Abstract
Purpose:
Use of magnetic resonance imaging (MRI)/transrectal ultrasound fusion biopsies to determine the accuracy of multiparametric MRI (mpMRI), using Prostate Imaging-Reporting and Data System version 2 (PI-RADSv2), for detecting clinically significant prostate cancer in the overall gland and specifically the peripheral zone (PZ) and transitional zone (TZ).
Methods:
A retrospective analysis of patients who underwent fusion biopsy identified 137 men with 231 prostate lesions was approved by the Institutional Review Board. Subjects initially classified under PI-RADSv1 criteria were regraded using PI-RADSv2 by a radiologist blinded to PI-RADSv1 score and biopsy results. Spearman correlation, chi-squared, and logistic regression analysis were performed.
Results:
There was positive correlation between PI-RADSv2 and Gleason scores (P < 0.001). In the PZ, mpMRI demonstrated 100% sensitivity, 100% negative predictive value, and 35.9% positive predictive value, compared to 100%, 100%, and 27.1%, respectively, for TZ lesions. When predicting clinically significant prostate cancer, the PI-RADSv2 area under the curve for TZ lesions was 0.844 (95% CI: 0.753-0.935, P < 0.001) and 0.769 (95% CI: 0.684-0.854, P < 0.001) for PZ lesions. Combining PI-RADSv2 with additional risk factors (body mass index, prostate-specific antigen density, digital rectal examination) improved the area under curve.
Conclusions:
PI-RADSv2 achieves excellent sensitivity and negative predictive value for both PZ and TZ lesions.
Introduction
Prostate cancer (PCa) is the second most common cancer among men worldwide.1 In the USA, Canada, and Europe, current expert opinion and guidelines recommend a 10-12 core transrectal ultrasound (TRUS) guided biopsy for men with elevated prostate-specific antigen (PSA) or abnormal digital rectal examination (DRE).2-4 However, the false-negative rate of 12-core biopsies can exceed 30%,5 and saturation prostate biopsy as an initial screening strategy does not significantly improve cancer detection.6 Multiparametric magnetic resonance imaging (mpMRI) of the prostate coupled with MRI/TRUS fusion biopsy has been proposed to improve PCa detection rates.7
MpMRI combines T2-weighted, diffusion-weighted (DWI), apparent diffusion coefficient mapping, dynamic contrast enhancement, or spectroscopy to evaluate the prostate gland. Prostate mpMRI is a useful tool for tumor detection,8,9 particularly in patients with higher-grade PCa,10 and for identifying potential active surveillance patients.11,12 Compared to TRUS alone, fusing mpMRI and TRUS data during prostate biopsy can increase cancer detection rates.13,14
Given the lack of uniform mpMRI protocols and prostate reporting, the European Society of Urogenital Radiology (ESUR) produced a set of standardized guidelines in 2012.15 These guidelines, Prostate Imaging-Reporting and Data System version 1 (PI-RADSv1), have previously been validated as a risk stratification model with high PCa detection accuracy.16-20 When used to guide biopsies, mpMRI using PI-RADSv1 can help detect a larger proportion of clinically significant PCa (csPCa) than standard TRUS-biopsy.7,12 However, mpMRI-guided biopsy alone has been shown to miss some PCa that would be detected by random TRUS-biopsy; and authors have found best results with combined guided and random TRUS-biopsy.7,21
Nonetheless, as discussed by Barentsz et al,22 PI-RADSv1 has its shortcomings. In 2014 the American College of Radiology (ACR) and the ESUR steering committee introduced a revised scoring system, PI-RADS version 2 (PI-RADSv2).23 PI-RADSv2 was developed to improve standardization and efficacy of the PI-RADS scoring system, focusing on csPCa by addressing the evaluation of PI-RADS score 3 lesions and the establishment of a global PI-RADS score.17,24,25 Significant changes from PI-RADS version 1 to version 2 involved the establishment of DWI as the primary sequence determining scores in the peripheral zone, (PZ) with dynamic contrast enhancement serving to influence PI-RADSv2 scores of 3; and T2 as the primary sequence for the transitional zone, (TZ) with DWI influencing PI-RADSv2 scores of 3. PI-RADSv2 eliminated the use of MR spectroscopy in the paradigm.26,27
Although prior studies have contributed to validating PI-RADSv2 as an accurate method to detect PCa,7,26,27 few studies, such as Cash et al,7 have used the added benefit of MRI/TRUS fusion biopsy for radiologic-pathologic correlation, or further stratified accuracy and risk into PZ or TZ lesions.19,20,28
We hypothesize that PI-RADSv2 scores have a high diagnostic accuracy for csPCa and a positive correlation with Gleason score. Thus, the purpose of our study is to use a combination biopsy method (12-core plus MRI/TRUS fusion biopsies) to determine and compare the accuracy of mpMRI, using PI-RADSv2, for detecting csPCa in the PZ and TZ.
Materials and Methods
A retrospective study protocol with strict adherence to the United States Health Insurance Portability and Accountability Act (HIPAA) policies was reviewed and approved by the Institutional Review Board. The informed consent requirement was waived by the Institutional Review Board.
In this single center retrospective study, we reviewed the records of 206 consecutive patients who underwent prostate mpMRI, owing to elevated PSA, abnormal DRE, or other concerning clinical findings at the discretion of our urologists from September 2014 through November 2015. Subject inclusion criteria for this study were: males having undergone mpMRI with subsequent 12-core Artemis 3D TRUS (Eigen, Grass Valley, CA) and MRI/TRUS fusion biopsy using Artemis and ProFuse software (Eigen, Grass Valley, CA) at our institution. Subjects were excluded from the analysis if they did not undergo both 12-core and MRI/TRUS fusion biopsy, or if complete follow-up data was unavailable. Subjects younger than 40 years old were considered outliers from the clinically encountered presentation of PCa and excluded from the analysis.
A total of 137 men (Table 1), having a mean age of 65.2 years (age range: 41–96 y) with a total of 231 identified prostate lesions on mpMRI, were included in this study.
TABLE 1.
Baseline characteristics of patient cohort
| Median age in years (IQR) | 65.0 (60–71) |
| Median BMI in kg/m2 (IQR) | 27.0 (24.6–29.7) |
| Median PSA in ng/mL (IQR) | 6.8 (4.6–9.4) |
| Median prostate volume in mm3 (IQR) | 50 (36–75) |
| Median PSA density in ng/mL*mm3 (IQR) | 0.12 (0.08–0.19) |
| % Abnormal DRE | 13.0 (17/137) |
The baseline clinical characteristics of the patient cohort of this study (n = 136). PSA density was calculated using the following formula: PSA density = PSA/prostate volume on MRI.
IQR = interquartile range.
PI-RADSv2 Evaluation
All mpMRI data were reviewed and scored under PI-RADS v2 criteria by a fellowship-trained, board-certified radiologist (R.H.), with 5 + years of experience with prostate MRI. The radiologist was given access to prebiopsy clinical data, per usual routine, but blinded to biopsy results to prevent bias. The reader was blinded to prior mpMRI reports, including any previously reported lesions.
Definition of Terms Used in Analysis
A DRE was considered abnormal if there was any palpable nodule. A positive 12-core or fusion biopsy was defined as Gleason score ≥6 lesion with in the same sector as a corresponding lesion identified on mpMRI. A positive concordant 12-core biopsy was a Gleason score ≥6 lesion that lateralized to the corresponding lesion identified on mpMRI. A positive combination biopsy was defined as any identified mpMRI lesion having a corresponding positive 12-core or fusion biopsy.
Our study defined csPCa as biopsies with a Gleason score ≥7.
A negative mpMRI study for csPCa of the prostate was defined as an overall PI-RADSv2 score 1-2, whereas a positive study was defined as a PI-RADSv2 score 3-5. Our initial experience with PI-RADSv2 score 1-2 lesions demonstrated only negative biopsies and as such we defined those lesions as low suspicion or negative for csPCa with no clinical indication for fusion biopsy. A subset of lesions (n = 39) was given a PI-RADSv2 score of 1 or 2, but were still biopsied owing to clinical factors at the discretion of the performing urologist.
Statistical Tests
Statistical analysis was performed with SPSS version 22.0 (IBM, Armonk, New York). PI-RADSv2 score was redefined into binary values for chi-squared analysis. Given that Gleason score is the major basis of our definition of csPCa, the Spearman rank-order correlation test was used to evaluate the association between Gleason score and overall PI-RADSv2 score. The higher Gleason score of the two biopsy methods (12-core or MRI/TRUS fusion biopsy) was ued for Spearman correlation. Logistic regression models with receiver operating characteristic curve and area under the curve (AUC) analysis were used to assess the relationship between csPCa and overall PI-RADSv2 score, adjusting for variables such as patient age, PSA, body mass index (BMI), PSA density, and DRE findings. Statistical significance was defined as P < 0.05.
Results
The baseline clinical characteristics of the study cohort are presented in Table 1.
mpMRI, TRUS-Guided 12-Core Biopsy, and MRI/TRUS Fusion Biopsy Results
The mean PI-RADSv2 scores of PZ, TZ, and all lesions on mpMRI were 3.4 (n = 142), 3.2 (n = 89), and 3.3 (n = 231), respectively. A total of 192/231 (83.1%) identified lesions were considered positive on mpMRI. The median number of days between the subject’s mpMRI and prostate biopsy was 35 days (interquartile range: 14.0-64.5 d).
No patient had a history of prior positive prostate biopsy. For all TRUS-guided 12-core biopsies, 82/231 (35.5%) lesions were positive, and 42/231 (18.2%) lesions were found to be csPCa. In the PZ, 59/142 (41.5%) lesions were positive on 12-core biopsy, and 31/142 (21.8%) were found to be csPCa. In the TZ, 23/89 (25.8%) lesions were positive on 12-core biopsy, and 11/89 (12.4%) were found to be csPCa.
When assessing the MRI/TRUS fusion biopsy results, a total of 77/231 (33.3%) lesions were positive, and 50/231 (21.6%) were found to be csPCa. Of the biopsied PZ lesions, 55/142 (38.7%) were positive on MRI/TRUS fusion biopsy, and 36/142 (25.3%) were found to be csPCa. Of the biopsied TZ lesions, 22/89 (24.7%) were positive on MRI/TRUS fusion biopsy, and 14/89 (15.7%) lesions were found to be csPCa.
When considering the dual TRUS-guided 12-core and MRI/TRUS fusion biopsies as a single combination biopsy method, 98/231 (42.4%) lesions were positive, and 58/231 (25.1%) were found to be csPCa. For PZ, 68/142 (47.9%) lesions were positive on combination biopsy, and 42/142 (29.6%) were found to be csPCa. Of the biopsied TZ lesions, 30/89 (33.7%) were positive on combination biopsy, and 16/89 (18.0%) lesions were found to be csPCa.
Of note, 17 patients underwent prostatectomy with 100% concordance between the prostatectomy and the combination biopsy specimens. Similar to Siddiqui et al,29 our high concordance rate suggests combination biopsy is a suitable surrogate for prostatectomy in assessing lesions identified on mpMRI.
The percentage of combination biopsies yielding csPCa, regardless of the location, increased as the overall PI-RADSv2 score increased. Of the 39 identified prostate lesions with PI-RADSv2 scores of 1-2, none yielded csPCa.
Correlation Between PI-RADSv2 Score and Gleason Score
The mean Gleason scores of TZ, PZ, and all positive prostate lesions were 6.83 (n = 68), 6.73 (n = 30), and 6.81 (n = 98), respectively. Preliminary analysis, by visual inspection of scatter-plots, determined the relationship between overall PI-RADSv2 scores (PZ only, TZ only, and all lesions) and Gleason scores to be monotonic. Spearman rank-order correlation showed a positive correlation between PI-RADSv2 score of PZ lesions and Gleason score (p = 0.501, P < 0.001), TZ lesions and Gleason score (p = 0.550, P < 0.001), and both TZ and PZ (all) lesions and Gleason score (p = 0.528, P < 0.001).
Diagnostic Accuracy
The combination biopsy method was used for determining diagnostic accuracy of mpMRI using PI-RADSv2. For csPCa, a positive mpMRI using PI-RADSv2 criteria had an negative predictive value (NPV), positive predictive value (PPV), sensitivity, and specificity of 100% (95% CI: 88.8%-100%), 30.2% (95% CI: 23.9%-37.3%), 100% (95% CI: 92.3%-100%), and 22.5% (95% CI: 16.7%-29.6%), respectively (Table 2).
TABLE 2.
NPV, PPV, sensitivity, specificity of PI-RADSv2 mpMRI for PCa
| PI-RADSv2 score | NPV (CI) | PPV (CI) | Sensitivity (CI) | Specificity (CI) | Accuracy | |
|---|---|---|---|---|---|---|
| For clinically significant PCa | ||||||
| 3–5 | 100% (88.8–100) | 30.2% (23.9–37.3) | 100% (92.3–100) | 22.5% (16.7–29.6) | 42.0% | |
| 4–5 | 90.7% (84.3–94.8) | 49.5% (38.9–60.1) | 77.6% (64.4–87.1) | 73.4% (66.1–79.7) | 74.5% | |
| 5 | 80.5% (74.2–85.6) | 61.3% (42.3–77.6) | 32.8% (21.4–46.5) | 93.1% (87.9–96.2) | 77.9% |
Breakdown of negative predictive value (NPV), positive predictive value (PPV), sensitivity, specificity of PI-RADSv2 mpMRI for any prostate cancer (PCa) and clinically significant PCa, regardless of lesion location, based on different mpMRI PI-RADSv2 score thresholds for a positive mpMRI study.
CI = 95% CI.
In the TZ, the NPV, PPV, sensitivity, and specificity for csPCa was 100% (95% CI: 85.9%-100%), 27.1% (95% CI: 16.7%-40.5%), 100% (95% CI: 75.9%-100%), and 41.1% (95% CI: 29.9%-53.2%), respectively. In the PZ, the NPV, PPV, sensitivity, and specificity for csPCa was 100% (95% CI: 62.9%-100%), 31.6% (95% CI: 23.9%-40.3%), 100% (95% CI: 89.6%-100%), and 9% (95% CI: 4.5%-16.8%), respectively. Furthermore, for each different threshold score, the accuracy of mpMRI was generally greater for TZ than PZ lesions. (Tables 3 and 4).
TABLE 3.
NPV, PPV, sensitivity, specificity of PI-RADSv2 mpMRI for PCa in the PZ
| PI-RADSv2 score | NPV (CI) | PPV (CI) | Sensitivity (CI) | Specificity (CI) | Accuracy | |
|---|---|---|---|---|---|---|
| For clinically significant PCa | ||||||
| TZ 3–5 | 100% (85.9–100) | 27.1% (16.7–40.5) | 100% (75.9–100) | 41.1% (29.9–53.2) | 51.7% | |
| TZ 4–5 | 94.8% (84.7–98.7) | 41.9% (25.1–60.7) | 81.3% (53.7–95.0) | 75.3% (63.6–84.4) | 76.4% | |
| TZ 5 | 89.2% (79.3–94.9) | 53.3% (27.4–77.7) | 50.0% (25.5–74.5) | 90.4% (80.7–95.7) | 71.9% |
Breakdown of negative predictive value (NPV), positive predictive value (PPV), sensitivity, specificity of PI-RADSv2 mpMRI for any prostate cancer (PCa) and clinically significant PCa in the PZ, based on different mpMRI PI-RADSv2 score thresholds for a positive mpMRI study.
CI = 95% CI.
TABLE 4.
NPV, PPV, sensitivity, specificity of PI-RADSv2 mpMRI for PCa in the TZ
| PI-RADSv2 score | NPV (CI) | PPV (CI) | Sensitivity (CI) | Specificity (CI) | Accuracy | |
|---|---|---|---|---|---|---|
| For clinically significant PCa | ||||||
| PZ 3–5 | 100% (62.9–100) | 31.6% (23.9–40.3) | 100% (89.6–100) | 9.0% (4.5–16.8) | 35.9% | |
| PZ 4–5 | 87.8% (78.3–93.7) | 53.3% (40.1–66.1) | 76.2% (60.2–87.4) | 72% (62.0–80.3) | 73.2% | |
| PZ 5 | 75.4% (66.8–82.4) | 68.8% (41.2–87.9) | 26.2% (14.4–42.3) | 95% (88.2–98.1) | 74.6% |
Breakdown of negative predictive value (NPV), positive predictive value (PPV), sensitivity, specificity of PI-RADSv2 mpMRI for any prostate cancer (PCa) and clinically significant PCa in the TZ, based on different mpMRI PI-RADSv2 score thresholds for a positive mpMRI study.
CI = 95% CI.
Cofactor Analysis for csPCa
Age, BMI, prostate volume, PSA density, DRE result, and overall PI-RADSv2 score were all associated (P < 0.01) with csPCa on univariate analysis; whereas PSA, scanner manufacturer (Phillips 3T or Siemens 3T), and b-value of apparent diffusion coefficient mapping were not associated with csPCa (P > 0.05). PSA and prostate volume were removed from the multivariate model because PSA density is the single score that takes both of these variables into account. Furthermore, on multivariate analysis, age, PSA density, DRE result, and overall PI-RADSv2 score were all independent predictors (P < 0.001) for csPCa. BMI was not an independent predictor on multivariate analysis, and thus, was removed from the final multivariate model. The AUC for PI-RADSv2 alone, when evaluating csPCa, was 0.797 (95% CI: 0.734-0.859, P < 0.001). Adding overall PI-RADSv2 score to the multivariate model (age, PSA density, DRE result) increased the AUC for csPCa from 0.764 to 0.886 (95% CI: 0.030–0.039, P < 0.01) (Fig 1).
FIG 1.
ROC curve for csPca multivariate model with and without PI-RADSv2 score. Area under the curve (AUC) of PI-RADSv2 alone (AUC = 0.797), the multivariate model (age, prostate-specific antigen density, digital rectal examination result) with PI-RADSv2 score (AUC = 0.886) and without PI-RADSv2 score (AUC = 0.764).(Color version of figure is available online.)
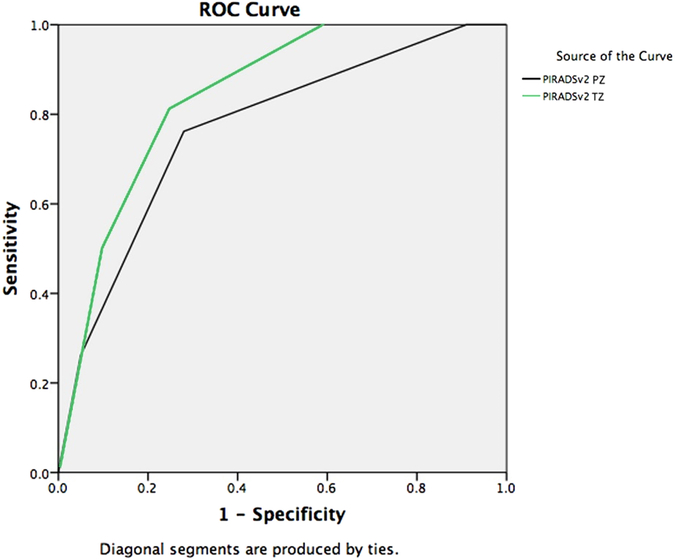
For PZ lesions, age, DRE result, PSA density, and overall PI-RADSv2 score were all associated (P < 0.05) with csPCa on univariate analysis; however, BMI, prostate volume, PSA, scanner manufacturer, and b-value were not. For TZ lesions, BMI, prostate volume, PSA density, and overall PI-RADSv2 score were associated (P < 0.05) with csPCa on univariate analysis, whereas age, DRE result, PSA, scanner manufacture, and b-value were not. The AUC for PI-RADSv2 score for PZ lesions was 0.769 (95% CI: 0.684-0.854, P < 0.001), compared to AUC = 0.844 (95% CI: 0.753-0.935, P < 0.001) for TZ lesions (Fig 2).
FIG 2.
ROC curve PI-RADSv2 score for clinically significant PZ and TZ lesions. Area under the curve (AUC) the PI-RADSv2 score for clinically significant peripheral zone and transitional zone lesions, AUC = 0.769 and 0.844 (respectively).(Color version of figure is available online.)
Discussion
This retrospective study assessed the utility of PI-RADSv2 for identification of PCa when using a TRUS-12 core and fusion combination biopsy. When assessing for csPCa, PI-RADSv2 achieved 100% sensitivity and NPV in both the TZ and PZ, suggesting a high efficacy for detection of clinically significant malignancy. Moreover, PI-RADSv2 demonstrates an AUC of 0.797 Breakdown of negative predictive value (NPV), positive predictive value (PPV), sensitivity, specificity of PI-RADSv2 mpMRI for any prostate cancer (PCa) and clinically significant PCa in the TZ, based on different mpMRI PI-RADSv2 score thresholds for a positive mpMRI study. CI = 95% CI. for global prostate lesions. This finding is consistent with current literature regarding PI-RADSv2, which have demonstrated high AUC for detection of PCa in the global gland with an AUC range of 0.71-0.9.26-28,30,31 Other authors have also addressed differences in cancer detection with the PZ vs the TZ. Auer et al28 found an AUC of 0.90 for the whole gland, 0.92 in the PZ, and 0.90 in the TZ. However, this study retrospectively evaluated imaging findings to evaluate a-priori selected lesions on prostatectomy sections. We feel that our analysis better reflects the clinical use of mpMRI. Polenec et al30 also assessed PI-RADSv2 in the PZ and TZ, finding a global AUC of 0.71–0.75, an AUC of 0.61–0.63 in the PZ, and 0.810.84 in the TZ. However, this group only performed MR-guided biopsy. They did not use the combination 12-core and MR fusion biopsy method, which has been shown to improve detection, nor did they use a 3.0 Tesla magnet as in our study.29
Differently from prior studies, we also investigated the addition of known predictive cofactors for csPCa to create a multivariate analysis of csPCa detection. We have identified cofactors, which augment the accuracy of PI-RADSv2 for csPCa identification, raising the AUC from 0.797-0.886. As the recent prospective analysis by Mertan et al25 found only moderate accuracy for PI-RADSv2, the addition of predictive cofactors may be a method to improve csPCa detection in the clinical setting.
Furthermore, as PI-RADSv2 scores increased, the PPV and the specificity increased concomitantly. These findings suggest that PI-RADSv2 may be an effective tool in evaluating mpMRI of the prostate for csPCa and may lend itself to prospective lesion screening and biopsy guidance.
This study further supports previous studies that indicate a high diagnostic accuracy of PI-RADSv2 and suggest prebiopsy mpMRI could markedly reduce the number of unnecessary prostate biopsies.26-28,30-35 Within our cohort, PI-RADSv2 evaluation detected all cases of csPCa, demonstrating a high sensitivity and NPV. The use of prebiopsy mpMRI with PI-RADSv2 in routine clinical practice may improve detection and limit unnecessary biopsies of benign lesions or indolent tumors. A decreased number of unnecessary biopsies may provide the additional benefit of reduced total complications, such as infection, bleeding, and urinary retention.34
PI-RADSv2 scores demonstrate excellent utility for PCa screening; however, when only the PI-RADSv2 score of 3 is assessed, overall specificity and PPV remain low. Furthermore, there is a large difference in specificity when assessing csPCa in PZ (9.8%) and TZ (43.5%) lesions; the AUC analysis predictably reflected this disparity, showing a 0.091 difference (0.860 for TZ and 0.769 for PZ) in AUCs. These findings may in part be owing to the significant overlap of benign and malignant features, such as the confounding appearance of prostatitis, for PZ lesions with PI-RADSv2 score of 3 as well as the improved PI-RADSv2 exclusion criteria of benign TZ lesions.
Our results indicate that as PI-RADSv2 score increases within both the TZ and PZ, the scoring classification becomes more accurate at predicting PCa. When increasing the positive/negative mpMRI threshold to PI-RADSv2 scores of 4-5, the accuracy for csPCa detection increases, yielding an accuracy of 74.5%, 73.2%, and 76.4%, for the whole gland, PZ, and TZ respectively. These improvements in accuracy come with modest decrease in sensitivity when compared to a threshold of PI-RADSv2 score 3-5. From these trends, we conclude that the PI-RADSv2 criteria for scores of 4 and 5 are most predictive of csPCa.
There were limitations to our study. First, the lack of multiple image readers limits the application of these results to a standard clinical practice; however, other studies have shown that PI-RADSv1 and PI-RADSv2 have an inherent moderate interobserver variability, among experienced prostate imagers.36–38 Second, as a tertiary academic center, many of our patients are referred for higher-level care. Our patient population may not accurately represent patient populations encountered in common practice. As such, patients with higher-grade tumors are likely over-represented in our practice. Third, this is a retrospective analysis, which limits our selection of adequate controls. Fourth, whole-cohort prostatectomy was not performed as part of the study protocol as this would not be within the clinical and ethical standard of care for all patients. Thus, biopsy result served as our diagnostic standard rather than prostatectomy. Fifth, there is no standard definition of csPCa, which makes interstudy comparison challenging. Our definition of csPCa as lesions with a Gleason score >7 was chosen to reflect a moderate inclusivity based on the current body of literature.19,23,39-42
The use of combined fusion and 12-core biopsy technique limits the evaluation of PI-RADSv2 for specific lesions. We feel this more accurately reflects the real clinical practice and the technically feasible methodology. Small regions of interest identified on mpMRI may be too-small to definitively biopsy by current methods and, as proposed by Siddiqui et al,29 likely may intersect with corresponding sector biopsy on 12-core technique; necessitating combined method and explaining its increased sensitivity for PCa. Lastly, only data obtained by 3 T magnet without endorectal coil was assessed. The reproducibility of our findings may vary from 1.5 T or 3 T with endorectal coil protocol. A future comparative study assessing PI-RADSv2 scoring using these 3 protocols is warranted.
In conclusion, using a combination of TRUS 12-core and MRI/ TRUS fusion biopsies, we have found PI-RADSv2 to be a highly sensitive and accurate PCa detection examination. By incorporating additional cofactors predictive for csPCa into a multivariate model with PI-RADSv2, accuracy may be further improved. These characteristics of the PI-RADSv2 paradigm suggest that it may have significant utility as a prospective screening tool, to not only determine if prostate lesions require biopsy, but also to guide subsequent fusion biopsies. Additional prospective research is needed to evaluate PI-RADSv2 in this role and to strengthen overall specificity through improvement of prostate lesion characterization on mpMRI. Future studies are needed to focus on further development of a multivariable predictive model for csPCa.
Acknowledgments
Research efforts of M. Nguyentat were supported by a Radiological Society of North America Research Medical Student Grant.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2013;65:124–37. [DOI] [PubMed] [Google Scholar]
- 3.Bjurlin MA, Carter HB, Schellhammer P, et al. Optimization of initial prostate biopsy in clinical practice: Sampling, labeling and specimen processing. J Urol 2013;189:2039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Hakim A, Moussa S. CUA guidelines on prostate biopsy methodology. Can Urol Assoc J 2010;4:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serefoglu EC, Altinova S, Ugras NS, et al. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J 2013;7:E293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JS, Patel A, Schoenfield L, et al. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006;175:485–8. [DOI] [PubMed] [Google Scholar]
- 7.Cash H, Maxeiner A, Stephan C, et al. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J Urol 2016;34:525–32. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection–Histopathologic correlation. Radiology 2010;255:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Futterer JJ, Briganti A, De Visschere P, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015;68:1045–53. [DOI] [PubMed] [Google Scholar]
- 10.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190:1721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turkbey B, Mani H, Aras O, et al. Prostate cancer: Can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology 2013;268:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urol Oncol 2015;33 17 e1–e7. [DOI] [PubMed] [Google Scholar]
- 13.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer 2016;122:884–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Rooij M, Hamoen EH, Futterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2013;202: 343–51. [DOI] [PubMed] [Google Scholar]
- 17.Hamoen EH, de Rooij M, Witjes JA, et al. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: A diagnostic meta-analysis. Eur Urol 2015;67:1112–21. [DOI] [PubMed] [Google Scholar]
- 18.Portalez D, Mozer P, Cornud F, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multi-parametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol 2012;62:986–96. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JE, Moses D, Shnier R, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: A prospective study. J Urol 2013;192:67–74. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JE, van Leeuwen PJ, Moses D, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol 2016;195:1428–35. [DOI] [PubMed] [Google Scholar]
- 21.Cash H, Gunzel K, Maxeiner A, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: Reasons for targeted biopsy failure. BJU Int 2016;118:35–43. [DOI] [PubMed] [Google Scholar]
- 22.Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 2016;69:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging–Reporting and Data System: 2015, version 2. Eur Urol 2016;69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junker D, Quentin M, Nagele U, et al. Evaluation of the PI-RADS scoring system for mpMRI of the prostate: A whole-mount step-section analysis. World J Urol 2015;33:1023–30. [DOI] [PubMed] [Google Scholar]
- 25.Mertan FV, Greer MD, Shih JH, et al. Prospective evaluation of the Prostate Imaging Reporting and Data System Version 2 for prostate cancer detection. J Urol 2016;196:690–6. [DOI] [PubMed] [Google Scholar]
- 26.Baldisserotto M, Neto EJ, Carvalhal G, et al. Validation of PI-RADS v.2 for prostate cancer diagnosis with MRI at 3T using an external phased-array coil. J Magn Reson Imaging 2016;44(5):1354–9. [DOI] [PubMed] [Google Scholar]
- 27.Kasel-Seibert M, Lehmann T, Aschenbach R, et al. Assessment of PI-RADS v2 for the detection of prostate cancer. Eur J Radiol 2016;85:726–31. [DOI] [PubMed] [Google Scholar]
- 28.Auer T, Edlinger M, Bektic J, et al. Performance of PI-RADS version 1 versus version 2 regarding the relation with histopathological results. World J Urol 2017;35(5):687–93. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. J Am Med Assoc 2015;313:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polanec S, Helbich TH, Bickel H, et al. Head-to-head comparison of PI-RADS v2 and PI-RADS v1. Eur J Radiol 2016;85:1125–31. [DOI] [PubMed] [Google Scholar]
- 31.Park SY, Jung DC, Oh YT, et al. Prostate cancer: PI-RADS version 2 helps preoperatively predict clinically significant cancers. Radiology 2016;280: 108–16. [DOI] [PubMed] [Google Scholar]
- 32.Lotan Y, Haddad AQ, Costa DN, et al. Decision analysis model comparing cost of multiparametric magnetic resonance imaging vs. repeat biopsy for detection of prostate cancer in men with prior negative findings on biopsy. Urol Oncol 2015;33 266 e9–16. [DOI] [PubMed] [Google Scholar]
- 33.Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound fusion-targeted prostate biopsy in men with previous negative biopsies: Impact on repeat biopsy strategies. Urology 2015;86:1192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez CM, Averch T, Boyd L. AUA/SUNA white paper on the incidence, prevention and treatment of complications related to prostate needle biopsy. Am Urol Assoc 2012. [Google Scholar]
- 35.Hutchinson RC, Costa DN, Lotan Y. The economic effect of using magnetic resonance imaging and magnetic resonance ultrasound fusion biopsy for prostate cancer diagnosis. Urol Oncol 2016;34:296–302. [DOI] [PubMed] [Google Scholar]
- 36.Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: Interobserver agreement and accuracy with the revised Prostate Imaging Reporting and Data System at multiparametric MR imaging. Radiology 2015;277:741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schimmoller L, Quentin M, Arsov C, et al. Inter-reader agreement of the ESUR score for prostate MRI using in-bore MRI-guided biopsies as the reference standard. Eur Radiol 2013;23:3185–90. [DOI] [PubMed] [Google Scholar]
- 38.Rosenkrantz AB, Margolis DJ. Commentary regarding the inter-reader reproducibility of PI-RADS version 2. Abdom Radiol (NY) 2016;41:907–9. [DOI] [PubMed] [Google Scholar]
- 39.Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011;185:121–5. [DOI] [PubMed] [Google Scholar]
- 40.Bangma CH, Bul M, van der Kwast TH, et al. Active surveillance for low-risk prostate cancer. Crit Rev Oncol Hematol 2013;85:295–302. [DOI] [PubMed] [Google Scholar]
- 41.Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. J Am Med Assoc 1994;271:368–74. [PubMed] [Google Scholar]
- 42.Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: A systematic review. Eur Urol 2015;68:8–19. [DOI] [PubMed] [Google Scholar]