Abstract
Type 2 diabetes is a risk factor for fracture independent of FRAX® probability. We directly compared four proposed methods to improve the performance of FRAX for type 2 diabetes: (1) the rheumatoid arthritis (RA) input to FRAX; (2) the trabecular bone score (TBS)-adjustment to FRAX; (3) reducing the femoral neck T-score input to FRAX by 0.5 SD; (4) increasing the age input to FRAX by 10 years. We examined major osteoporotic fractures (MOF) and hip fractures (HF) over mean 8.3 years observation among 44,543 women and men 40 years of age or older (4136 with diabetes) with baseline lumbar spine and hip DXA during 1999-2016. Controlled for unadjusted FRAX probability, diabetes was associated with increased risk for MOF and HF. All four FRAX adjustments attenuated the effect of diabetes, but a residual effect of diabetes was seen on MOF risk after TBS adjustment, and on HF risk after the RA and TBS adjustments. Among those with diabetes, unadjusted FRAX risk underestimated MOF (observed/predicted ratio 1.15, 95% CI 1.03–1.28) but this was no longer significant after applying the diabetes adjustments. HF risk was more severely underestimated (observed/predicted ratio 1.85, 95% CI 1.51–2.20) and was only partially corrected with the diabetes adjustments (still significant for the RA and TBS adjustments). Among those with diabetes there was moderate reclassification based upon a fixed MOF cutoff of 20% (4.1–7.1%) or fixed HF cutoff of 3% (5.7–16.5%). Net reclassification improvement (NRI) increased for MOF with each of the diabetes adjustments (range 3.9–5.6% in the diabetes subgroup). In conclusion, each of the proposed methods for addressing limitations in the ability of FRAX to assess fracture risk in individuals with diabetes was found to improve performance, though no single method was optimal in all settings.
Keywords: Osteoporosis, DXA, Diabetes, Fracture risk assessment, FRAX, Trabecular bone score
Introduction
The World Health Organization (WHO) defines osteoporosis conceptually as a systemic skeletal disease characterized by low bone mass (decreased quantity) and microarchitectural deterioration of bone tissue (decreased quality) with a consequent increase in bone fragility and susceptibility to fracture (1). Despite the ability of bone mineral density (BMD) measurements from dual-energy X-ray absorptiometry (DXA) to stratify fracture risk, it has low sensitivity (2). In fact, most fractures occur in individuals who do not have a BMD below the threshold for osteoporosis, implying that factors other than BMD influence bone strength and fracture risk (3, 4). This has stimulated the development of risk algorithms that integrate multiple risk factors for fracture and also interest in new techniques for bone quality assessment. The most widely used tool for fracture risk assessment is FRAX®, developed by the Collaborating Centre for Metabolic Bone Diseases at Sheffield, UK, a computer-based algorithm that computes the 10-year probability of major osteoporotic fracture (MOF) (hip, clinical spine, forearm and humerus fracture) and hip fracture (HF) in the presence of competing mortality (5). Fracture risk is computed from easily assessed clinical risk factors for fracture and (optionally) femoral neck BMD. FRAX is country specific and is currently calibrated for over 60 countries (6).
Notwithstanding the strengths of FRAX, concerns have been raised regarding its performance in those with type 2 diabetes which is not a direct input variable to FRAX (7). Despite being associated with higher bone mineral density (BMD), type 2 diabetes is a risk factor for osteoporotic fracture independent of FRAX probability (8, 9). The underlying mechanisms are unclear, but are clearly multifactorial and include impaired muscle strength and quality, falls, greater skeletal impact forces related to a fall, and alterations in bone strength (10, 11).
The foregoing has given rise to several proposals on how to improve the performance of FRAX for those with type 2 diabetes, but these have not been directly compared (12). The current study was performed to directly compare several proposed methods using a large clinical registry that includes all DXA tests for the Province of Manitoba, Canada, providing results applicable to the clinical practice setting.
Methods
Study population
We performed a registry-based cohort study to examine MOF and HF outcomes factors among women and men 40 years of age or older who had undergone baseline DXA of the lumbar spine and hip during 1999-2016. In the Canadian province of Manitoba, health services are provided to nearly all residents through a single public health care system (13). For each health system contact, information is recorded to document the patient's demographics, date and type of service, and diagnostic code(s). Hospital discharge abstracts (diagnoses and procedures) are coded using the International Classification of Diseases (ICD), 9th revision, Clinical Modification [i.e., ICD-9-CM] prior to 2004 and the 10th revision of ICD, Canadian version [i.e., ICD-10-CA] thereafter). Physician billing claims are coded using ICD-9-CM for all data years as previously described (14, 15). In previous analyses we saw no evidence that there was any unexpected change in fracture rates straddling the period of transition from ICD-9-CM to ICD-10-CA for hospitalization data, even in the case of hip fractures which are identified solely from hospitalization codes (16). Medication use is obtained from the provincial pharmacy system (17). DXA testing through the Manitoba Density Program has been managed as an integrated program since 1997 (18). The Manitoba Density Program maintains a database of all DXA results that can be linked with other population-based databases through an anonymous personal identifier. The associated database exceeds 99% in terms of completeness and accuracy (19). The study was approved by the Research Ethics Board of the University of Manitoba and the Health Information Privacy Committee of Manitoba Health.
Bone densitometry, trabecular bone score and fracture probability
All spine and hip DXA scans were performed with a fan-beam DXA configuration (Prodigy or iDXA, GE Healthcare, Madison, WI, USA) and analyzed in accordance with manufacturer recommendations. Femoral neck BMD T-scores were calculated using the NHANES III white female reference values (20). The DXA instruments used were cross-calibrated using anthropomorphic phantoms and no clinically significant differences were identified (T-score differences < 0.1). Short-term reproducibility (coefficient of variation [CV]) for femoral neck BMD from the multiple technologists was 2.3% (over 400 repeat hip DXA scans performed within 28 days).
Among the clinically applicable techniques developed for bone quality assessment, trabecular bone score (TBS) has been most extensively studied (21, 22). TBS can help enhance fracture prediction when used in conjunction with FRAX probability estimated with BMD (23). TBS measurements were performed in the Bone Disease Unit at the University of Lausanne, Switzerland (TBS iNsight Software, Version 2.1, Med-Imaps, Merignac, France), using anonymized spine DXA files to ensure blinding of the Swiss investigators to all clinical parameters and outcomes. We excluded women with body mass index (BMI) outside the range 15-37 kg/m2 as recommended by the TBS manufacturer (22). No significant calibration differences in mean TBS levels were seen for the DXA scanners used. Lumbar spine TBS CV from the multiple technologists was 2.1% (92 repeat spine DXA scans performed within 28 days).
Ten-year probability of a major fracture and hip fracture with femoral neck BMD was calculated for each subject using the Canadian FRAX tool (FRAX® Desktop Multi-Patient Entry, version 3.8). The Canadian FRAX tool was calibrated using nationwide hip fracture and mortality data (17). The Manitoba BMD Registry was not used in the creation or calibration of the FRAX tool. Weight and height were measured at the time of DXA, and BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Prior fracture and other FRAX input variables were assessed using linkage to the population-based research registry that includes hospital discharge abstracts and physician billing claims as previously described (24). We defined prior fragility fracture as any non-traumatic MOF that occurred before the baseline DXA test using records back to 1987. We did not include other fracture sites but note that MOF represent the majority of fragility fractures (after excluding head/neck, hand/foot, ankle) and are more strongly associated with recurrent fracture than the remaining sites (25). Prolonged oral corticosteroid use (>90 days dispensed in the 1 year prior to DXA) was obtained from the provincial pharmacy system (17). Parental hip fracture was by self-report from 2005 onwards and from linkage to parental hospitalization records in earlier years (26). Current smoking was by self-report from 2005 onwards and from a proxy variable in earlier years (chronic obstructive lung disease codes). High alcohol use from 2012 onwards and from a proxy variable in earlier years (alcohol substance abuse codes). FRAX predictions with the Canadian FRAX tool agree with observed fracture probability in this cohort and in the Canadian population (FRAX with BMD area under the curve for MOF prediction ~0.69 and for hip fracture prediction ≥0.80) (24, 27).
Diabetes mellitus case definition and risk adjustments
Diabetes diagnosed prior to the baseline DXA was ascertained from the presence of at least two physician billing claims with a diabetes diagnosis within 2 years or at least one hospitalization with a diabetes diagnosis. These definitions have been well-validated in our population and used as the basis for nationwide diabetes surveillance reporting (28, 29). The duration of diabetes was based upon the time since the earliest qualifying ICD-9-CM or ICD-10-CA diagnosis code, and was included in the analysis plan since longer duration of diabetes has been shown to increase fracture risk and the extent to which FRAX underestimates this risk (12, 30). More than 90% of the cohort had health coverage exceeding 10 years (mean 32 ± 11 years). Individuals with possible type 1 diabetes (diagnosed before age 50 years, insulin-dependent within 2 years of diagnosis, and no use of oral agents) were excluded. Women without diabetes were retained in the analysis as a referent comparison population.
We compared four options to enhance the performance of FRAX in patients with diabetes. First, we used the rheumatoid arthritis (RA) input to FRAX as a proxy for the effect of diabetes; this is justified by the similar weights accorded RA and type 2 diabetes in the QFracture algorithm (31, 32). Second, we used the TBS-adjustment to the FRAX score; this is justified by the observation in several studies that TBS is lower in those with type 2 diabetes than in the general population (33–37). Initially the TBS adjustment was applied to patients with diabetes since the TBS adjustment to FRAX was developed and validated for use in the general population, we secondarily considered its effect for the entire population including those without diabetes (38, 39). Third, we reduced the femoral neck T-score input to FRAX by 0.5 SD in patients with diabetes; this follows from the observation that a T-score in a woman with DM is associated with hip fracture risk equivalent to a woman without DM with a T-score of approximately 0.5 units lower (8). Finally, we increased the age input to FRAX by 10 years in patients with diabetes; this is comparable to the femoral neck BMD loss of 0.5 SD expected over 10 years.
Assessment of incident fractures
Longitudinal health service records were assessed between April 1, 1987 and March 31, 2016 for the presence of fracture not associated with codes indicative of severe trauma (i.e., external injury) using validated definitions (14). Fragility fracture codes were assessed using hospital discharge abstracts (coded ICD-10-CA) and physician billing claims (coded ICD-9-CM) (Supplementary Table 1). Hip and forearm fractures were required to have a site-specific fracture reduction, fixation or casting code. To minimize misclassification of prevalent and incident fractures at the same skeletal site, we required that there be no hospitalization or physician visit(s) with the same fracture type in the 6 months preceding an incident fracture. There was no time restriction on prior and incident fractures involving different skeletal sites.
Statistical analyses
Statistical analyses were performed with Statistica (Version 13.0, StatSoft Inc, Tulsa, OK). Descriptive statistics for demographic and baseline characteristics are presented as mean ± SD for continuous variables or number (%) for categorical variables. Cox proportional hazards models were used to study time to first fracture, with diabetes as the covariate of interest, controlled for the effect of FRAX probability before and after application of the proposed diabetes adjustments. We initially considered diabetes as a binary variable (present vs absent [referent]) and then stratified according to duration (<5 years, 5-10 years, >10 years vs absent [referent]). Unadjusted and adjusted FRAX scores were log-transformed due to a skewed distribution. Reduction in the model Chi2 statistic for diabetes was used as an ancillary measure of attenuation in the diabetes effect with the adjustment being tested. Risk gradients for the various fracture probability measurements were also estimated and are presented as hazard ratio (HR) per SD decrease with 95% confidence intervals (CI). We also computed calibration ratios (observed vs predicted 10-year fracture probability with 95% CI), overall and for the diabetes subgroup. Observed 10-year fracture probability was derived from the cumulative incidence function (CIF) for MOF and hip fracture up to 10 years incorporating competing mortality risk (40, 41). Observed fracture probabilities were compared with those predicted from the various fracture probability measurements. An optimal method for capturing and accounting for the fracture risk associated with diabetes would result in an HR for diabetes close to unity (~1.00), negligible model Chi2 statistic for diabetes (~0), and an observed/predicted calibration ratio close to unity (~1.00).
We also examined reclassification rates and categorical net reclassification improvement (NRI) from using the diabetes adjustments applied to FRAX-based probabilities based upon fixed intervention cutoffs as recommended by the National Osteoporosis Foundation (MOF 20% and HF 3%) (42). NRI was computed separately for individuals with and without incident fractures, and for overall reclassification improvement (43, 44). For individuals who sustain a fracture in follow up, NRI fracture is the probability of moving to a higher FRAX risk category minus the probability of moving to a lower FRAX risk category. Conversely, for individuals who remain fracture-free in follow up, NRI non-fracture is the probability of moving into a lower FRAX risk category minus the probability of moving into a higher FRAX risk category. Values of NRI fracture and NRI non-fracture greater than zero indicate an improvement in risk classification, whereas negative values indicate worse risk classification. An asymptotic test of significance for the null hypothesis of NRI=0 based upon the multinomial distribution was performed (44).
Results
Baseline characteristics are shown in Table 1. The study cohort consisted of 44,543 individuals, mean age 63.9 ± 11.0 years, predominantly women but 4484 men. Diagnosed diabetes was present in 4,136 (9.3%). Individuals with diabetes tended to be older, men, with greater BMI, greater femoral neck T-score, lower lumbar spine TBS and greater fracture probability even prior to application of the proposed adjustments which further increased mean fracture probability among those with diabetes (Supplementary Table 2). The prevalence of RA was low and similar among those with diabetes and without diabetes (3.0% vs 2.6%, P = 0.067).
Table 1. Study population baseline characteristics according to incident major osteoporotic fracture (MOF) or incident hip fracture (HF).
| VARIABLE | Overall | No MOF | Incident MOF | p-value | No HF | Incident HF | p-value |
|---|---|---|---|---|---|---|---|
| N = 44,543 | N = 40,597 | N = 3946 | N = 43,381 | N = 1162 | |||
| Age (years) | 63.9 ± 11.0 | 63.4 ± 10.8 | 68.6 ± 11.0 | <0.001 | 63.6 ± 10.9 | 73.3 ± 9.5 | <0.001 |
| Sex (men) | 4484 (10.1) | 4166 (10.3) | 318 (8.1) | <0.001 | 4391 (10.1) | 93 (8.0) | 0.018 |
| BMI (kg/m2) | 26.4 ± 4.4 | 26.4 ± 4.4 | 25.8 ± 4.4 | <0.001 | 26.4 ± 4.4 | 25.1 ± 4.2 | <0.001 |
| Prior fracture | 6658 (14.9) | 5693 (14.0) | 965 (24.5) | <0.001 | 6359 (14.7) | 299 (25.7) | <0.001 |
| Femoral neck T-score | -1.4 ± 1.0 | -1.3 ± 1.0 | -1.9 ± 0.9 | <0.001 | -1.3 ± 1 | -2.2 ± 0.8 | <0.001 |
| FRAX 10-year MOF fracture risk (%) a | 9.5 ± 6.8 | 9.2 ± 6.5 | 13.3 ± 8.4 | <0.001 | 9.4 ± 6.7 | 16.5 ± 8.7 | <0.001 |
| FRAX 10-year HF risk (%) a | 2.2 ± 3.7 | 2.0 ± 3.5 | 4.0 ± 4.8 | <0.001 | 2.1 ± 3.6 | 5.7 ± 5.2 | <0.001 |
| Lumbar spine TBS | 1.262 ± 0.123 | 1.267 ± 0.121 | 1.211 ± 0.125 | <0.001 | 1.264 ± 0.122 | 1.188 ± 0.121 | <0.001 |
| Diabetes | 4136 (9.3) | 3750 (9.2) | 386 (9.8) | 0.260 | 3997 (9.2) | 139 (12.0) | 0.001 |
FRAX score computed with femoral neck BMD but without TBS. Data are mean ± SD or N (percent). BMI, body mass index. TBS, trabecular bone score
During 8.3 ± 4.7 years observation, one or more incident MOF were identified in 3,946 (8.9%) of the cohort, including 1,162 (2.6%) with an incident hip fracture. The prevalence of diabetes was non-significantly greater in those with vs without incident MOF (9.8% vs 9.2%, P = 0.260) but was significantly greater for incident hip fracture (12.0% vs 9.2%, P = 0.001). Older age, lower BMI, prior fracture, lower femoral neck T-score, lower lumbar spine TBS and higher fracture probability were all significantly associated with incident MOF or incident HF.
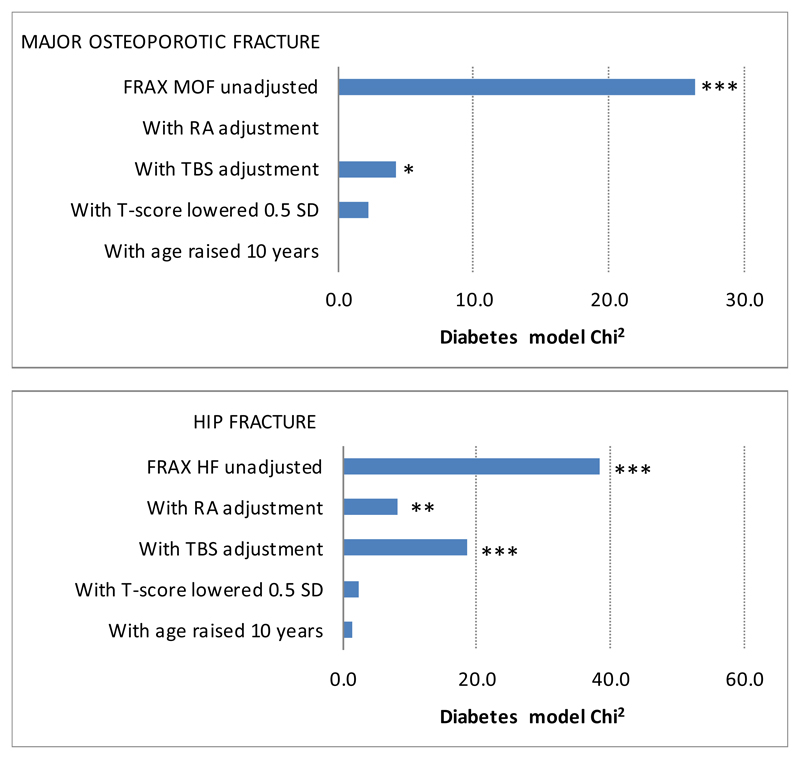
After controlling for the standard unadjusted FRAX probability, diabetes (all durations combined) was associated with significantly increased risk for incident MOF (HR 1.32, 95% CI 1.19 – 1.46) and incident HF (HR 1.76, 95% CI 1.47 – 2.10). The four FRAX adjustments evaluated all attenuated the effect of diabetes (Table 2). However, there was a significant residual effect of diabetes on MOF risk after TBS adjustment, and on HF risk after the RA and TBS adjustments. Stratification by duration of diabetes controlled for the standard unadjusted FRAX score demonstrated a gradient of increasing risk with longer duration, statistically significant for MOF in those with duration exceeding 10 years and significant for all durations of HF. The diabetes adjustments attenuated but did not eliminate the MOF risk associated with diabetes exceeding 10 years. All methods successfully negated the effect of HF risk on duration of diabetes < 10 years but only partially attenuated this for diabetes duration exceeding 10 years. Figure 1 shows the relative importance of diabetes in the model, with the model Chi square for diabetes reduced by over half in all scenarios.
Table 2. Effect of diabetes (hazard ratio [HR] with 95% confidence interval [CI]) on incident major osteoporotic fracture (MOF) or incident hip fracture (HF) controlled for fracture probability using unadjusted FRAX (referent) and four adjustments applied to those with diabetes.
| Diabetes, any duration N = 4136 |
Diabetes <5 years N = 1323 |
Diabetes 5-10 years N = 922 |
Diabetes >10 years N = 1891 |
|
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| MOF prediction, controlled for: | ||||
| FRAX MOF unadjusted (referent) | 1.32 (1.19-1.46) | 1.08 (0.89-1.31) | 1.24 (1.00-1.55) | 1.54 (1.34-1.78) |
| With RA adjustment | 1.02 (0.91-1.13) | 0.83 (0.69-1.01) | 0.96 (0.77-1.20) | 1.19 (1.03-1.37) |
| With TBS adjustment | 1.12 (1.01-1.24) | 0.92 (0.76-1.11) | 1.05 (0.84-1.31) | 1.31 (1.13-1.51) |
| With T-score lowered 0.5 SD | 1.08 (0.98-1.21) | 0.89 (0.73-1.08) | 1.03 (0.82-1.28) | 1.26 (1.09-1.46) |
| With age raised 10 years | 1.01 (0.91-1.12) | 0.82 (0.68-1.00) | 0.95 (0.76-1.18) | 1.19 (1.03-1.37) |
| HF prediction, controlled for: | ||||
| FRAX HF unadjusted (referent) | 1.76 (1.47-2.10) | 1.41 (1.01-1.97) | 1.53 (1.05-2.25) | 2.12 (1.67-2.68) |
| With RA adjustment | 1.30 (1.09-1.55) | 1.04 (0.75-1.45) | 1.13 (0.77-1.65) | 1.57 (1.25-1.99) |
| With TBS adjustment | 1.48 (1.24-1.77) | 1.19 (0.85-1.65) | 1.29 (0.88-1.90) | 1.78 (1.41-2.26) |
| With T-score lowered 0.5 SD | 1.15 (0.96-1.38) | 0.92 (0.66-1.28) | 1.01 (0.69-1.47) | 1.40 (1.11-1.77) |
| With age raised 10 years | 1.12 (0.94-1.34) | 0.88 (0.63-1.22) | 0.99 (0.67-1.44) | 1.37 (1.08-1.73) |
Boldface indicates p-value <0.05. RA, rheumatoid arthritis. TBS, trabecular bone score.
Figure 1.
Diabetes effect (model Chi2) on incident major osteoporotic fracture (MOF) or incident hip fracture (HF) controlled for unadjusted FRAX (referent) and after four adjustments applied to those with diabetes. Smaller values are preferred, with zero indicating that the effect of diabetes has been completely captured by the adjustment used.
* p-value <0.05, ** p-value <0.01, *** p-value <0.001. RA, rheumatoid arthritis. TBS, trabecular bone score.
The calibration analysis in Table 3 showed excellent agreement between observed and predicted MOF in individuals without diabetes (observed/predicted ratio 1.00, 95% CI 0.97 – 1.04) but slight underestimation in hip fracture risk (ratio 1.17, 95% CI 1.08 – 1.25). Among those with diabetes, the standard unadjusted FRAX risk significantly underestimated MOF (ratio 1.15, 95% CI 1.03 – 1.28) but this was no longer significant after applying the diabetes adjustments. HF risk was even more severely underestimated (observed/predicted ratio 1.85, 95% CI 1.51 – 2.20) and was partially but not completely corrected with the diabetes adjustments (still significant for the RA and TBS adjustments).
Table 3. Calibration ratios for observed versus predicted 10-year major osteoporotic fracture (MOF) probability percent or hip fracture (HF) probability percent using unadjusted FRAX (referent) and four adjustments applied to those with diabetes.
| No diabetes | Diabetes, any duration | |||||
|---|---|---|---|---|---|---|
| N = 40,407 | N = 4136 | |||||
| Predicted | Observed | Calibration ratio | Predicted | Observed | Calibration ratio | |
| MOF prediction | ||||||
| FRAX MOF unadjusted | 9.5 | 9.5 | 1.00 (0.97-1.04) | 10.3 | 11.9 | 1.15 (1.03-1.28) |
| With RA adjustment | 13.1 | 11.9 | 0.91 (0.81-1.01) | |||
| With TBS adjustment | 11.7 | 11.9 | 1.02 (0.91-1.12) | |||
| With T-score lowered 0.5 SD | 12.4 | 11.9 | 0.96 (0.86-1.06) | |||
| With age raised 10 years | 12.9 | 11.9 | 0.93 (0.83-1.03) | |||
| HF prediction | ||||||
| FRAX HF unadjusted | 2.2 | 2.5 | 1.17 (1.08-1.25) | 2.5 | 4.5 | 1.85 (1.51-2.20) |
| With RA adjustment | 3.4 | 4.5 | 1.35 (1.10-1.60) | |||
| With TBS adjustment | 2.8 | 4.5 | 1.63 (1.33-1.93) | |||
| With T-score lowered 0.5 SD | 3.7 | 4.5 | 1.22 (0.99-1.44) | |||
| With age raised 10 years | 3.7 | 4.5 | 1.22 (0.99-1.44) | |||
Boldface indicates p-value <0.05. RA, rheumatoid arthritis. TBS, trabecular bone score. 10-year fracture probability includes competing mortality.
Reclassification statistics are shown in Table 4. Among the diabetes subgroup there was a moderate amount of reclassification (predominantly upwards) for MOF based upon a fixed intervention cutoff of 20% (4.1 – 7.1%) and for HF based upon a fixed intervention cutoff of 3% (5.7 – 16.5%). The improvement in NRI among fracture cases exceeded the reduction in NRI among fracture non-cases, resulting in a significant improvement in overall NRI for MOF with each of the diabetes adjustments (range 3.9 – 5.6% in the diabetes subgroup). There was a numerical increase in NRI for HF but this was not statistically significant (range 1.5 – 4.7% among the diabetes subgroup).
Table 4. Reclassification and net reclassification improvement (NRI) for incident major osteoporotic fracture (MOF) or incident hip fracture (HF) using four adjustments applied to those with diabetes (referent unadjusted FRAX). Analyses limited to the diabetes subgroup.
| Reclassified %, total (up, down) |
NRI %, fracture cases |
NRI %, fracture non-cases |
NRI %, overall |
|
|---|---|---|---|---|
| MOF 20% | ||||
| With RA adjustment | 7.1 (7.1, 0.0) | 12.2*** | -6.5*** | 5.6** |
| With TBS adjustment | 4.1 (3.5, 0.6) | 6.5*** | -2.5*** | 3.9** |
| With T-score lowered 0.5 SD | 6.1 (6.1, 0.0) | 11.1*** | -5.5*** | 5.6*** |
| With age raised 10 years | 6.7 (6.3, 0.4) | 9.8*** | -5.4*** | 4.4* |
| HF 3% | ||||
| With RA adjustment | 8.4 (8.4, 0.0) | 11.5*** | -8.3*** | 3.2 |
| With TBS adjustment | 5.7 (5.2, 0.4) | 7.2** | -4.7*** | 2.5 |
| With T-score lowered 0.5 SD | 11.5 (11.5, 0.0) | 12.9*** | -11.4*** | 1.5 |
| With age raised 10 years | 16.5 (16.4, 0.1) | 20.9*** | -16.2*** | 4.7 |
* p-value <0.05, ** p-value <0.01, *** p-value <0.001. Boldface indicates p-value <0.05. RA, rheumatoid arthritis. TBS, trabecular bone score.
The gradient of risk for MOF and HF prediction was not appreciably different when the diabetes adjustment was only performed in individuals with diabetes (Supplementary Table 3). The TBS adjustment to FRAX (but not the other methods) is also applicable to those without diabetes. When applied to the overall population, the TBS adjustment resulted in a small increase in gradient of risk for both MOF and HF (Supplementary Table 3), a larger number with risk reclassification (2.8% overall for MOF and 4.0% overall for HF), and a larger improvement in NRI for MOF (3.1%, P < 0.001) and for HF (2.3%, P = 0.002) (Supplementary Table 4).
Supplementary Table 5 shows the calibration analyses according to duration of diabetes. The diabetes adjustments tended to overcorrect MOF risk among those with diabetes duration < 5 years (non-significant for the TBS adjustment). For HF all methods showed non-significant miscalibration for diabetes duration < 10 years but underestimated risk for those with diabetes duration exceeding 10 years (non-significant with lowering the femoral neck T-score by 0.5).
We performed supplementary analyses to examine for an effect of regular insulin or thiazolidinedione use (medication possession ratio ≥0.5 in the year prior to BMD testing). After adjusting for the standard unadjusted FRAX score and diabetes (stratified by duration), neither of these had any detectable effect on MOF or hip fracture risk (all P >0.4). We also tested for two-way interactions (adjusted for referent FRAX probability) and confirmed that there are no significant differences for diabetes with age, sex, TBS or BMD (all P >0.05).
Discussion
To our knowledge, this is the first study that has directly compared the performance of proposed methods to improve the prediction of fracture risk among individuals with type 2 diabetes when using the FRAX tool. Although individual differences in performance of these adjustment methods are noted, in general each approach represented a significant improvement in the performance of FRAX by reducing or in some cases eliminating the effect of diabetes on incident MOF and HF.
Notably, no single method was optimal for all fracture outcomes and durations of diabetes. Furthermore, only one method (TBS adjustment) can be used in the general population, whereas the others are restricted to use among individuals with diabetes. Therefore, although the TBS adjustment was somewhat less effective in the diabetes subgroup, it had a greater benefit when applied to the overall population which included those without diabetes. Miscalibration (underestimation in risk) has been the primary limitation with using FRAX in those with type 2 diabetes (8, 9), and it follows that this is an important measure to examine in any proposed adjustments. Based upon the calibration ratio (Supplementary Table 5), which considers competing mortality, the TBS adjustment may be preferred for MOF (non-significant miscalibration for all durations of diabetes) while lowering the femoral neck T-score by 0.5 may be the preferred method for HF (non-significant miscalibration for all durations of diabetes), although the performance of raising age by 10 years was almost equivalent. Using the age adjustment may be less satisfactory in older individuals, however, as the effect of competing mortality may paradoxically reduce fracture probability and could differ between populations since FRAX incorporates population-specific mortality data. Lower TBS is associated with increased mortality, and likely explains why the TBS adjustment gave accurate calibration for diabetes (any duration) from 10-year fracture probability which includes competing mortality, while there was a significantly increased hazard ratio for diabetes from the Cox regression model (39). Conversely, the RA adjustment was quite effective for both MOF and HF at attenuating the effect of diabetes. Additional clinical considerations are the ease with which a method can be applied, availability of the TBS software, and prevalence of RA in the population (since this can only be applied when RA and diabetes do not coexist in the same individual). The data reported here may help to inform future position statements and practice guidelines aimed at enhancing the care of diabetic patients.
Limitations of this analysis are acknowledged. The clinical source of the study cohort is recognized, and referred individuals are likely to be at higher perceived risk of osteoporosis and likelihood of fracture. This is particularly likely to affect referral of men for BMD testing. However, since we included all individuals within the geographic region referred for BMD testing, our results are likely to be broadly generalizable to postmenopausal women and older men in clinical practice who are referred for BMD testing. Our study cohort was 98% Caucasian and underpowered to examine the effect of race/ethnicity; other cohorts would be required to address this question. Although we did not have access to x-rays to confirm fractures, particularly vertebral fractures, the definitions for fracture used have been validated and adopted for national surveillance of osteoporosis and related fractures (14, 15). Definitive differentiation of type 1 and type 2 diabetes within administrative data is not possible, but excluding those with diabetes who were insulin-dependent, diagnosed before age 50 years and had never used an oral anti-diabetes agent would remove almost all individuals with type 1 diabetes. Importantly, none of the methods we tested have been proposed for use in type 1 diabetes which differs in terms of pathophysiology and fracture risk, particularly for hip fractures, which are much higher in type 1 diabetes (45, 46).
In conclusion, each of the proposed methods for addressing limitations in the ability of FRAX to assess fracture risk in individuals with type 2 diabetes was found to improve performance. No single method was optimal in all settings, however. Ultimately, incorporating diabetes directly into FRAX would likely be the preferred method, though there are challenges to implementing this approach (7). Meanwhile, clinical practitioners can choose from among these currently available options to enhance the performance of FRAX.
Supplementary Material
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017- 29). The results and conclusions are those of the authors and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Healthy Living, and Seniors, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee.
Funding:
This study had no external funding body.
Funding: No funding support was received for this research project.
Footnotes
ORCID: 0000-0002-1056-1691.
Disclosures:
William Leslie, Helena Johansson declare that they have no conflict of interest.
Eugene McCloskey: Nothing to declare for FRAX and the context of this paper, but numerous ad hoc consultancies/ speaking honoraria and/or research funding from Amgen, Bayer, General Electric, GSK, Hologic, Lilly, Merck Research Labs, Novartis, Novo Nordisk, Nycomed, Ono, Pfizer, ProStrakan, Roche, Sanofi-Aventis, Servier, Tethys, UBS and Warner-Chilcott.
Nicholas Harvey: Nothing to declare for FRAX and the context of this paper, but has received consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Radius, Consilient Healthcare and Internis Pharma.
John A. Kanis: Grants from Amgen, grants from Lilly, non-financial support from Medimaps, grants from Unigene, non-financial support from Asahi, grants from Radius Health, outside the submitted work; and Dr Kanis is the architect of FRAX but has no financial interest. Governmental and NGOs: National Institute for health and clinical Excellence (NICE), UK; International Osteoporosis Foundation; INSERM, France; Ministry of Public Health, China; Ministry of Health, Australia; Ministry of Health, Abu Dhabi; National Osteoporosis Guideline Group, UK; WHO.
Didier Hans: Co-ownership in the TBS patent. Stock options or royalties: Med-Imaps. Research grants: Amgen, Radius Pharma, Agnovos, GE Healthcare.
Roles:
Authors' roles: conception, design, data analysis, drafting the article (WDL), interpretation of data (All Authors); critically revising the article for important intellectual content (All Authors); final approval of the version to be published (All Authors); and agreement to be accountable for all aspects of the work (All Authors). WDL had full access to all the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Marshall D, Hailey D, Jonsson E. Health policy on bone density measurement technology in Sweden and Australia. Health Policy. 1996;35(3):217–28. doi: 10.1016/0168-8510(95)00785-7. [DOI] [PubMed] [Google Scholar]
- 3.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177(6):575–80. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–54. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA. Technical Report. Published by the University of Sheffield; 2007. Assessment of osteoporosis at the primary health-care level. Accessible at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. [Google Scholar]
- 6.Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, et al. A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie WD, Rubin MR, Schwartz AV, Kanis JA. Type 2 diabetes and bone. J Bone Miner Res. 2012;27(11):2231–7. doi: 10.1002/jbmr.1759. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27(2):301–8. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 10.Lecka-Czernik B, Fowlkes JL. Diabetic Bone Disease Basic and Translational Research and Clinical Applications. Published Cham : Springer International Publishing; 2015. [Google Scholar]
- 11.Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 12.Schacter GI, Leslie WD. Diabetes and Bone Disease. Endocrinol Metab Clin North Am. 2017;46(1):63–85. doi: 10.1016/j.ecl.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Roos NP, Shapiro E. Revisiting the Manitoba Centre for Health Policy and Evaluation and its population-based health information system. Med Care. 1999;37(6 Suppl):JS10–JS4. doi: 10.1097/00005650-199906001-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lix LM, Azimaee M, Osman BA, Caetano P, Morin S, Metge C, et al. Osteoporosis-related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell S, Canadian Chronic Disease Surveillance System Osteoporosis Working G Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporos. 2013;8:143. doi: 10.1007/s11657-013-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie WD, Sadatsafavi M, Lix LM, Azimaee M, Morin S, Metge CJ, et al. Secular decreases in fracture rates 1986-2006 for Manitoba, Canada: a population-based analysis. Osteoporos Int. 2011;22(7):2137–43. doi: 10.1007/s00198-010-1470-4. [DOI] [PubMed] [Google Scholar]
- 17.Kozyrskyj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32(11):1152–7. doi: 10.1345/aph.18117. [DOI] [PubMed] [Google Scholar]
- 18.Leslie WD, Metge C. Establishing a regional bone density program: lessons from the Manitoba experience. J Clin Densitom. 2003;6(3):275–82. doi: 10.1385/jcd:6:3:275. [DOI] [PubMed] [Google Scholar]
- 19.Leslie WD, Caetano PA, Macwilliam LR, Finlayson GS. Construction and validation of a population-based bone densitometry database. J Clin Densitom. 2005;8(1):25–30. doi: 10.1385/jcd:8:1:025. [DOI] [PubMed] [Google Scholar]
- 20.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 21.Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture Risk Prediction by Non-BMD DXA Measures: the 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J Clin Densitom. 2015;18(3):309–30. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Martineau P, Leslie WD, Johansson H, Oden A, McCloskey EV, Hans D, et al. Clinical Utility of Using Lumbar Spine Trabecular Bone Score to Adjust Fracture Probability: The Manitoba BMD Cohort. J Bone Miner Res. 2017;32(7):1568–74. doi: 10.1002/jbmr.3124. [DOI] [PubMed] [Google Scholar]
- 24.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25(11):2350–8. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 25.Morin SN, Lix LM, Leslie WD. The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J Bone Miner Res. 2014;29(7):1675–80. doi: 10.1002/jbmr.2204. [DOI] [PubMed] [Google Scholar]
- 26.Lix LM, Leslie WD, Yang S, Yan L, Walld R, Morin SN, et al. Accuracy of Offspring-Reported Parental Hip Fractures: A Novel Population-Based Parent-Offspring Record Linkage Study. Am J Epidemiol. 2017:1–8. doi: 10.1093/aje/kww197. [DOI] [PubMed] [Google Scholar]
- 27.Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. Fracture prediction and calibration of a Canadian FRAX(R) tool: a population-based report from CaMos. Osteoporos Int. 2011;22(3):829–37. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchard JF, Ludwig S, Wajda A, Dean H, Anderson K, Kendall O, et al. Incidence and prevalence of diabetes in Manitoba, 1986-1991. Diabetes Care. 1996;19(8):807–11. doi: 10.2337/diacare.19.8.807. [DOI] [PubMed] [Google Scholar]
- 29.Lix L, Yogendran M, Shaw S, Burchill C, Metge C, Bond R. Population-based data sources for chronic disease surveillance. Chronic Dis Can. 2008;29(1):31–8. [PubMed] [Google Scholar]
- 30.Majumdar SR, Leslie WD, Lix LM, Morin SN, Johansson H, Oden A, et al. Longer Duration of Diabetes Strongly Impacts Fracture Risk Assessment: The Manitoba BMD Cohort. J Clin Endocrinol Metab. 2016;101(11):4489–96. doi: 10.1210/jc.2016-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ. 2012;344:e3427. doi: 10.1136/bmj.e3427. [DOI] [PubMed] [Google Scholar]
- 32.Leslie WD, Hough S. Fracture risk assessment in diabetes. In: Lecka-Czernik B, Fowlkes JL, editors. Diabetic Bone Disease Basic and Translational Research and Clinical Applications. Cham: Cham : Springer International Publishing; 2015. pp. 45–69. [Google Scholar]
- 33.Leslie WD, Aubry-Rozier B, Lamy O, Hans D, Manitoba Bone Density P TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98(2):602–9. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 34.Dhaliwal R, Cibula D, Ghosh C, Weinstock RS, Moses AM. Bone quality assessment in type 2 diabetes mellitus. Osteoporos Int. 2014;25(7):1969–73. doi: 10.1007/s00198-014-2704-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Choi HJ, Ku EJ, Kim KM, Kim SW, Cho NH, et al. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100(2):475–82. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 36.Choi YJ, Ock SY, Chung YS. Trabecular Bone Score (TBS) and TBS-Adjusted Fracture Risk Assessment Tool are Potential Supplementary Tools for the Discrimination of Morphometric Vertebral Fractures in Postmenopausal Women With Type 2 Diabetes. J Clin Densitom. 2016;19(4):507–14. doi: 10.1016/j.jocd.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Holloway KL, De Abreu LLF, Hans D, Kotowicz MA, Sajjad MA, Hyde NK, et al. Trabecular Bone Score in Men and Women with Impaired Fasting Glucose and Diabetes. Calcif Tissue Int. 2017 doi: 10.1007/s00223-017-0330-z. [DOI] [PubMed] [Google Scholar]
- 38.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J Bone Miner Res. 2016;31(5):940–8. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 39.McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, et al. Adjusting fracture probability by trabecular bone score. Calcif Tissue Int. 2015;96(6):500–9. doi: 10.1007/s00223-015-9980-x. [DOI] [PubMed] [Google Scholar]
- 40.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229–35. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leslie WD, Lix LM, Wu X, Manitoba Bone Density P Competing mortality and fracture risk assessment. Osteoporos Int. 2013;24(2):681–8. doi: 10.1007/s00198-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 42.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160(2):122–31. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 44.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 45.Hough FS, Pierroz DD, Cooper C, Ferrari SL, Bone IC, Diabetes Working G Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174(4):R127–38. doi: 10.1530/EJE-15-0820. [DOI] [PubMed] [Google Scholar]
- 46.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.