this study were to define detailed, comprehensive methods for cleaning and disinfecting HMERs and PAPRs when challenged with influenza virus in the presence of soiling agents, and subsequently assess their effectiveness. These methods are largely based on existing practices recommended by OSHA and RPD manufacturers, while addressing guidance gaps to ensure these procedures are being performed in a safe and effective manner. Five HMER models and 3 PAPR models were contaminated with H1N1 influenza and artificial skin oil, then were either cleaned only or cleaned and disinfected using the methods defined as part of this study.
MATERIALS AND METHODS
H1N1 influenza
H1N1 influenza A/PR/8/34 (ATCC VR-1469) was propagated in embryonic chicken eggs (Charles River Premium Specific Pathogen Free Eggs 10100326) using standard World Health Organization (WHO) protocols.28 Virus titers were determined by 50% tissue culture infectious dose (TCID50) assay. Madin-Darby canine kidney cells (ATCC CCL-34) were passaged and maintained using WHO-approved cell culture techniques.
Test respirators
Five commercially available HMER models and 3 commercially available PAPR models were tested for this study (Table 1). RPD models were selected based on a combination of a National Institute of Occupational Safety and Health survey, Veterans Health Administration use of HMERs, and HMERs used by Ciconte and Danyluk.10 Each model has a unique design with different surface types that could influence cleaning efficiency. To account for this, multiple surface types were inoculated for each respirator model. Additionally, the same PAPR hood model was used for both 3M PAPR systems tested as part of this study. It should be noted the 3M Air-Mate PAPR model was discontinued by the manufacturer as of June 30, 2017, but replacement parts will be available until June 30, 2019 (personal communication).
Table 1.
Reusable respiratory protection devices (RPDs) selected for this study
| RPD type | RPD model |
|---|---|
| Half-mask elastomeric respirator | 3M* 6200 |
| 3M* 7502 | |
| Scott† Xcel | |
| Sperian by Honeywell‡ Survivair Blue 1H | |
| North by Honeywell‡ 7700 | |
| Powered air-purifying respirator | 3M* Air-Mate |
| 3M* Breathe Easy Turbo | |
| Syntech§ Maxair 78SP series |
3M, St Paul, MN.
Scott Safety, Monroe, NC.
Honeywell Inc, Morris Plains, NJ.
Syntech, Stilwell, KS.
HMER reprocessing studies
Nine replicates of each HMER model were aseptically inoculated with 10 1-μL droplets per surface, totaling 107 log10 TCID50 H1N1 influenza for each surface tested. HMERs were inoculated on 4 surfaces: the exterior of the facemask nose and mouth, head strap, and adjustment strap. HMER models were also inoculated on the filter cartridge cover, except the North 7700 model, which did not have protective filter covers. Inoculated surfaces were allowed to dry at room temperature for approximately 20 minutes. After the inoculum dried, synthetic sebum (Scientific Services S/D, Sparrow Bush, NY) was applied over each inoculum to act as a protective factor and soiling agent. The sebum overlay was prepared by pipetting 2.5 mL liquefied sebum into a 100-mm Petri dish that was rotated to spread the synthetic skin oil evenly. A triangle-shaped spreader was used to collect the sebum from the Petri dish. The collected sebum was then applied to the inoculum area at a density of approximately 2.5 mg/cm2.
Three inoculated HMER replicates were used for each test: 1 was left untreated and served as a control mask to quantify the challenge concentration, 1 was cleaned only, and 1 was cleaned and disinfected (bleach soak, see below). Procedures for HMER cleaning and disinfecting were based on procedures recommended by OSHA.13 Reprocessors donned a disposable lab coat, eye protection, and used a double-glove technique, changing the outer gloves when potentially contaminated. After inoculating the HMERs with both influenza and sebum, HMERs were aseptically transported to a class I biological safety cabinet where cartridges, if present, were removed from the mask and placed in an empty reservoir. HMERs and cartridge covers were placed in a 5 L Nalgene pan (ThermoFisher Scientific, Irvine, CA) with 1 L 32°C-43°C 0.5% Neutrawash detergent solution (Getinge USA, Inc, Rochester, NY) and wiped with a sterile sponge. The external face of the mask was wiped first, and then the sponge was folded over each strap for wiping; the inside of the mask was wiped last. Each HMER and cartridge cover was then rinsed with 1 L 32°C-43°C nonsterile water over the same pan. The external face of the mask and the straps were rinsed first and then the inside of the mask was rinsed. For cartridges, the front side of each cartridge was wiped with a sponge soaked in 0.5% Neutrawash solution and then wiped with a sponge soaked in water only to remove any detergent. Overall, the cleaning process required about 2–3 minutes to complete.
For HMER disinfection, HMERs and cartridge covers were transferred to a separate 5 L Nalgene pan containing 3 L 0.1% household bleach solution (Clorox Bleach, The Clorox Co, Oakland, CA). Each side of the HMERs and cartridge covers were immersed in the bleach solution for 2 minutes. Each HMER and cartridge cover was then rinsed with 1 L 32°C-43°C tap water to remove any bleach. For cartridge disinfection, a Super SaniCloth (PDI, Orangeburg, NY) with an alcohol quaternary antimicrobial was used to wipe the exterior surfaces and allowed to dry at room temperature for approximately 2 minutes.
Cleaning and disinfection steps required 21 minutes per batch (3 respirators per batch) and the drying period was approximately 20 minutes for the HMER body, but more than 6 hours for the HMER straps. It is important to note that these time estimates do not include the time required to sterilize potentially contaminated materials used during reprocessing (ie, cleaning pan and sponges).
After cleaning and/or disinfecting, each inoculated surface (including untreated) was sampled using a sterile polyester swab moistened with serum-free Eagle’s minimum essential medium (EMEM).29 Each swab was placed in a 50-mL tube containing 10 mL serum-free EMEM and vortexed for 5 minutes to extract the influenza virus. Extracts were subsequently serially diluted in serumfree EMEM and plated in quadruplicate in 24-well plates with confluent monolayers of Madin-Darby canine kidney cells according to the WHO protocol for a TCID50 assay.28 Plates were then incubated at 37°C in 5% carbon dioxide for 7 days. After the incubation period, each well was observed under the microscope for cytopathic effects, generally demonstrated by a disruption of the cell monolayer. Plates were subsequently stained with crystal violetglutaraldehyde to confirm the presence of cytopathic effects.
PAPR reprocessing studies
Nine replicates of each PAPR model were aseptically inoculated with 10 1-μL droplets per surface, totaling 107 log10 TCID50 H1N1 influenza for each surface tested. Due to the different system designs, the inoculation locations varied between PAPR models. The 3M Air-Mate model was inoculated on the motor blower unit, belt, belt clip, and breathing tube. The 3M Breathe Easy model was inoculated on the motor blower unit, filter cartridge, belt, belt clip, battery, and breathing tube. The same PAPR hood (3M BE-10) was used for both 3M PAPR models and was inoculated on the visor, Tychem (DuPont, Wilmington, DE), and breathing tube connection. The Syntech MAXAIR model was inoculated on the helmet, visor, and battery pack. Procedures for PAPR cleaning and disinfecting were based on a combination of procedures recommended by OSHA and Oregon Health and Science University.13,30 PPE used for PAPR reprocessing was the same as PPE used for HMER reprocessing described above.
Three inoculated PAPR replicates were used for each test: 1 was left untreated and served as a control mask, 1 was cleaned only, and 1 was cleaned and disinfected. All external surfaces of each PAPR model were wiped once with a sterile sponge moistened with a 42°C, 0.5% Neutrawash detergent solution and subsequently wiped with another sterile sponge soaked in 42°C nonsterile water to remove any residual detergent. PAPRs to be disinfected were then wiped with a Super SaniCloth and allowed to dry for 2 minutes. The 3M Breathe Easy PAPR motor was wiped around the cartridges, on the sides and back of the motor, on the external surfaces of the battery avoiding the switch, and on the belt clip. For the 3M Air-Mate, the front of the blower unit was wiped first, followed by the back and the sides, and then the belt and belt clip were wiped. The 3M hoods were first wiped on the crown of the hood, then the clear visor and breathing tube insert were wiped. Long wipes were made down the hood while rotating the hood, making sure all areas were wiped. The external surfaces of the breathing tubes were wiped using the same methods as the PAPR blower motors and hoods. The 3M Breathe Easy breathing tube was stretched and held in place by clamps attached to a ring stand for cleaning, disinfecting, and sampling. The Syntech Maxair was first wiped first across the top of the helmet and then across the clear visor. The battery was wiped last, taking care to avoid the plug for the battery cable. Similar virus sampling and quantification methods used in the HMER reprocessing studies were also used for PAPRs.
The time required to clean and disinfect an entire single unit was approximately 15 minutes, not including drying time. Additionally, preparation and cleanup steps combined required approximately 20 minutes to complete. These time estimates do not include the time required to sterilize potentially contaminated materials used during reprocessing (ie, cleaning pan and sponges).
Data analysis
To determine the level of viable virus recovered from each sampled location, the Spearman-Karber formula was used to interpret the TCID50 assay data.31 To perform statistical analyses, Environmental Protection Agency guidance using half the detection limit (0.20 log10 TCID50) for below detection limit values was followed.32 A 1-way analysis of variance with a Dunnett’s posttest was used to determine statistical significance when comparing differences between control and treated respirators for each RPD model. If needed, a 2-tailed unpaired t test was used to determine the P value for significant differences identified by the analysis of variance.
RESULTS
HMER reprocessing studies
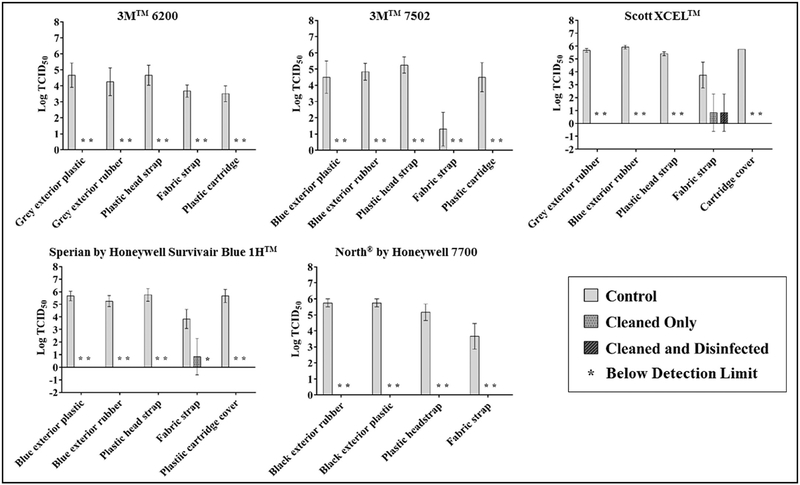
The mean viable influenza recovered from all untreated HMER surfaces was 4.76 ± 1.23 log10 TCID50 (Fig 1). A mean recovery of 5.15 ± 0.79 log10 TCID50 was achieved for nonporous surfaces and 3.24 ± 1.23 log10 TCID50 was recovered from porous surfaces (fabric straps). For cleaned-only surfaces, the mean log reduction was 4.55 ± 0.79, and only 2 of 24 surfaces demonstrated recoverable viable virus: 0.97 ± 1.33 log10 TCID50 from the fabric strap of the Scott model (Scott Safety, Monroe, NC) and 0.97 ± 1.33 log10 TCID50 from the fabric strap of the Honeywell Sperian model. For cleaned and disinfected surfaces, the mean log reduction was 4.66 ± 0.44 and only 1 surface demonstrated recoverable virus: 0.97 ± 1.33 log10 TCID50 from the fabric strap of the Scott model. For all surfaces tested, viable virus from treated surfaces was significantly lower than their respective untreated surfaces (P < .05), except for the treated Scott Xcel fabric straps (P = .10) and the treated 3M 7502 fabric straps (P = .14). Additionally, there was no significant difference between cleaned only and cleaned and disinfected surfaces (P = .92).
Fig 1.
Mean log recovery of sebum-covered H1N1 influenza from multiple surfaces of 5 half-mask elastomeric respirators models manufactured by 3M (St Paul, MN), Scott Safety (Monroe, NC), and Honeywell (Morris Plains, NJ).
PAPR reprocessing studies
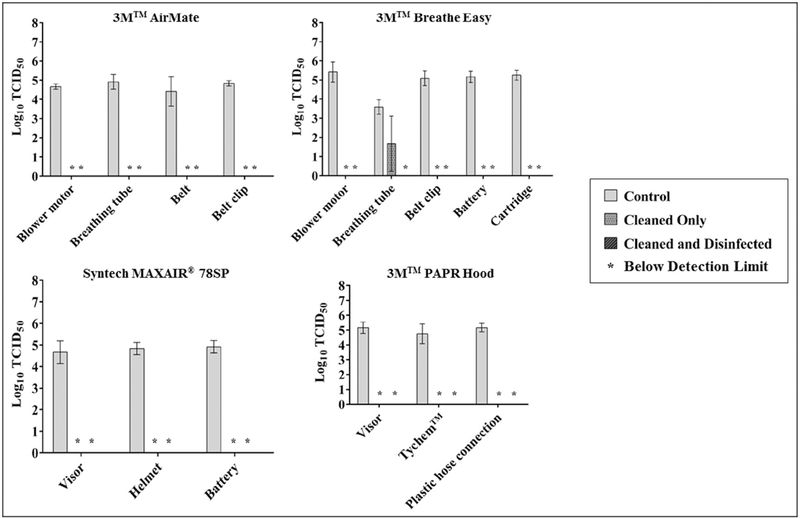
The mean viable influenza recovery from all untreated PAPR surfaces was 4.87 ± 0.42 log10 TCID50 (Fig 2); all PAPR surfaces tested were nonporous. For cleaned-only surfaces, 1 of 17 surfaces demonstrated recoverable viable virus: 1.73 ± 1.33 log10 TCID50 from the breathing tube of the 3M Breathe Easy model. For cleaned and disinfected surfaces, no detectable viable virus was recovered. For all surfaces tested, virus recovery from treated surfaces was significantly lower than their respective untreated surfaces (P < .05), except for the breathing tube of the 3M Breathe Easy model (P = .08). Additionally, there was no significant difference between cleaned only and cleaned and disinfected surfaces (P = .67).
Fig 2.
Mean log recovery of sebum-covered H1N1 influenza from multiple surfaces of 3 powered-air purifying respirator models manufactured by 3M (St Paul, MN) and Syntech (Stilwell, KS).
DISCUSSION
This study demonstrates the decontamination effectiveness of the RPD reprocessing protocols defined as part of this study against H1N1 influenza in the presence of a heavy soiling agent. Twentyfour different HMER surfaces and 17 different PAPR surfaces were evaluated to account for differences in material properties and surface types. All treated surfaces demonstrated a mean log reduction of 4.54 ± 0.97 log10 TCID50, indicating this reprocessing approach may significantly reduce fomite transmission of viable influenza from RPDs during a pandemic.
Of the 41 unique surfaces tested, viable virus ranging from 0.971.73 log10 TCID50 was recovered from only 3 surfaces: Scott fabric strap, Sperian fabric strap, and 3M Breathe Easy breathing tube, after being inoculated with a significant challenge of 107 log10 TCID50 influenza virus covered in artificial skin oil.
Both the Scott and Sperian fabric straps are hydrophilic porous surfaces, which may have influenced their ability to be disinfected. Across the board, significantly lower levels of virus were extracted from porous surfaces compared with nonporous surfaces(P< .0001).Although nonporous, the 3M Breathe Easy breathing tube has accordion-style grooves and ridges, making it difficult to make contact with a sponge or soft cloth when reprocessing. Although the 3M 7502 fabric strap demonstrated a relatively low log reduction for both treatment types (1.10), this is largely due to the significantly lower recovery value from the control straps (1.30 ± 1.04 log10 TCID50) compared with other surfaces tested (P < .0001).
Cleaning alone was demonstrated to be similarly effective as cleaning and disinfection for most surfaces. Both reprocessing approaches produced a mean 4.5-log reduction for RPD surfaces tested. Only 2 cleaned and disinfected surfaces, the Sperian fabric strap and 3M Breathe Easy breathing tube, demonstrated lower virus recoveries compared with the respective cleaned-only surfaces. These results indicate cleaning alone as a reprocessing approach may be effective in removing/killing viable influenza. Considerations of the material type and design must be made before implementing a similar approach for reprocessing RPDs. Adoption of such an approach may be necessary if disinfectant (eg, bleach) is not available due to potential supply shortages during a pandemic.
The disinfection requirements for medical devices vary based on their classification as either critical, semicritical, or noncritical. RPDs are not currently identified in terms of the Spaulding classification and thus their disinfection requirements have not been defined. The reprocessing methods used in this study solely focus on their effectiveness against pandemic influenza, indicated by the log reduction in viable virus. The log reduction achieved by both treatment types used in this study is likely limited by a number of factors. The inoculum size sets the upper boundary for the log reduction achievable. For this study, a high titer suspension prepared in embryonic chicken eggs (109 log10 TCID50/mL) was applied as 10 1-μL droplets to represent contamination via small droplets during actual respirator use. The resulting inoculum size (107 log10 TCID50) limits the log reduction achievable to a maximum of 7-log reduction. There are no published data on influenza contamination levels of FFRs in hospitals. However, Fisher et al33 validated a predictive model for estimating the level of influenza contamination on FFRs and surgical masks resulting from aerosols in health care settings. The estimated contamination level for the entire external surface of an FFR ranged from 101-105 viruses depending on different scenarios using airborne influenza concentrations published in the literature. Lindsley et al34 evaluated the amount of airborne influenza virus in human coughs and found a median value of 15.8 ± 29.3 viral copies per cough based on 48 subjects using quantitative real-time reverse transcription polymerase chain reaction. These data provide an indication that the challenge concentration used in this study represents a worst-case scenario.
Additionally, the log reduction achievable is decreased when the sampling efficiency is accounted for, which as the data indicates, can vary considerably based on surface type. Additionally, the detection limit of the TCID50 assay sets the lower boundary of the log reduction achievable. This is a log-based assay with a detection limit of approximately 0.4 log10 TCID50 for the testing performed for this study. Another factor potentially limiting the log reduction is the level of sebum used. Pochi and Strauss35 measured casual sebum levels of 51 male subjects with and without acne to determine a cause-and-effect relationship. They found that subjects with severe acne had a mean density of 0.18 ± 0.08 mg/cm2 sebum on their face based on the samples taken. Compared with the sebum level of subjects with acne, the sebum challenge used in this study is approximately a 14-fold increase. Despite undefined disinfection requirements for reusable RPDs, the data from this study demonstrated a mean 4.5-log reduction in viable influenza under rather extreme challenge conditions compared with the contamination events that would likely occur in the real world, indicating these reprocessing methods would be effective for RPD reprocessing during an influenza pandemic.
Although the reprocessing protocols used in this study demonstrated effectiveness against viable influenza contamination, implementation of these processes into a hospital presents some logistic challenges. Based on the protocols used in this study, the time required to perform HMER or PAPR reprocessing could be substantial in a health care facility. Ciconte and Danyluk10 also determined that manual reprocessing of HMERs was more time consuming than originally believed, in large part due to the HMERs floating in the soaking solutions, prompting a separate soak period for each side of the respirator. Another consideration is the reprocessor will require great attention to detail, ensuring all surfaces are properly scrubbed and cleaned. This procedure would also need to take place within a containment device to prevent potential contamination or infection of the reprocessor via aerosolized droplets or potentially contaminated washwater produced as a result of the cleaning process. To address these issues, a follow-up study is being performed evaluating the effectiveness and compatibility of automated methods for emergency reprocessing of influenzacontaminated reusable RPDs. Additionally, influenza contamination of HMERs and PAPRs through the aerosol route has the potential to reach surfaces not easily accessible by a sponge or brush. Future studies evaluating the effectiveness of reprocessing procedures for HMERs and PAPRs contaminated with influenza through the aerosol route and/or the hazard posed by these hard-to-access potentially contaminated surfaces would likely be beneficial for infection control practitioners.
Guidance provided to HCWs for reprocessing reusable RPDs needs to be clear and provide adequate definition for the task to be performed safely and effectively. The guidance provided by OSHA or RPD manufacturers for the models tested in this study did not specify the appropriate PPE to wear or containment considerations, as confirmed by Bessesen et al.21 The OSHA guidance is also not compatible with all RPD components. Often, reprocessing instructions provided by the HMER manufacturers did not define all of the key components of the process, instead omitting key information entirely or providing ambiguous generalizations (eg, wash with a cleaner-sanitizer solution). For PAPRs, the reprocessing guidance provided by manufacturers can be more complicated and even less defined than for HMERs. Each main component of the PAPR system (hood, motor blower unit, and battery) can have separate IFUs according to the manufacturer, but vary in the level of detail provided. Overall, better guidance is needed for HCWs to perform HMER and PAPR reprocessing, especially if these devices are used during a pandemic event. This study helps fill that gap by providing detailed cleaning and disinfection methods for both HMERs and PAPRs and then demonstrating their effectiveness, prompting their potential adoption as standard reprocessing guidance for the industry.
CONCLUSIONS
The effectiveness demonstrated by the reprocessing protocols evaluated as part of this study indicates that HMERs and PAPRs can be effectively disinfected when challenged with a pandemic influenza strain in the presence of soiling agents. Of 41 surfaces tested, only 1 demonstrated recoverable viable virus after being both cleaned and disinfected, indicating that the likelihood of these devices acting as fomites after proper use of the reprocessing protocols evaluated here is low. The data from this study also demonstrate similar efficacies between an approach that uses just cleaning methods (eg, neutral detergent) and an approach that uses both cleaning and disinfection methods (eg, neutral detergent and hypochlorite) for most surfaces. However, material characteristics and designs may decrease cleaning efficiency, an important consideration because supplies other than RPDs may experience a shortage during an influenza pandemic (eg, bleach). In general, guidance provided by HMER and PAPR manufacturers needs more clarity and definition for HCWs to effectively reprocess these devices, especially during a pandemic event. In the interim, the method used in this research could be considered by regulators or standards development organizations in their efforts to develop guidance or criteria.
Acknowledgments
Supported by the US Food and Drug Administration Medical Countermeasures Initiative Regulatory Science Extramural Research Program (contract No. HHSF223201400158C).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health or the Food and Drug Administration.
Conflicts of interest: None to report.
References
- 1.Talbot TR, Babcock H, Caplan AL, Cotton D, Maragakis LL, Poland GA, et al. Revised SHEA position paper: influenza vaccination of healthcare personnel. Infect Control Hosp Epidemiol 2010;31:987–95. [DOI] [PubMed] [Google Scholar]
- 2.Occupational Safety and Health Administration. 2009. Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers. OSHA 3328–05R. Available from: https://www.osha.gov/Publications/OSHA_pandemic_health.pdf. Accessed August 24, 2016.
- 3.Gosch ME, Shafer RE, Eagan AE, Roberge RJ, Davey VJ, Radonovich LJ. B95: a new respirator for health care personnel. Am J Infect Control 2013;41:1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailar JC, Burke DS, Brosseau LM, Cohen HJ, Gallagher EJ, Gensheimber KF, et al. Reusability of Facemasks During an Influenza Pandemic. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 5.Carias C, Rainisch G, Shankar M, Adhikari BB, Swerdlow DL, Bower WA, et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis 2015;60(Suppl 1):S42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebmann T, Alexander S, Bartley J, Cain T, Citarella B, Cloughessy M, et al. 2009. APIC Position Paper: Extending the Use and/or Reusing Respiratory Protection in Healthcare Settings During Disasters. Association for Professionals in Infection Control and Epidemiology. Available from: http://www.apic.org/Resource_/TinyMceFileManager/Position_Statements/APIC_Position_Ext_the_Use_and_or_Reus_Resp_Prot_in_Hlthcare_Settings1209l.pdf. Accessed August 24, 2016.
- 7.Murray M, Grant J, Bryce E, Chilton P, Forrester L. Facial protective equipment, personnel, and pandemics: impact of the pandemic (H1N1) 2009 virus on personnel and use of facial protective equipment. Infect Control Hosp Epidemiol 2010;31:1011–6. [DOI] [PubMed] [Google Scholar]
- 8.Beckman S, Materna B, Goldmacher S, Zipprich J, D’Allesandro M, Novak D, et al. Evaluation of respiratory protection programs and practices in California hospitals during the 2009–2010 H1N1 influenza pandemic. Am J Infect Control 2013;41:1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan A, Jernign D, Liedtke L, Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a National Survey of Infectious Diseases Consultants. Clin Infect Dis 2004;39:272–4. [DOI] [PubMed] [Google Scholar]
- 10.Ciconte R, Danyluk Q. Assessment and Determination of Practical Considerations for Wide-Scale Utilization of Elastomeric Half-facepiece Respirators during a Pandemic or Outbreak Situation. WorkSafe BC. May 2013. Available from: https://www.worksafebc.com/en/resources/about-us/research/assessment-and-determination-of-practical-considerations-for-wide-scale-utilization-of-elastometric-half-facepiece-respirators-during-a-pandemic-or-outbreak-situation?lang=en. Accessed January 27, 2017.
- 11.Casanova L, Alfano-Sobsey E, Rutala WA, Weber DJ, Sobsey M. Virus transfer from personal protective equipment to healthcare employees’ skin and clothing. Emerg Infect Dis 2008;14:1291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimbuch BK, Wallace WH, Balzli CL, Laning ML, Harnish DA, Wander JD. Bioaerosol exposure to personnel in a clinical environment absent patients. J Occup Environ Hyg 2016;13:D11–5. [DOI] [PubMed] [Google Scholar]
- 13.Occupational Safety and Health Administration. Respiratory Protection. OSHA Technical Manual Section VIII: Chapter 2. Available from: https://www.osha.gov/dts/osta/otm/otm_viii/otm_viii_2.html Accessed August 26, 2016.
- 14.Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities. Healthcare Infection Control Practices Advisory Committee. 2008. Available from: https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf. Accessed August 28, 2017.
- 15.3M™. Cleaning Reusable Respirators and Powered Air Purifying Respirator Assemblies. January 2015. Available from: http://multimedia.3m.com/mws/media/988556O/cleaning-reusable-respirators-and-powered-air-purifying-respirators-after-possible-ebola-exposure.pdf. Accessed January 26, 2017.
- 16.3M™. Half Facepiece Respirator 6000 Series User Instructions. Available from: http://multimedia.3m.com/mws/media/96751O/3m-6000-series-half-facepiece-respirator-user-instructions.pdf. Accessed January 26, 2017.
- 17.3M™. Half Facepiece Respirator 7500 Series User Instructions. Available from: http://multimedia.3m.com/mws/media/147813O/3m-7500-series-half-facepiece-respirator-user-instructions-for-silicone.pdf. Accessed January 26, 2017.
- 18.Scott Health & Safety. Scott® XCEL™. Twin cartridge half facepiece respirator user’s instructions. Available from: https://www.scottsafety.com/en/us/DocumentandMedia1/10011421_B.pdf. Accessed January 26, 2017.
- 19.Honeywell Sperian Blue 1H™ Series Half Mask. Operating and Maintenance Instruction Manual 2012.
- 20.North Safety Products. 5500 & 7700 series half mask air purifying respirator operating and maintenance instruction manual. 2001. Available from: www.uaf.edu/files/safety/industrial_hygiene/north_7700_manual.pdf. Accessed January 26, 2017.
- 21.Bessesen MT, Adams JC, Radonovich L, Anderson J. Disinfection of reusable elastomeric respirators by health care workers: a feasibility study and development of standard operating procedures. Am J Infect Control 2015; 43:629–34. [DOI] [PubMed] [Google Scholar]
- 22.3M™. Air-Mate™ belt-mounted high efficiency powered air purifying respirator user instructions. Available from: http://multimedia.3m.com/mws/media/92956O/3m-air-mate-belt-mounted-high-efficiency-papr-respirator-user-instructions.pdf. Accessed January 26, 2017.
- 23.Assembly User Instructions. Available from: http://www.approvedgasmasks.com/3mBreathEasymanual.pdf. Accessed July 31, 2017.
- 24.Syntech™. Maxair Systems User’s Instructions. Available from: http://maxair-systems.net/ManualsUIMIFU/78SP_Rev_F.pdf. Accessed July 31, 2017.
- 25.US Food & Drug Administration. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. Available from: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253010.pdf. Accessed January 26, 2017.
- 26.Wizner K, Stradtman L, Novak D, Shaffer R. Prevalence of respiratory protection devices in U.S. health care facilities: implications for emergency preparedness. Workplace Health Saf 2016;64:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subhash SS, Cavaiuolo M, Radonovich LJ, Eagan A, Lee ML, Campbell S, et al. Effectiveness of common healthcare disinfectants against H1N1 influenza virus on reusable elastomeric respirators. Infect Control Hosp Epidemiol 2014;35:894–7. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. Published 2002. http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf. Accessed January 26, 2017.
- 29.Julian TR, Tamayo FJ, Leckie JO, Boehm AB. Comparison of surface sampling methods for virus recovery from fomites. Appl Environ Microbiol 2011;77:6918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oregon Health & Science University. 3M Air-Mate HEPA Training for Users. Available from: http://www.ohsu.edu/xd/health/for-healthcare-professionals/infection-control/patient-isolation-and-de-isolation/upload/Airmate-HEPA-Training-for-Users.pdf. Accessed January 26, 2017.
- 31.Finney DJ. Statistical methods in biological assays. 2nd ed. New York: Hafner; 1964. [Google Scholar]
- 32.Singh A, Nocerino J. Robust estimation of mean and variance using environmental data sets with below detection limit observations. Chemometr Intell Lab Syst 2002;60:69–86. [Google Scholar]
- 33.Fisher EM, Noti JD, Lindsley WG, Blachere FM, Shaffer RE. Validation and application of models to predict facemask influenza contamination in healthcare settings. Risk Anal 2014;34:1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS ONE 2010;5:e15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pochi PE, Strauss JS. Sebum production, casual sebum levels, titratable acidity of sebum, and urinary fractional 17-ketosteroid excretion in males with acne. J Invest Dermatol 1964;43:383–8. [DOI] [PubMed] [Google Scholar]